1. Introduction
Cancer is a malignant disease characterized by uncontrolled proliferation of cells in the body, causing damage to the tissue of origin and potentially spreading to surrounding and/or distant tissues [
1]. Cancer develops due to environmental and genetic factors. Environmental causes include exposure to radiation and chemicals, viruses, poor nutrition, and smoking, while genetic causes encompass hereditary changes, other genetic factors, hormonal elements, and immune disorders [
2]. Cancer is one of the most significant health issues affecting society worldwide. According to World Health Organization data, the global cancer rate increased to 19.3 million new cases and 10.0 million fatalities in 2020. One in every five individuals worldwide will develop cancer during their lifetime [
3]. Cancer affects not only adults but also children. Cancer types occurring in the 0-19 age range are referred to as childhood cancers. Childhood cancers constitute 2-4% of all cancers. Annually, at least 300,000 children aged 0-19 are diagnosed with cancer worldwide. In Turkey, this figure is approximately 2500-3000 [
4]. Disciplined efforts in cancer treatment have led to significant developments, with major advancements achieved in the last 30 years. Survival rates for childhood cancers have now reached 70-80% [
5]. There are four main types of treatment commonly used in childhood cancers: chemotherapy, radiotherapy, stem cell transplantation, and surgical treatment [
6]. Treatments may be applied individually or in combination, as determined by the physician. After diagnosis and treatment plan determination, a traumatic process and extended treatment period begin for both the family and the child [
7]. While cancer is a medical and physical illness, it also encompasses psychological and psychosocial aspects. Children undergoing cancer treatment may exhibit physical symptoms such as vomiting, weight loss, fatigue, and hair and eyebrow loss. In addition to physical symptoms, they also experience psychological symptoms [8, 9]. The manifestation of the disease can vary depending on the age at diagnosis. In young children, the predominant features are concerns about pain and fear of separation from parents. School-age children begin to experience feelings of loneliness. Adolescents may experience fear of death and stress related to physical changes [10-11]. Kübler-Ross et al [
12] described this process in five stages based on interviews with two hundred patients: denial, anger, bargaining, depression, and acceptance. The stages in the Kübler-Ross model do not always occur sequentially; some stages may recur or not be experienced at all. Every patient and their family members invariably experience at least two stages [
12].
Depression and anxiety are frequently observed in patients diagnosed with cancer. Some symptoms of depression include intense feelings of helplessness and hopelessness. There is generally a pessimistic outlook on life, with the belief that nothing in life can be good and the situation will never improve. Loss of interest in daily activities emerges [
13]. Anxiety, also known as anxiety disorder, is characterized by the emergence of anxiety without the presence of danger, its prolonged duration, and its intense experience. These feelings are similar to fear, stress, and worry. The individual may constantly think about the future and feel anxious and concerned about various issues. A person with anxiety disorder may not want to do anything in their daily life because they cannot enjoy activities due to their thoughts. They may have difficulty communicating with family and individuals in their environment, leading to disruption of social life [
14]. An individual who cannot communicate may eventually become withdrawn, and the degree of anxiety disorder may increase. Patients diagnosed with cancer should be encouraged to freely express their anger reactions experienced during these stages and to discuss their thoughts about the illness; they should be assisted in improving their quality of life by facilitating psychological and social adaptation and strengthening the relationship between the patient, family, and social interaction areas [
15]. It is believed that distancing from play and school environments, impact on school performance, loss of self-confidence, future anxiety, pessimism, hopelessness, observing other patients undergoing similar treatment and anxious family members in the hospital environment while peers and friends continue their social lives, lack of communication or socialization areas other than nurses, doctors, and ward staff, spending days constantly in bed, and complete isolation from the external world can lead to the loss or reduction of some psychological, social, and physical characteristics of the patient while making medical progress, and trigger psychological disorders such as anxiety and depression. The impact of psychological distress on individuals has been a field of study for years. In many studies in this area, researchers have stated that exercise is beneficial for mental disorders. It has been expressed that sports can be beneficial for certain disorders [
16].
Regular exercise in cancer patients improves physical function, aerobic capacity, strength, and flexibility; helps maintain and improve body composition (preserves or increases muscle mass); provides psychological well-being; reduces stress, depression, and anxiety; increases bone mineral density, improves symptoms such as nausea, pain, sleep difficulties, and diarrhea; reduces hospital stay duration and strengthens the immune system. Due to the increase in physical capacity and the reduction in symptoms related to side effects, the level of fatigue experienced by the patient decreases, thus leading to an increase in the patient's quality of life [
17,
18]. In light of all this information, we can observe that physical activity/exercise has positive effects on patients undergoing cancer treatment.
2. Materials and Methods
Research Model
In the study, experimental model was performed. The experiments with the highest scientific value are those conducted with true experimental models. The common characteristics of true experimental models are the use of multiple groups and the formation of these groups through random assignment (sampling). Thus, each study includes at least one experimental group and one control group. These are considered "equalized" in terms of other control variables.
Three of the true experimental models are:
Pretest-posttest control group model,
Posttest control group model, and
Solomon four-group model.
Pretest-Posttest Control Group Model: In the pretest-posttest control group model, there are two groups formed by random assignment. One of these is used as the experimental group and the other as the control group. Measurements are taken in both groups before and after the experiment. The synbolic view of the model was presented at
Table 1.
The presence of pretests in the model helps to determine the degree of similarity between the groups before the experiment and to adjust the posttest results accordingly. In this model, to determine the extent of "X"'s effectiveness, both pretest and posttest measurement results are used. For this purpose:
Percentage increases in pretest-posttest scores for each group are found and average increases are compared, or
Covariance analysis is performed using pretest scores as the covariate with posttest scores, or
Pretest scores (O1.1, O2.1) are compared, and if there is no significant difference, only posttest scores (O1.2, O2.2) are used to test the differences between means [
19].
Sample Groups
In this study, the universe of the study consisted of cancer patients who were diagnosed with childhood cancers between the ages of 9-17 who were undergoing treatment at Erciyes University Mustafa Eraslan and Fevzi Mercan Children's Hospital, Hematology-Oncology unit. These patients had not previously received a psychiatric diagnosis, had not used antidepressants or their derivatives, had been diagnosed with a psychological disorder following their cancer diagnosis or were receiving support from the hospital's psychological counseling service, had been undergoing cancer treatment and were receiving outpatient treatment. For creating a sample group, children with cancer were informed about the content of the study. 40 children with cancer agreed to participate in the study voluntarily. The volunteers were divided into two groups, control and experimental, each consisting of 20 people. The study was began with 20 patients in both groups. While experimental group was completed with 14 patients due to various factors such as voluntary withdrawal, disease progression, and mortality, some patients in the control group chose to withdraw from the study, declining to participate in the final assessment and concluded with 8 participants. The sociodemographic and clinical characteristics of the control and experimental group are shown in
Table 2
An examination of
Table 2 reveals the demographic and clinical characteristics of the control and experimental groups participating in the study. In the control group, the gender distribution was 62.5% male and 37.5% female. The age distribution showed that 50% were aged 12-13, 12.5% were 14-15, and 37.5% were 16 and above. Educational status was evenly split between middle school and high school students at 50% each. Regarding the number of siblings, 37.5% had one sibling, 25.0% had two siblings, and 37.5% had three or more siblings. Disease diagnosis in this group was distributed as follows: 25% leukemia, 37.5% lymphoma, and 37.5% other conditions. The experimental group exhibited some differences in demographic and clinical characteristics. Gender distribution was equal, with 50% male and 50% female participants. Age distribution showed 35.7% aged 12-13, 42.9% aged 14-15, and 21.4% aged 16 and above. In terms of educational status, 42.9% were middle school students, while 57.1% were high school students. The sibling distribution indicated that 28.6% had one sibling, 35.7% had two siblings, and 35.7% had three or more siblings. Disease diagnosis in the experimental group was as follows: 50% leukemia, 28.6% lymphoma, and 21.4% other conditions.
Preparetion of Exercise Program
When creating the content of the prepared exercise program, literature reviewed. Exercise plans previously implemented for cancer patients were referenced. Aerobic exercise (light-moderate intensity), anaerobic exercise (very light, light), walking, and strength exercises targeting large muscle groups were identified as the main components of the program. Step aerobics, rhythm and dance (light, moderate intensity), educational games, and stretching/relaxation movements, which were thought to be supportive and motivating for pediatric patients, were also included. The duration, frequency, intensity and type of exercises were adjusted under the guidance of the specialist physician participating in the study, by taking into account the medical health conditions of the patients, and by taking into account the opinions of the faculty members of the Faculty of Sports Sciences. Before starting the exercise program, The family was informed about the content, process and possible situations of the study, and a parental consent form was obtained. The exercise program is presented in the
Table 3.
As it seen in table 3, the experimental group patients undergoing cancer treatment participated in 24 exercise sessions over 8 weeks, with sessions lasting 50 minutes each, three days a week.
Procedure:
As the first step of the study, Control and experimental groups were performed the patient voluntary consent form, socio demographic information form, Kovacs depression, Beck anxiety and Child form of the pediatric cancer quality of life scales were administered in the presence of the psychologist of the relevant hospital unit.
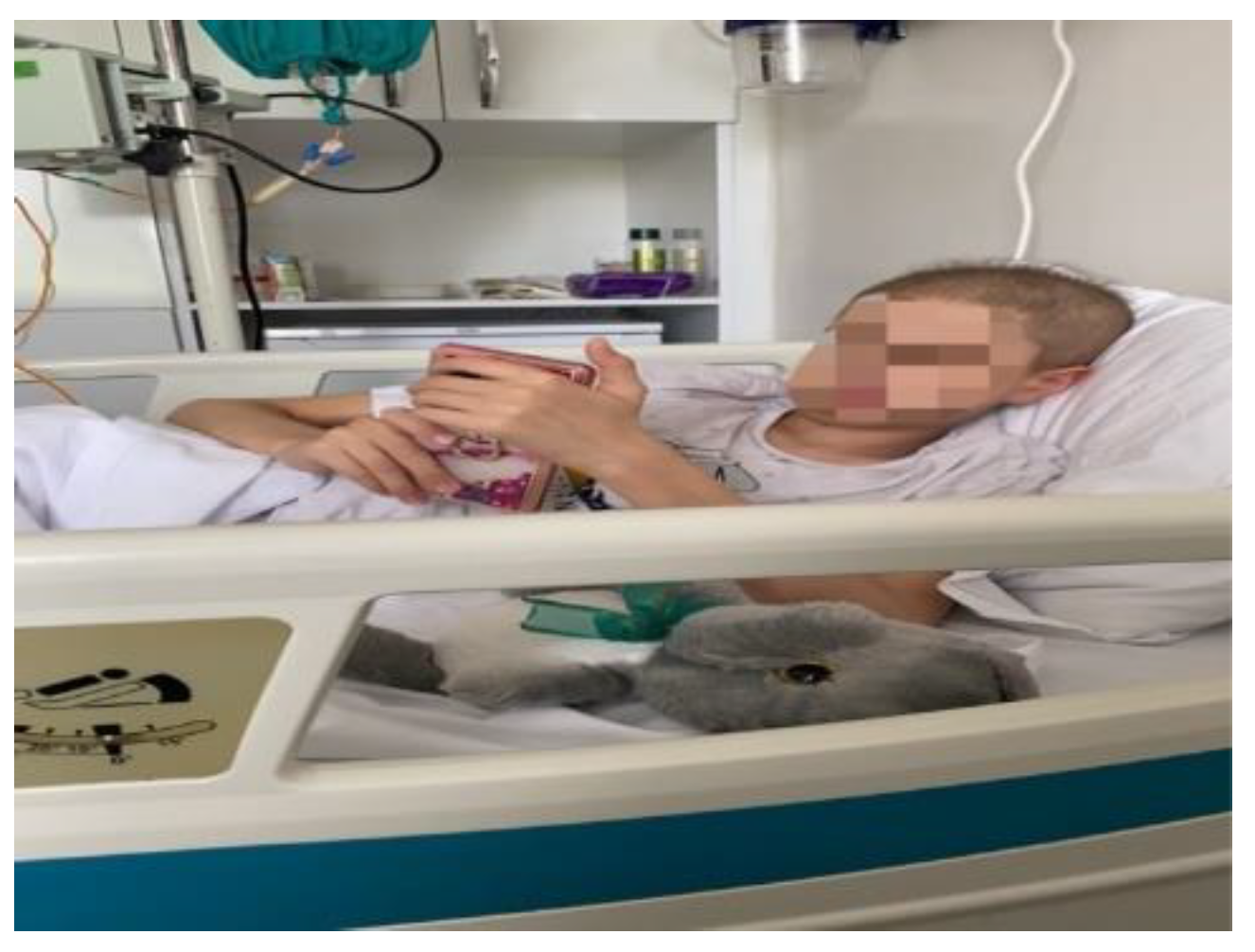
Figure 1.
An example image of the control group performing pre test scales.
Figure 1.
An example image of the control group performing pre test scales.
In the second step of the study, The exercise program was conducted in the sports halls of Erciyes University's Faculty of Sports Sciences or in areas suitable for sports that would not create infection and similar problems for the experimental group children. Before starting the exercise, the area where sports would be performed was ventilated, and necessary hygiene conditions were ensured. The sessions in the first five weeks of the program were conducted face-to-face by the researchers under the supervision of oncology doctors and psychologists.
Considering the need to establish a standard for the comparison of the parameters to be used in determining the physical conditions of children and exercise programs for the applied exercises, the modified borg scale, which is generally used in such studies, was used to determine the intensity of the exercise. Modified Borg Scale (MBS), MBS was developed by Borg in 1970 to measure the effort expended during physical exercise. However, it is one of the most reliable scales for determining the severity of dyspnea at rest and during exertion. It consists of 10 items describing the severity of dyspnea according to its degree. It is also easier for patients to apply and has been shown to correlate with pulmonary function tests. MBS is also superior to other scales in terms of long-term reproducibility. Studies in the literature were examined, and the 2-3 point range, which is considered mild to moderate, was taken as a reference after consultation with oncologists and sports physicians. The scale was taught to the children and placed in a place where they could see it during exercise and they were asked to report when they felt the severity in the range of 2-3 points and the exercise was interrupted. [
20,
21,
22]
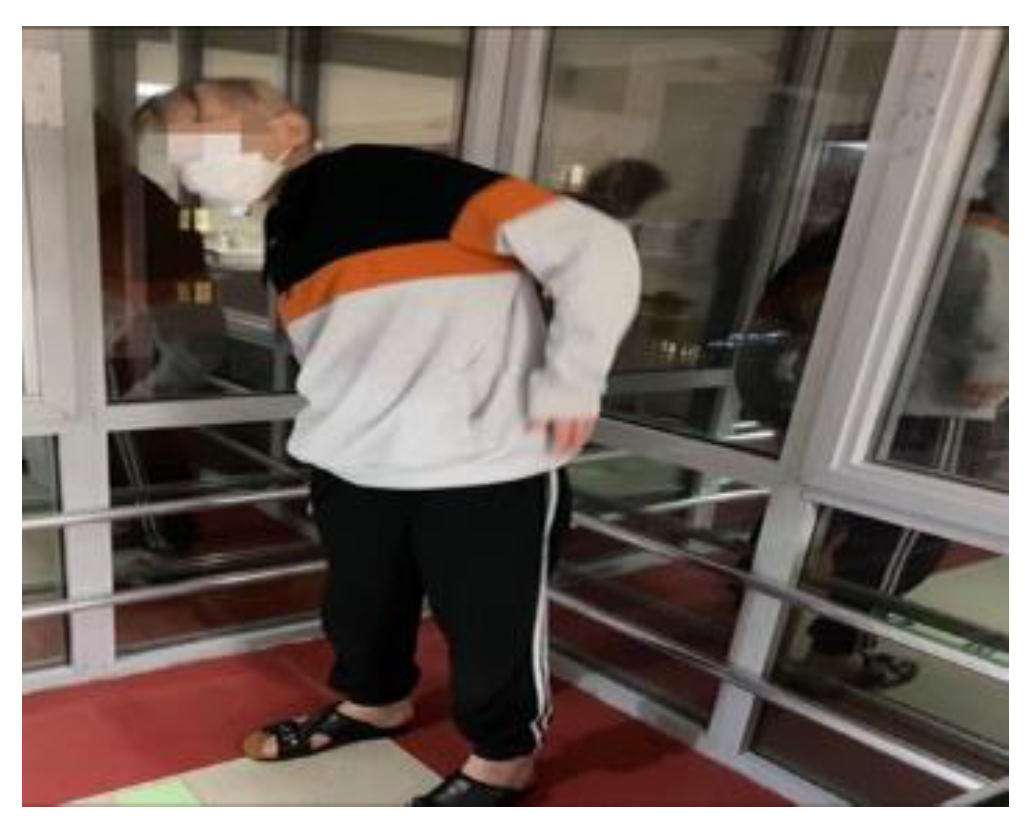
Figure 2.
An example image of the experimental group exercising face to face.
Figure 2.
An example image of the experimental group exercising face to face.
At the beginning of the sixth week, due to precautions taken in response to the COVID-19 pandemic, the study switched to the Zoom online video platform. Patients in the experimental group participated in video conferences organized by the researcher at consistent intervals. The researcher first demonstrated the exercises, then watched a video replay of the movements. The children then completed the program under supervision by doing the exercises. Patients in the experimental group were viewed from a wide-angle perspective.
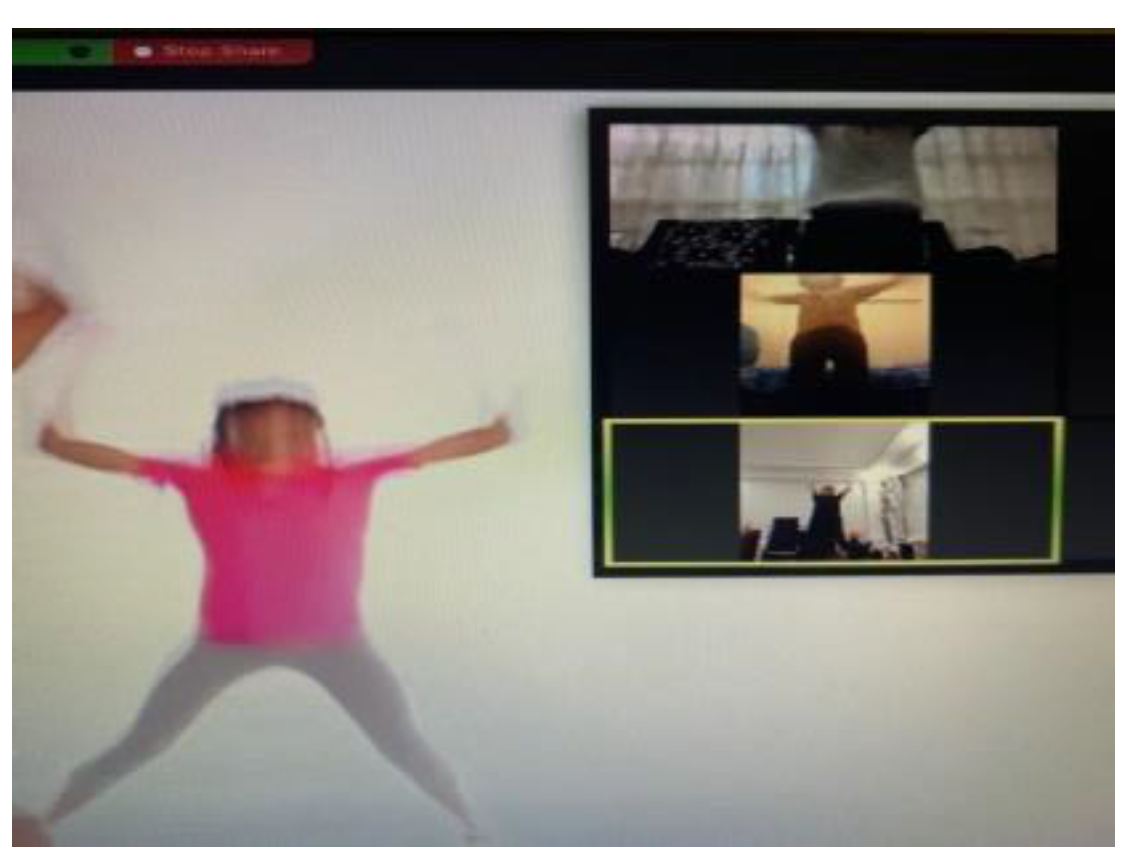
Figure 3.
An example image of the experimental group exercising in person and via Zoom.
Figure 3.
An example image of the experimental group exercising in person and via Zoom.
As the third and final step of the study, control and experimental groups were performed the Kovacs depression, Beck anxiety and Child form of the pediatric cancer quality of life scales were administered in the presence of the psychologist of the relevant hospital unit again.
Figure 4.
An example image of the experimental group performing post test scales.
Figure 4.
An example image of the experimental group performing post test scales.
Kovacs Depression Scale
The Scale is a self-assessment tool developed by Kovacs in 1980 [
23] based on the Beck depression inventory. This scale, which evaluates the severity of depressive symptoms, is applicable to children and adolescents aged 6-17 years. The scale consists of 26 items, with each item scored 0, 1, or 2 points based on symptom severity. The maximum total score is 54, with a recommended cut-off point of 19. Reverse items in the scale are scored inversely. Higher scores indicate more severe depression [23, 24]. The validity and reliability study for Turkish culture was conducted by Öy [
24]. Öy [
24] reported that the test-retest reliability of the Children's depression scale was reported as 0.70, with an internal consistency of 0.80.
Beck Anxiety Inventory
The Beck anxiety ınventory was developed by Beck et al in 1988 [
25]. The scale aims to determine the frequency and severity of anxiety symptoms experienced by individuals. It consists of 21 items, with a maximum possible score of 63. The validity and reliability study for Turkish culture was conducted by Ulusoy et al in 1998 [
26]. Ulusoy et al. [
26] was determined the Cronbach's Alpha internal consistency score of the scale as 0.93.
Pediatric Cancer Quality of Life Inventorty in 7- 18 Years Old Children
The scale developed by Öztürk in 2008 in line with the determined characteristics of quality of life scales and disease-specific quality of life scales in the literature, the Quality of life scale for child cancer patients, consisting of 41 items [
27]. The scale is a 5-point likert type. Öztürk [
27] was reported that Cronbach alfa for Child scale is 0,88.
According to
Table 4, Cronbach's Alpha values obtained from the scales show that the overall scales are sufficiently reliable. The data provided by the participants to the relevant scale shows an acceptable level of consistency within itself.
Data Analysis
Socio-demographic ınformation form, Kovacs depression scale, Beck anxiety inventory, and Child forms of the pediatric cancer quality of life were administered, and the results were transferred to the SPSS 22.00 program developed by IBM for necessary calculations. Descriptive scores of volunteer information and scales which applied by volunteers, were presented as frequency (f) and percentage (%). The normality of data distribution was determined by examining skewness-kurtosis values. Normality test results of participants were presented in table 5 and 6.
In
Table 5, the results of the normality analysis for the variables were found to be between ±1.5. There are some studies in the literature stating that values between ±1.5 [
28] or ±2 [
29] are acceptable for normal distribution. In light of this information, it was observed that the data showed a normal distribution and was found to be suitable for parametric tests. In this direction, "Independent Sample T" and "Paired Samples T Test" statistics were used for comparing the pre-tests and post-tests of the control and experimental groups
3. Results
Table 6.
Comparison of Depression Scale Pre-test and Post-test Scores for Experimental and Control Groups.
Table 6.
Comparison of Depression Scale Pre-test and Post-test Scores for Experimental and Control Groups.
| Scale |
Groups |
Test |
N |
M±Sd |
t |
p |
| Kovacs Depression |
Experimental |
Pre-test |
14 |
12,14±6,47 |
1,348 |
,201 |
| Post-test |
14 |
10,57±5,27 |
| Control |
Pre-test |
8 |
15,88±6,62 |
-,628 |
,550 |
| Post-test |
8 |
16,75±8,07 |
An examination of the pre-test and post-test means and standard deviations of the participants reveals that the experimental group's depression scores were 12.14±6.47 for the pre-test and 10.57±5.27 for the post-test, with the difference between these scores yielding t=1.348 and p=.201. For the control group, the depression scores were 15.88±6.62 for the pre-test and 16.75±8.07 for the post-test, with the difference between these scores yielding t=-.628 and p=.550.
Table 7.
Comparison of Depression Scale Scores Between Experimental and Control Groups.
Table 7.
Comparison of Depression Scale Scores Between Experimental and Control Groups.
| Scale |
Test |
Groups |
N |
M±Sd |
t |
p |
| Kovacs Depression |
Pre-test |
Experimental |
14 |
12,14±6,47 |
-1,283 |
,220 |
| Control |
8 |
15,88±6,62 |
| Post Test |
Experimental |
14 |
10,57±5,27 |
-1,942 |
,041* |
| Control |
8 |
16,75±8,07 |
Analysis of the participants' pre-test scores revealed that the mean and standard deviation for the experimental group were 12.14±6.47, while for the control group, they were 15.88±6.62. The difference between these scores was found to be t=-1.283, p=.220. Examination of the post-test scores showed that the experimental group scored 10.57±5.27, while the control group scored 16.75±8.07. The difference between these post-test scores was determined to be t=-1.942, p=.041. A significant difference was identified between the post-test scores of the experimental group and the control group.
Table 8.
Comparison of Anxiety Scale Pre-test and Post-test Scores for Experimental and Control Groups.
Table 8.
Comparison of Anxiety Scale Pre-test and Post-test Scores for Experimental and Control Groups.
| Scale |
Groups |
Test |
N |
M±Sd |
t |
p |
| Beck Anxiety |
Experimental |
Pre-test |
14 |
20,86±5,92 |
7,990 |
,000** |
| Post-test |
14 |
8,14±7,92 |
| Control |
Pre-test |
8 |
27,25±10,46 |
-1,042 |
,332 |
| Post-test |
8 |
29,38±7,58 |
Examination of the participants' pre-test and post-test means and standard deviations revealed that the experimental group's anxiety scores were 20.86±5.92 for the pre-test and 8.14±7.92 for the post-test, with the difference between these scores yielding t=7.990, p=.000. For the control group, the anxiety scores were 27.25±10.46 for the pre-test and 29.38±7.58 for the post-test, with the difference between these scores yielding t=-1.042, p=.332. A significant difference was identified between the pre-test and post-test scores of the experimental group.
Table 9.
Comparison of Anxiety Scale Scores Between Experimental and Control Groups.
Table 9.
Comparison of Anxiety Scale Scores Between Experimental and Control Groups.
| Scale |
Test |
Groups |
N |
M±Sd |
t |
p |
| Beck Anxiety |
Pre-test |
Experimental |
14 |
20,86±5,92 |
-1,590 |
,080 |
| Control |
8 |
27,25±10,46 |
| Post Test |
Experimental |
14 |
8,14±7,92 |
-6,140 |
,000** |
| Control |
8 |
29,38±7,58 |
Analysis of the participants' pre-test scores indicated that the mean and standard deviation for the experimental group were 20.86±5.92, while for the control group, they were 27.25±10.46. The difference between these scores was found to be t=-1.590, p=.080. Examination of the post-test scores showed that the experimental group scored 8.14±7.92, while the control group scored 29.38±7.58. The difference between these post-test scores was determined to be t=-6.140, p=.000. A significant difference was identified between the post-test scores of the experimental group and the control group.
Table 10.
Comparison of Quality of Life Scale Pre-test and Post-test Scores for Experimental and Control Groups.
Table 10.
Comparison of Quality of Life Scale Pre-test and Post-test Scores for Experimental and Control Groups.
| Scale |
Groups |
Test |
N |
M±Sd |
t |
p |
| Child Form of The Pediatric Cancer Quality of Life |
Experimental |
Pre-test |
14 |
28,21±8,20 |
-3,935 |
,002** |
| Post-test |
14 |
38,14±8,24 |
| Control |
Pre-test |
8 |
34,00±5,86 |
-,652 |
,535 |
| Post-test |
8 |
34,88±5,82 |
Analysis of the participants' pre-test and post-test means and standard deviations revealed that the experimental group's quality of life scores were 28.21±8.20 for the pre-test and 38.14±8.24 for the post-test, with the difference between these scores yielding t=-3.935, p=.002. For the control group, the quality of life scores were 34.00±5.86 for the pre-test and 34.88±5.82 for the post-test, with the difference between these scores yielding t=-.652, p=.535. A significant difference was identified between the pre-test and post-test scores of the experimental group.
Table 11.
Comparison of Quality of Life Scale Scores Between Experimental and Control Groups.
Table 11.
Comparison of Quality of Life Scale Scores Between Experimental and Control Groups.
| Scale |
Test |
Groups |
N |
M±Sd |
t |
p |
| Child Form of The Pediatric Cancer Quality of Life |
Pre-test |
Experimental |
14 |
28,21±8,20 |
-1,659 |
,088 |
| Control |
8 |
33,75±6,09 |
| Post Test |
Experimental |
14 |
38,14±8,24 |
1,085 |
,292 |
| Control |
8 |
34,88±5,82 |
Examination of the participants' pre-test scores indicated that the mean and standard deviation for the experimental group were 28.21±8.20, while for the control group, they were 33.75±6.09. The difference between these scores was found to be t=-1.659, p=.088. Analysis of the post-test scores showed that the experimental group scored 38.14±8.24, while the control group scored
4. Discussion
This study implemented a practical program to investigate the effects of moderate exercise on depression, anxiety, and quality of life in pediatric cancer patients. Regarding the depression variable, initial pre-test results showed no significant difference between control and experimental groups. Post-intervention analysis revealed a significant difference between groups, favoring the experimental group. Literature review indicates:
Studies demonstrate the positive effects of exercise on individuals. For instance, regular exercise has been shown to reduce tension and effectively decrease depression [30, 31, 32, 33], contribute to feelings of well-being and happiness [34, 35], and increase psychological well-being [
36]. Malchow et al. [
37] found that adolescents engaging in regular physical activity had lower depression scores, while those with minimal or no exercise participation showed significantly higher depression levels. Eime et al. [
38] reported fewer depressive symptoms in children who exercised regularly. Janssen and LeBlanc [
39] noted that an 8-12 week exercise program positively affected at least one depressive symptom. Rothon et al. [
40] observed that each additional hour of weekly exercise reduced depressive symptoms by 8%.
The World Health Organization (WHO) and the UK National Institute for Health and Clinical Excellence (NICE) recommend exercise as a treatment method for depression. Scientific studies have confirmed the antidepressant effect of exercise on both depressive symptoms and diagnosed cases of depression of varying intensities [41, 42, 43]. Various sources in the literature indicate that regular physical activity and sports participation prevent the development of certain cancer types [44, 45] or reduce the negative effects of cancer [46, 47, 48, 49, 50, 51, 52].
Bar-Or and Rowland [
53] evaluated the effect of twice-weekly hospital-based aerobic exercise on 10 post-pubertal cancer survivors who had not received chemotherapy or radiation therapy for at least a year. The results showed positive effects on anxiety, depression, stress management, and quality of life. The present study also demonstrates that exercise contributes to reducing depression levels in pediatric cancer patients. It is thought that physiological and hormonal changes resulting from exercise help alleviate depression by distancing individuals from the negative effects of cancer to some extent.
Regarding the anxiety variable, initial pre-test results showed no significant difference between control and experimental groups. Post-intervention analysis revealed a significant difference between groups, favoring the experimental group. Literature review indicates:
Chang et al. [
16] found that AML patients undergoing chemotherapy who participated in a 3-week exercise program (12 minutes daily, at least 5 days a week) experienced less depression and anxiety compared to the control group. Blaauwbroek et al. [
54] demonstrated the positive effect of weekly yoga exercises for 7 weeks on anxiety in lymphoma patients receiving or having received chemotherapy within the last 12 months. Shore and Shepard [
55] observed significant improvements in physical fitness and anxiety after a 12-week, 30-minute daily aerobic exercise program supervised by an expert and family members. Courneya et al. [
17] concluded that a 12-week regular aerobic exercise program for 122 lymphoma patients in Canada increased happiness and reduced anxiety. These studies align with our research findings. It can be inferred that various stretching and relaxation exercises during the intervention helped cancer patients relax, reduce stress, and consequently, moderate their anxiety levels.
Regarding the quality of life variable, initial pre-test results showed no significant difference between control and experimental groups. Post-intervention analysis revealed a positive, though not statistically significant, difference between groups, favoring the experimental group. However, a significant difference was observed between the experimental group's pre- and post-test results, favoring the post-test.
Kürtüncü and Kuğuoğlu [
56] reported statistically significant differences in mean quality of life scores between groups after a 3-month exercise program for children with acute lymphocyetic leukemia. Another study examined the effect of twice-weekly hospital-based aerobic exercise on post-pubertal cancer survivors who had not received treatment or therapy for at least a year [
53]. San Juan et al. [
57] demonstrated significant improvements in muscle strength, functional mobility, physical fitness, and quality of life in children aged 8-16 who underwent hematopoietic stem cell transplantation and participated in an 8-week aerobic and resistance training program.
Polat et al [
58] and de Almeida and Noll [
59] identified a significant relationship between physical activity levels and quality of life. Conversely Barakou et al [
60] and Luca et al [
61] found that physical activity is one of the most important factors in preventing chronic diseases and improving quality of life.
Some studies have shown that exercise positively affects quality of life, while others have found no significant effect. It is evident that there is no consensus on this matter, and more research is needed. Although our study results were not statistically significant, the positive increase in mean scores may be attributed to people finding relaxation and value in such activities during the pandemic period.
In conclusion, in intragroup comparisons, significant differences were observed in favor of the post-test scores within the experimental group for both the anxiety scale and the quality of life children's form. In intergroup comparisons, significant differences were detected in favor of the experimental group regarding the post-test scores of depression and anxiety scales. This phenomenon is attributed to the beneficial impact of physical activity in alleviating the psychological distress experienced by pediatric cancer patients.
Recommendations:
Considering this study and existing literature, exercise should be evaluated as a potential supportive element in treatment procedures.
Oncology hospitals could incorporate activities such as music therapy, exercise, and art therapy in addition to primary treatment elements to enhance patients' motivation and psychological resilience.
Extensive long-term pilot studies could be conducted with volunteers of different cancer types and age groups.
Based on the results, relevant authorities could implement additional remedial measures for individuals with poor quality of life, depression, and anxiety conditions.
Medical school curricula could be expanded to include topics on patient psychology and alternative supportive treatments.
Author Contributions
Conceptualization, F.N.Ş, K.KO. and MS.Y.; methodology, O.P, İ.D. and G.B.B.; software, T.A. and F.N.Ş ; validation, Ö.A. and B.K, K.KA. and T.A.; formal analysis, K.KO and Ş.Ü.; investigation, O.P. and L.C.; resources, MS.Y.and H.K.; data curation, Ö.A and K.KO; writing—original draft preparation, O.P. and T.A; writing—review and editing, İ.D.,O.P., K.KO and L.C.; visualization, K.KO and Ş.Ü.; supervision, K.KO, B.K. and H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was produced from a master's thesis which was numbered 786173. Within the scope of the study, approval was obtained from Erciyes University Health Sciences Ethics Committee with the decision dated 28/07/2021 and numbered 214. The studies were conducted in accordance with the local legislation and institutional requirements. This study was prepared to comply with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from the parents of all subjects participating in the study. Written informed consent was obtained from the parents of the patients for the publication of this article.
Data Availability Statement
Data are available on request due to privacy and ethics restrictions.
Acknowledgments
We would like to thank Suleyman Demirel University Department of Foreign Languages for providing us with certified professional English translation and editing services. We also wish health and well-being to all children with cancer and their families who participated in our study and express our gratitude for their contributions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- National Cancer Institute. Childhood Acute Lymphoblastic Leukemia Treatment (PDQ®)–Patient Version. URL: https://www.cancer.gov/types/leukemia/patient/child-all-treatment-pdq, Erişim Tarihi: 10.05.2022.
- American Academy of Long-term follow-up care for pediatric cancer survivors. Pediatrics; 2009;123 (7): 906-915.
- Global Cancer Observatory (GCO), ‘’Cancer Today’’ https://gco.iarc.fr/today/home. Erişim tarihi: 10 Ekim 2021.
- Kebudi R. Pediatric oncology in Turkey. Journal of pediatric hematology / oncology. 2012; 34: 12-4.
- Oeffinger KC, Robison LL. Childhood cancer survivors, late effects, and a new model for understanding survivorship. JAMA 2007; 297(24): 2762-2764. [CrossRef]
- Zawrzykraj, M., Deptuła, M., Kondej, K., Tymińska, A., & Pikuła, M. (2023). The effect of chemotherapy and radiotherapy on stem cells and wound healing. Current perspectives and challenges for cell-based therapies. Biomedicine & Pharmacotherapy, 168, 115781. [CrossRef]
- Lu, Y., Bai, X., & Pan, C. (2024). Impact of exercise interventions on quality of life and depression in lung cancer patients: A systematic review and meta-analysis. The International Journal of Psychiatry in Medicine, 59(2), 199-217. [CrossRef]
- Sepahvand, F., Valizadeh, F., Karami, K., Abdolkarimi, B., & Ghasemi, F. (2024). Falling and rising in the vortex of cancer: children’s adaptation with cancer: a qualitative study. BMC psychology, 12(1), 221. [CrossRef]
- Cai, J., Cheung, Y. T., & Hudson, M. M. (2024). Care Models and Barriers to Long-Term Follow-Up Care Among Childhood Cancer Survivors and Health Care Providers in Asia: A Literature Review. JCO Global Oncology, 10, e2300331. [CrossRef]
- Thomassen, R. A., Kvammen, J. A., Bentsen, B. S., Solheim, A., Størdal, K., Henriksen, C., & Brun, A. C. (2025). Impact of parenteral nutrition on quality of life, the family and gastrointestinal symptoms in children with intestinal failure. Journal of Pediatric Gastroenterology and Nutrition, 80(1), 69-79. [CrossRef]
- Arpaci, T., & Altay, N. (2024, April). Qualitative analysis of school re-entry experiences of turkish survivors of childhood and adolescent cancer: Parental perspective. In Seminars in Oncology Nursing (Vol. 40, No. 2, p. 151613). WB Saunders. [CrossRef]
- Kübler-Ross E, Wessler S, Avioli LV. On death and dying. JAMA 1972;221:174–179.
- Whyte F, Smith, L. A literature review of adolescence and cancer. European Journal of Cancer Care, 1997;6(2): 137-146. [CrossRef]
- Hirschfeld RM. The comorbidity of major depression and anxiety disorders: Recognition and management in primary care. Primary Care Companion to the Journal of Clinical Psychiatry, 2001; 3: 244-254.
- Csuka, Sára Imola, Magda Rohánszky, and Barna Konkolÿ Thege. "Gender differences in the predictors of quality of life in patients with cancer: A cross sectional study." European Journal of Oncology Nursing 68 (2024): 102492. [CrossRef]
- Rethorst, C. D, Wipfli, B. M, and Landers, D. M. The antidepressive effects of exercise: a meta-analysis of randomized trials. 2009; Sports Med. 39, 491–511.
- Chang PH, Lai YH, Shun SC, Lin LY, Chen ML, Yang Y, Tsai JC, Huang GS, Cheng SY: Effects of a walking intervention on fatigue-related experiences of hospitalized acute myelogenous leukemia patients undergoing chemotherapy: a randomized controlled trial. Journal of Pain and Symptom Management, 2008;35: 524-534.
- Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddie CJ, Friedenreich CM, Tankel K, Basi S, Chua N, Mazurek A, Reiman T. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. Journal of Clinical Oncology 2009;27: 4605-4612. [CrossRef]
- Karasar, N. Bilimsel Araştırma Yöntemleri, Nobel Yayınevi 2014; 97.
- Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, et al. Pulmonary rehabilitation: joint ACCP/ AACVPR evidence-based clinical practice guidelines: update. Chest 2007; 131 (5 Suppl): 4-42.
- Borg GAV. Psychophysical basis of perceived exertion. Medicine and Science in Sports and Exercise 1982; 14: 377-81.
- Karakuş, M., Çelenk, Ç., Kaya, M., Sucan, S., vd. Çocuklarda 12 Haftalık Yüzme Egzersizinin Bazı Fiziksel Fizyolojik Parametrelere Etkisi. Mediterranean Journal of Sport Science, 2018; 1(1), 50-57.
- Kovacs M. The Children’s Depression Inventory (CDI), Psychopharmacol Bull 1981; 21: 995-8.
- Öy B. Çocuklar için depresyon ölçeği: Geçerlik ve güvenirlik çalışması. Türk Psikiyatri Dergisi 1991; 2: 132-7.
- Beck, A.T., Epstein, N., Brown, G., and Steer, R. A. An Inventory for Measuring Clinical Anxiety: Psychometric Properties. Journal of Consulting and Clinical Psychology 1988, 56,893-897.
- Ulusoy M, Şahin N, Erkmen H. Turkish Version of the Beck Anxiety Inventory: Psychometric Properties. Journal of Cognitive Psychotherapy, 1998;12: 163-172.
- Öztürk O, Ruh Sağlığı ve Bozuklukları. Feryal Matbaası, 9.Basım, 2002; Ankara 90.
- Tabachnick, B. G., & Fidell, L. S. (2015). Using multivariate statistics. (sixth ed). Allyn & Bacon/Pearson Education.
- George D, Mallery P. IBM SPSS Statistics 23 Step by Step. 13th ed. Roudledge, New York; 2016.
- Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Preventive Medicine 2003; 36:698-703. [CrossRef]
- Carek, P. J., Laibstain, S. E., & Carek, S. M. Exercise for the treatment of depression and anxiety. The international journal of psychiatry in medicine, 2011; 41(1), 15-28. [CrossRef]
- Craft, L. L., & Perna, F. M. The benefits of exercise for the clinically depressed. Primary Care Companion To The Journal Of Clinical Psychiatry,2004; 6(3), 104.
- Brosse, A. L., Sheets, E. S., Lett, H. S., & Blumenthal, J. A. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Medicine, 2002; 32, 741-760.
- Khazaee-Pool, M., Sadeghi, R., Majlessi, F., & Rahimi Foroushani, A. Effects of physical exercise programme on happiness among older people. Journal of psychiatric and mental health nursing, 2015; 22(1), 47-57. [CrossRef]
- Denny K.G. & Steiner H. External andinternal factors influencing happiness in elitecollegiate athletes. Child Psychiatry and HumanDevelopment 2009; 40, 55–72.
- Delextrat AA, Warner S, Graham S, Neupert E. An 8-week exercise intervention based on Zumba improves aerobic fitness and psychological well-being in healthy women. Journal of Physical Activity and Health, 2016;13(2):131-139. [CrossRef]
- Malchow, B., Reich-Erkelenz, D., Oertel-Knöchel, V., Keller, K., Hasan, A., Schmitt, A., Scheewe, T.W., Cahn, W., Kahn, R.S., Falkai, P. The effects of physical exercise in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci 263, 451–467 (2013). [CrossRef]
- Eime RM, Young JA, Harvey JT, Charity MJ, Payne WR. A systematic review of the psychological and social benefits of participation in sport for children and adolescents: informing development of a conceptual model of health through sport. Int J Behav Nutr Phys Act, 2013;3: 10:98. [CrossRef]
- Janssen I, LeBlanc AG. Systematic review of the health benefits of physical activity and fitness in school-agedchildren and youth. Int J Behav Nutr Phys Act, 2010;7:40-47.
- Rothon, C., Edwards, P., Bhui, K., Viner, R. M., Taylor, S., & Stansfeld, S. A. Physical activity and depressive symptoms in adolescents: a prospective study. BMC medicine, 2010; 8, 1-9. [CrossRef]
- Kvam, S., Kleppe, C. L., Nordhus, I. H., & Hovland, A. Exercise as a treatment for depression: a meta-analysis. Journal of affective disorders, 2016; 202, 67-86. [CrossRef]
- Josefsson, T., Lindwall, M., & Archer, T. Physical exercise intervention in depressive disorders: Meta-analysis and systematic review. Scandinavian journal of medicine & science in sports, 2014; 24(2), 259-272. [CrossRef]
- Silveira, H., Moraes, H., Oliveira, N., Coutinho, E. S. F., Laks, J., & Deslandes, A. Physical exercise and clinically depressed patients: a systematic review and meta-analysis. Neuropsychobiology, 2013; 67(2), 61-68. [CrossRef]
- Kruk, J. Physical activity in the prevention of the most frequent chronic diseases: an analysis of the recent evidence. Asian Pacific Journal of Cancer Prevention, 2007; 8(3), 325.
- Patel, A. V., Friedenreich, C. M., Moore, S. C., Hayes, S. C., Silver, J. K., Campbell, K. L., Einters-Stone, K., Gerber, L.H., George, S.M., Fulton, J.E., Denlinger, C., Morris, G.S., Hue, T., Schmitz, K.H., & Matthews, C. E. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Medicine and science in sports and exercise, 2019; 51(11), 2391. [CrossRef]
- Braam, K.I., van Dijk, E.M., Veening, M.A., Bierings, M.B., Merks, J.H.M., Grootenhuis, M. A., Chinapaw, M.J.M, Sinnema. G., Takken. T., Huisman. J., Kaspers. G.J.L., & van Dulmen-den Broeder, E. Design of the Quality of Life in Motion (QLIM) study: a randomized controlled trial to evaluate the effectiveness and cost-effectiveness of a combined physical exercise and psychosocial training program to improve physical fitness in children with cancer. BMC Cancer, 2010; 10, 1-9. [CrossRef]
- Thorsteinsson, T., Helms, A. S., Adamsen, L., Andersen, L. B., Andersen, K. V., Christensen, K. B., Halse. H., Heilmann. C., Hejgaard. N., Johansen. C., Madsen. M., Madsen, S.A., Simovska. V., Strange, B., Thing, F.T., Wehner, P.S. Schmiegelow, K. & Larsen, H. B. Study protocol: rehabilitation including social and physical activity and education in children and teenagers with cancer (RESPECT). BMC cancer, 2013;13, 1-7. [CrossRef]
- Ness KK, Baker KS, Dengel DR, Youngren N, Sibley S, Mertens AC. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer, 2007; 49, 975- 981. [CrossRef]
- Lucia A, Ramírez M, San Juan AF, Fleck SJ, García-Castro J, Madero L. Intrahospital supervised exercise training: a complementary tool in the therapeutic armamentarium against childhood leukemia. Leukemia. 2005 Aug;19(8):1334-7. PMID: 15931268. [CrossRef]
- Battaglini CL, Hackney AC, Garcia R, Groff D, Evans E, Shea T. The effects of an exercise program in leukemia patients. Integrative Cancer Therapies, 2009;8(2): 130-138. [CrossRef]
- Paxton, R. J., Jones, L. W., Rosoff, P. M., Bonner, M., Ater, J. L., & Demark-Wahnefried, W. (2010). Associations between leisure-time physical activity and health-related quality of life among adolescent and adult survivors of childhood cancers. Psycho-oncology, 19(9), 997-1003. [CrossRef]
- Galvão, D. A., & Newton, R. U. Review of exercise intervention studies in cancer patients. Journal Of Clinical Oncology, 2005; 23(4), 899-909. [CrossRef]
- Bar-Or, O., & Rowland, T. W. Physiologic and perceptual responses to exercise in the healthy child. Pediatric Exercise Medicine–From Physiologic Principles to Health Care Application, 2004; 03-58.
- Blaauwbroek R, Bouma MJ, Tuinier W, et al. The effect of exercise counselling with feedback from a pedometer on fatigue in adult survivors of childhood cancer: a pilot study. Support Care Cancer 2009; 17:1041-8. [CrossRef]
- Shore, S., & Shepard, R. J. Immune responses to exercise in children treated for cancer. Journal of sports medicine and physical fitness, 1999; 39(3), 240.
- Kürtüncü Tanır M, Kuğuoğlu S. Impact of exercise on the lower activity levels in children with acute lymphoblastic leukemia: a randomised controlled trial from Turkey. Rehabilitation Nursing 2013; 38 (1): 48-59.
- San Juan AF, Chamorro-Vina C, Moral S, et al. Benefits of intrahospital exercise training after pediatric bone marrow transplantation. Int J Sports Med 2008; 29:439-46. [CrossRef]
- Polat, K., Karadibak, D., Güç, Z. G. S., Yavuzşen, T., & Öztop, İ. (2024). The Relationship between Exercise Capacity and Muscle Strength, Physical Activity, Fatigue and Quality of Life in Patients with Cancer Cachexia. Nutrition and Cancer, 76(1), 55-62. [CrossRef]
- de Almeida, A.A.; Noll, M. Physical Activity and Lifestyle Behaviors in Children and Adolescents. Children 2024, 11, 1403. [CrossRef]
- Barakou, I., Seves, B. L., Abonie, U. S., Finch, T., Hackett, K. L., & Hettinga, F. J. (2025). Health-related quality of life associated with fatigue, physical activity and activity pacing in adults with chronic conditions. BMC Sports Science, Medicine and Rehabilitation, 17(1), 1-15. [CrossRef]
- Luca, A.C.; T, arcă, E.; Tănase, V.-G.; Păduret, , I.-A.; Dragoiu, T.-S.; Butnariu, L.I.; Ros,u, S.T.; Roca, I.C.; Mîndru, D.-E. Benefits of Physical Activity in Children with Cardiac Diseases—A Concise Summary for Pediatricians. Children 2024, 11, 1432. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).