Submitted:
12 February 2025
Posted:
13 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. CMR Acquisition
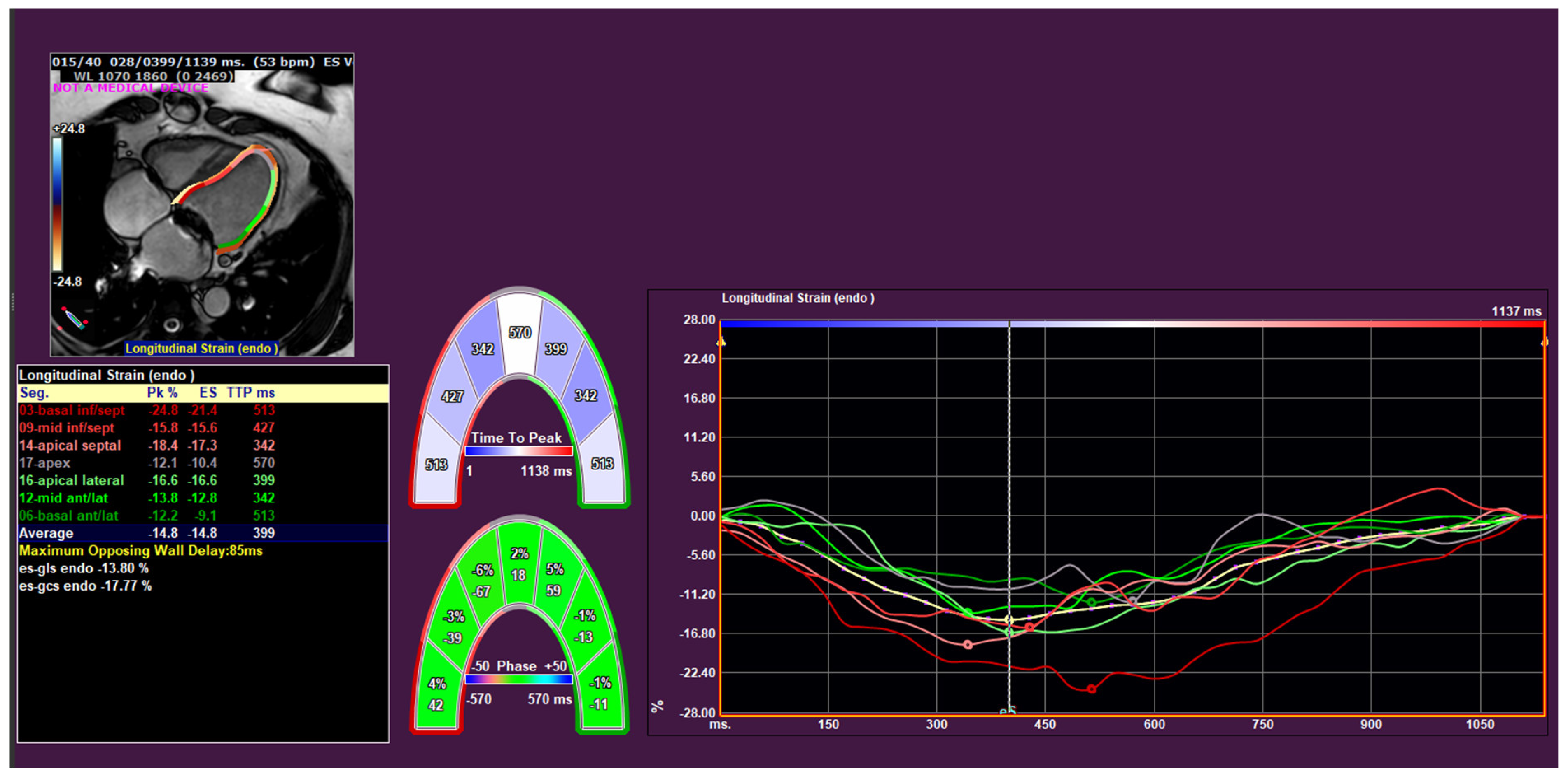
2.3. CMR Analysis
2.4. Echocardiographic Analysis and Definition of CRT Response
2.5. Statistical Analysis
3. Results
3.1. Basic CMR Parameters and CRT Response
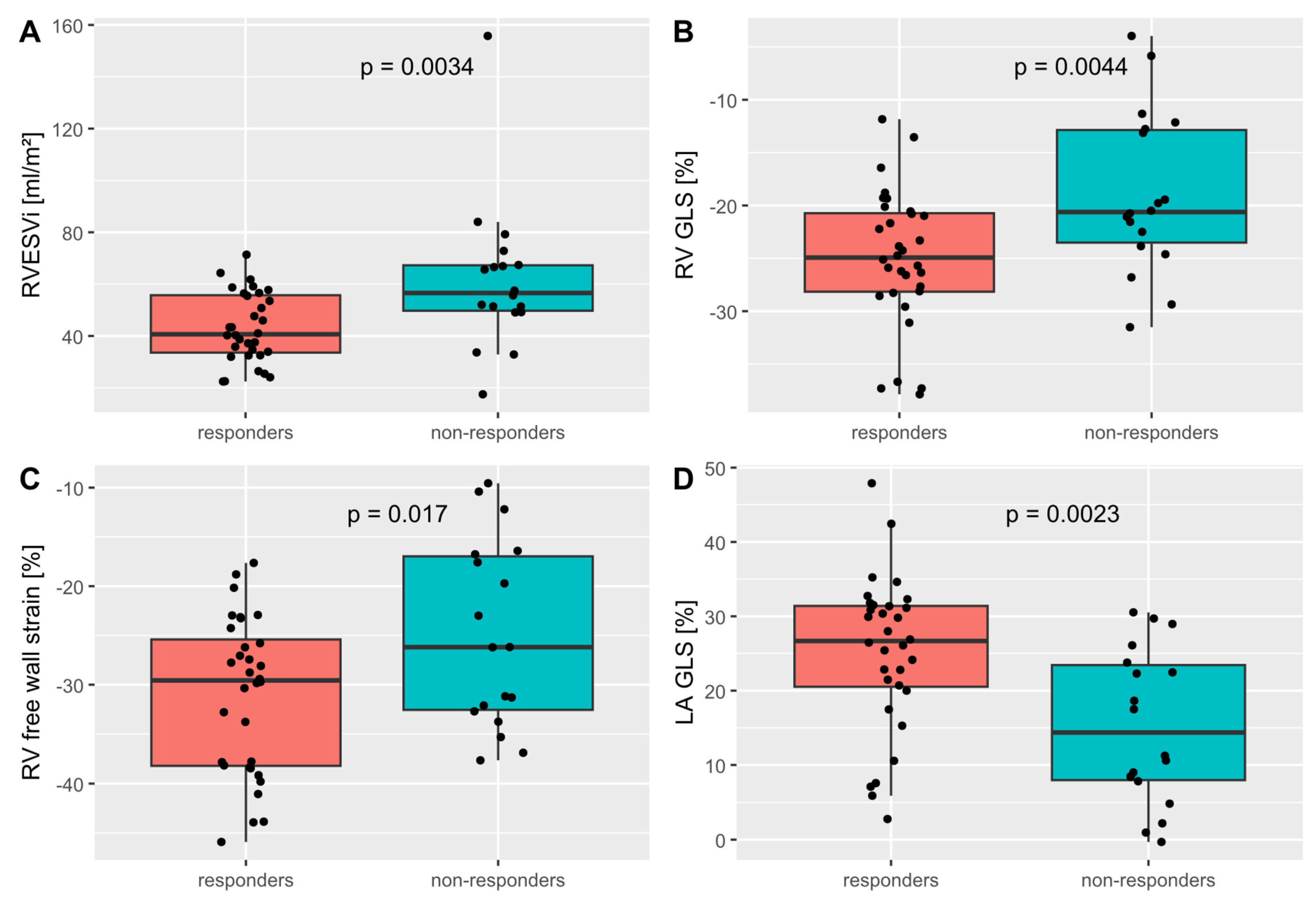
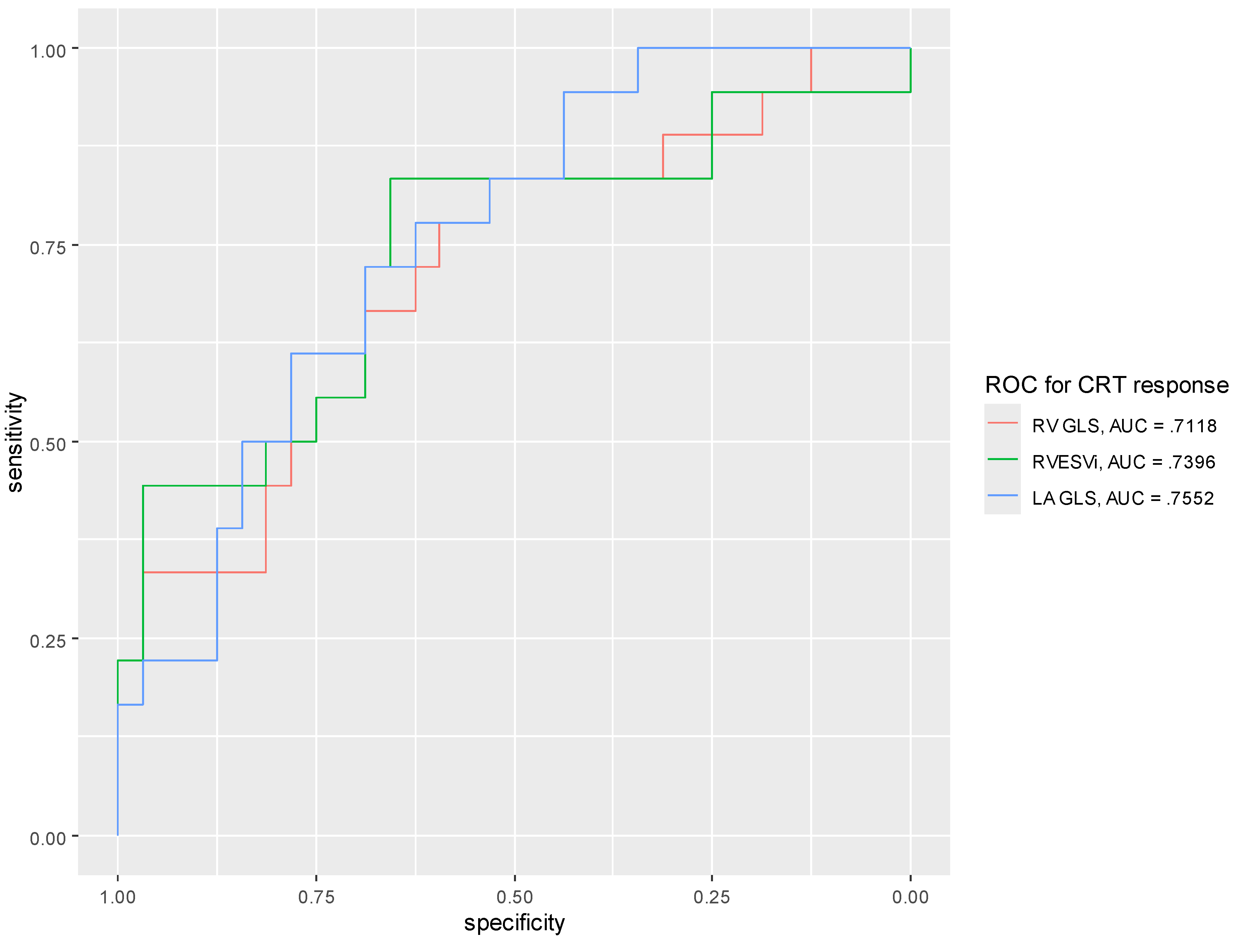
3.2. Strain Measurements and CRT Response
4. Discussion
- (1)
- RV volumes and strain measurements correlated with CRT response
- (2)
- LA area and strain measurements correlated with CRT response
- (3)
- Left ventricular strain parameters and the presence and location of scar did not correlate with CRT response.
4.1. LV Strain and CRT Response
4.2. The RV and CRT Response
4.3. The LA and CRT Response
4.4. Scar and CRT Response
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMR | Cardiac magnetic resonance |
| CRT | Cardiac resynchronization therapy |
| CS | Circumferential strain |
| ECV | Extracellular volume |
| FT | Feature tracking |
| GCS | Global circumferential strain |
| GLS | Global longitudinal strain |
| LA | Left atrium |
| LBBB | Left bundle branch block |
| LGE | Late gadolinium enhancement |
| LS | Longitudinal strain |
| LV | Left ventricule |
| LVEDd | Left ventricular end-diastolic diameter |
| LVEDV | Left ventricular end-diastolic volume |
| LVEDVi | Left ventricular end-diastolic volume index |
| LVEF | Left ventricular ejection fraction |
| LVESV | Left ventricular end-systolic volume |
| NYHA | New York Heart Association |
| RA | Right atrium |
| ROC | Receinver operator characteristic |
| RV | Right ventricle |
| RVEDVi | Right ventricular end-diastolic volume index |
| RVEF | Right ventricular ejection fraction |
| RVESVi | Right ventricular end-systolic volume index |
| SD | Standard deviation |
| T | Tesla |
| TAPSE | Tricuspid annular plane systolic excursion |
References
- Chung, M.K.; Patton, K.K.; Lau, C.P.; Dal Forno ARJ, Al-Khatib, S. M.; Arora, V.; et al. 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure. Heart Rhythm. 2023, 20, e17–e91. [Google Scholar] [CrossRef] [PubMed]
- Ypenburg, C.; van Bommel, R.J.; Borleffs, C.J.; Bleeker, G.B.; Boersma, E.; Schalij, M.J.; Bax, JJ. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol. 2009, 53, 483–90. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Leon, A.R.; Tavazzi, L.; Sun, J.P.; Nihoyannopoulos, P.; Merlino, J.; et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008, 117, 2608–16. [Google Scholar] [CrossRef] [PubMed]
- Bordachar, P.; Lafitte, S.; Réant, P.; Reuter, S.; Clementy, J.; Mletzko, R.U.; et al. Low value of simple echocardiographic indices of ventricular dyssynchrony in predicting the response to cardiac resynchronization therapy. Eur J Heart Fail. 2010, 12, 588–92. [Google Scholar] [CrossRef] [PubMed]
- Gorcsan, J.; Anderson, C.P.; Tayal, B.; Sugahara, M.; Walmsley, J.; Starling, R.C.; Lumens, J. Systolic Stretch Characterizes the Electromechanical Substrate Responsive to Cardiac Resynchronization Therapy. JACC: Cardiovascular Imaging. 2019, 12, 1741–52. [Google Scholar] [CrossRef] [PubMed]
- Zweerink, A.; Nijveldt, R.; Braams, N.J.; Maass, A.H.; Vernooy, K.; de Lange, F.J.; et al. Segment length in cine (SLICE) strain analysis: a practical approach to estimate potential benefit from cardiac resynchronization therapy. J Cardiovasc Magn Reson. 2021, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Zweerink, A.; van Everdingen, W.M.; Nijveldt, R.; Salden OAE, Meine, M. ; Maass, A.H.; et al. Strain imaging to predict response to cardiac resynchronization therapy: a systematic comparison of strain parameters using multiple imaging techniques. ESC Heart Fail. 2018, 5, 1130–40. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bax, J.J.; Vallakati, A.; Goel, S.; Lavie, C.J.; Kassotis, J.; et al. Meta-Analysis of the Relation of Baseline Right Ventricular Function to Response to Cardiac Resynchronization Therapy. Am J Cardiol. 2016, 117, 1315–21. [Google Scholar] [CrossRef] [PubMed]
- Manca, P.; Cossa, S.; Matta, G.; Scalone, A.; Tola, G.; Schintu, B.; et al. Right ventricular function assessed by cardiac magnetic resonance predicts the response to resynchronization therapy. J Cardiovasc Med (Hagerstown). 2020, 21, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Alpendurada, F.; Guha, K.; Sharma, R.; Ismail, T.F.; Clifford, A.; Banya, W.; et al. Right ventricular dysfunction is a predictor of non-response and clinical outcome following cardiac resynchronization therapy. J Cardiovasc Magn Reson. 2011, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Zegard, A.; Okafor, O.; Moody, W.; Marshall, H.; Qiu, T.; Stegemann, B.; et al. Right ventricular function and long-term clinical outcomes after cardiac resynchronization therapy: A cardiovascular magnetic resonance study. Pacing Clin Electrophysiol. 2022, 45, 1075–84. [Google Scholar] [CrossRef] [PubMed]
- Feneon, D.; Behaghel, A.; Bernard, A.; Fournet, M.; Mabo, P.; Daubert, J.C.; et al. Left atrial function, a new predictor of response to cardiac resynchronization therapy? Heart Rhythm. 2015, 12, 1800–6. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Oger, E.; Aalen, J.M.; Duchenne, J.; Larsen, C.K.; Sade, E.; et al. Left atrial strain is a predictor of left ventricular systolic and diastolic reverse remodelling in CRT candidates. Eur Heart J Cardiovasc Imaging. 2022, 23, 1373–82. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, Z.; Song, Y.; Wang, J.; Yang, S.; Yu, S.; et al. CMR feature tracking-based left atrial mechanics predicts response to cardiac resynchronization therapy and adverse outcomes. Heart Rhythm. 2024, 21, 1354–62. [Google Scholar] [CrossRef] [PubMed]
- Aalen, J.M.; Donal, E.; Larsen, C.K.; Duchenne, J.; Lederlin, M.; Cvijic, M.; et al. Imaging predictors of response to cardiac resynchronization therapy: left ventricular work asymmetry by echocardiography and septal viability by cardiac magnetic resonance. Eur Heart J. 2020, 41, 3813–23. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.K.; Smiseth, O.A.; Duchenne, J.; Galli, E.; Aalen, J.M.; Lederlin, M.; et al. Cardiac Magnetic Resonance Identifies Responders to Cardiac Resynchronization Therapy with an Assessment of Septal Scar and Left Ventricular Dyssynchrony. J Clin Med. 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.J.; Umar, F.; Panting, J.R.; Stegemann, B.; Leyva, F. Left ventricular lead position, mechanical activation, and myocardial scar in relation to left ventricular reverse remodeling and clinical outcomes after cardiac resynchronization therapy: A feature-tracking and contrast-enhanced cardiovascular magnetic resonance study. Heart Rhythm. 2016, 13, 481–9. [Google Scholar] [PubMed]
- Militaru, S.; Panovsky, R.; Hanet, V.; Amzulescu, M.S.; Langet, H.; Pisciotti, M.M.; et al. Multivendor comparison of global and regional 2D cardiovascular magnetic resonance feature tracking strains vs tissue tagging at 3T. Journal of Cardiovascular Magnetic Resonance 2021, 23, 54. [Google Scholar] [CrossRef] [PubMed]
- Doeblin, P.; Shing, C.; Li, J.; Werhahn, S.; Beyer, R.; Estepa, M.; et al. Right ventricular and left atrial strain predict Volumetric response to cardiac resynchronization therapy. Journal of Cardiovascular Magnetic Resonance 2025, 27, 101241. [Google Scholar] [CrossRef]
| Parameter | Sample size (n) | All subjects | Responders (n=32) | Non-responders (n=18) | P-value |
| Age (years) | 50 | 68.1 ±10.5 | 67.7 ±11.4 | 68.9 ±9.0 | .71 |
| Male gender (n) | 50 | 33 (66%) | 19/32 (59%) | 14/18 (78%) | .23 |
| BMI (kg/m2) | 50 | 27.0 ±4.3 | 26.4 ±3.9 | 28.0 ±4.9 | .19 |
| Hypertension (n) | 50 | 29 (58%) | 19/32 (59%) | 10/18 (56%) | >.99 |
| Diabetes mellitus (n) | 50 | 14 (28%) | 8/32 (25%) | 6/18 (33%) | .53 |
| Chronic kidney disease (n) | 50 | 23 (46%) | 12/32 (38%) | 11/18 (61%) | .14 |
| Atrial fibrillation (n) | 50 | 26 (52%) | 15/32 (47%) | 11/18 (61%) | .39 |
| Valve surgery (n) | 50 | 11 (22%) | 5/32 (16%) | 6/18 (33%) | .17 |
| NYHA II (n) | 48 | 30 (62.5%) | 22/31 (71%) | 8/17 (47%) | .13 |
| NYHA III (n) | 48 | 18 (36%) | 9/31 (29%) | 9/17 (53%) | |
| LBBB (n) | 50 | 41 (82%) | 26/32 (81%) | 15/18 (83%) | >.99 |
| QRS duration (ms) | 50 | 150.3 ±27.6 | 150.3 ±27.5 | 150.3 ±28.6 | >.99 |
| Biventricular pacing (%) | 44 | 97.1 ±3.8 | 97.5 ±3.4 | 96.5 ±4.6 | .72† |
| Baseline echo LVEDV (ml) | 50 | 202.6 ±66.9 | 200 ±65.9 | 207.3 ±70.3 | .71 |
| Baseline echo LVESV (ml) | 50 | 142.4 ±59.0 | 140.7 ±57.9 | 145.4 ±62.5 | .79 |
| Baseline echo LVEF (%) | 50 | 31.0 ±11.4 | 30.5 ±12.2 | 31.9 ±10.1 | .69 |
| LVEDV change (%) | 50 | -16.9 ±26.1 | -31.6 ±17.8 | 9.3 ±15.5 | <.001 |
| LVESV change (%) | 50 | -19.1 ±35.8 | -40.5 ±17.5 | 19.0 ±27.1 | <.001 |
| Parameter | Sample size (n) | All subjects | Responders (n=32) | Non-responders (n=18) | P-value |
| LVEDd (mm) | 50 | 64.3 ±8.7 | 63.1 ±9 | 66.4 ±7.8 | .18 |
| Septal thickness (mm) | 50 | 12.3 ±3.1 | 12.7 ±2.9 | 11.6 ±3.3 | .11 |
| Posterior thickness (mm) | 50 | 7.7 ±1.6 | 7.8 ±1.5 | 7.6 ±1.7 | .19 |
| LVEDVi (ml/m2) | 50 | 122.5 ±37.7 | 115.7 ±37.1 | 134.7 ±36.5 | .06 |
| LVEF (%) | 50 | 32.9 ±9.6 | 33.6 ±9.5 | 31.7 ±9.9 | .69 |
| RVEDVi (ml/m2) | 50 | 81.8 ±25.6 | 74.5 ±19.5 | 94.8 ±30.2 | .006 |
| RVESVi (ml/m2) | 50 | 49.8 ±21.9 | 43.2 ±13.3 | 61.6 ±28.8 | .003 |
| RVEF (%) | 50 | 40.0 ±11.8 | 41.8 ±11.1 | 36.9 ±12.5 | .17 |
| LA area (cm²) | 50 | 26.8 ±8.3 | 24.8 ±6.8 | 30.4 ±9.5 | .020 |
| RA area (cm²) | 50 | 23.9 ±8.0 | 22.5 ±5.9 | 26.4 ±10.6 | .10 |
| Septal flash (n) | 49 | 35 (71.4%) | 23/31 (74%) | 12/18 (67%) | .74 |
| Apical rocking (n) | 49 | 31 (63.3%) | 18/31 (58%) | 13/18 (72%) | .37 |
| Parameter | All subjects (n=49) | Responders (n=32) | Non-responders (n=17) | P-value |
|---|---|---|---|---|
| Any scar (n) | 27 (55%) | 17 (53%) | 10 (59%) | .77 |
| Ischemic scar (n) | 15 (31%) | 8 (25%) | 7 (41%) | .33 |
| Septal scar (n) | 13 (27%) | 9 (28%) | 4 (24%) | >.99 |
| Lateral scar (n) | 10 (20%) | 5 (16%) | 5 (29%) | .29 |
| Parameter | All subjects (n=50) | Responders (n=32) | Non-responders (n=18) | P-value |
|---|---|---|---|---|
| LV GCS (%) | -14.5 ±6.6 | -14.9 ±6.0 | -13.8 ±6.0 | .56 |
| Peak septal CS (%) | -14.9 ±8.9 | -16.0 ±8.8 | -12.9 ±9.0 | .23 |
| Peak lateral CS (%) | -22.3 ±10.5 | -23.9 ±11.5 | -19.5 ±7.9 | .16 |
| End-systolic septal CS (%) | -10.3 ±10.2 | -11.3 ±9.6 | -8.5 ±11.3 | .37 |
| End-systolic lateral CS (%) | -19.3 ±10.1 | -20.0 ±11.3 | -17.9 ±7.5 | .48 |
| Parameter | All subjects (n=50) | Responders (n=32) | Non-responders (n=18) | P-value |
|---|---|---|---|---|
| LV GLS (%) | -10.4 ±3.9 | -11.1 ±3.8 | -9.2 ±4.0 | .10 |
| Peak septal LS (%) | -10.7 ±5.1 | -10.9 ±4.6 | -10.3 ±6.2 | .67 |
| Peak lateral LS (%) | -24.8 ±9.4 | -26.1 ±10.1 | -22.4 ±7.5 | .18 |
| End-systolic septal LS (%) | -6.2 ±7.7 | -6.4 ±7.8 | -5.9 ±7.7 | 82 |
| End-systolic lateral LS (%) | -21.2 ±11.4 | -22.2 ±13.2 | -19.3 ±7.0 | .38 |
| RV GLS (%) | -22.8 ±7.4 | -25.0 ±6.5 | -18.9 ±7.6 | .004 |
| RV free wall GLS (%) | -28.9 ±8.9 | -31.1 ±7.9 | -24.9 ±9.5 | .017 |
| LA GLS (%) | 21.6 ±11.3 | 25.1 ±10.4 | 15.6 ±10.4 | .002 |
| LA GCS (%) | 24.0 ±15.0 | 27.9 ±14.7 | 17.1 ±13.1 | .012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





