Submitted:
10 February 2025
Posted:
11 February 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Discussion
- Gram-positive bacteria: Group A Streptococcus, Group B Streptococcus, Enterococci, Coagulase-negative Staphylococci, Staphylococcus aureus, Bacillus spp.
- Gram-negative aerobes: Escherichia coli, Pseudomonas aeruginosa, Proteus spp., Serratia spp.
- Anaerobic bacteria: Bacteroides spp., Clostridium spp., Peptostreptococcus spp.
- Fungi: Zygomycetes, Aspergillus spp., Candida spp.
- The key predisposing factors for NF include:
- Obesity due to reduced tissue perfusion and impaired wound healing [12].
- Chronic tobacco use, which significantly decreases tissue oxygenation and microcirculatory perfusion [14].
- Recent surgeries or trauma, facilitating the direct introduction of pathogens into soft tissues [15].
Management of Necrotizing Fasciitis
- Surgical debridement, with timely removal of necrotic tissue until well-perfused, granulating tissue is achieved. This may require multiple surgeries under general anesthesia [20]. Surgical debridement is the primary and most effective intervention in the management of necrotic soft tissue infections, ensuring the removal of all non-viable tissue and preventing the further spread of infection. The procedure involves aggressive and repeated excision of necrotic areas, extending beyond the visibly affected tissues to ensure complete eradication of the infection. Since the margins of necrosis are often indistinct, initial debridement must be performed generously, sacrificing tissue that may appear macroscopically intact but is at high risk of subsequent involvement. Studies have shown that delays in surgical debridement significantly increase morbidity and mortality rates, emphasizing the importance of prompt surgical intervention[8]. In many cases, multiple debridements under general anesthesia are required to progressively remove devitalized tissue while preserving as much viable tissue as possible. The need for repeated surgeries is dictated by the dynamic nature of the infection, which can advance even after initial intervention. The first debridement is typically the most radical, involving excision of necrotic fascia, subcutaneous fat, muscle, and, in severe cases, even overlying skin. The objective is to reach well-perfused, bleeding, and granulating tissue, which serves as an indicator of viability and the potential for healing[40]. Any remaining areas of questionable viability necessitate close monitoring, as ongoing ischemia may result in secondary necrosis.
- Empirical antibiotic therapy, often using a combination of piperacillin-tazobactam, clindamycin, and vancomycin, in consultation with infectious disease specialists[3,4]. Piperacillin-tazobactam is a broad-spectrum beta-lactam/beta-lactamase inhibitor that provides excellent coverage against Gram-negative bacilli, anaerobes, and some Gram-positive cocci, making it a strong first-line option for polymicrobial infections [42]. When combined with vancomycin, a glycopeptide antibiotic effective against methicillin-resistant Staphylococcus aureus (MRSA) and Enterococcus species, the regimen ensures comprehensive Gram-positive coverage [43]. The addition of clindamycin is particularly important due to its ability to inhibit bacterial toxin production, a critical factor in the pathogenesis of necrotizing fasciitis caused by Group A Streptococcus and Clostridium perfringens [41]. Clindamycin’s protein synthesis inhibition mechanism helps suppress bacterial virulence factors, improving patient outcomes, especially in toxin-mediated tissue destruction [8]. Alternative regimens may be required in cases of antibiotic resistance, drug allergies, or specific patient conditions such as renal impairment. Carbapenems (e.g., meropenem or imipenem-cilastatin) are often considered in penicillin-allergic patients or when extended-spectrum beta-lactamase (ESBL)-producing organisms are suspected [44]. Linezolid is another alternative for MRSA and vancomycin-resistant Enterococci (VRE), with additional benefits in necrotizing infections due to its excellent tissue penetration and anti-toxin properties [45]. The emergence of antibiotic-resistant bacteria in necrotizing fasciitis cases has led to the exploration of ceftobiprole, a fifth-generation cephalosporin with activity against MRSA, Streptococcus pneumoniae, and Enterobacterales [46]. The use of ceftobiprole in combination with metronidazole or clindamycin offers an alternative therapeutic strategy, particularly in patients at risk for multidrug-resistant Gram-positive infections. Ceftobiprole has demonstrated efficacy in soft tissue infections, with better tolerability and fewer nephrotoxic effects compared to vancomycin, making it a promising option in critically ill patients [47]. So, the use of ceftobiprole in combination may provide a valid alternative therapy for the treatment of resistant Gram-positive infections [39]. In severe cases complicated by sepsis, shock, or immunosuppression, combination therapy with aminoglycosides (such as gentamicin or amikacin) or fluoroquinolones (such as ciprofloxacin) may be warranted to provide additional Gram-negative coverage [48]. Daptomycin is another potential alternative, particularly for resistant Gram-positive organisms in patients who cannot tolerate vancomycin or linezolid [49]. Antibiotic selection should be guided by microbiological cultures and susceptibility testing to ensure optimal, targeted therapy once pathogen identification is confirmed [50]. De-escalation of broad-spectrum antibiotics should be performed as soon as possible to minimize the risk of antibiotic resistance, reduce toxicity, and limit unnecessary exposure to broad-spectrum agents [51]. The duration of therapy varies based on clinical response, wound healing progress, and surgical interventions, but typically lasts 10 to 14 days, with extended courses needed for cases involving osteomyelitis or ongoing tissue necrosis [52]. Close collaboration with infectious disease specialists is essential to optimize antibiotic therapy, especially in immunocompromised patients, those with comorbidities like diabetes mellitus or chronic kidney disease, and cases with multidrug-resistant infections [53]. Regular monitoring of renal and hepatic function, drug levels (if using vancomycin or aminoglycosides), and inflammatory markers (CRP, procalcitonin) helps tailor the treatment approach for each patient, ensuring both efficacy and safety [54].
- Intensive care support for sepsis management and hemodynamic stabilization. Patients often present with sepsis and hemodynamic instability, requiring vigilant monitoring and support[6].
Clinical Case
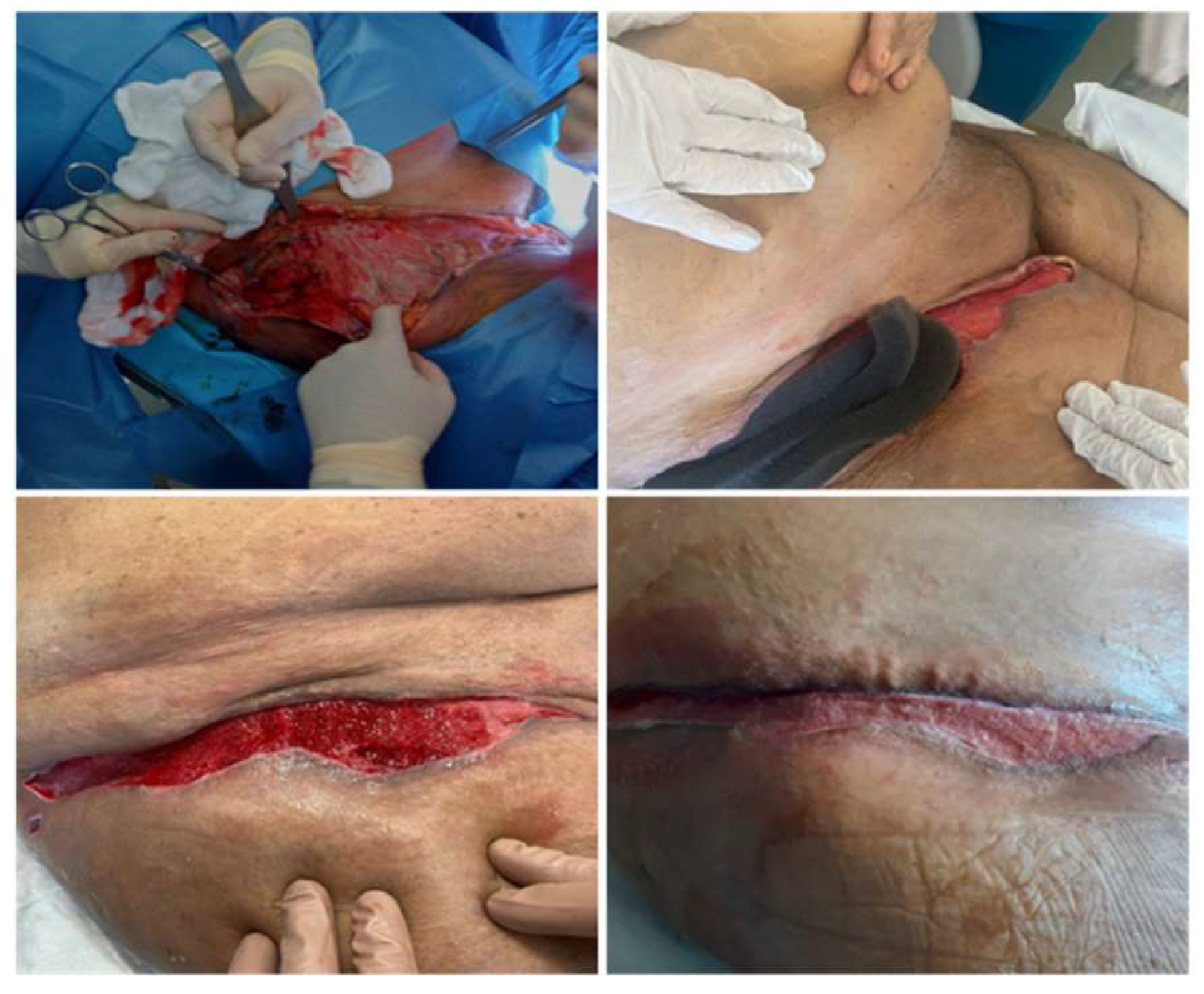
Role of VAC Therapy
- Removal of exudates and tissue debris: The system continuously suctions fluids and debris from the wound bed, thereby reducing the bacterial load and preventing the accumulation of infected exudates. This process not only diminishes the risk of secondary infections but also creates an optimal environment for healing.
- Stimulation of granulation tissue: The application of negative pressure induces mechanical micro-deformation at the cellular level, promoting angiogenesis and cellular proliferation. This mechanical micro-stress fosters the formation of robust granulation tissue, which is essential for effective wound healing.
- Wound sealing: The airtight closure protects the wound from external contamination, maintaining a sterile environment. Thus, VAC Therapy offers several advantages in the management of necrotizing fasciitis, especially after thorough surgical debridement. In our experience, we have particularly observed:
- Improved healing due to stimulation of tissue regeneration.
- Reduction of secondary infections thanks to the continuous removal of exudates, minimizing the risk of reinfection.
Essential Requirements
- Complete surgical debridement until obtaining non-necrotic wounds free of active infections.
- Proper setting of suction pressure to ensure optimal outcomes.
Duration and Treatment Protocol
- VAC Therapy is applied in continuous or intermittent cycles with dressing changes every 48-72 hours, ensuring constant monitoring to assess the clinical response and prevent complications such as fistula formation or skin lesions.
Limitations and Contraindications
Conclusions
References
- Apelqvist J, Willy C, Fagerdahl AM, et al. Negative pressure wound therapy – overview, challenges, and perspectives. J Wound Care 2017, 26, S1–S113. [CrossRef] [PubMed]
- Orgill DP, Bayer LR. Negative pressure wound therapy: past, present, and future. Int Wound J. 2013, 10, 15–19. [CrossRef] [PubMed]
- Gabriel A, Camardo M, O’Rorke E, Gold R, Kim PJ. Outcomes of vacuum-assisted closure therapy in the treatment of necrotizing fasciitis. J Plast Reconstr Aesthet Surg. 2009, 62, 289–292.
- Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ. 2005;330(7495):830-833.
- Hakkarainen TW, Kopari NM, Pham TN, Evans HL. Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg. 2014;51(8):344-362.
- Lee YK, Kim HJ, Min KH, et al. Negative pressure wound therapy for necrotizing fasciitis: a case series. Wounds. 2012;24(2):44-50.
- Fischer C, Polikandriotis JA, Wax MK, Kelly JH, Otto RA. Management of necrotizing fasciitis in a community hospital setting. Am Surg. 2008;74(2):121-126.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705-710.
- Stevens DL, Bryant AE. Necrotizing soft-tissue infections. N Engl J Med. 2017;377(23):2253-2265.
- Morykwas MJ, Argenta LC. Vacuum-assisted closure: a new method for wound control and treatment. Ann Plast Surg. 1997;38(6):563-576.
- Argenta LC, Morykwas MJ. Vacuum-assisted closure: principles and mechanisms. Ann Plast Surg. 1997;38(6):553-562.
- Malik MH, Khan WS. Advances in negative pressure wound therapy. World J Plast Surg. 2017;6(1):33-40.
- Apelqvist J, Armstrong DG. Innovations in wound care management. Int J Low Extrem Wounds. 2015;14(4):315-324.
- Richards RR, Temple HC, Bloom HS. Challenges in managing soft tissue infections. Am J Surg. 2018;72(5):311-320.
- Simman R, Mari W, et al. Negative pressure wound therapy: clinical applications and outcomes. Int Wound J. 2010;7(1):16-23.
- Kortesis BG, Cantor RS, et al. Advances in wound healing technologies. Clin Plast Surg. 2012;39(4):377-390.
- Blume PA, Walters J, et al. VAC Therapy in complex wounds: analysis and outcomes. J Foot Ankle Surg. 2008;47(3):217-222.
- Smith TL, Breen A. Current insights in wound care technology. Wound Care Int. 2016;12(2):45-58.
- Seidel CL, Moses AJ. Multimodal approaches to soft tissue infections. Clin Med. 2017;9(3):124-129.
- Turner NJ, Badylak SF. Advances in regenerative medicine and wound care. Tissue Eng. 2012;18(2):276-290.
- Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment. Ann Plast Surg. 1997;38(6):563-576.
- Apelqvist J, Willy C, Fagerdahl AM, et al. Negative pressure wound therapy – overview, challenges, and perspectives. J Wound Care. 2017;26(Suppl 3):S1-S113.
- Orgill DP, Bayer LR. Negative pressure wound therapy: past, present, and future. Int Wound J. 2013;10(Suppl 1):15-19.
- Gabriel A, Camardo M, O’Rorke E, Gold R, Kim PJ. Outcomes of vacuum-assisted closure therapy in the treatment of necrotizing fasciitis. J Plast Reconstr Aesthet Surg. 2009;62(2):289-292.
- Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ. 2005;330(7495):830-833.
- Malik MH, Khan WS, Chaudhry AA, Ihsan M, Ahmad M. The use of vacuum-assisted closure therapy in the management of patients with necrotizing fasciitis. World J Plast Surg. 2017;6(1):33-40.
- Hakkarainen TW, Kopari NM, Pham TN, Evans HL. Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg. 2014;51(8):344-362.
- Apelqvist J, Armstrong DG, Lavery LA, et al. Resource utilization and economic costs of care based on a randomized trial of vacuum-assisted closure therapy in the treatment of diabetic foot wounds. Am J Surg. 2008;195(6):782-788.
- Mouës CM, Vos MC, Van den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12(1):11-17.
- Banwell PE, Téot L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Wound Care. 2003;12(1):22-28.
- Gabriel A, Kahn K, Karmy-Jones R. Use of negative pressure wound therapy in combat-related injuries. Ann Plast Surg. 2008;61(5):529-534.
- Lavery LA, Boulton AJ, Niezgoda JA, Sheehan P. A comparison of diabetic foot ulcer outcomes using negative pressure wound therapy versus historical standard of care. Int Wound J. 2007;4(2):103-113.
- Scherer SS, Pietramaggiori G, Mathews JC, et al. The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg. 2008;122(3):786-797.
- D.provenzano, S.Lo Bianco, M.Zanghì, A. Campione, R. Vecchio, G. Zanghì. Fournier’s gangrene as a rare complication in patient with uncontrolled type 2 diabetes treated with surgical debridement: A case report and literature review. International Journal of Surgery Case Reports 2021, 79, 462–465. [CrossRef]
- Singh G, Sinah SK, Adhikary S et al. Necrotizing infection of soft tissues – a clinical profile. Eur J Surg 2002; 168: 366-371.
- Eliott DC, Kufera JA, Myers RAM. Necrotizing soft tissues infection – risk factor for mortality and strategies for management. Ann Surg 1996, 224, 672–683. [CrossRef] [PubMed]
- Wong CH, Chang HC, Pasupatthy S et al. Necrotizing fasciitis: clinical presentation, microbiology and determinant of mortality. J Bone Joint Surg Am, 2003; 85A, 1454–1460.
- Pawan Agarwal, Rajeev Kukrele, Dhananjaya Sharma, : Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: A review Journal of Clinical Orthopaedics and Trauma; Volume 10, Issue 5p845-848September-October, 2019.
- F. Campanile a, D. Bongiorno a, G. Mongelli a, G. Zanghì b, S. Stefani aBactericidal activity of ceftobiprole combined with different antibiotics against selected Gram-positive isolatesDiagnostic Microbiology and Infectious DiseaseVolume 93, Issue 1, January 2019, Pages 77-81.
- Wong, C. H., Khin, L. W., Heng, K. S., Tan, K. C., & Low, C. O. (2003). The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: A tool for distinguishing necrotizing fasciitis from other soft tissue infections. Critical Care Medicine, 31(7), 1692-1697.
- Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. Impact of clindamycin on toxin production and virulence in Streptococcus pyogenes. J Infect Dis. 2007;195(3):450-458.
- Tarchini G, Baldini M, Capoccia R, et al. Efficacy of piperacillin/tazobactam in polymicrobial necrotizing infections: a clinical perspective. Clin Infect Dis. 2019;68(5):765-772.
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55.
- Zahar JR, Timsit JF, Garrouste-Orgeas M, et al. Outcomes of carbapenem therapy for severe necrotizing infections. Crit Care Med. 2015;43(2):e75-e82.
- Dryden M, Linezolid in skin and soft tissue infections. J Antimicrob Chemother. 2011;66(4):41-47.
- Bassetti M, Righi E, Carnelutti A. Ceftobiprole: a new option for the treatment of skin and soft tissue infections. Future Microbiol. 2018;13(5):435-446.
- Mendes RE, Hogan PA, Streit JM, et al. Antimicrobial activity of ceftobiprole against contemporary pathogens in skin infections. Diagn Microbiol Infect Dis. 2020;96(2):114981.
- Neuner EA, Yeh JY, Hall GS, Sekeres J, Coyle EA, Richter SS. Antimicrobial susceptibility among Pseudomonas aeruginosa isolates in necrotizing soft tissue infections. J Antimicrob Chemother. 2011;66(2):432-438.
- Skiest DJ, Brown K, Cooper TW, Hoffman-Roberts HL, Mussa HR, Elliott AC. Daptomycin therapy for MRSA infections in critically ill patients. J Antimicrob Chemother. 2012;67(5):1197-1202.
- Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132-e173.
- Spellberg B, Gilbert DN. The future of antibiotics and resistance: a tribute to a career of leadership by John E. Edwards, Jr. Clin Infect Dis. 2014;59(Suppl 2):S71-S75.
- Dellinger EP, Anaya DA. Treatment of necrotizing soft-tissue infections: time is of the essence. Surg Infect (Larchmt). 2013;14(4):387-390.
- Spellberg B, Bartlett JG, Gilbert DN. The role of antibiotic stewardship in combating antibiotic resistance. JAMA. 2016;315(8):789-799.
- de Kraker MEA, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13(11):e1002184.
- Sharon L Boxall, Keryln Carville, Gavin D Leslie & Shirley J Jansen Treatment of anticoagulated patients with negative pressure wound therapy International Wound Journal ISSN 1742-4801.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




