1. Introduction
Postbiotics, a relatively recent concept in microbiology and skincare, refer to microbial metabolites, including short-chain fatty acids, functional proteins, extracellular metabolites, cell lysates, and other products derived from probiotics [
1]. These compounds can influence the microbiome's composition and provide numerous health and skincare benefits. With growing consumer demand for natural and effective skincare solutions, the adoption of postbiotic-infused products is on the rise [
2]. Clinical studies highlight the benefits of postbiotic formulations, including enhanced skin hydration, improved epithelial tone, reduced wrinkle depth, and acceptability for sensitive skin [
3]. Unlike live probiotics, which face stability and shelf-life challenges in skincare formulations, postbiotics offer a stable, innovative alternative, making them a promising advancement in the cosmetics market.
The postbiotics market is expanding rapidly, with the personal care and cosmetics sector playing a pivotal role [
4]. According to Markets and Markets, the microbiome cosmetic products market is projected to reach USD 76.16 million by 2030 [
5], reflecting a surge in consumer interest in products supporting skin microbiome health. Postbiotics are celebrated for their extended shelf life, safety, and diverse benefits, including anti-inflammatory, immunomodulatory, antioxidant, and antimicrobial properties [
6]. These attributes position postbiotic-based formulations as effective solutions for various skin concerns, particularly acne-related conditions [11].
Acne vulgaris (AV), a widespread skin disorder affecting the pilosebaceous units, is most common during puberty but can persist across different life stages. Globally, it is ranked as the eighth most common skin disease, impacting 9.4% of the population, with young adults comprising 85% of cases [
7]. Acne manifests through comedones, papules, pustules, nodules, and scars, caused by increased sebum production, follicular hyperkeratinization, and
Propionibacterium acnes (or
Cutibacterium acnes) proliferation. A similar condition, Malassezia folliculitis (MF), results from an overgrowth of
Malassezia yeast. MF typically presents as itchy papules and pustules resembling acne, making it challenging to differentiate between the two conditions. Furthermore, MF and AV can coexist, with excessive sebum production being a shared contributing factor [
8]. Although not life-threatening, acne can leave lasting scars, contributing to psychological distress, social withdrawal, and depression. Treatment typically involves topical agents like benzoyl peroxide, retinoids, and clindamycin for AV [10], or antifungal treatments such as azoles for MF [12]. However, these treatments can cause side effects like dryness, irritation, and antimicrobial resistance [
9]. Misdiagnosis or improper treatment can exacerbate symptoms, underscoring the need for a natural, dual-action solution with antibacterial and antifungal properties.
Our research and development team at Breww Therapeutics Pvt. Ltd. had already developed and patented a formulation FERMENZA® [13], which is infused with pomegranate and beetroot yeast fermented extract, having proven results to be a natural alternative to ketoconazole and zinc pyrithione for managing dandruff and scalp disorders caused by Malessezia yeast present in our skin microbiome [15]. This present study has been undertaken to develop FERMENZA®as postbiotic gel formulation with pomegranate and beetroot yeast fermented extract as active ingredient. The antimicrobial activity and minimum inhibitory concentration of the formulation was assessed against Propionibacterium acnes (MTCC 1951). The mechanism of action of the active was studied followed by time killing assay and nucleotide efflux assay with respect to a marketed product (clindamycin 2% gel). A consumer research trial study was performed for organoleptic evaluation and skin irritation test of the investigational product. Efficacy of the formulation was evaluated based on subject self-evaluation questionnaire. This study will be the first time report of a dual action postbioic gel, as a natural solution for treatment of acne symptoms whether the condition is Acne vulgaris (AV) or Malassezia folliculitis (MF).
2. Materials and Methods
2.1. Preparation and Characterization of the Active Postbiotic Ingredient
Pomegranate and beetroot combinations were finely chopped, ground into a uniform paste, and fermented in a fermentor or bioreactor using commercially available Saccharomyces cerevisiae yeast powder. The fermentation process was designed and optimized using the Design of Experiment (DoE) or Quality by Design (QbD) methodology [14]. This approach ensured the development of an efficient, scalable, cost-effective, and patented method for producing high-quality, natural postbiotic extracts suitable for health and personal care products. Metabolite profiling of the postbiotic was done according to Li et al. [16] through LC-MS analysis at TBI, IISER Mohali, Punjab. The raw postbiotic was centrifuged and filtered before sample analysis. The mass spectrometer operated in electrospray ionization (ESI) mode. Samples were injected onto a Symmetry C18 column (150 × 2.1 mm, 5 µm particle size) with a BioBasic stationary phase. The mobile phase comprised solvent A (5% acetonitrile with 0.1% formic acid in deionized water) and solvent B (95% acetonitrile with 0.1% formic acid). Positive and negative ion mode was used with an ion trap analyzer, scanning a mass range of 50–2500 m/z at a flow rate of 200 µL/min, with a spray voltage of 4 kV, capillary voltage of 30 V, and capillary temperature of 300°C. Detection employs electrospray ionization in positive and negative modes, capturing full scan (m/z 50–2500) and MS/MS data acquisition was performed using Thermo-Xcalibur™ software (Thermo Scientific). Further data analysis identifies the probable compounds from matching retention times with MS/MS data validating through METLIN database.
2.2. Development of FERMENZA® Postbiotic Gel for Acne
The yeast fermented extracts from pomegranate and beetroot was the active postbiotic ingredient which is formulated with all GRAS (generally regarded as safe as per USFDA) status excipients and developed into FERMENZA® anti-acne gel as detailed in Indian Patent No. 459674 [13]. A simple preparation method is outlined in Table 2. Ingredients were accurately measured, mixed uniformly, and heated in an aqueous base (water, hydroxyl ethyl cellulose, propylene glycol, glycerin) to 80–90°C. Active extracts and sodium benzoate were added under stirring for 1 hour to ensure complete mixing. The initial formulation was packaged into bottles upon cooling for further use as anti-acne gel. The postbiotics in pomegranate and beetroot fermented extract demonstrated remarkable antioxidant properties, making them highly effective for antinflammatory skin care and the formulation were designed for industrial scalability and cost-effectiveness as detailed in our previous study [14].
2.3. Anti-Microbial Potency of the Investigational Product
2.3.1. Anti-Bacterial Susceptibility Assay Against P. acnes (MTCC 1951)
Microbial strain Propionibacterium acnes (MTCC 1951) was procured from Microbial Type Culture Collection (MTCC), Chandigarh. The antibacterial susceptibility was assessed using the agar well diffusion technique against P. acnes (MTCC 1951) according to previous studies [14,17] . Sterile cork borers were used to create four 6 mm wells in LB (Luria Bertani media) agar plates, into which 50 µL of each fermented extract and final FERMENZA® gel formulation was added. Positive controls consisted of 50 µL of clindamycin (2% w/v) as active pharmaceutical ingredient and 50 µL of propylene glycol was used as negative control. The plates were incubated at 37°C in a humidified environment for 24 hours. Zones of inhibition (ZOI) were measured with a digital caliper, and the percentage of growth inhibition was calculated using the formula:
% Growth Inhibition = [(Zc - Zt) / Zc] × 100
where Zc represents the ZOI for the control and Zt denotes the ZOI for the test sample.
2.3.2. Minimum INHIBITORY Concentration Assay Against P. acnes (MTCC 1951)
For the determination of MIC, the agar diffusion method was followed as described previously [15] in our studies against M. furfur (MTCC 1374). Mueller- Hinton Agar (MHA) was used as the growth medium. A bacterial suspension of Propionibacterium acnes (MTCC 1951) in its logarithmic phase (OD620 = 1) was prepared as the inoculum. The suspension was evenly spread across the MHA agar plates using a sterile cotton swab to create a uniform bacterial lawn. Wells of 6 mm diameter were punched into the agar using a sterile borer. Each well was loaded with 50 μl of FERMENZA® gel formulation with postbiotic concentrations ranging from 0.50 mg/ml to 0.05 mg/ml. As a positive control, clindamycin IP active (2% w/v) in propylene glycol was added to separate wells. Gel formulation without the postbiotic ingredient served as the negative control. The plates were incubated at 37°C for 24 hours. After incubation, the zones of inhibition (ZOI) were measured in millimeters (mm) using a digital Vernier caliper. The MIC50 value was determined as the lowest concentration of FERMENZA® gel formulation that produced a ZOI with a diameter greater than 50% of the ZOI observed for the positive control. The measurements were conducted in triplicate for each concentration, and the average ZOI was calculated. Results were compared to the control group treated with clindamycin to evaluate the efficacy of the FERMENZA® gel.
2.4. Mechanism of Action of the Investigational Product
2.4.1. Time- Killing Assay
A time-kill assay was conducted to assess the antibacteria properties of FERMENZA® against P. acnes (MTCC 1951) and compared with a marketed anti-acne gel (Clindamycin phosphate 2%) according to the method described by Balakrishna et al. [18] This method determines whether the test agent exhibits bacteriostatic or bactericidal effects over time. A standardized suspension of P. acnes was prepared by culturing the organism in Mueller-Hinton broth for optimal growth. The inoculum was adjusted to approximately 6.0 log CFU/mL (1.0 × 10⁶ CFU/mL). FERMENZA® was tested at concentrations equal to twice the MIC50 level (2× MIC50). Aliquots of the bacterial suspension were exposed to FERMENZA® and marketed anti-acne product (Clindamycin 2%) separately, while a control group without any test agent was maintained to track natural growth. At specific time intervals (0, 4, 8, 12, 16, 20 and 24 hours), 100 µL samples were withdrawn, serially diluted in Luria Bertani broth, and plated onto Nutrient agar plates. The plates were incubated at 37°C for 24 hours to allow colony formation. Bacterial growth was quantified as CFU/mL, and the reduction in bacterial cell count over time was graphically analyzed.
2.4.2. Nucleotide Efflux Assay
The nucleotide efflux assay was also performed as per the methodology described by Balkrishna et al. [18] to evaluate the effect of FERMENZA® postbiotic gel on Propionibacterium acnes (MTCC 1951). Bacterial cultures were grown to the exponential phase, washed, and resuspended in phosphate-buffered saline (PBS). FERMENZA® postbiotic gel were prepared and added to bacterial suspensions at MIC with 1:1 ratio. After incubation at 37°C, cultures were withdrawn at different time intervals from 0-12 hours. Samples were centrifuged to remove bacterial cells, and the supernatant was collected. Extracellular nucleotide release was measured by UV absorbance at 260 nm. Untreated bacterial culture served as controls. Increased nucleotide levels in treated samples compared to controls indicated membrane damage, suggesting antimicrobial activity of the postbiotics.
2.5. Physicochemical Evaluation of FERMENZA® Postbiotic Gel
Physico-chemical and microbial evaluations were conducted at DN Laboratory, Panchkula, Haryana, India, an FDA-approved and ISO 9001:2015, GLP-certified facility. Using pharmacopoeial and in-house methods, the product was analyzed for pH, colour, consistency, homogeneity, specific gravity, polyphenol and gallic acid content (used as marker compound for phenolic acids), viscosity, extrudability, spreadability, preservative levels, heavy metals, and microbial counts (total aerobic and fungal).
2.6. Stability Assessment of FERMENZA® Postbiotic Gel
To assess potential changes in physicochemical properties, FERMENZA® postbiotic gel sample bottles in HDPE packaging, were stored at 40 °C ±75% RH (relative humidity) over 180 day period. Centrifugal testing of the FERMENZA® postbiotic gel formulation was conducted at room temperature, where 5 g of the sample was placed into a 15 mL centrifuge tube and subjected to 5000 rpm for 20 minutes (split into two cycles of 10 minutes each) using an bench top centrifuge (REMI Labs, India). After centrifugation, the tubes were inspected for phase separation. Observations regarding physicochemical changes and phase separation at this storage conditions were recorded after 30 days, 90 days and 180 days during the period [17].
2.7. FERMENZA® Postbiotic Gel Efficacy Assessment
A consumer trial was conducted under expert supervision, involving 24 volunteers aged 18-35 years with moderate to severe acne. The participants were randomly divided into two groups, in which each participant were dispensed with either 30 g sample of FERMENZA® postbiotic gel or a marketed anti-acne gel with 2% clindamycin active. They were instructed to apply the provided gel twice daily while refraining from using any other anti-acne products, except a neutral cleanser which is provided to all the participants. The trial lasted 10 days, with participants being reviewed every two days.
2.7.1. Primary Skin Irritation Test of FERMENZA® Postbiotic Gel
A quantity of 0.5 g of the gel formulation was applied evenly over a 6 cm² section of hairless, normal skin and then covered with a semi-occlusive dressing for 1 hour. Following the application period, the dressing was removed, the gel was carefully scraped away, and the skin was inspected for any visible signs of irritation or rash. This process was carried out for 7 consecutive days, and the outcomes were recorded in grades [17].
2.7.2. Self-Assessment Questionnaire
Assessments were conducted using a self-assessment questionnaire according to the study described by Yogesh et al. [19], and the product's efficacy was evaluated on a 0-4 scale across the following parameters: How effective do you feel the product is at reducing acne?; How effective do you feel the product is at reducing redness around acne lesions?; How effective do you feel the product is at fading acne spots?; How effective do you feel the product is at preventing acne recurrence?; How effective do you feel the product is at alleviating pain over acne lesions? The scoring criteria were ranged between no improvement; mild improvement; moderate improvement and marked improvement.
2.7.3. Organoleptic Evaluation
FERMENZA
® was evaluated for sensory attributes and safety using an eight-parameter scoring system according to our previous study [14]. Parameters included appearance, odor, stickiness, oiliness, ease of spreading, dryness, allergic reactions, and burning sensation. Each parameter was rated on a 0-5 scale, where [0] indicated no effect and [
5] indicated the highest intensity. Sensory assessment focused on product color, consistency, and user experience, while safety evaluation captured adverse reactions such as redness, swelling, or discomfort.
2.8. Statistical Analysis
All in- vitro experiments were performed in triplicate, and the results were expressed as mean ± standard deviation (SD). The statistical significance of differences between experimental groups was evaluated using one-way analysis of variance (ANOVA). A significance level of p < 0.05 was set as the threshold for statistical significance, indicating that differences observed between methods were not due to random chance.
3. Results and Discussion
3.1. Metabolite Profile of the Post-Biotic with Potential for Anti-Acne Formulation
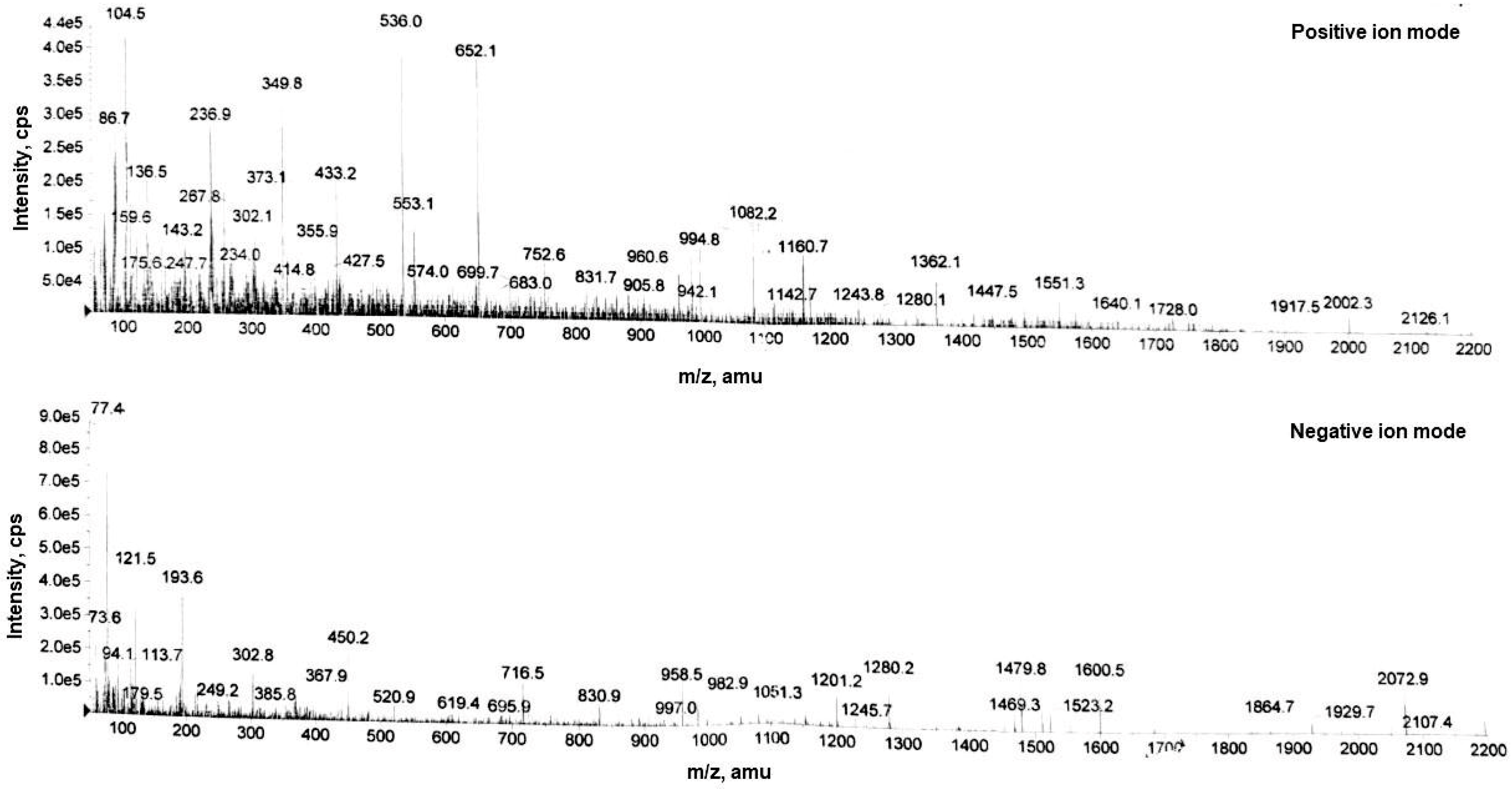
The LC-MS analysis of pomegranate-beetroot fermented extract reveals a diverse phytochemical profile (
Table 1 and
Figure 1) that positions it as a promising ingredient for anti-acne cosmeceutical formulations. Phenolic acids, present in the postbiotic extract including protocatechuic acid (m/z 136.5), caffeic acid (m/z 159.6), gallic acid (m/z 171.2) and ferulic acid (m/z 193.6), exhibit potent antioxidant and anti-inflammatory properties, which are critical in mitigating oxidative stress and inflammation associated with acne pathogenesis as demonstrated in recent studies [20,21]. Hydrolyzable tannins which are present in the postbiotic, including ellagic acid (m/z 302.1), hexagalloylglucose (m/z 960.6), and ellagitannin oligomers (m/z 2002.3), possess antimicrobial activity against
Propionibacterium acnes, the bacterium commonly implicated in acne [22,24]. Their astringent properties help regulate sebum production and tighten pores, reducing the likelihood of pore blockages and inflammation. The postbiotic is also rich in flavonoids like epigallocatechin (m/z 373.1) and anthocyanins such as cyanidin-3-glucoside (m/z 433.2) further enhance the extract's cosmeceutical potential by protecting skin cells from free radical damage and promoting wound healing, aiding in the repair of acne lesions [23,24]. The small alpha-hydroxy acids present in the extract like glycolic acid (m/z 86.7) also support exfoliation and skin renewal, preventing the buildup of dead skin cells that contribute to acne formation [27]. This metabolite-rich postbiotic extract combines antimicrobial, anti-inflammatory, and skin-renewing properties, making it an ideal candidate for developing FERMENZA
® postbiotic gel, natural anti-acne formulation as described in
Table 2.
3.2. Anti-Microbial Potential of FERMENZA® Postbiotic Gel Against P. acne (MTCC 1951)
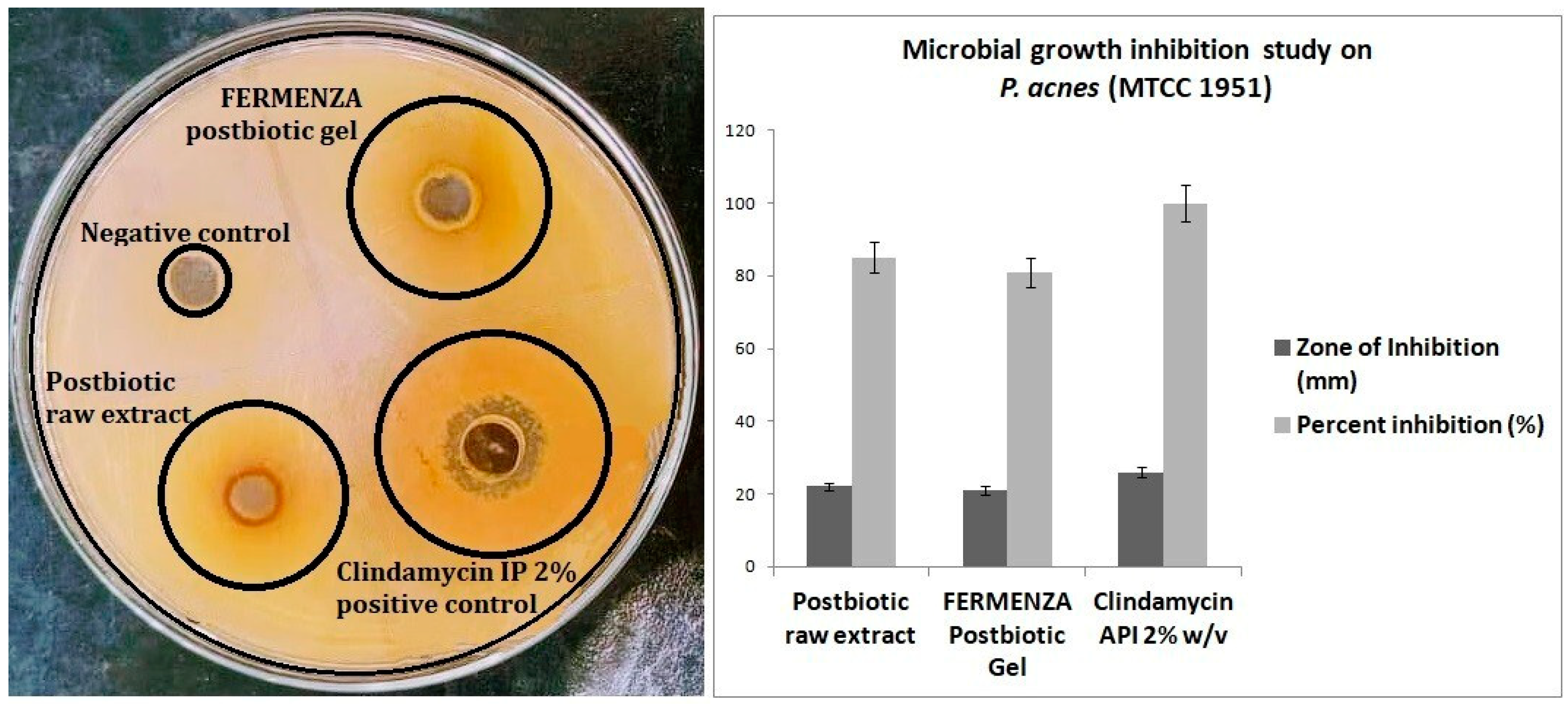
The antimicrobial activity of a postbiotic extract rich in polyphenolic compounds from pomegranate and beetroot, as well as the FERMENZA
® postbiotic gel formulated with this extract, was evaluated against
Propionibacterium acnes (MTCC 1951). The postbiotic raw extract and FERMENZA gel exhibited significant antimicrobial efficacy, with inhibition percentages of 85% and 81%, respectively, with respect to clindamycin IP 2% w/v as active pharmaceutical ingredient (
Figure 2a and 2b). Propylene glycol showed no growth inhibition against
P. acne (MTCC 1951). The slight reduction in efficacy in the FERMENZA
® postbiotic gel, relative to the raw extract, can be attributed to the gel formulation affecting the bioavailability or release of the active compounds as previously reported by other researchers [22,24]. Despite this, both formulations demonstrated substantial antimicrobial activity, suggesting that the polyphenolic-rich postbiotic extract could serve as an effective therapeutic option [25]. The growing concerns over antibiotic resistance and side effects associated with long-term antibiotic use like clindamycin, underscore the importance of exploring such natural alternatives [
10,26]. Further clinical trials are needed to validate these findings and explore broader applications of FERMENZA
® postbiotic gel in dermatology.
3.3. Minimum Inhibitory Concentration of FERMENZA® Postbiotic Gel Against P. acnes (MTCC 1951)
The results as depicted in
Table 3 indicate that FERMENZA
® postbiotic gel effectively inhibits the growth of
P. acnes, with inhibitory activity increasing in a concentration-dependent manner. At lower concentrations (0.05–0.15 mg/ml), the postbiotic gel exhibited moderate inhibitory activity, with ZOI values ranging from average 8.1 to 11.1 mm. These values correspond to 31–42% of the activity observed for clindamycin 2% API, suggesting the need for higher concentrations to achieve substantial inhibition. At concentrations of 0.25 mg/ml and above, the gel displayed significant antimicrobial activity, with ZOI values exceeding 13.8 mm and corresponding to more than 50% of the efficacy of clindamycin. Hence the MIC
50 value against
P. acnes was determined as 0.25mg/ml (or 2.5% w/v). In our previous studies, the MIC potency of the fermented extract was assessed against
M. furfur (MTCC 1374) compared to ketoconazole, which also peaked at 8 mg/mL [14]. The highest postbiotic concentration tested (0.50 mg/ml or 5% w/v) achieved 81% of the ZOI produced by clindamycin IP 2% active, indicating strong potential for clinical applications as an alternative or adjunctive treatment for acne vulgaris or malessezia folliculitis. The progressive increase in % ZOI with respect to clindamycin highlights the dose-dependent antimicrobial action of the postbiotic gel. This effect is likely attributable to the synergistic antimicrobial and anti-inflammatory properties of the pomegranate and beetroot-derived fermented postbiotic extracts, which are rich in bioactive compounds such as polyphenols, phenolic acids and hydrolysable tannins [23,28].
3.4. Mechanism of Action of FERMENZA® Postbiotic Gel
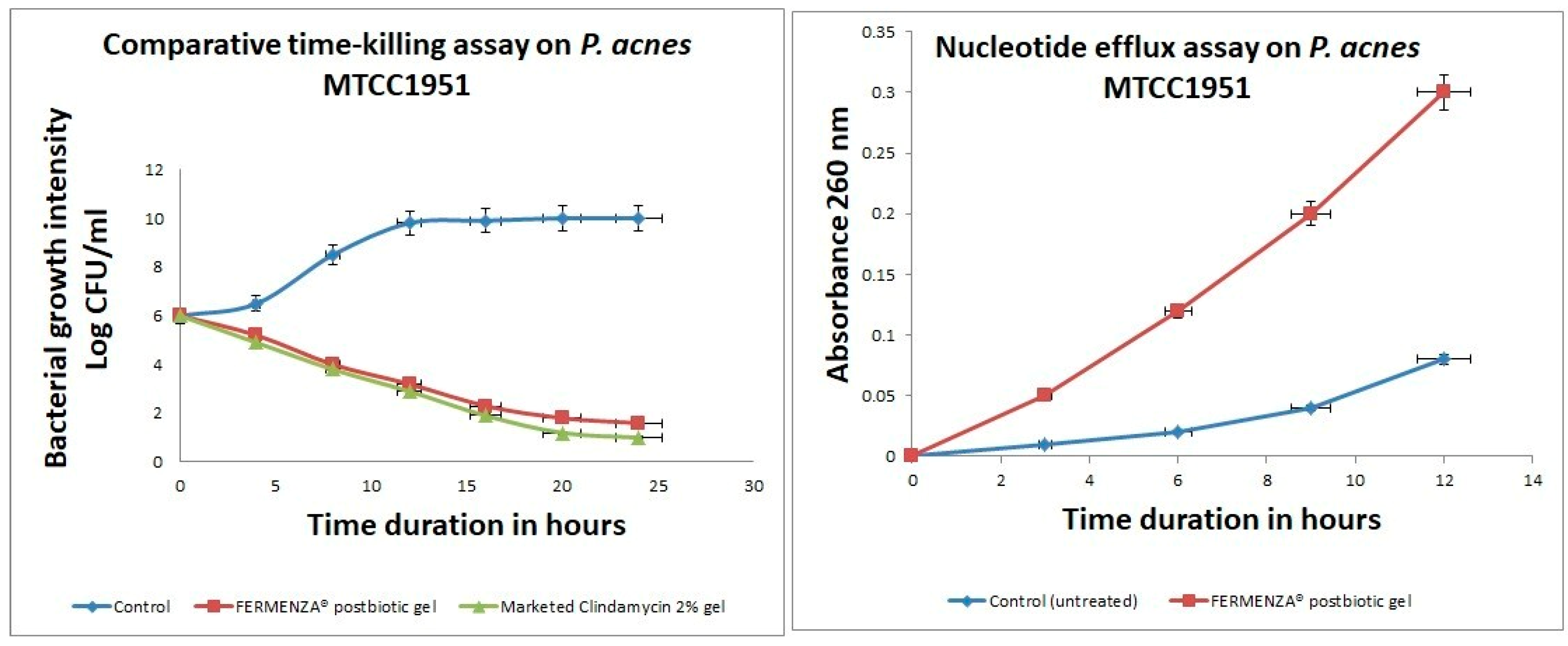
The time-kill assay (
Figure 3a) demonstrates the antimicrobial efficacy of FERMENZA
® postbiotic gel compared to a marketed clindamycin 2% gel and an untreated control against
Propionibacterium acnes (MTCC 1951). The untreated control showed negligible reduction in microbial load, remaining stable from 6 log CFU/ml at 0 hours to 10 log CFU/ml at 24 hours, indicating no antimicrobial effect. FERMENZA
® postbiotic gel significantly reduced bacterial counts over time, achieving 5.2 log CFU/ml at 4 hours, 3.2 log CFU/ml at 12 hours, and 1.6 log CFU/ml at 24 hours. The marketed clindamycin gel exhibited slightly higher efficacy, with bacterial counts dropping to 4.9 log CFU/ml at 4 hours, 2.9 log CFU/ml at 12 hours, and 1 log CFU/ml at 24 hours. The nucleotide efflux assay (
Figure 3b), measured by absorbance at 260 nm, reflects membrane disruption and cellular lysis caused by FERMENZA
® postbiotic gel. Untreated control samples showed minimal nucleotide release, while FERMENZA
® postbiotic gel caused a significant nucleotide efflux, with absorbance rising to 0.05 at 3 hours, 0.2 at 9 hours, and peaking at 0.3 at 12 hours, indicating progressive cell membrane damage. The progressive increase in nucleotide efflux suggests that the postbiotic components compromise bacterial membrane integrity, causing leakage of intracellular contents. This is consistent with the findings of Kamran et al. [29], who reported that postbiotic metabolites disrupt bacterial membranes by altering lipid bilayers. These results align with recent studies highlighting the role of postbiotic metabolites in enhancing membrane permeability [30]. While clindamycin demonstrated slightly faster bacterial killing, FERMENZA
® gel achieved similar efficacy by 24 hours, reducing bacterial counts to 1.6 log CFU/ml. The bioactive compounds derived from fermented pomegranate and beetroot extracts, such as gallic acid, ellagic acid, and ellagitannins, likely contribute to the synergistic antimicrobial effect of FERMENZA
® postbiotic gel. Unlike clindamycin, which targets bacterial protein synthesis, the postbiotic gel acts via a multi-target approach, making it less susceptible to resistance development [
7,
10].
3.5. Physicochemical Properties and Stability of FERMENZA® Postbiotic Gel
The stability study of FERMENZA
® postbiotic gel over six months demonstrated that the formulation remained stable and within acceptable limits for all tested physicochemical and microbiological parameters (
Table 4). The visual appearance of the gel remained unchanged throughout the study, retaining its reddish-brown color, suggesting no significant oxidation or degradation of polyphenolic compounds. This is in accordance with recent findings [31,32] where polyphenol-rich postbiotic formulations exhibited color stability due to their antioxidant potential. The pH values showed a slight decline from 6.0 to 5.93 over six months, indicating minimal acidification. This stability is crucial for topical applications, as pH plays a key role in maintaining skin compatibility and microbiome balance [27]. The viscosity and specific gravity remained relatively stable, confirming consistency in formulation density, a critical factor for uniform application and user experience. Extrudability of more than 90% and spreadability with very minor variations implied the gel’s usability over time. These results are in agreement with studies indicating that postbiotic formulations exhibit stable rheological properties due to their structured bioactive matrix [33]. The homogeneity and consistency parameters remained unchanged, with no observable phase separation or lumps, suggesting robust formulation integrity. Additionally, the polyphenol content showed a slight increase from 10.05% to 10.65%, likely due to continued release of bound polyphenols from postbiotic metabolites, as reported in previous fermentation-based studies [16,18]. Gallic acid content was measured as a phenolic acids marker and its content (50.42–50.87 ppm) remains stable, indicating no significant degradation during the stability study period. The combination of gallic acid and polyphenols can act synergistically, amplifying the antimicrobial and anti-inflammatory effects [25,31]. Notably, the gradual decrease in ethyl alcohol content (0.0024% to 0.001%) suggests volatilization over time, a common phenomenon in water-based formulations. Heavy metals were within undetectable limits, and the gel exhibited robust microbiological stability, with no detectable pathogens and low aerobic and fungal counts (<10 cfu/ml). Overall, FERMENZA
® postbiotic gel demonstrated exceptional physicochemical stability, making it a promising candidate for commercial application. The results corroborate the stability trends observed in postbiotic-based gels and emulsions [35]. These findings align with previous studies on postbiotic formulations, which highlight their inherent stability due to bioactive metabolites [33].
3.6. Efficacy of FERMENZA® Postbiotic Gel
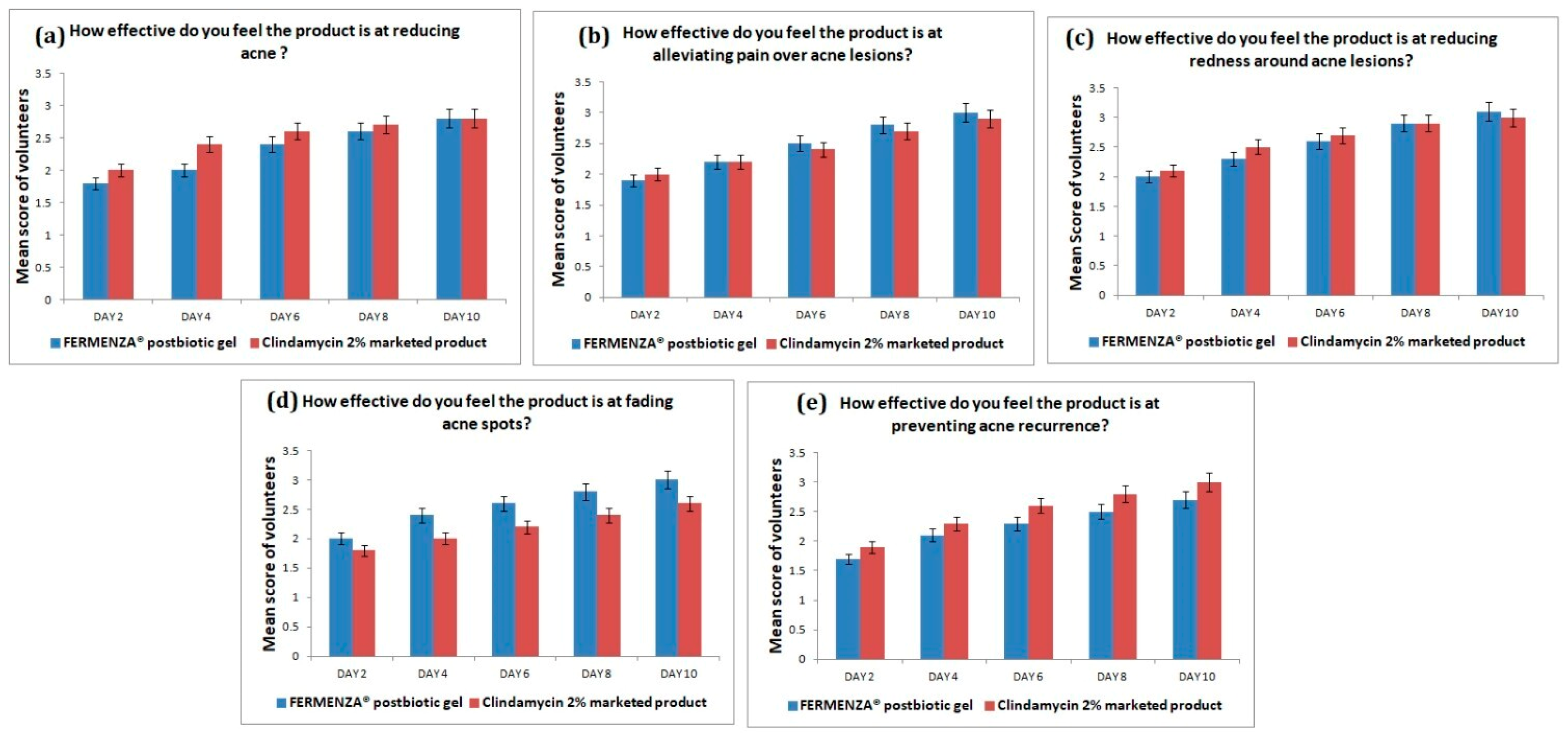
The results of the consumer trial suggest that FERMENZA
® postbiotic gel is an effective acne treatment, with significant improvements in reducing acne lesions, alleviating pain, decreasing redness, fading acne spots, and preventing recurrence. Over the 10-day period, participants reported consistent and gradual improvements, achieving marked improvement by Day 10 (
Figure 4). Although the Clindamycin 2% marketed product demonstrated slightly higher initial effectiveness in some areas (e.g., acne reduction and prevention of recurrence), FERMENZA
® postbiotic gel caught up quickly and surpassed in certain areas such as pain relief and fading of acne spots. These findings are consistent with previous studies that highlight the positive effects of postbiotics in supporting skin health and reducing inflammation [34]. A key factor in FERMENZA
®'s effectiveness is the presence of phenolic acids, particularly gallic acid, along with high polyphenol content, which provide anti-inflammatory, antioxidant, and antimicrobial benefits. These compounds help regulate skin microbiota, reduce inflammation, and inhibit the growth of acne-causing bacteria [36]. Unlike antibiotics such as clindamycin, which can lead to antibiotic resistance over time, FERMENZA
® postbiotic gel modulates the skin's natural defenses without disrupting microbial balance, potentially reducing the risk of resistance. Long-term use of FERMENZA
® could offer a sustainable acne treatment by harnessing the natural properties of phenolic acids, providing continued effectiveness without the risks associated with antibiotic therapies. These characteristics make it a valuable alternative for those seeking to manage acne without contributing to antibiotic resistance. Further studies involving a larger sample size and longer treatment duration would be valuable to confirm these findings and to explore the long-term benefits of FERMENZA
® postbiotic gel in acne treatment.
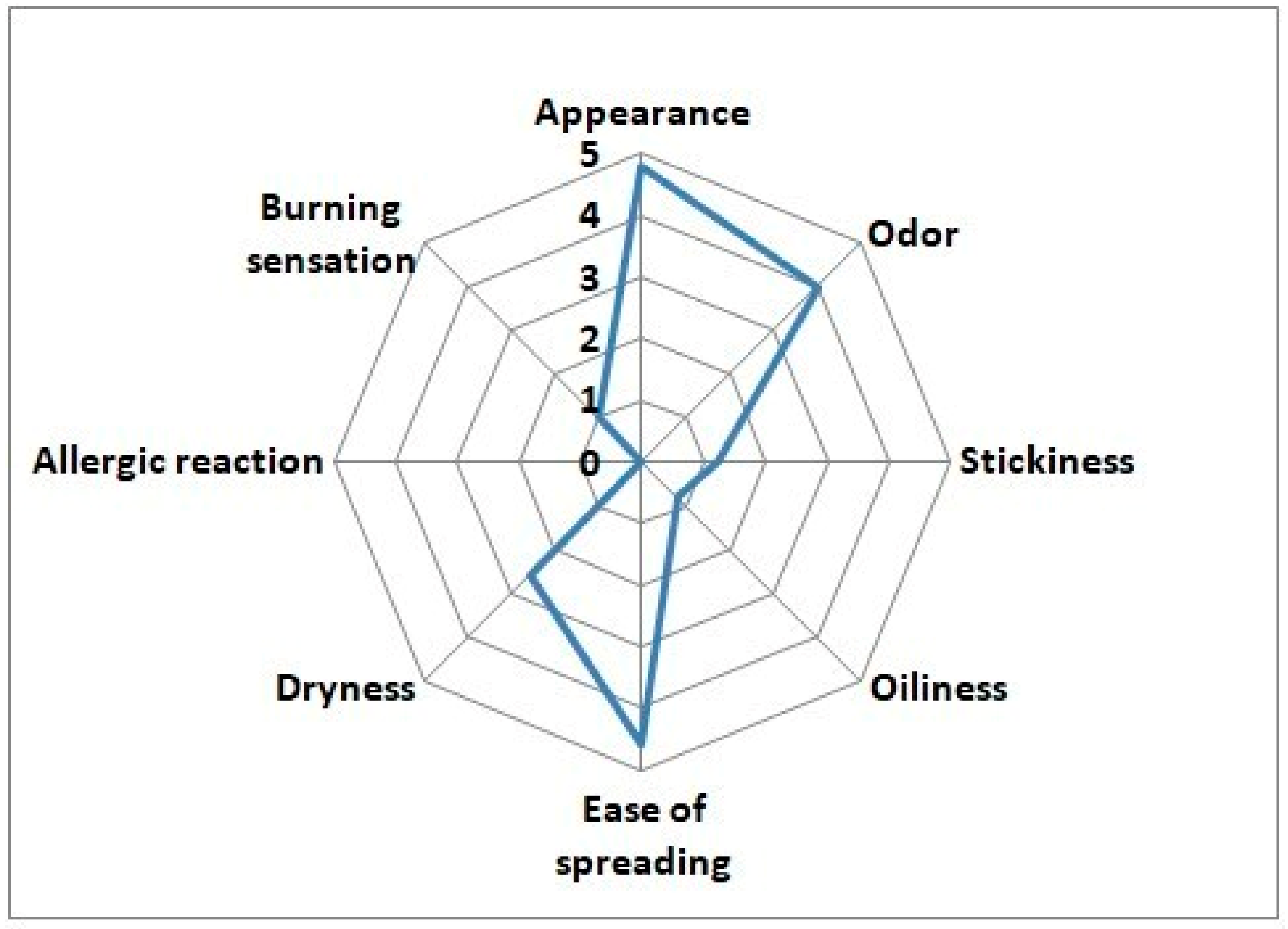
3.7. Sensory and Safety Profile of FERMENZA® Postbiotic Gel
Prior to the start of the trial, the investigational product did not cause any visible skin irritation during the initial patch test conducted on the volunteers. The sensory and safety profile of FERMENZA
® postbiotic gel was generally well-received by participants in the trial, demonstrating favorable attributes in several key areas (
Figure 5). The product scored highly in terms of appearance (4.8) and ease of spreading (4.6), indicating that users found it visually appealing and easy to apply. The gel also had a mild odor (4), which was not reported as bothersome. In terms of texture, the gel was notably non-sticky (1.2) and non-oily (0.8), enhancing its user-friendliness and comfort during application. While dryness was reported at a moderate level (2.6), it was not considered excessive, suggesting that the gel does not overly dry the skin. Importantly, FERMENZA
® postbiotic gel demonstrated excellent safety, with no allergic reactions and mild burning sensations due to presence of phenolic acids as reported by the participants, confirming its mild and gentle formulation. These findings suggest that FERMENZA
® postbiotic gel offers a positive sensory experience while maintaining a high safety profile, making it suitable for long-term use in individuals with acne-prone skin.
4. Conclusion
In conclusion, FERMENZA® postbiotic gel represents an innovative treatment for Acne vulgaris and Malassezia folliculitis, expanding upon its previous success in hair and scalp conditions. Formulated with a fermented extract of pomegranate and beetroot, the gel harnesses the power of bioactive metabolites such as phenolic acids, flavonoids, and hydrolyzable tannins to offer a dual-action antimicrobial and anti-inflammatory solution. Its unique mechanism of action, which targets key pathogens like Propionibacterium acnes and Malessezia furfur, provides an effective alternative to traditional antimicrobials, minimizing the risk of resistance development. Additionally, its favorable stability profile, ease of application, and minimal skin irritation make it a promising candidate for long-term use in acne management. With positive consumer feedback indicating significant improvements in acne symptoms, FERMENZA® postbiotic gel stands out as a sustainable and safe option for addressing common skin concerns. Further clinical research will be essential to fully understand its long-term efficacy and explore its potential in broader dermatological applications.
Acknowledgement
We would like to acknowledge, incubation support through IISER, Mohali and Punjab State Council for Science and Technology, Punjab, India.
Conflict of interest
The authors declare that they have no conflict of interest.
Declaration statement
During the preparation of this work the author(s) used ChatGPT (
https://openai.com/index/chatgpt/) in order to improve the language quality of the manuscript. After using this tool, the author(s) reviewed and edited the content as needed and take full responsibility for the content of the published article. The authors have seen and approved the manuscript and it has not been published or considered for publication elsewhere.
Ethics approval and consent to participate
This study was conducted in accordance with the written informed consent obtained from all participants prior to their inclusion in the study.
References
- Aguilar-Toalá, J.; Garcia-Varela, R.; Garcia, H.; Mata-Haro, V.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114.
- Theodorou, I.M.; Kapoukranidou, D.; Theodorou, M.; Tsetis, J.K;, Menni, A.E.; Tzikos, G.; Bareka, S.; Shrewsbury, A.; Stavrou, G.; Kotzampassi, K. Cosmeceuticals: A Review of Clinical Studies Claiming to Contain Specific, Well-Characterized Strains of Probiotics or Postbiotics. Nutrients. 2024, 16(15):2526. [CrossRef]
- Liang, B.; Xing, D. The Current and Future Perspectives of Postbiotics. Probiotics Antimicrob. Proteins. 2023, 15(6):1626-1643. [CrossRef]
- https://www.globenewswire.com/news-release/2023/03/06 /Microbiome-Cosmetic-Products-Market-Size- by-2030.
- https://www.marketsandmarkets.com/Market-Reports/postbiotics-market-Global Forecast to 2029.
- Duarte, M.; Oliveira, A.L.; Oliveira, C.; Pintado, M.; Amaro, A.; Madureira, A.R. Current postbiotics in the cosmetic market-an update and development opportunities. Appl Microbiol Biotechnol. 2022, 106(18):5879-5891. [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Mundkur, L.; Rajalakshmi, H.R.; Shah, K.; Beede, K. Novel Topical Application of a Postbiotic, LactoSporin®, in Mild to Moderate Acne: A Randomized, Comparative Clinical Study to Evaluate its Efficacy, Tolerability and Safety. Cosmetics. 2020, 7(3):70. [CrossRef]
- Paichitrojjana, A.; Chalermchai, T. The Prevalence, Associated Factors, and Clinical Characterization of Malassezia folliculitis in Patients Clinically Diagnosed with Acne Vulgaris. Clinical, Cosmetic and Investigational Dermatology. 2022, 2647-2654. [CrossRef]
- Nast, A.; Dréno, B.; Bettoli, V.; Bukvic Mokos, Z.; Degitz, K.; Dressler, C.; Finlay, A.Y.; Haedersdal, M.; Lambert, J.; Layton, A.; Lomholt, H.B.; López-Estebaranz, J.L.; Ochsendorf, F.; Oprica, C.; Rosumeck, S.; Simonart, T.; Werner, R.N.; Gollnick, H. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016, 30(8):1261-8. [CrossRef]
- Fluhr, J.W.; Degitz, K. Antibiotics, azelaic acid and benzoyl peroxide in topical acne therapy. J. Dtsch. Dermatol. Ges. 2010, 8 (Suppl. 1), S24–S30.
- Duarte, M.; Carvalho, M.J.; de Carvalho, N.M.; Azevedo-Silva, J.; Mendes, A.; Ribeiro, I.P.; Fernandes, J.C.; Oliveira, A.L.S.; Oliveira, C.; Pintado, M.; Amaro, A.; Madureira, A.R. Skincare potential of a sustainable postbiotic extract produced through sugarcane straw fermentation by Saccharomyces cerevisiae. Biofactors. 2023, 49(5):1038-1060. [CrossRef]
- Green, M.; Feschuk, A.M.; Kashetsky, N.; Maibach, H.I. Clinical characteristics and treatment outcomes of Pityrosporum folliculitis in immunocompetent patients. Arch Dermatol Res. 2023, 315(6):1497-1509. [CrossRef]
- Ghosh, S.; Bhattacharya, M. Fermented Cider compositions and method of preparation thereof. 2023. Indian patent No. 459674. https://www.ipindia.gov.in/.
- Ghosh, S.; Bhattacharya, M. FERMENZA®: A patented bioactive fermented product developed through process optimization. 16 January 2025, PREPRINT (Version 1) available at Research Square . [CrossRef]
- Ghosh, S.; Bhattacharya, M. FERMENZA®: A Patented Natural Alternative to Ketoconazole and Zinc Pyrithione for Managing Dandruff and Scalp Disorders. Indian J Dermatol.2024 (Under Process).
- Li, J.; Xu, R.; Zong, L.; Brake, J.; Cheng, L.; Wu, J.; Wu, X. Dynamic Evolution and Correlation between Metabolites and Microorganisms during Manufacturing Process and Storage of Fu Brick Tea. Metabolites. 2021, 11(10):703. [CrossRef]
- Ansong, J.A.; Asante, E.; Johnson, R.; Boakye-Gyasi, M.E.; Kuntworbe, N.; Owusu, F.W.A.; Ofori-Kwakye, K. Formulation and Evaluation of Herbal-Based Anti-acne Gel Preparations. Biomed Res Int. 2023 Dec 18;2023:7838299. [CrossRef]
- Balkrishna, A.G.; Gupta, A.; Singh, A.; Singh, P.; Tomar, K.; Rajagopal, M. D. Antibacterial Activity and Mechanism of Action of an Ayurvedic formulation Khadirarishta. J Herbal Med. 2021, 32. 100509. 10.1016/j.hermed.2021.100509.
- Yogesh, H.R.; Gajjar, T.; Patel, N.; Kumawat, R. Clinical study to assess efficacy and safety of Purifying Neem Face Wash in prevention and reduction of acne in healthy adults. J Cosmet Dermatol. 2022, 21:2849–2858. [CrossRef]
- Ramsis, T.; Selim, H.M.; Elseedy, H.; Fayed, E.A. The role of current synthetic and possible plant and marine phytochemical compounds in the treatment of acne. RSC Advances. 2024, 14(33):24287-321.
- Sun, C.; Na, Y.; Wang, Z.; Zhu, T.; Liu, X. Phytochemicals, promising strategies combating Cutibacterium acnes. Front. Pharmacol. 2024 15:1476670. [CrossRef]
- Piazza, S., Martinelli, G., Maranta, N., Pozzoli, C., Fumagalli, M., Nicolaci, V., et al. Investigation into the anti-acne effects of Castanea sativa mill leaf and its pure ellagitannin castalagin in HaCaT cells infected with Cutibacterium acnes. Int. J. Mol. Sci. 2024, 25 (9), 4764. [CrossRef]
- Lee, C.J.; Chen, L.G.; Liang, W.L.; Wang, C.C. Multiple activities of Punica granatum Linne against acne vulgaris. Int. J. Mol. Sci. 2017, 18 (1), 141. [CrossRef]
- Muddathir, A.M.; Yamauchi, K.; Mitsunaga, T. Anti-acne activity of tannin-related compounds isolated from Terminalia laxiflora. J Wood Science, 2013, 59(5), 426–431. [CrossRef]
- Hatem, S.; Moftah, N.H.; Ragai, M.H.; El-Maghawry, E. Development of gallic acid loaded composite nanovesicles for the topical treatment of acne: optimization, characterization, and clinical investigation. Pharm Development Technol, 2024, 29(8), 899–911. [CrossRef]
- Nguyen, N.N.T.; Nguyen, T.T.D.; Vo, D.L.; Than, D.T.M.; Tien, G.P.; Pham, D.T. Microemulsion-based topical hydrogels containing lemongrass leaf essential oil (Cymbopogon citratus (DC.) Stapf) and mango seed kernel extract (Mangifera indica Linn) for acne treatment: Preparation and in-vitro evaluations. PLoS ONE. 2024, 19(10): e0312841. [CrossRef]
- Valle-González, E.R.; Jackman, J.A.; Yoon, B.K. et al. pH-Dependent Antibacterial Activity of Glycolic Acid: Implications for Anti-Acne Formulations. Sci. Rep. 2020, 10, 7491. [CrossRef]
- da Silva Vale, A.; de Melo Pereira, G.V.; de Oliveira, A.C.; de Carvalho Neto, D.P.; Herrmann, L.W.; Karp, S.G.; Soccol, V.T.; Soccol, C.R. Production, Formulation, and Application of Postbiotics in the Treatment of Skin Conditions. Fermentation. 2023, 9(3):264. [CrossRef]
- Kamran, M. et al. Microbiome-Based Therapeutics in Immunological Disorders. In: Khurshid, M., Akash, M.S.H. (eds) Human Microbiome. 2024, Springer, Singapore. [CrossRef]
- Bhawal, H.; Kumari, A.; Rana, S.; Kapila, S.; Kapila, R. Scope of bacterial surface effector molecules beyond probiotics. Food Bioscience. 2023, 56, 103180. [CrossRef]
- Park, H.H.; Ko, S.C.; Oh, G.W.; Jang, Y.M.; Kim, Y.M.; Park, W.S.; Choi, I.W.; Jung, W.K. Characterization and biological activity of PVA hydrogel containing chitooligosaccharides conjugated with gallic acid. Carbohydrate Polymers. 2018, 198: 197-205.
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules. 2022, 4;12(11):1640. [CrossRef]
- Prajapati, N.; Patel, J.; Singh, S.; Yadav, V. K.; Joshi, C.; Patani, A.; Prajapati, D.; Sahoo, D.K.; Patel, A. Postbiotic production: harnessing the power of microbial metabolites for health applications. Frontiers in Microbiol. 2023,14, 1306192. [CrossRef]
- De Almeida, C.V.; Antiga, E.; Lulli, M. Oral and Topical Probiotics and Postbiotics in Skincare and Dermatological Therapy: A Concise Review. Microorganisms. 2023, 27; 11(6):1420. [CrossRef]
- Zhao, X.; Liu, S.; Li, S.; Jiang, W.; Wang, J.; Xiao, J.; Chen, T.; Ma, J.; Khan, M.Z.; Wang, W.; Li, M.; Li, S.; Cao, Z. Unlocking the power of postbiotics: A revolutionary approach to nutrition for humans and animals, Cell Metabolism, 2024, 36, 4: 725-744. [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed Pharmacotherapy. 2021, 133: 110985. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).