Submitted:
07 February 2025
Posted:
10 February 2025
You are already at the latest version
Abstract
Oxidative stress represents a significant contributing factor to male infertility and sperm dysfunction. In this condition, an increase in ROS production exceeds the body's antioxidant defences, resulting in a decline in spermatozoa quality and fertilising capacity. Furthermore, excessive ROS production has been linked to the promotion of genomic damage, lipid peroxidation, inflammation, altered enzyme activity, and ultimately, irreversible alterations, cell death, and a decline in seminal parameters associated with male infertility. It is established that physical activity (PA), acting on inflammatory parameters and improving antioxidant defence, can alleviate the negative effects caused by free radicals, offering numerous health benefits and positively influencing sperm quality. The objective of this review is to highlight the mechanisms of ROS production, the physiological and pathophysiological roles of ROS in relation to the male reproductive system, and recent knowledge on the impact of some protocols of PA on these systems and the molecular mechanisms involved.
Keywords:
1. Introduction
2. Spermatogenesis
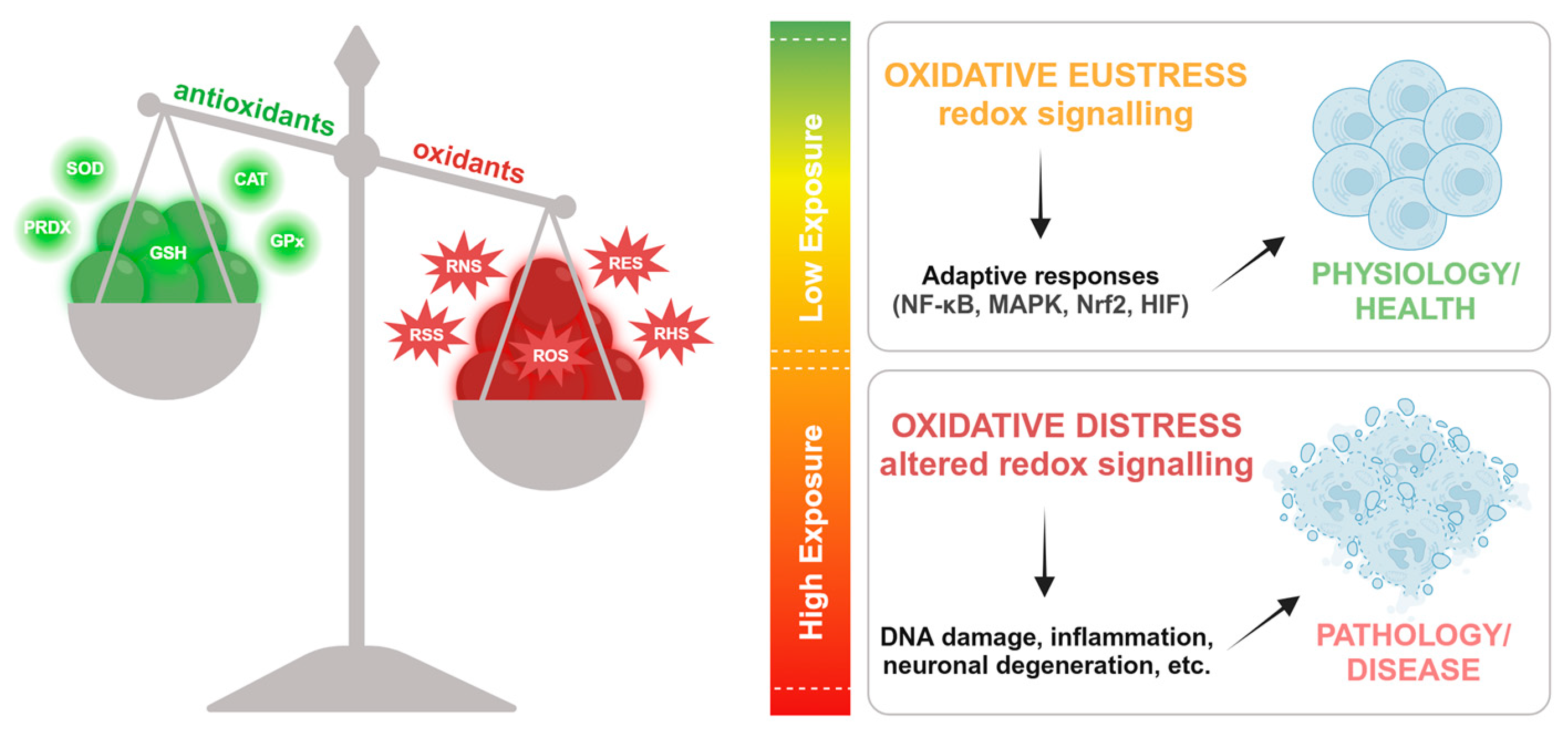
3. Oxidative stress
4. Redox homeostasis and physio-pathological conditions
5. The Role of Oxidative Stress in Male Infertility
5.1. Testicular Cells and Oxidative Stress
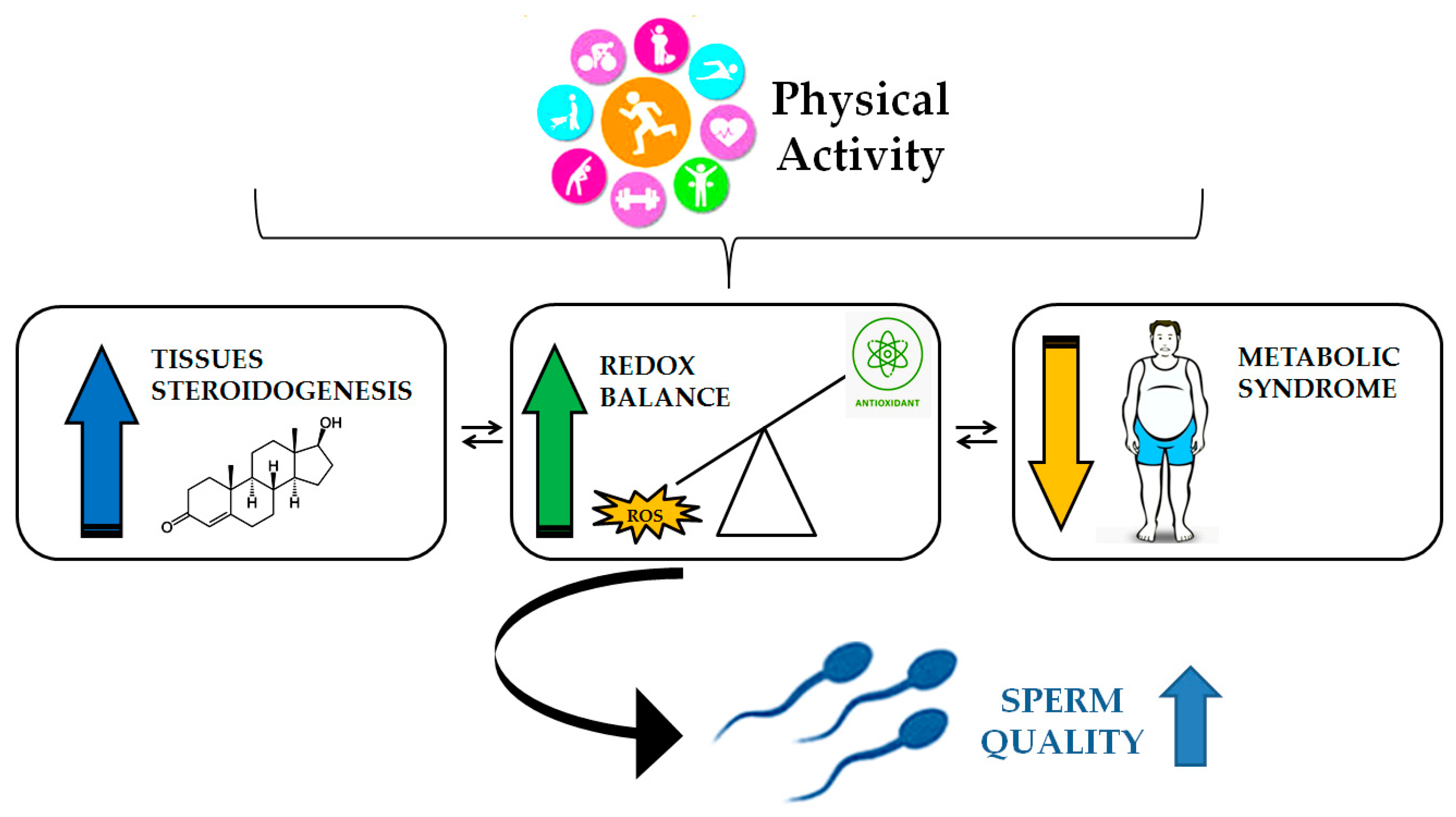
6. Spermatogenesis and Physical Activity: Role for Oxidative Stress Control.
6.1. Impact of Physical Activity on Spermatogenesis
7. Conclusion and Future Perspectives
Author Contributions
Conflicts of Interest
Abbreviations
| cAMP | cyclic adenosine monophosphate |
| CAT | catalase |
| CO | carbon monoxide |
| Cx43 | connexin-43 |
| FSH | follicle-stimulating hormone |
| GPx | glutathione peroxidase |
| GnRH | gonadotropin-releasing hormone |
| H2O2 | hydrogen peroxide |
| H2 | hydrogen |
| LCS | leukocytospermia |
| LH | luteinizing hormone |
| NH3 | ammonia |
| PKA | protein kinase A |
| PA | physical activity |
| Prdx2 | peroxiredoxin 2 |
| PUFA | polyunsaturated fatty acids |
| RES | reactive electrophile species |
| RNS | reactive nitrogen species |
| RHS | reactive halogen species |
| REDOX | oxidation–reduction |
| ROS | reactive oxygen species |
| SSCs | spermatogonial stem cells |
| SOD | superoxide dismutase |
| SPI | and spermiogenesis indices |
| TBARS | thiobarbituric acid-reactive substances |
| TDI | tubular differentiation |
References
- Rivero, M.-J.; Kulkarni, N.; Thirumavalavan, N.; Ramasamy, R. Evaluation and Management of Male Genital Tract Infections in the Setting of Male Infertility: An Updated Review. Current Opinion in Urology 2023, 33, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J Mens Health 2019, 37, 296. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.; Hetherington, L.; Aitken, R.J. Identification of SRC as a Key PKA-Stimulated Tyrosine Kinase Involved in the Capacitation-Associated Hyperactivation of Murine Spermatozoa. Journal of Cell Science 2006, 119, 3182–3192. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ghanghas, P.; Kaushal, N.; Kaur, J.; Kaur, P. Epigenetics and Oxidative Stress: A Twin-edged Sword in Spermatogenesis. Andrologia 2019, 51. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A. The Role of the Natural Antioxidant Mechanism in Sperm Cells. Reprod. Sci. 2022, 29, 1387–1394. [Google Scholar] [CrossRef]
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D.; Lauro, D. The Molecular Link between Oxidative Stress, Insulin Resistance, and Type 2 Diabetes: A Target for New Therapies against Cardiovascular Diseases. Current Opinion in Pharmacology 2022, 62, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Adelowo, O.E.; Akindele, B.M.; Adegbola, C.A.; Oyedokun, P.A.; Akhigbe, T.M.; Akhigbe, R.E. Unraveling the Complexity of the Impact of Physical Exercise on Male Reproductive Functions: A Review of Both Sides of a Coin. Front. Physiol. 2024, 15, 1492771. [Google Scholar] [CrossRef]
- Ramírez-López, C.J.; Barros, E.; Vidigal, P.M.; Okano, D.S.; Gomes, L.L.; Carvalho, R.P.R.; De Castro, A.G.; Baracat-Pereira, M.C.; Guimarães, S.E.F.; Guimarães, J.D. Oxidative Stress Associated with Proteomic and Fatty Acid Profiles of Sperm from Nellore Bulls at Rest. Biology of Reproduction 2023, 109, 878–891. [Google Scholar] [CrossRef]
- Dunleavy, J.E.M.; O’Bryan, M.K.; Stanton, P.G.; O’Donnell, L. The Cytoskeleton in Spermatogenesis. Reproduction 2019, 157, R53–R72. [Google Scholar] [CrossRef]
- Sperry, A.O. The Dynamic Cytoskeleton of the Developing Male Germ Cell. Biology of the Cell 2012, 104, 297–305. [Google Scholar] [CrossRef]
- Boguenet, M.; Bouet, P.-E.; Spiers, A.; Reynier, P.; May-Panloup, P. Mitochondria: Their Role in Spermatozoa and in Male Infertility. Human Reproduction Update 2021, 27, 697–719. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.E.; Lopez, K.H. Human Reproductive Biology, 4. ed.; Elsevier: Amsterdam Heidelberg, 2014; ISBN 978-0-12-382184-3. [Google Scholar]
- O’Shaughnessy, P.J.; Mitchell, R.T.; Monteiro, A.; O’Hara, L.; Cruickshanks, L.; Der Grinten, H.C.; Brown, P.; Abel, M.; Smith, L.B. Androgen Receptor Expression Is Required to Ensure Development of Adult Leydig Cells and to Prevent Development of Steroidogenic Cells with Adrenal Characteristics in the Mouse Testis. BMC Dev Biol 2019, 19, 8. [Google Scholar] [CrossRef]
- Wong, W.J.; Khan, Y.S. Histology, Sertoli Cell. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2025. [Google Scholar]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biology 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Eustress and Oxidative Distress: Introductory Remarks. In Oxidative Stress; Elsevier, 2020; pp. 3–12 ISBN 978-0-12-818606-0.
- Sies, H. On the History of Oxidative Stress: Concept and Some Aspects of Current Development. Current Opinion in Toxicology 2018, 7, 122–126. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox Homeostasis: The Golden Mean of Healthy Living. Redox Biology 2016, 8, 205–215. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat Rev Mol Cell Biol 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.N.; Rios, N.; Trujillo, M.; Radi, R.; Denicola, A.; Alvarez, B. Detection and Quantification of Nitric Oxide–Derived Oxidants in Biological Systems. Journal of Biological Chemistry 2019, 294, 14776–14802. [Google Scholar] [CrossRef]
- Nakamura, T.; Lipton, S.A. Nitric Oxide-Dependent Protein Post-Translational Modifications Impair Mitochondrial Function and Metabolism to Contribute to Neurodegenerative Diseases. Antioxidants & Redox Signaling 2020, 32, 817–833. [Google Scholar] [CrossRef]
- Olson, K.R. Reactive Oxygen Species or Reactive Sulfur Species: Why We Should Consider the Latter. Journal of Experimental Biology 2020, 223, jeb196352. [Google Scholar] [CrossRef]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox Signaling by Reactive Electrophiles and Oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxidants & Redox Signaling 2020, 32, 957–981. [Google Scholar] [CrossRef]
- Santolini, J.; Wootton, S.A.; Jackson, A.A.; Feelisch, M. The Redox Architecture of Physiological Function. Current Opinion in Physiology 2019, 9, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Strategies of Antioxidant Defense. European Journal of Biochemistry 1993, 215, 213–219. [Google Scholar] [CrossRef]
- López-Otín, C.; Kroemer, G. Hallmarks of Health. Cell 2021, 184, 33–63. [Google Scholar] [CrossRef] [PubMed]
- Ayres, J.S. The Biology of Physiological Health. Cell 2020, 181, 250–269. [Google Scholar] [CrossRef]
- Demirovic, D.; Rattan, S.I.S. Establishing Cellular Stress Response Profiles as Biomarkers of Homeodynamics, Health and Hormesis. Experimental Gerontology 2013, 48, 94–98. [Google Scholar] [CrossRef]
- Lloyd, D.; Aon, M.A.; Cortassa, S. Why Homeodynamics, Not Homeostasis? The Scientific World JOURNAL 2001, 1, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. Stress and Distress. Compr Ther 1975, 1, 9–13. [Google Scholar]
- Lu, S.; Wei, F.; Li, G. The Evolution of the Concept of Stress and the Framework of the Stress System. CST 2021, 5, 76–85. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Jones, D.P.; Sies, H. The Redox Code. Antioxidants & Redox Signaling 2015, 23, 734–746. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; Van Goor, H.; et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxidants & Redox Signaling 2017, 27, 684–712. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; Du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J Mens Health 2014, 32, 1. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Reactive Oxygen Species in Seminal Plasma as a Cause of Male Infertility. Journal of Gynecology Obstetrics and Human Reproduction 2018, 47, 565–572. [Google Scholar] [CrossRef]
- Raj, C.J.; Aishwarya, C.V.S.; Mounika, K.V.S.S.N.; Mishra, B.; Sumithra, B.; Vishal, B.; Mandal, S.K. Deciphering the Nexus Between Oxidative Stress and Spermatogenesis: A Compendious Overview. In Oxidative Stress and Toxicity in Reproductive Biology and Medicine; Roychoudhury, S., Kesari, K.K., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, 2022; Volume 1391, pp. 1–16. ISBN 978-3-031-12965-0. [Google Scholar]
- Tremellen, K. Oxidative Stress and Male Infertility—a Clinical Perspective. Human Reproduction Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K. Impact of Environmental Factors on Human Semen Quality and Male Fertility: A Narrative Review. Environ Sci Eur 2022, 34, 6. [Google Scholar] [CrossRef]
- Agarwal, A.; Prabakaran, S.A. Mechanism, Measurement, and Prevention of Oxidative Stress in Male Reproductive Physiology. Indian J Exp Biol 2005, 43, 963–974. [Google Scholar]
- Tortolero, I.; Duarte Ojeda, J.M.; Pamplona Casamayor, M.; Alvarez González, E.; Arata-Bellabarba, G.; Regadera, J.; Leiva Galvis, O. [The effect of seminal leukocytes on semen quality in subfertile males with and without varicocele]. Arch Esp Urol 2004, 57, 921–928. [Google Scholar]
- Iommiello, V.M.; Albani, E.; Di Rosa, A.; Marras, A.; Menduni, F.; Morreale, G.; Levi, S.L.; Pisano, B.; Levi-Setti, P.E. Ejaculate Oxidative Stress Is Related with Sperm DNA Fragmentation and Round Cells. International Journal of Endocrinology 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Kurkowska, W.; Bogacz, A.; Janiszewska, M.; Gabryś, E.; Tiszler, M.; Bellanti, F.; Kasperczyk, S.; Machoń-Grecka, A.; Dobrakowski, M.; Kasperczyk, A. Oxidative Stress Is Associated with Reduced Sperm Motility in Normal Semen. Am J Mens Health 2020, 14, 1557988320939731. [Google Scholar] [CrossRef]
- Aitken, R.J.; Roman, S.D. Antioxidant Systems and Oxidative Stress in the Testes. In Molecular Mechanisms in Spermatogenesis; Cheng, C.Y., Ed.; Advances in Experimental Medicine and Biology; Springer New York: New York, NY, 2009; ISBN 978-0-387-79990-2. [Google Scholar]
- Asadi, N. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving It: A Review. JCDR 2017. [Google Scholar] [CrossRef]
- Henkel, R.R. Leukocytes and Oxidative Stress: Dilemma for Sperm Function and Male Fertility. Asian J Androl 2011, 13, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Vahedi Raad, M.; Firouzabadi, A.M.; Tofighi Niaki, M.; Henkel, R.; Fesahat, F. The Impact of Mitochondrial Impairments on Sperm Function and Male Fertility: A Systematic Review. Reprod Biol Endocrinol 2024, 22, 83. [Google Scholar] [CrossRef]
- Chandra, S.; Romero, M.; Shatanawi, A.; Alkilany, A.; Caldwell, R.; Caldwell, R. Oxidative Species Increase Arginase Activity in Endothelial Cells through the RhoA/Rho Kinase Pathway. British J Pharmacology 2012, 165, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Dare, B. Role of Antioxidant in Testicular Integrity. ARRB 2014, 4, 998–1023. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress That Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef]
- Hu, R.; Yang, X.; He, J.; Wu, S. Oxidative Stress and Autophagy: Unraveling the Hidden Threat to Boars’ Fertility. Antioxidants 2024, 14, 2. [Google Scholar] [CrossRef]
- Washburn, R.L.; Hibler, T.; Kaur, G.; Dufour, J.M. Sertoli Cell Immune Regulation: A Double-Edged Sword. Front. Immunol. 2022, 13, 913502. [Google Scholar] [CrossRef]
- Zhang, P.; Li, F.; Zhang, L.; Lei, P.; Zheng, Y.; Zeng, W. Stage-specific Embryonic Antigen 4 Is a Membrane Marker for Enrichment of Porcine Spermatogonial Stem Cells. Andrology 2020, 8, 1923–1934. [Google Scholar] [CrossRef]
- Aly, H.A.A.; Khafagy, R.M. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD)-Induced Cytotoxicity Accompanied by Oxidative Stress in Rat Sertoli Cells: Possible Role of Mitochondrial Fractions of Sertoli Cells. Toxicology and Applied Pharmacology 2011, 252, 273–280. [Google Scholar] [CrossRef]
- Yilmaz, B.; Yildizbayrak, N.; Aydin, Y.; Erkan, M. Evidence of Acrylamide- and Glycidamide-Induced Oxidative Stress and Apoptosis in Leydig and Sertoli Cells. Hum Exp Toxicol 2017, 36, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Quan, C.; Huang, W.-T.; Yang, K. [PI3K-Akt/LKB1-AMPK-mTOR-p70S6K/4EBP1 signaling pathways participate in the regulation of testis development and spermatogenesis: An update]. Zhonghua Nan Ke Xue 2016, 22, 1016–1020. [Google Scholar] [PubMed]
- Cinthya Riris, A.A.I.D.; I’tishom, R.; Khaerunnisa, S. Role of Antioxidant to Protect Leydig Cells Induced by Reactive Oxygen Species: A Literature Review. Qanun Medika 2021, 5, 49. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig Cells: Effects of Aging and Environmental Factors. Reproduction 2017, 154, R111–R122. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Oxidative Stress, Sperm Survival and Fertility Control. Molecular and Cellular Endocrinology 2006, 250, 66–69. [Google Scholar] [CrossRef]
- Cafe, S.L.; Nixon, B.; Dun, M.D.; Roman, S.D.; Bernstein, I.R.; Bromfield, E.G. Oxidative Stress Dysregulates Protein Homeostasis Within the Male Germ Line. Antioxidants & Redox Signaling 2020, 32, 487–503. [Google Scholar] [CrossRef]
- Cafe, S.L.; Nixon, B.; Ecroyd, H.; Martin, J.H.; Skerrett-Byrne, D.A.; Bromfield, E.G. Proteostasis in the Male and Female Germline: A New Outlook on the Maintenance of Reproductive Health. Front Cell Dev Biol 2021, 9, 660626. [Google Scholar] [CrossRef]
- Duong, L.D.; West, J.D.; Morano, K.A. Redox Regulation of Proteostasis. Journal of Biological Chemistry 2024, 300, 107977. [Google Scholar] [CrossRef]
- Hipp, M.S.; Hartl, F.U. Interplay of Proteostasis Capacity and Protein Aggregation: Implications for Cellular Function and Disease. Journal of Molecular Biology 2024, 436, 168615. [Google Scholar] [CrossRef]
- Long, S.; Kenworthy, S. Round Cells in Diagnostic Semen Analysis: A Guide for Laboratories and Clinicians. Br J Biomed Sci 2022, 79, 10129. [Google Scholar] [CrossRef]
- Pavuluri, H.; Bakhtiary, Z.; Panner Selvam, M.K.; Hellstrom, W.J.G. Oxidative Stress-Associated Male Infertility: Current Diagnostic and Therapeutic Approaches. Medicina 2024, 60, 1008. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J Hum Reprod Sci 2019, 12, 4–18. [Google Scholar] [CrossRef]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative Stress: Role of Physical Exercise and Antioxidant Nutraceuticals in Adulthood and Aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomedicine & Pharmacotherapy 2023, 162, 114606. [Google Scholar] [CrossRef]
- Bo, H.; Jiang, N.; Ji, L.L.; Zhang, Y. Mitochondrial Redox Metabolism in Aging: Effect of Exercise Interventions. Journal of Sport and Health Science 2013, 2, 67–74. [Google Scholar] [CrossRef]
- Meng, Q.; Su, C.-H. The Impact of Physical Exercise on Oxidative and Nitrosative Stress: Balancing the Benefits and Risks. Antioxidants 2024, 13, 573. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? Journal of Sport and Health Science 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L.; Boldogh, I.; Koltai, E. Exercise, Oxidants, and Antioxidants Change the Shape of the Bell-Shaped Hormesis Curve. Redox Biology 2017, 12, 285–290. [Google Scholar] [CrossRef]
- Assidi, M. Infertility in Men: Advances towards a Comprehensive and Integrative Strategy for Precision Theranostics. Cells 2022, 11, 1711. [Google Scholar] [CrossRef]
- Greco, F.; Guarascio, G.; Giannetta, E.; Oranges, F.P.; Quinzi, F.; Emerenziani, G.P.; Tarsitano, M.G. The Influence of an Intense Training Regime in Professional and Non-Professional Athletes on Semen Parameters: A Systematic Review. JCM 2025, 14, 201. [Google Scholar] [CrossRef]
- Sgrò, P.; Di Luigi, L. Sport and Male Sexuality. J Endocrinol Invest 2017, 40, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Di Luigi, L.; Gentile, V.; Pigozzi, F.; Parisi, A.; Giannetti, D.; Romanelli, F. Physical Activity as a Possible Aggravating Factor for Athletes with Varicocele: Impact on the Semen Profile. Hum Reprod 2001, 16, 1180–1184. [Google Scholar] [CrossRef]
- Abedpoor, N.; Taghian, F.; Hajibabaie, F. Exploring the Dynamics of Exercise Intensity on Male Fertility and Reproductive Health: Advancements and Implications for Fertility Research. Front. Reprod. Health 2024, 6, 1423916. [Google Scholar] [CrossRef] [PubMed]
- Minas, A.; Fernandes, A.C.C.; Maciel Júnior, V.L.; Adami, L.; Intasqui, P.; Bertolla, R.P. Influence of Physical Activity on Male Fertility. Andrologia 2022, 54. [Google Scholar] [CrossRef]
- Lavín-Pérez, A.M.; Collado-Mateo, D.; Villafaina, S.; Calle-Guisado, V. The Role of Exercise to Reduce the Impact of Diabetes in the Seminal Quality: A Systematic Review. Medicina 2021, 57, 159. [Google Scholar] [CrossRef] [PubMed]
- Antinozzi, C.; Duranti, G.; Ceci, R.; Lista, M.; Sabatini, S.; Caporossi, D.; Di Luigi, L.; Sgrò, P.; Dimauro, I. Hydrogen Peroxide Stimulates Dihydrotestosterone Release in C2C12 Myotubes: A New Perspective for Exercise-Related Muscle Steroidogenesis? IJMS 2022, 23, 6566. [Google Scholar] [CrossRef]
- Lyons, H.E.; Gyawali, P.; Mathews, N.; Castleton, P.; Mutuku, S.M.; McPherson, N.O. The Influence of Lifestyle and Biological Factors on Semen Variability. J Assist Reprod Genet 2024, 41, 1097–1109. [Google Scholar] [CrossRef]
- Mehri, K.; Hamidian, G.; Babri, S.; Farajdokht, F.; Zavvari Oskuye, Z. Exercise and Insulin Glargine Administration in Mothers with Diabetes during Pregnancy Ameliorate Function of Testis in Offspring: Consequences on Apelin-13 and Its Receptor. Life Sciences 2024, 342, 122517. [Google Scholar] [CrossRef]
- Morano, S.; Gatti, A.; Mandosi, E.; Tiberti, C.; Fallarino, M.; Cipriani, R.; Buchetti, B.; Gandini, L.; Sgrò, P.; Jannini, E.A.; et al. Circulating Monocyte Oxidative Activity Is Increased in Patients With Type 2 Diabetes and Erectile Dysfunction. Journal of Urology 2007, 177, 655–659. [Google Scholar] [CrossRef]
- Zampieri, N. The Effect of Aerobic Training on Serum Levels of Adiponectin, Hypothalamic-Pituitary-Gonadal Axis and Sperm Quality in Diabetic Rats. Urology Journal 2019. [Google Scholar] [CrossRef]
- Antinozzi, C.; Lista, M.; Caponecchia, L.; Salacone, P.; Minganti, C.; Battaglia, F.A.; Di Luigi, L.; Sgrò, P. Exploratory Analysis in the Differences in Blood Serum and Seminal Plasma of Adipose-Tissue Related Peptides in Obese and Non-Obese Men and Their Correlations With Semen Parameters. Front. Endocrinol. 2021, 12, 681939. [Google Scholar] [CrossRef]
- Green, D.J.; Chasland, L.C.; Yeap, B.B.; Naylor, L.H. Comparing the Impacts of Testosterone and Exercise on Lean Body Mass, Strength and Aerobic Fitness in Aging Men. Sports Med Open 2024, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Belladelli, F.; Basran, S.; Eisenberg, M.L. Male Fertility and Physical Exercise. World J Mens Health 2023, 41, 482. [Google Scholar] [CrossRef]
- Di Luigi, L.; Sgrò, P.; Fierro, V.; Bianchini, S.; Battistini, G.; Magini, V.; Jannini, E.A.; Lenzi, A. Prevalence of Undiagnosed Testosterone Deficiency in Aging Athletes: Does Exercise Training Influence the Symptoms of Male Hypogonadism? The Journal of Sexual Medicine 2010, 7, 2591–2601. [Google Scholar] [CrossRef]
- Jóźków, P.; Rossato, M. The Impact of Intense Exercise on Semen Quality. Am J Mens Health 2017, 11, 654–662. [Google Scholar] [CrossRef]
- Hajizadeh Maleki, B.; Tartibian, B.; Chehrazi, M. The Effects of Three Different Exercise Modalities on Markers of Male Reproduction in Healthy Subjects: A Randomized Controlled Trial. Reproduction 2017, 153, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Gebreegziabher, Y.; Marcos, E.; McKinon, W.; Rogers, G. Sperm Characteristics of Endurance Trained Cyclists. Int J Sports Med 2004, 25, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Duclos, M.; Tabarin, A. Exercise and the Hypothalamo-Pituitary-Adrenal Axis. In Frontiers of Hormone Research; Lanfranco, F., Strasburger, C.J., Eds.; S. Karger AG, 2016; Vol. 47, pp. 12–26 ISBN 978-3-318-05868-0.
- Brownlee, K.K.; Moore, A.W.; Hackney, A.C. Relationship between Circulating Cortisol and Testosterone: Influence of Physical Exercise. J Sports Sci Med 2005, 4, 76–83. [Google Scholar]
- Duclos, M.; Guinot, M.; Le Bouc, Y. Cortisol and GH: Odd and Controversial Ideas. Appl. Physiol. Nutr. Metab. 2007, 32, 895–903. [Google Scholar] [CrossRef]
- Snyder, A.C.; Hackney, A.C. The Endocrine System in Overtraining. In Endocrinology of Physical Activity and Sport; Constantini, N., Hackney, A.C., Eds.; Humana Press: Totowa, NJ, 2013; pp. 523–534. ISBN 978-1-62703-313-8. [Google Scholar]
- Sansone, M.; Sansone, A.; Borrione, P.; Romanelli, F.; Di Luigi, L.; Sgrò, P. Effects of Ketone Bodies on Endurance Exercise. Curr Sports Med Rep 2018, 17, 444–453. [Google Scholar] [CrossRef]
- Walke, G.; Gaurkar, S.S.; Prasad, R.; Lohakare, T.; Wanjari, M. The Impact of Oxidative Stress on Male Reproductive Function: Exploring the Role of Antioxidant Supplementation. Cureus 2023. [Google Scholar] [CrossRef]
- Torres-Arce, E.; Vizmanos, B.; Babio, N.; Márquez-Sandoval, F.; Salas-Huetos, A. Dietary Antioxidants in the Treatment of Male Infertility: Counteracting Oxidative Stress. Biology 2021, 10, 241. [Google Scholar] [CrossRef]
- Yavari, A.; Javadi, M.; Mirmiran, P.; Bahadoran, Z. Exercise-Induced Oxidative Stress and Dietary Antioxidants. Asian J Sports Med 2015, 6, e24898. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




