Submitted:
11 February 2025
Posted:
12 February 2025
You are already at the latest version
Abstract
We describe the case of a 24-year-old woman who developed a swelling under her collarbone, along with fever, swollen lymph nodes, tiredness, and weight loss. These symptoms started about three months after she had a moderate COVID-19 infection. Blood tests showed a high white blood cell count, with more neutrophils and fewer lymphocytes, as well as high levels of inflammation markers. Serological and molecular diagnostics confirmed Epstein-Barr virus (EBV) reactivation.. Despite receiving antiviral and supportive treatment, her condition worsened. Advanced imaging showed that her lymph nodes remained swollen and started to break down. Doctors ruled out other possible causes, such as bacterial infections, blood cancers, autoimmune diseases, and cancer spread. A needle biopsy, combined with a special genetic test (PCR), confirmed the presence of Mycobacterium tuberculosis, the bacteria that cause tuberculosis. She started a standard tuberculosis treatment, which led to major improvement within two months. Her swollen lymph nodes shrank, and her inflammation markers returned to normal. This case shows how COVID-19-related immune system changes, EBV reactivation, and tuberculosis infection can be connected. It highlights the need for careful monitoring of patients with long-lasting swollen lymph nodes, especially those with weakened immune systems.
Keywords:
1. Introduction
2. Case Description
2.1. Clinical Manifestations and Disease Course
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deveci, H.S.; Kule, M.; Kule, Z.A.; Habesoglu, T.E. Diagnostic challenges in cervical tuberculous lymphadenitis: A review. Northern clinics of Istanbul. 2016, 3, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Gopalaswamy, R.; Dusthackeer, V.N.; Kannayan, S.; Subbian, S. Extrapulmonary Tuberculosis—An Update on the Diagnosis, Treatment and Drug Resistance. Journal of Respiration. 2021, 1, 141–164. [Google Scholar] [CrossRef]
- Odumade, O.A.; Hogquist, K.A.; Balfour, H.H., Jr. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clinical microbiology reviews. 2011, 24, 193–209. [Google Scholar] [CrossRef]
- Bernal, K.D.; Whitehurst, C.B. Incidence of Epstein-Barr virus reactivation is elevated in COVID-19 patients. Virus research. 2023, 334, 199157. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Q.; Zhang, B.; Dai, Y.; Gao, Y.; Li, C.; et al. Epstein–Barr Viruses: Their Immune Evasion Strategies and Implications for Autoimmune Diseases. International journal of molecular sciences. 2024, 25, 8160. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, Z.; Prajapati, M.; Li, Y. Lymphopenia Caused by Virus Infections and the Mechanisms Beyond. Viruses. 2021, 13. [Google Scholar] [CrossRef]
- Bartleson, J.M.; Radenkovic, D.; Covarrubias, A.J.; Furman, D.; Winer, D.A.; Verdin, E. SARS-CoV-2, COVID-19 and the Ageing Immune System. Nature aging. 2021, 1, 769–782. [Google Scholar] [CrossRef]
- Geng, L.; Wang, X. Epstein-Barr Virus-associated lymphoproliferative disorders: experimental and clinical developments. International journal of clinical and experimental medicine. 2015, 8, 14656–14671. [Google Scholar]
- Quintanilla-Martinez, L.; Swerdlow, S.H.; Tousseyn, T.; Barrionuevo, C.; Nakamura, S.; Jaffe, E.S. New concepts in EBV-associated B, T, and NK cell lymphoproliferative disorders. Virchows Archiv : an international journal of pathology. 2023, 482, 227–244. [Google Scholar] [CrossRef]
- Marrella, V.; Nicchiotti, F.; Cassani, B. Microbiota and Immunity during Respiratory Infections: Lung and Gut Affair. International journal of molecular sciences. 2024, 25. [Google Scholar] [CrossRef]
- Sankar, P.; Mishra, B.B. Early innate cell interactions with Mycobacterium tuberculosis in protection and pathology of tuberculosis. Frontiers in immunology. 2023, 14, 1260859. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Najafi-Fard, S.; Goletti, D. Initial immune response after exposure to Mycobacterium tuberculosis or to SARS-COV-2: similarities and differences. Frontiers in immunology. 2023, 14, 1244556. [Google Scholar] [CrossRef] [PubMed]
- Petakh, P.; Kamyshna, I.; Oksenych, V.; Kamyshnyi, O. Metformin Alters mRNA Expression of FOXP3, RORC, and TBX21 and Modulates Gut Microbiota in COVID-19 Patients with Type 2 Diabetes. Viruses. 2024, 16. [Google Scholar] [CrossRef]
- Carabalí-Isajar, M.L.; Rodríguez-Bejarano, O.H.; Amado, T.; Patarroyo, M.A.; Izquierdo, M.A.; Lutz, J.R.; et al. Clinical manifestations and immune response to tuberculosis. World journal of microbiology & biotechnology. 2023, 39, 206. [Google Scholar]
- Sia, J.K.; Rengarajan, J. Immunology of Mycobacterium tuberculosis Infections. Microbiology spectrum. 2019, 7. [Google Scholar] [CrossRef]
- Thakkar, K.; Ghaisas, S.M.; Singh, M. Lymphadenopathy: Differentiation between Tuberculosis and Other Non-Tuberculosis Causes like Follicular Lymphoma. Frontiers in public health. 2016, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Cataño, J.C.; Robledo, J. . Tuberculous Lymphadenitis and Parotitis. Microbiology spectrum. 2016, 4. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Rasizadeh, R.; Sharaflou, S.; Aghbash, P.S.; Shamekh, A.; Jafari-Sales, A.; et al. Coinfection of EBV with other pathogens: a narrative review. Frontiers in Virology. 2024, 4. [Google Scholar] [CrossRef]
- Sausen, D.G.; Poirier, M.C.; Spiers, L.M.; Smith, E.N. Mechanisms of T cell evasion by Epstein-Barr virus and implications for tumor survival. Frontiers in immunology. 2023, 14, 1289313. [Google Scholar] [CrossRef]
- Paolucci, S.; Cassaniti, I.; Novazzi, F.; Fiorina, L.; Piralla, A.; Comolli, G.; et al. EBV DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2021, 104, 315–9. [Google Scholar] [CrossRef]
- Berger, L.; Wolf, J.; Kalbitz, S.; Kellner, N.; Lübbert, C.; Borte, S. Comparative Analysis of Lymphocyte Populations in Post-COVID-19 Condition and COVID-19 Convalescent Individuals. Diagnostics. 2024, 14, 1286. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; He, G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Annals of hematology. 2020, 99, 1421–1428. [Google Scholar] [CrossRef]
- Hosseinian, K.; Gerami, A.; Bral, M.; Venketaraman, V. Mycobacterium tuberculosis-Human Immunodeficiency Virus Infection and the Role of T Cells in Protection. Vaccines. 2024, 12. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; Muskat, K.; Tippalagama, R.; Sette, A.; Burel, J.; Arlehamn, C.S.L. Classical CD4 T cells as the cornerstone of antimycobacterial immunity. Immunological reviews. 2021, 301, 10–29. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.A.; Khayumbi, J.; Ongalo, J.; Tonui, J.; Campbell, A.; Allana, S.; et al. CD4 T Cells in Mycobacterium tuberculosis and Schistosoma mansoni Co-infected Individuals Maintain Functional TH1 Responses. Frontiers in immunology. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Buchynskyi, M.; Oksenych, V.; Kamyshna, I.; Budarna, O.; Halabitska, I.; Petakh, P.; et al. Genomic insight into COVID-19 severity in MAFLD patients: a single-center prospective cohort study. Frontiers in genetics. 2024, 15, 1460318. [Google Scholar] [CrossRef]
- Buchynskyi, M.; Oksenych, V.; Kamyshna, I.; Vorobets, I.; Halabitska, I.; Kamyshnyi, O. Modulatory Roles of AHR, FFAR2, FXR, and TGR5 Gene Expression in Metabolic-Associated Fatty Liver Disease and COVID-19 Outcomes. Viruses. 2024, 16. [Google Scholar] [CrossRef]
- Britton, W.J.; Fernando, S.L.; Saunders, B.M.; Sluyter, R.; Wiley, J.S. The genetic control of susceptibility to Mycobacterium tuberculosis. Novartis Foundation symposium. 2007, 281, 79–89. [Google Scholar]
- Weatherhead, J.E.; Clark, E.; Vogel, T.P.; Atmar, R.L.; Kulkarni, P.A. Inflammatory syndromes associated with SARS-CoV-2 infection: dysregulation of the immune response across the age spectrum. The Journal of clinical investigation. 2020, 130, 6194–6197. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Khatibi, S.M.H.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Sorbeni, F.G.; et al. COVID-19 infection: an overview on cytokine storm and related interventions. Virology journal. 2022, 19, 92. [Google Scholar] [CrossRef]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; et al. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina (Kaunas, Lithuania). 2022, 58(2). 2022, 58. [Google Scholar]
- Que, Y.; Hu, C.; Wan, K.; Hu, P.; Wang, R.; Luo, J.; et al. Cytokine release syndrome in COVID-19: a major mechanism of morbidity and mortality. International reviews of immunology. 2022, 41, 217–230. [Google Scholar] [CrossRef]
- Nazerian, Y.; Ghasemi, M.; Yassaghi, Y.; Nazerian, A.; Hashemi, S.M. Role of SARS-CoV-2-induced cytokine storm in multi-organ failure: Molecular pathways and potential therapeutic options. International immunopharmacology 2022, 113 (Pt B), 109428. [Google Scholar] [CrossRef] [PubMed]
- Müller-Durovic, B.; Jäger, J.; Bantug, G.R.; Hess, C. Epstein-Barr virus hijacks B cell metabolism to establish persistent infection and drive pathogenesis. Trends in immunology. 2025, 46, 7–16. [Google Scholar] [CrossRef]
- Weidner-Glunde, M.; Kruminis-Kaszkiel, E.; Latency, M.S.H. Herpesviral Latency—Common Themes. Pathogens (Basel, Switzerland) 2020, 9, 125. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, M. EBV-induced T-cell responses in EBV-specific and nonspecific cancers. Frontiers in immunology. 2023, 14. [Google Scholar] [CrossRef]
- Dropulic, L.K.; Lederman, H.M. Overview of Infections in the Immunocompromised Host. Microbiology spectrum. 2016, 4. [Google Scholar] [CrossRef]
- Baral, B.; Saini, V.; Kandpal, M.; Kundu, P.; Dixit, A.K.; Parmar, H.S.; et al. The interplay of co-infections in shaping COVID-19 severity: Expanding the scope beyond SARS-CoV-2. Journal of infection and public health. 2024, 17, 102486. [Google Scholar] [CrossRef] [PubMed]
- Kiazyk, S.; Ball, T.B. Latent tuberculosis infection: An overview. Canada communicable disease report = Releve des maladies transmissibles au Canada. 2017, 43, 62–66. [Google Scholar] [CrossRef]
- Dutta, N.K.; Latent tuberculosis infection, P.C.K. ; models, and molecular mechanisms. Microbiology and molecular biology reviews : MMBR. 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Palanivel, J.; Sounderrajan, V.; Thangam, T.; Rao, S.; Harshavardhan, S.; Parthasarathy, K. Latent Tuberculosis: Challenges in Diagnosis and Treatment, Perspectives, and the Crucial Role of Biomarkers. Current microbiology. 2023, 80. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.L.; Flynn, J.L. Understanding latent tuberculosis: a moving target. Journal of immunology (Baltimore, Md : 1950). 2010, 185, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.L.; Flynn, J.L. CD8 T cells and Mycobacterium tuberculosis infection. Seminars in immunopathology. 2015, 37, 239–249. [Google Scholar] [CrossRef]
- Chinna, P.; Bratl, K.; Lambarey, H.; Blumenthal, M.J.; Schäfer, G. The Impact of Co-Infections for Human Gammaherpesvirus Infection and Associated Pathologies. International journal of molecular sciences. 2023, 24. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Saidara, E.; Maes, M. Persistent SARS-CoV-2 Infection, EBV, HHV-6 and Other Factors May Contribute to Inflammation and Autoimmunity in Long COVID. Viruses. 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Naidu, A.S.; Wang, C.K.; Rao, P.; Mancini, F.; Clemens, R.A.; Wirakartakusumah, A.; et al. Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID. NPJ science of food. 2024, 8, 19. [Google Scholar] [CrossRef]
- Dormans, T.; Zandijk, E.; Stals, F. Late Tuberculosis Reactivation After Severe Covid-19. European journal of case reports in internal medicine. 2024, 11, 004406. [Google Scholar] [CrossRef]
- Alene, K.A.; Wangdi, K.; Clements, A.C. Impact of the COVID-19 Pandemic on Tuberculosis Control: An Overview. Tropical medicine and infectious disease. 2020, 5, 123. [Google Scholar] [CrossRef]
- Hoerter, A.; Arnett, E.; Schlesinger, L.S.; Pienaar, E. Systems biology approaches to investigate the role of granulomas in TB-HIV coinfection. Frontiers in immunology. 2022, 13, 1014515. [Google Scholar] [CrossRef]
- Yang, H.; Lei, X.; Chai, S.; Su, G.; Du, L. From pathogenesis to antigens: the key to shaping the future of TB vaccines. Frontiers in immunology. 2024, 15, 1440935. [Google Scholar] [CrossRef]
- Booysen, P.; Wilkinson, K.A.; Sheerin, D.; Waters, R.; Coussens, A.K.; Wilkinson, R.J. Immune interaction between SARS-CoV-2 and Mycobacterium tuberculosis. Frontiers in immunology. 2023, 14, 1254206. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Menzies, D.; Schwartzman, K. Tuberculosis screening of travelers to higher-incidence countries: a cost-effectiveness analysis. BMC public health. 2008, 8, 201. [Google Scholar] [CrossRef]
- Bhatt, A.; Syed, Z.Q.; Singh, H. Converging Epidemics: A Narrative Review of Tuberculosis (TB) and Human Immunodeficiency Virus (HIV) Coinfection. Cureus. 2023, 15, e47624. [Google Scholar] [CrossRef]
- Cioboata, R.; Biciusca, V.; Olteanu, M.; Vasile, C.M. COVID-19 and Tuberculosis: Unveiling the Dual Threat and Shared Solutions Perspective. Journal of clinical medicine. 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, E.; López-Varela, E.; Broderick, C.; Seddon, J.A. Examining the Complex Relationship Between Tuberculosis and Other Infectious Diseases in Children. Frontiers in Pediatrics. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Elhatw, A.; Hegazy, M.; Albeyoumi, H.; Sakr, N.; Deyab, A.M.; et al. The Evaluation of Lymphadenopathy in a Resource-Limited Setting. Cureus. 2022, 14, e30623. [Google Scholar] [CrossRef]
- Mohseni, S.; Shojaiefard, A.; Khorgami, Z.; Alinejad, S.; Ghorbani, A.; Ghafouri, A. Peripheral lymphadenopathy: approach and diagnostic tools. Iranian journal of medical sciences. 2014, 39 (2 Suppl), 158–170. [Google Scholar]
- Petakh, P.; Kobyliak, N.; Kamyshnyi, A. Gut microbiota in patients with COVID-19 and type 2 diabetes: A culture-based method. Frontiers in cellular and infection microbiology. 2023, 13, 1142578. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Gao, Q.; Zhao, Y.; Lai, Y. Clinical and immunologic features of co-infection in COVID-19 patients, along with potential traditional Chinese medicine treatments. Frontiers in immunology. 2024, 15, 1357638. [Google Scholar] [CrossRef]
- SM, M.; Vijayaraghavan, A.; Sundaram, S.; Nair, S.; Sukumaran, S. Post-infectious Neurological Complications of COVID-19– A tertiary care centre experience. Journal of Clinical Virology Plus. 2023, 3, 100165. [Google Scholar]
- Petakh, P.; Oksenych, V.; Kamyshnyi, A. The F/B ratio as a biomarker for inflammation in COVID-19 and T2D: Impact of metformin. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2023, 163, 114892. [Google Scholar]
- Halabitska, I.; Babinets, L.; Oksenych, V.; Kamyshnyi, O. Diabetes and Osteoarthritis: Exploring the Interactions and Therapeutic Implications of Insulin, Metformin, and GLP-1-Based Interventions. Biomedicines. 2024, 12. [Google Scholar] [CrossRef] [PubMed]
- Callender, L.A.; Curran, M.; Bates, S.M.; Mairesse, M.; Weigandt, J.; Betts, C.J. The Impact of Pre-existing Comorbidities and Therapeutic Interventions on COVID-19. Frontiers in immunology. 2020, 11, 1991. [Google Scholar] [CrossRef]
- Lebu, S.; Kibone, W.; Muoghalu, C.C.; Ochaya, S.; Salzberg, A.; Bongomin, F.; et al. Soil-transmitted helminths: A critical review of the impact of co-infections and implications for control and elimination. PLoS neglected tropical diseases. 2023, 17, e0011496. [Google Scholar] [CrossRef]
- Halabitska, I.; Oksenych, V.; Kamyshnyi, O. Exploring the Efficacy of Alpha-Lipoic Acid in Comorbid Osteoarthritis and Type 2 Diabetes Mellitus. 2024. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Kamyshnyi, A. Gene expression of protein kinase AMP-activated catalytic subunit alpha 1 (PRKAA1), solute carrier family 2 member 1 (SLC2A1) and mechanistic target of rapamycin (MTOR) in metformin-treated type 2 diabetes patients with COVID-19: impact on inflammation markers. Inflammopharmacology. 2024, 32, 885–891. [Google Scholar]
- Buchynskyi, M.; Kamyshna, I.; Lyubomirskaya, K.; Moshynets, O.; Kobyliak, N.; Oksenych, V.; et al. Efficacy of interferon alpha for the treatment of hospitalized patients with COVID-19: A meta-analysis. Frontiers in immunology. 2023, 14, 1069894. [Google Scholar] [CrossRef]
- Diamanti, A.P.; Rosado, M.M.; Pioli, C.; Sesti, G. ; Laganà; B Cytokine Release Syndrome in COVID-19 Patients, A New Scenario for an Old Concern: The Fragile Balance between Infections and Autoimmunity. International journal of molecular sciences. 2020, 21, 3330. [Google Scholar] [CrossRef] [PubMed]
- Nahid, P.; Dorman, S.E.; Alipanah, N.; Barry, P.M.; Brozek, J.L.; Cattamanchi, A.; et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016, 63, e147–e95. [Google Scholar] [CrossRef]
- Li, F.; Chen, D.; Zeng, Q.; Du, Y. Possible Mechanisms of Lymphopenia in Severe Tuberculosis. Microorganisms. italic>2023, 11. [Google Scholar]
- Petakh, P.; Kamyshna, I.; Nykyforuk, A.; Yao, R.; Imbery, J.F.; Oksenych, V.; et al. Immunoregulatory Intestinal Microbiota and COVID-19 in Patients with Type Two Diabetes: A Double-Edged Sword. Viruses. 2022, 14. [Google Scholar] [CrossRef]
- Petakh, P.; Griga, V.; Mohammed, I.B.; Loshak, K.; Poliak, I.; Kamyshnyiy, A. Effects of Metformin, Insulin on Hematological Parameters of COVID-19 Patients with Type 2 Diabetes. Medical archives (Sarajevo, Bosnia and Herzegovina). 2022, 76, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Comberiati, P.; Di Cicco, M.; Paravati, F.; Pelosi, U.; Di Gangi, A.; Arasi, S.; et al. The Role of Gut and Lung Microbiota in Susceptibility to Tuberculosis. International journal of environmental research and public health. 2021, 18. [Google Scholar] [CrossRef]
- Halabitska, I.; Petakh, P.; Kamyshna, I.; Oksenych, V.; Kainov, D.E.; Kamyshnyi, O. The interplay of gut microbiota, obesity, and depression: insights and interventions. Cellular and molecular life sciences : CMLS. 2024, 81, 443. [Google Scholar] [CrossRef] [PubMed]
- Gęca, T.; Wojtowicz, K.; Guzik, P.; Góra, T. Increased Risk of COVID-19 in Patients with Diabetes Mellitus-Current Challenges in Pathophysiology, Treatment and Prevention. International journal of environmental research and public health. 2022, 19. [Google Scholar] [CrossRef]
- Halabitska, I.; Petakh, P.; Lushchak, O.; Kamyshna, I.; Oksenych, V.; Kamyshnyi, O. Metformin in Antiviral Therapy: Evidence and Perspectives. Viruses. 2024, 16, 1938. [Google Scholar] [CrossRef] [PubMed]
- Dass, S.A.; Balakrishnan, V.; Arifin, N.; Lim, C.S.; Nordin, F.; Tye, G.J. The COVID-19/Tuberculosis Syndemic and Potential Antibody Therapy for TB Based on the Lessons Learnt From the Pandemic. Frontiers in immunology. 2022, 13, 833715. [Google Scholar] [CrossRef]
- DeWolf, S.; Laracy, J.C.; Perales, M.A.; Kamboj, M.; van den Brink, M.R.; Vardhana, S. SARS-CoV-2 in immunocompromised individuals. Immunity. 2022, 55, 1779–1798. [Google Scholar] [CrossRef]
- Alemu, A.; Bitew, Z.W.; Seid, G.; Diriba, G.; Gashu, E.; Berhe, N.; et al. Tuberculosis in individuals who recovered from COVID-19: A systematic review of case reports. PloS one. 2022, 17, e0277807. [Google Scholar] [CrossRef]
- Fehily, S.R.; Al-Ani, A.H.; Abdelmalak, J.; Rentch, C.; Zhang, E.; Denholm, J.T.; et al. Review article: latent tuberculosis in patients with inflammatory bowel diseases receiving immunosuppression-risks, screening, diagnosis and management. Alimentary pharmacology & therapeutics. 2022, 56, 6–27. [Google Scholar]
- WHO Guidelines Approved by the Guidelines Review Committee. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management; World Health Organization: World Health Organization, 2018; Volume 2018. [Google Scholar]
- Goletti, D.; Pisapia, R.; Fusco, F.M.; Aiello, A.; Van Crevel, R. Epidemiology, pathogenesis, clinical presentation and management of TB in patients with HIV and diabetes. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2023, 27, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nature reviews Drug discovery. 2018, 17, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Yang, L.; Li, L.; Ye, Z. Gong, W. Mycobacterium tuberculosis: immune response, biomarkers, and therapeutic intervention. MedComm. 2024, 5, e419. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Paladin, F.; Mangini, G.; Tiso, D.; Gangemi, S. TBC and COVID: an interplay between two infections. Expert opinion on drug safety. 2023, 22, 303–311. [Google Scholar] [CrossRef]
- Sharan, R.; Bucşan, A.N.; Ganatra, S.; Paiardini, M.; Mohan, M.; Mehra, S.; et al. Chronic Immune Activation in TB/HIV Co-infection. Trends in microbiology. 2020, 28, 619–632. [Google Scholar] [CrossRef]
- Olivier, C.; Luies, L. . Metabolic insights into HIV/TB co-infection: an untargeted urinary metabolomics approach. Metabolomics : Official journal of the Metabolomic Society. 2024, 20, 78. [Google Scholar] [CrossRef]
- Herbert, C.; Luies, L.; Loots, D.T.; Williams, A.A. The metabolic consequences of HIV/TB co-infection. BMC infectious diseases. 2023, 23, 536. [Google Scholar] [CrossRef]
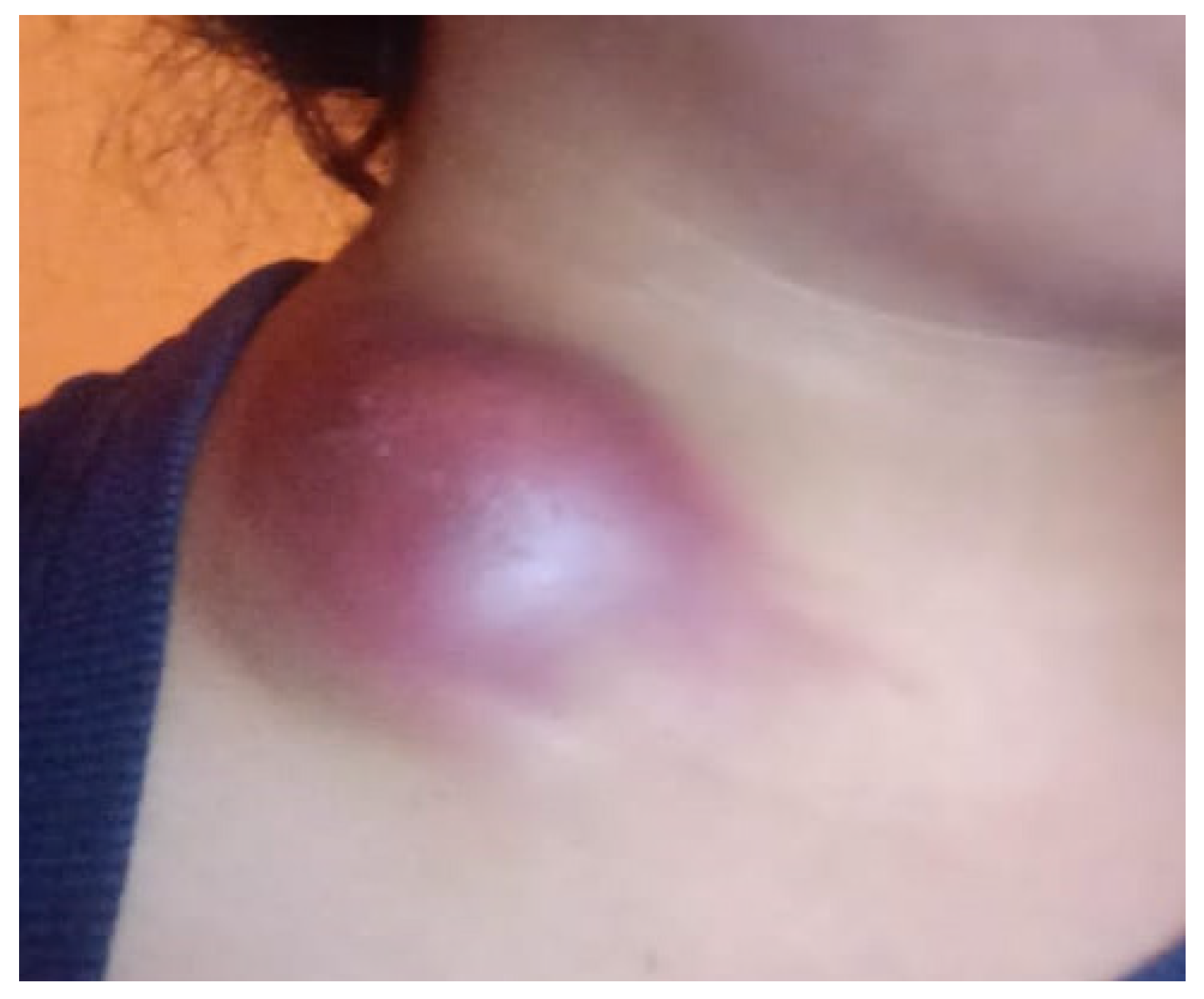
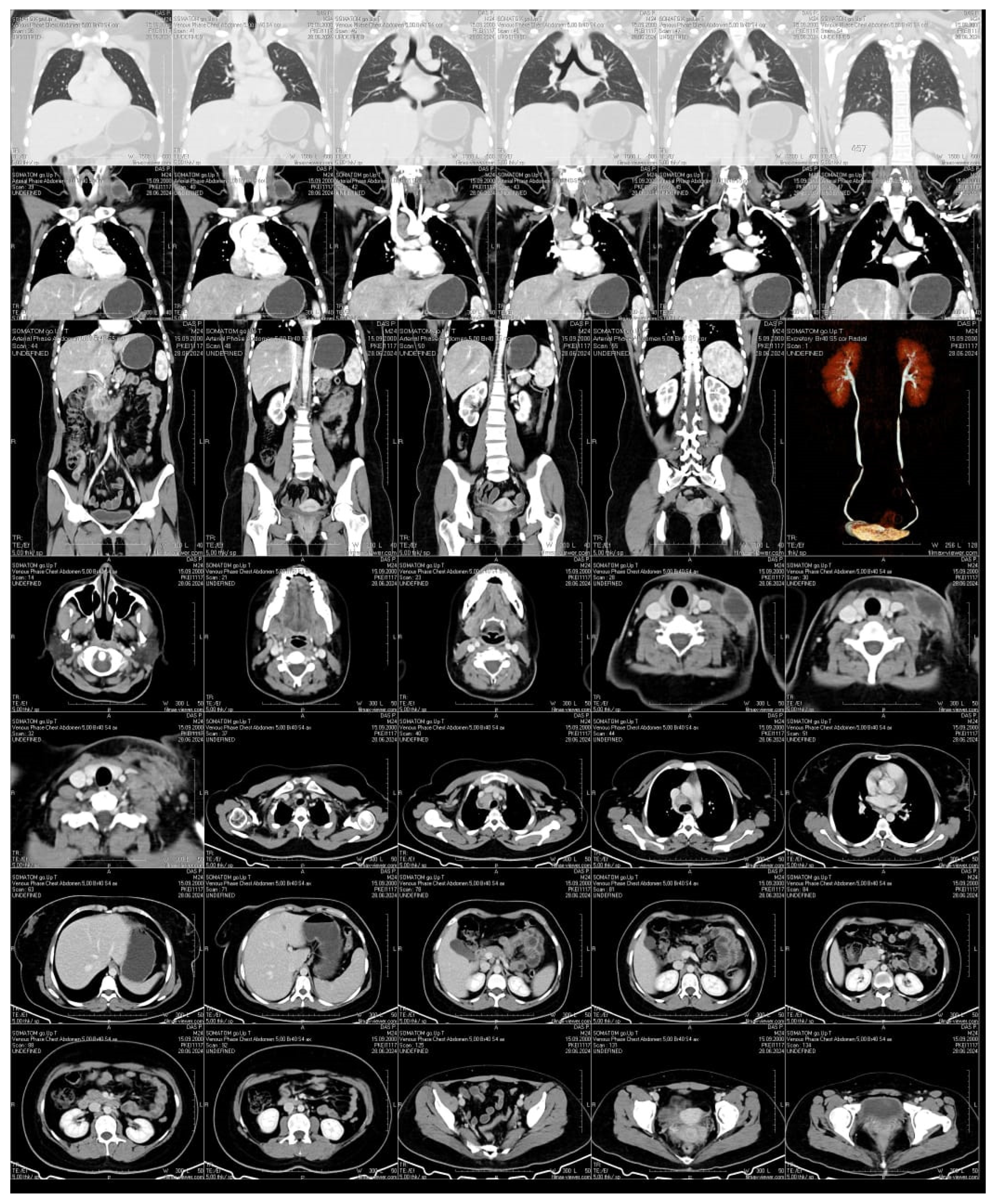
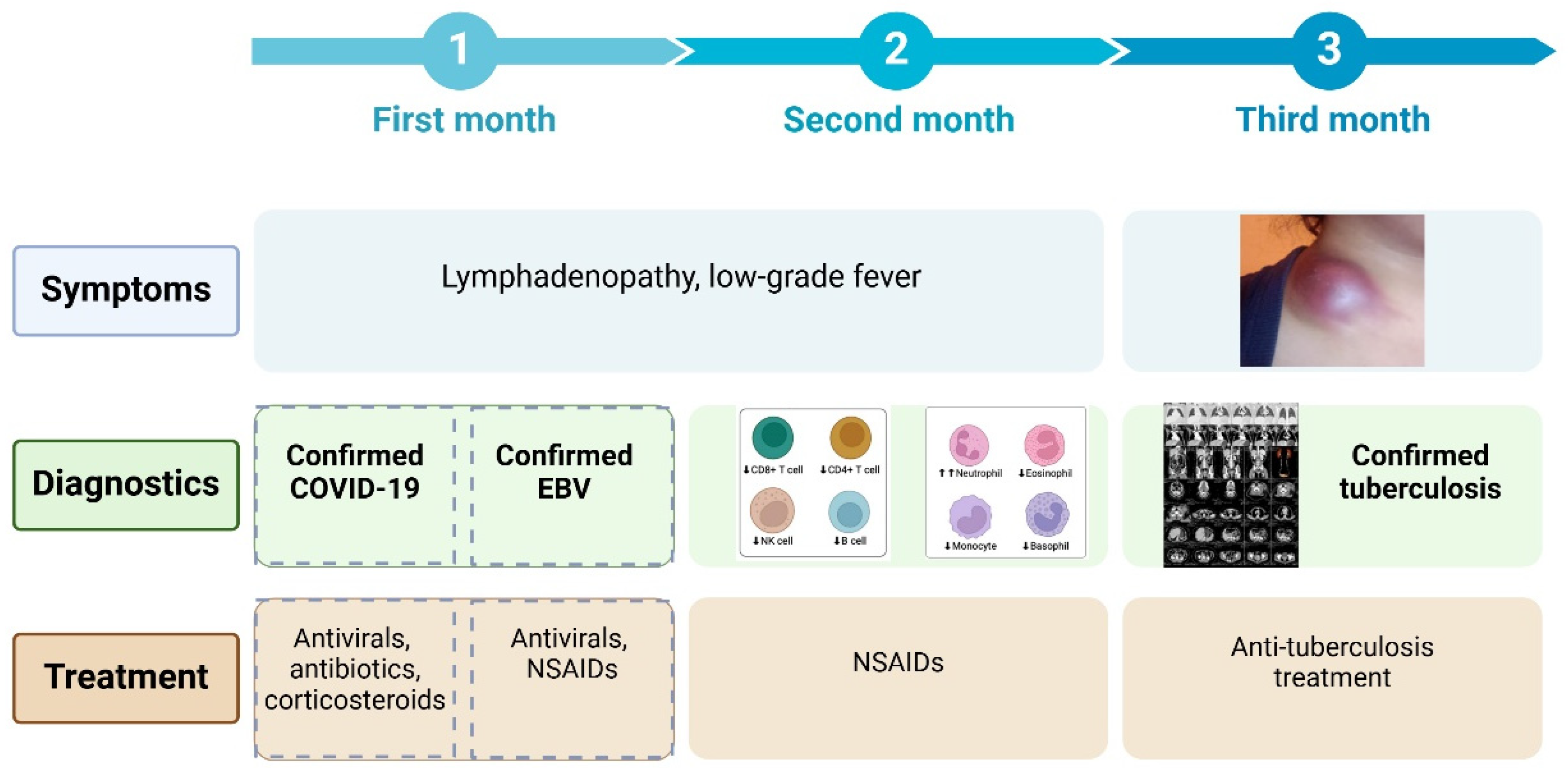
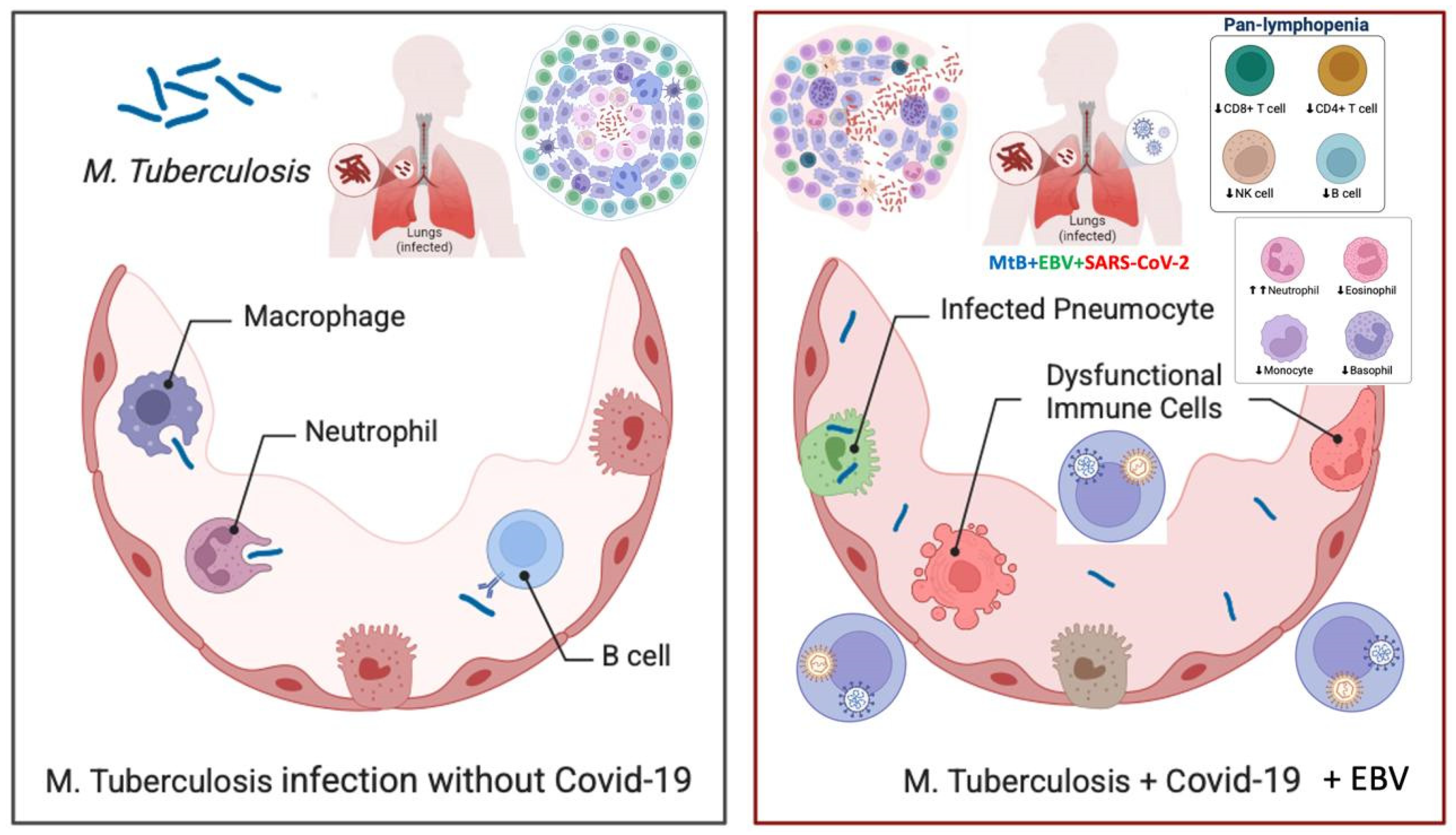
| Parameter | Normal Range | Before Diagnosis | After Initial Treatment | Worsening Symptoms | After Tuberculosis Treatment |
|---|---|---|---|---|---|
| Hemoglobin (g/L) | 120–160 | 118 | 110 | 102 | 115 |
| Erythrocytes (×10⁹/L) | 3.8–5.2 | 3.7 | 3.5 | 3.2 | 3.6 |
| Hematocrit (%) | 36–46 | 35 | 33 | 31 | 34 |
| Leukocytes (×10⁹/L) | 4.0–9.0 | 9.8 | 11.2 | 12.5 | 10.3 |
| Neutrophils (%) | 40–75 | 78 | 82 | 85 | 76 |
| Lymphocytes (%) | 20–45 | 15 | 12 | 9 | 14 |
| Monocytes (%) | 2–10 | 2 | 1.6 | 1.5 | 1.8 |
| Eosinophils (%) | 1–6 | 0.5 | 0.5 | 0.2 | 0.8 |
| Basophils (%) | 0–1 | 0.5 | 0.3 | 0.2 | 0.4 |
| ESR (mm/h) | < 20 | 25 | 32 | 40 | 28 |
| C-reactive protein (mg/L) | < 5 | 12 | 18 | 24 | 10 |
| Procalcitonin (ng/mL) | < 0.5 | 0.8 | 1.2 | 1.5 | 0.6 |
| D-dimer (µg/mL) | < 0.5 | 1.5 | 2.3 | 2.8 | 1.1 |
| Ferritin (ng/mL) | 30–400 | 450 | 500 | 550 | 470 |
| Pameter | Normal Range | After Initial Treatment | Worsening Symptoms | After Tuberculosis Treatment |
|---|---|---|---|---|
| CD4+ T lymphocytes (%) | 30–60 | 25 | 20 | 30 |
| CD8+ T lymphocytes (%) | 15–35 | 14 | 12 | 18 |
| B lymphocytes (%) | 5–20 | 3 | 2 | 5 |
| NK cells (%) | 5–15 | 4 | 4 | 6 |
| Complement C4 (mg/dL) | 18–40 | 10 | 8 | 15 |
| Complement C3 (mg/dL) | 90–180 | 110 | 100 | 130 |
| Immunoglobulin G (IgG, Serum) (g/L) | 7–16 | 7.0 | 6.8 | 7.8 |
| Immunoglobulin M (IgM, Serum) (g/L) | 0.4–2.3 | 0.6 | 0.8 | 0.7 |
| Immunoglobulin A (IgA, Serum) (g/L) | 0.7–4.0 | 1.1 | 0.9 | 1.3 |
| Immunoglobulin E (IgE, Total, Serum) (IU/mL) | <100 | 80 | 78 | 73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





