1. Introduction
Jaspine B is a naturally occurring cyclic anhyrophytosphingosine originally derived from marine sponges, Pachastrissamine sps. [1] and Jaspis sps. [2]. Initially isolated in 2002, Jaspine B shows a potent anticancer activity and has gathered much interest in recent years [3]. Jaspine B and its analogs are effective in various types of cancer cells in vitro [4-6].
Jaspine B exerts its anticancer effect through multiple mechanisms, primarily by disrupting sphingolipid mechanisms leading to apoptotic and non-apoptotic cell deaths [4,7]. Jaspine B inhibits sphingomyelin synthase, increasing the accumulation of ceramide and other sphingolipid metabolites [8]. In addition to sphingomyelin synthase, Jaspine B also inactivated sphingosine kinase 1,8 and inhibited Forkhead box O3 (FOXO3) to induce apoptosis [9].
Despite promising therapeutic potential, the clinical applications of Jaspine B are limited by its poor pharmacokinetic profile. The primary limitation of Jaspine B is its poor oral bioavailability. Previous studies have shown that the oral bioavailability of Jaspine B in rats is only about 6.2% [10]. The low bioavailability can be attributed to its poor aqueous solubility, extensive metabolism and rapid systemic clearance. Zhang et al. studied numerous chemical modifications to generate Jaspine B analogs to improve their anticancer activity [11]. Choi et al. used the taurocholate supplementation method to enhance the bioavailability of Jaspine B. Results showed that there was significant improvement in the pharmacokinetic profile of Jaspine B with taurocholate supplementation with over six-fold increase in bioavailability [10]. This pharmacokinetic improvement, however, was not evaluated for the correlation in pharmacodynamic effect.
As a novel approach to improve the efficacy of Jaspine B in vitro and in vivo, we developed a nano liposomal formulation of Jaspine B. We suggest that the improvement in the pharmacodynamic effects of liposomal Jaspine B is attributed to the improved pharmacokinetic properties of the formulation, as we observed in a previous study conducted in our lab, in which liposomal formulation was found to improve the therapeutic efficacy of Jaspine B in tumor-bearing mice [6]. This observation corresponds with previous reports emphasizing that liposomal formulations are promising drug delivery systems for oral water-insoluble drugs such as nifedipine, leading to a several-fold increase in their bioavailability [12,13].
2. Materials and Methods
2.1. Reagents
In-house synthesis and scale-up of Jaspine B was carried out by Dr. S Pashikanti (Idaho State University, Pocatello, ID, USA) based on the previously published method [14]. Cholesterol, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N- [amino (polyethylene glycol)-2000] carboxylic acid (DSPE-PEG2000-COOH) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Water, acetonitrile, methanol and formic acid used in the analysis were LC-MS grade and purchased from Fisher Scientific (Fair Lawn, NJ, USA).
2.2. LC-MS/MS system
LC-MS/MS system was composed of liquid chromatography (Shimadzu, MD, USA) with a binary pump (LC-30AD), an autosampler (SIL-30AC), a controller (CBM-20A), a degasser (DGU-20A5R), a column oven (CTO-20A), and an ABSciex QTRAP 5500 mass spectrometer (SCIEX, Foster City, CA, USA) with electron spray ionization (ESI) source. The chromatograms were monitored using Analyst 1.7 software, and the data were analyzed in MultiQuant 3.0 software (SCIEX, Foster City, CA, USA). The analytes were separated using Synergi™ Fusion-RP column (2.5 µm, 100 × 2 mm) obtained from Phenomenex (Torrance, CA, USA). All the analyses were performed in positive ion mode.
The mobile phase consisted of 0.1% formic acid in water (A) and acetonitrile (B) applied as a gradient at a flow rate of 0.2 ml/min. The gradient time program started with 10% B and increased to 25% over 2 minutes. The gradient was increased to 50%, 75% and 90% over the subsequent 2 minutes, after which it was held for 7 minutes. The analytes were detected using multiple reaction monitoring (MRM) in positive ion mode.
2.3. Preparation of working solutions and calibration standards
Jaspine B was dissolved in methanol to produce a stock solution of 1 mg/ml concentration. The stock solution was serially diluted with methanol at concentrations of 0.5, 1, 2, 4, 8 and 16 ng/ml to prepare the working solutions. Similarly, a 5 ng/ml working solution of Spisulosine was prepared by diluting the stock solution of 1 mg/ml Spisulosine in methanol. Calibration standards were prepared by spiking blank rat plasma with 100 µl of Jaspine B and Internal standard working solutions.
2.4. Sample preparations
An aliquot of 100 µl of rat plasma samples was mixed with 100 µl of 5 ng/ml solution of Spisulosine and mixed. 1 ml of cold methanol (-20 °C) was added to the mixture and vortexed for 30 seconds. 500 µl of cold acetonitrile (-20 °C) was further added to the mixture and vortexed to mix thoroughly. The mixture was then placed in a water bath at 60 °C for 10 min to allow complete dissolution of the analyte in the solution. After removing from the water bath, the mixture was shaken well and centrifuged for 20 min at 17,000g. The resulting supernatant was transferred to a glass test tube and dried under a gentle stream of nitrogen gas. The dried supernatant was reconstituted in 100 µl of methanol, and an aliquot of 10 µl was injected into the LC-MS/MS system.
2.5. Pharmacokinetic study
The pharmacokinetic study was performed on two groups of Sprague Dawley rats (N=3/group): Jaspine B suspension in a vehicle containing DMSO: PEG2000: PBS (2:4:4) and Jaspine B liposomes formulation. The rats were cannulated in the right jugular vein under anesthesia and allowed to recover overnight. The respective groups of rats were orally dosed with 5 mg/kg equivalent of Jaspine B using a gavage needle.
The blood samples were drawn serially at 15-, 30-, 60-, 120-, 360-, and 720-min post-dose through a jugular vein cannula. The cannula was flushed with normal saline after each drawing. At 24 hr post-dose, rats were euthanized with CO2, and the blood was drawn through cardiac puncture. Comparison between the two groups was carried out using a student's t-test, with p < 0.05 being considered statistically significant.
3. Results
3.1. LC-MS/MS analysis of Jaspine B in rat plasma
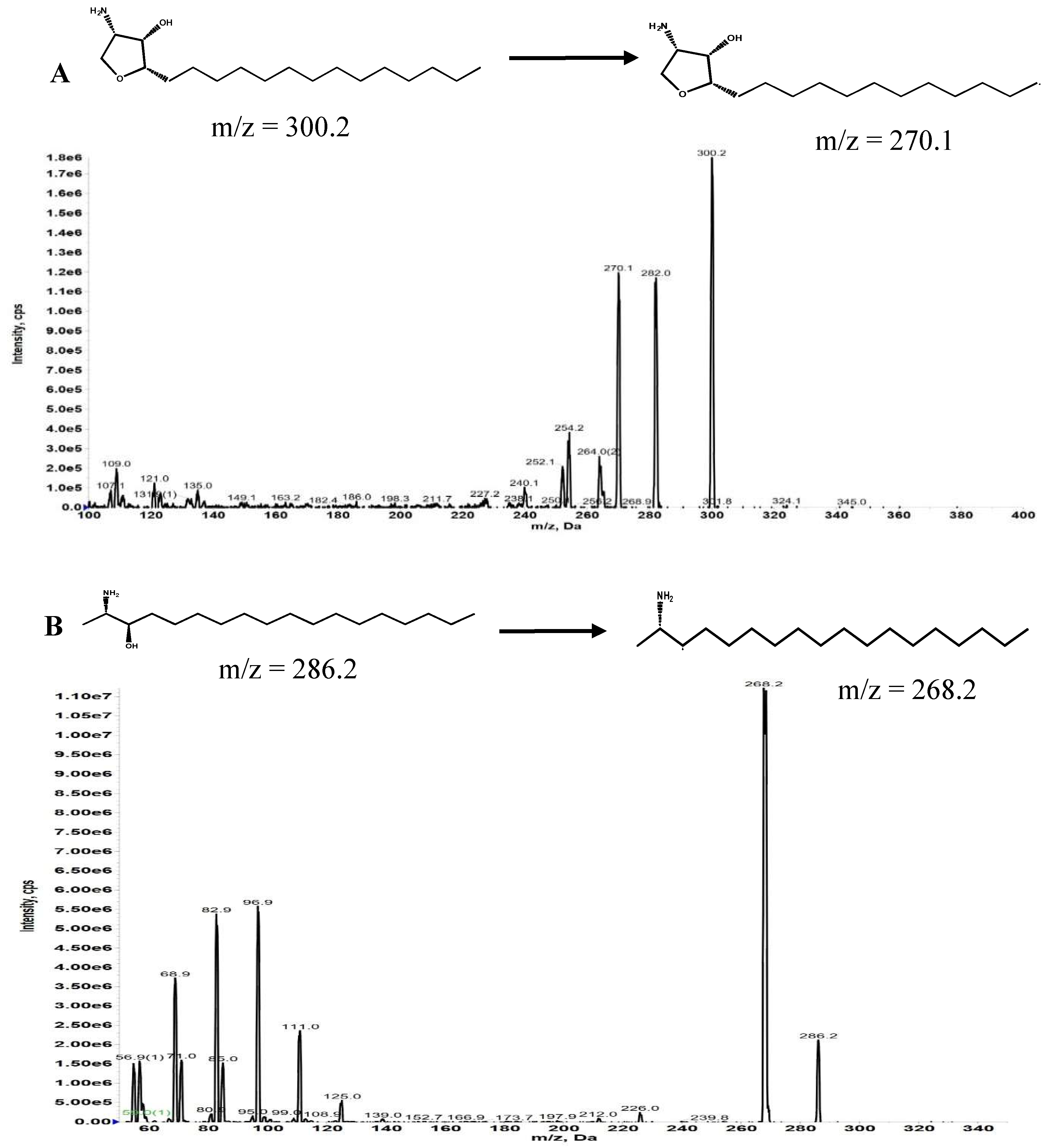
We developed and validated an analytical method to measure the concentration of Jaspine B in rat plasma using MRM mode in an LC-MS/MS system. The MS was optimized in a positive ion mode after direct injection of Jaspine B and Spisulosine methanolic solutions at 100 ng/ml concentration.
Figure 1.
(A) Product ion scan and multiple reaction monitoring (MRM) chromatogram of Jaspine B. (B) Product ion scan and multiple reaction monitoring (MRM) chromatogram of Spisulosine.
Figure 1.
(A) Product ion scan and multiple reaction monitoring (MRM) chromatogram of Jaspine B. (B) Product ion scan and multiple reaction monitoring (MRM) chromatogram of Spisulosine.
3.2. Analytical Method Validation
3.2.1. Specificity
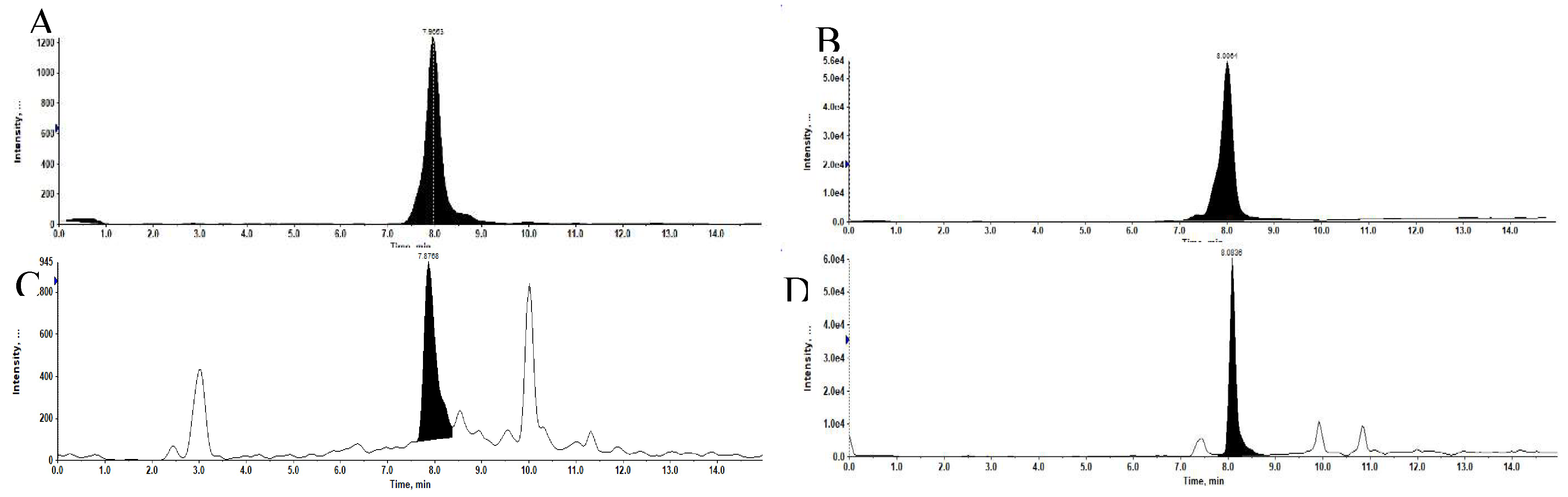
The specificity of the method was confirmed with five different rat blank plasma and rat plasma samples spiked with 1 ng/ml of Jaspine B and 5 ng/ml of Spisulosine as internal standard (IS).
Figure 2.
Representative extracted ion chromatograms (XIC). (A) Jaspine B at 1 ng/ml in Methanolic solution. (B) Spisulosine at 5 ng/ml in Methanolic solution. (C) of Jaspine B at 1 ng/ml in Plasma matrix. (D) of Spisulosine at 5 ng/ml in Plasma matrix.
Figure 2.
Representative extracted ion chromatograms (XIC). (A) Jaspine B at 1 ng/ml in Methanolic solution. (B) Spisulosine at 5 ng/ml in Methanolic solution. (C) of Jaspine B at 1 ng/ml in Plasma matrix. (D) of Spisulosine at 5 ng/ml in Plasma matrix.
3.2.2. Accuracy and Precision
A set of six concentrations of Jaspine B in rat plasma were analyzed to create calibration curves ranging from 0.5 ng/ml to 16 ng/ml. The results of intra-day and inter-day assays are shown in
Table 1. The intra-day assays showed an average recovery of analytes ranging from 92.23% to 108.28%, with the variance ranging from 0.77% to 8.76%. For inter-day assays, the average accuracy of the method ranged from 99.23% to 103.64%, with the variance ranging from 0.78% to 7.88%.
Table 1.
LC-MS/MS ion acquisition parameters (MRM mode) for identification and confirmation of Jaspine B and Spisulosine.
Table 1.
LC-MS/MS ion acquisition parameters (MRM mode) for identification and confirmation of Jaspine B and Spisulosine.
| Compound |
Q1 mass |
Q3 mass |
DP (volts) |
EP (volts) |
CE (volts) |
CXP (volts) |
| Jasine B |
300.2 |
270.1 |
100 |
10 |
30 |
15 |
| 300.2 |
282.0 |
100 |
10 |
30 |
15 |
| 300.2 |
254.2 |
100 |
10 |
30 |
15 |
| Spisulosine |
286.2 |
268.2 |
50 |
5 |
30 |
15 |
| 286.2 |
68.9 |
50 |
5 |
30 |
15 |
| 286.2 |
56.9 |
50 |
5 |
30 |
15 |
Table 2.
Intra-day and Inter-day precision with accuracy of Jaspine B in LC-MS/MS analysis.
Table 2.
Intra-day and Inter-day precision with accuracy of Jaspine B in LC-MS/MS analysis.
| Conc (ng/ml) |
Intra-day Precision |
Inter-day Precision |
| |
Average recovery (%) ± SD |
CV (%) |
Average recovery (%) ± SD |
CV (%) |
| |
|
|
|
|
| 0.5 |
108.28 ± 7.31 |
6.75 |
99.23 ± 7.82 |
7.88 |
| 1 |
92.25 ± 8.08 |
8.76 |
100.24 ± 5.81 |
5.80 |
| 2 |
96.88 ± 3.85 |
3.98 |
102.64 ± 5.65 |
5.51 |
| 4 |
97.69 ± 7.27 |
7.44 |
99.81 ± 0.78 |
0.78 |
| 8 |
100.87 ± 3.97 |
3.93 |
100.15 ± 5.08 |
5.07 |
| 16 |
100.07 ± 0.77 |
0.77 |
99.82 ± 1.09 |
1.10 |
3.3. Pharmacokinetics
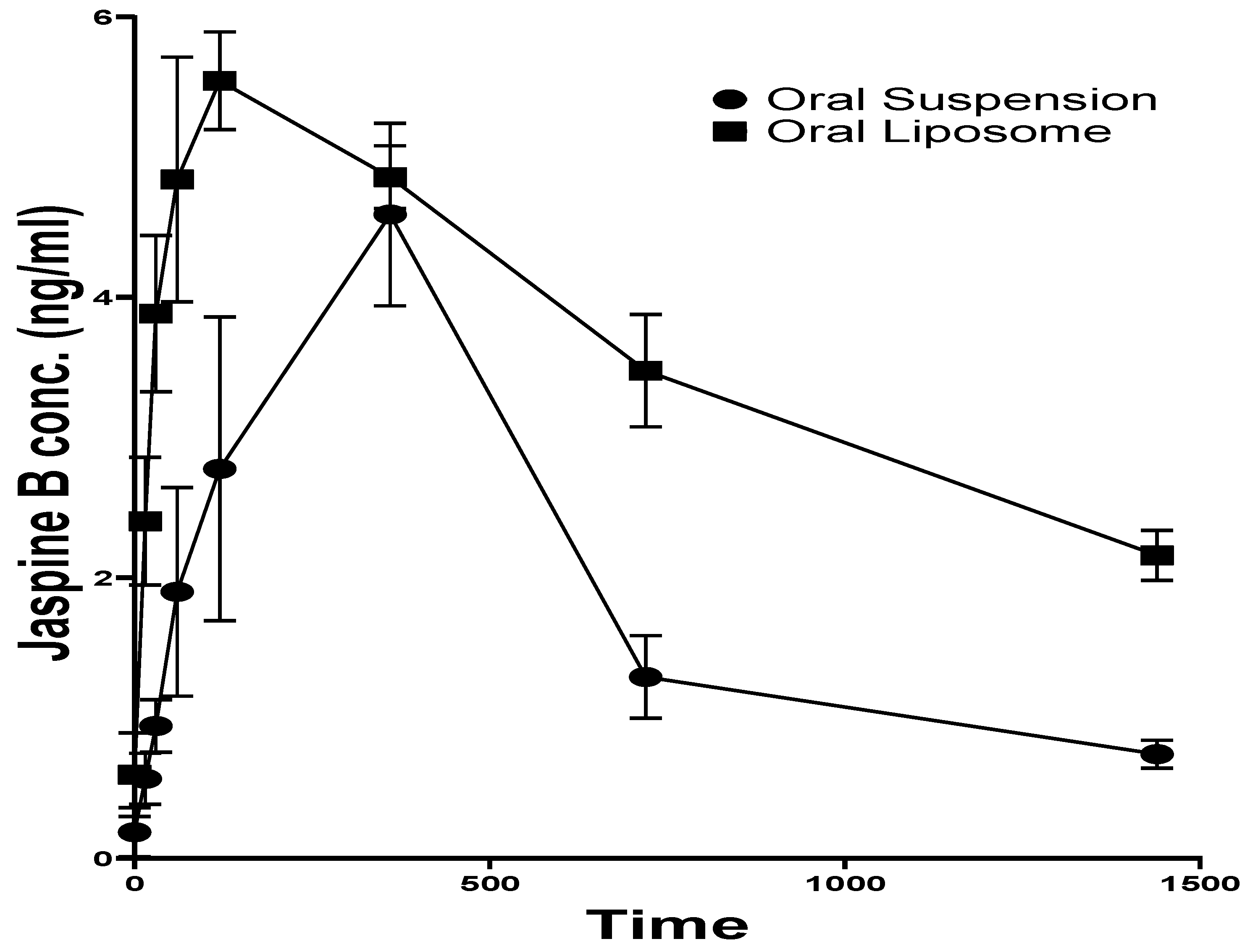
The plasma concentration of Jaspine B in the rats orally administered with Jaspine B suspension reached the maximum value (Cmax) of 4.59 ± 1.12 ng/ml at 6.00 ± 0.02 hr (tmax). Although there was no significant difference in the Cmax after administration of the liposomal formulation, its tmax was 2.00 ± 0.02 hr, which was achieved significantly faster than the administration of the drug alone (P > 0.0001).
Jaspine B's half-life (t1/2) was calculated as 7.89 ± 2.34 hr, slightly longer than the previously reported value of 5.5 ± 1.1 hr [10]. The liposomal formulation increased the t1/2 more than three-fold (26.67 ± 7.32 hrs, p = 0.0370).
The liposomal formulation of Jaspine B significantly impacted its body exposure (AUC0-t) and mean residence time (MRT). The AUC after 24 hr increased from 47.96 ± 12.81 ng.hr/ml to 88.20 ± 3.85 ng.hr/ml (p = 0.0303) and AUC0-∞ increased more than two-fold (p = 0.0244). The MRT of Jaspine B increased from 12.86 ± 3.65 to 39.13 ± 9.70 hr (p = 0.0202), indicating significantly longer circulation time after administration of liposomal formulation compared with plain Jaspine B formulation. This observation could explain the enhanced therapeutic outcome, which concurred with the pharmacodynamic effect of the Jaspine B liposomes in our previous study [6].
Figure 3.
Plasma concentration-time profile of Jaspine B in rats after oral administration of Jaspine B suspension or liposomal formulation.
Figure 3.
Plasma concentration-time profile of Jaspine B in rats after oral administration of Jaspine B suspension or liposomal formulation.
Table 3.
PK parameters after an oral dose of 5 mg/kg Jaspine B (JB) suspension or as liposomal formulation.
Table 3.
PK parameters after an oral dose of 5 mg/kg Jaspine B (JB) suspension or as liposomal formulation.
| Formulation |
Tmax (hr) |
Cmax (ng/ml) |
t1/2 (hr) |
AUC0-t**(ng. hr/mL)
|
AUC0-∞**(ng. hr/mL)
|
MRT**(hr)
|
| JB suspension |
6.00 ± 0.02 |
4.59 ± 1.12 |
7.89 ± 2.34 |
47.96 ± 12.81 |
56.77 ± 12.30 |
12.86 ± 3.65 |
| JB Liposomes |
2.00 ± 0.02 |
5.54 ± 0.61 |
26.67 ± 7.32 |
88.20 ± 3.85 |
139.69 ± 27.21 |
39.13 ± 9.70 |
| P value |
<0.0001 |
0.2840 |
0.0370 |
0.0303 |
0.0244 |
0.0202 |
4. Discussion
The low bioavailability of Jaspin B is a challenge to its effective application as a therapeutic option and anticancer agent. The current pharmacokinetic study demonstrated that the liposomal formulation of Jaspin B significantly improved its bioavailability compared to the plain oral suspension. The results showed a marked increase in its half-life, indicating sustained systemic exposure and a higher AUC, reflecting enhanced overall drug absorption. These findings highlight the effectiveness of the liposomal delivery system in overcoming the limitations of Jaspin B's poor bioavailability and support its potential for improving clinical outcomes through enhanced pharmacokinetic properties.
A sensitive, specific and robust analytical method for Jaspine B using LC-MS/MS was developed and validated. Sample preparation was carried out using the protein precipitation method to create a vigorous process with a limit of quantification as low as 0.5 ng/ml. This method is more sensitive than the methods developed in previous studies that are limited by their higher limit of quantification (25 ng/ml) [
10,
15]. Additionally, the internal standard (spisulosine) used in our study has a similar molecular structure to the analyte, leading to improved accuracy, linearity and precision.
Developing a liposomal formulation of Jaspine B promises a significant advancement in addressing the pharmacokinetic challenges associated with this promising natural product. Our results suggest that the liposomal formulation has a superior pharmacokinetic profile compared to the drug alone.
The significantly shorter time (T
max) taken by the liposomal formulation (2.00±0.0 hrs) compared to the jaspine B suspension (6.00±0.00 hrs) indicates rapid absorption of the drug. This faster absorption rate could lead to an earlier onset of therapeutic action crucial in cancer treatment. Our findings are consistent with several studies that suggest liposomal formulations reduce the absorption time, resulting in lower T
max [
16,
17,
18].
Although there wasn't a significant difference between the maximum concentration of Jaspine B in both the groups, the liposomal formulation increased the half-life of Jaspine B by more than three-folds, from 7.89 ± 2.34 hrs to 26.67 ± 7.32 hrs. This extended half-life suggests that the liposomal formulation provides a slower release of Jaspine B, protecting it from degradation throughout the body. In addition to the half-life, the similar increase in MRT confirms that the formulation spends more time in circulation than the drug itself, making it available for a longer duration of action. These findings align with the numerous studies that have established that liposomal formulations enhance the half-life and residence time in the blood [
19,
20,
21]. This property may be attributed to the ability of the PEGylated liposomes to repel the plasma components such as opsonins and thereby avoid being taken up by reticuloendothelial cells [
22,
23]. Consequently, this reduces the metabolism of the liposomes and allows them to stay in circulation for extended periods.
As a measure of total drug exposure, the significant increase in AUC could be another factor contributing to the improved therapeutic outcome of liposomal formulation we observed in our previous study [
6]. The combination of faster absorption, prolonged circulation time, and increased total exposure makes the liposomal formulation a promising approach to improve the therapeutic activity of drugs that suffer from poor pharmacokinetic profiles. The added advantage that liposomal formulations offer may be extrapolated to its potential to utilize lower and less frequent dosing of the cytotoxic effects, thereby reducing the adverse effects. These drugs may have enhanced availability to the tumors due to their longer residence time and the passive uptake of liposomes through enhanced permeation and retention (EPR) effect [
24]. In such scenarios, the drug's anticancer efficacy will be improved, and the rate of resistance to drug therapy will be reduced.
5. Conclusions
In this study, we developed and validated a sensitive, selective and robust LC-MS/MS method for the assay of Jaspine B in liposomal formulation and rat plasma. The developed method was applied to study the comparative pharmacokinetics of the liposomal formulation of Jaspine B in rats.
Our results show that liposomal encapsulation significantly improved the pharmacokinetic parameters of Jaspine B by reducing the time required to reach maximum plasma concentration
, increasing oral bioavailability, total body exposure, circulation time, and the drug's half-life, leading to improved therapeutic outcomes. This observation was in concert with our pharmacodynamic study result [
6].
Author Contributions
Conceptualization, A.A.-H.; methodology, A.A.-H., B.G., and P.G.; formal analysis, B.G., and P.G.; investigation, A.A.-H., and B.G.; resources, A.A.-H., and S.P.; data curation, A.A.-H., and B.G..; writing—original draft preparation, B.G., and A.A.-H.; writing—review and editing, A.A.-H., and B.G.; visualization, B.G., and A.A.-H.; supervision, A.A.-H.; project administration, A.A.-H.; funding acquisition, A.A.-H., and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding and was supported by the ISU startup fund.
Institutional Review Board Statement
Idaho State University's Institutional Animal Care Committee approved the animal study protocol under legal and ethical standards established by the National Research Council and published in the Guide for the Care and Use of Laboratory Animals (protocol #798).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kuroda, I.; Musman, M.; Ohtani, I.I.; Ichiba, T.; Tanaka, J.; Gravalos, D.G.; Higa, T. Pachastrissamine, a Cytotoxic Anhydrophytosphingosine from a Marine Sponge, Pachastrissa sp. Journal of Natural Products 2002, 65, 1505–1506. [Google Scholar] [CrossRef] [PubMed]
- Ledroit, V.; Debitus, C.; Lavaud, C.; Massiot, G. Jaspines A and B: two new cytotoxic sphingosine derivatives from the marine sponge Jaspis sp. Tetrahedron Letters 2003, 44, 225–228. [Google Scholar] [CrossRef]
- Abraham, E.; Davies, S.G.; Roberts, P.M.; Russell, A.J.; Thomson, J.E. Jaspine B (pachastrissamine) and 2-epi-jaspine B: synthesis and structural assignment. Tetrahedron: Asymmetry 2008, 19, 1027–1047. [Google Scholar] [CrossRef]
- BOGDANOVA, A.; KELLO, M.; MACEJOVA, A.; NOSALOVA, N.; PETIK, P.; TAKAC, P.; MARTINKOVA, M.; MEZEIOVA, E.; MIROSSAY, L.; GAL, P.; et al. Jaspine B Hydrochloride-induced Apoptosis in HeLa Cells Is Associated With Disrupted Sphingolipid Metabolism and Ceramide Overload. Anticancer Research 2021, 41, 2875–2883. [Google Scholar] [CrossRef]
- Xu, F.; Xie, Q.; Li, Y.-w.; Jing, Q.-q.; Liu, X.-j.; Xu, Y.-c.; Wang, X.; Liu, L.; Kim, G.; Choi, Y.; et al. Suppression of JNK/ERK dependent autophagy enhances Jaspine B derivative-induced gastric cancer cell death via attenuation of p62/Keap1/Nrf2 pathways. Toxicology and Applied Pharmacology 2022, 438, 115908. [Google Scholar] [CrossRef]
- Khajeh pour, S.; Mateen, S.; Pashikanti, S.; Barrott, J.J.; Aghazadeh-Habashi, A. Formulation, Characterization, and In Vitro/In Vivo Efficacy Studies of a Novel Liposomal Drug Delivery System of Amphiphilic Jaspine B for Treatment of Synovial Sarcoma. Marine Drugs 2022, 20, 509. [Google Scholar] [CrossRef]
- Cingolani, F.; Simbari, F.; Abad, J.L.; Casasampere, M.; Fabrias, G.; Futerman, A.H.; Casas, J. Jaspine B induces non-apoptotic cell death in gastric cancer cells independently of its inhibition of ceramide synthase. J Lipid Res 2017, 58, 1500–1513. [Google Scholar] [CrossRef]
- Salma, Y.; Lafont, E.; Therville, N.; Carpentier, S.; Bonnafé, M.-J.; Levade, T.; Génisson, Y.; Andrieu-Abadie, N. The natural marine anhydrophytosphingosine, Jaspine B, induces apoptosis in melanoma cells by interfering with ceramide metabolism. Biochemical Pharmacology 2009, 78, 477–485. [Google Scholar] [CrossRef]
- Yoo, H.; Lee, Y.S.; Lee, S.; Kim, S.; Kim, T.-Y. Pachastrissamine from Pachastrissa sp. Inhibits Melanoma Cell Growth by Dual Inhibition of Cdk2 and ERK-mediated FOXO3 Downregulation. Phytotherapy Research 2012, 26, 1927–1933. [Google Scholar] [CrossRef]
- Choi, M.-K.; Lee, J.; Nam, S.J.; Kang, Y.J.; Han, Y.; Choi, K.; Choi, Y.A.; Kwon, M.; Lee, D.; Song, I.-S. Pharmacokinetics of Jaspine B and Enhancement of Intestinal Absorption of Jaspine B in the Presence of Bile Acid in Rats. Marine Drugs 2017, 15, 279. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, S.; Li, L.-L.; Hua, Y.-G.; Yue, J.-F.; Li, J.-F.; Jin, C.-Y. Discovery of novel jaspine B analogues as autophagy inducer. Bioorganic & Medicinal Chemistry Letters 2018, 28, 497–502. [Google Scholar] [CrossRef]
- Lee, M.-K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef]
- Bi, Y.; Lv, B.; Li, L.; Lee, R.J.; Xie, J.; Qiu, Z.; Teng, L. A Liposomal Formulation for Improving Solubility and Oral Bioavailability of Nifedipine. Molecules 2020, 25, 338. [Google Scholar] [CrossRef] [PubMed]
- Pashikanti, S.; Ukani, R.; David, S.A.; Datta, A. Total Synthesis and Structure–Activity Relationship Studies of the Cytotoxic Anhydrophytosphingosine Jaspine B (Pachastrissamine). Synthesis 2017, 49, 2088–2100. [Google Scholar] [CrossRef]
- Song, I.-S.; Jeon, J.-H.; Lee, J.; Lim, D.Y.; Lee, C.H.; Lee, D.; Choi, M.-K. Development of Jaspine B analysis using LC-MS/MS and its application: Dose-independent pharmacokinetics of Jaspine B in rats. Analytical Science and Technology 2021, 34, 37–45. [Google Scholar]
- Kim, J.H.; Shin, D.H.; Kim, J.-S. Preparation, characterization, and pharmacokinetics of liposomal docetaxel for oral administration. Archives of Pharmacal Research 2018, 41, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Al-Meshal, M.A.; Khidr, S.H.; Bayomi, M.A.; Al-Angary, A.A. Oral administration of liposomes containing cyclosporine: a pharmacokinetic study. International Journal of Pharmaceutics 1998, 168, 163–168. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, M.; Zhang, J.; Peng, W.; Firempong, C.K.; Deng, W.; Wang, Q.; Wang, S.; Shi, F.; Yu, J.; et al. Improved oral bioavailability of capsaicin via liposomal nanoformulation: preparation, in vitro drug release and pharmacokinetics in rats. Archives of Pharmacal Research 2015, 38, 512–521. [Google Scholar] [CrossRef]
- Bally, M.B.; Nayar, R.; Masin, D.; Hope, M.J.; Cullis, P.R.; Mayer, L.D. Liposomes with entrapped doxorubicin exhibit extended blood residence times. Biochimica et Biophysica Acta (BBA) - Biomembranes 1990, 1023, 133–139. [Google Scholar] [CrossRef]
- Shehata, T.; Ogawara, K.-i.; Higaki, K.; Kimura, T. Prolongation of residence time of liposome by surface-modification with mixture of hydrophilic polymers. International Journal of Pharmaceutics 2008, 359, 272–279. [Google Scholar] [CrossRef]
- Gabizon, A.; Papahadjopoulos, D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proceedings of the National Academy of Sciences 1988, 85, 6949–6953. [Google Scholar] [CrossRef] [PubMed]
- Senior, J.; Delgado, C.; Fisher, D.; Tilcock, C.; Gregoriadis, G. Influence of surface hydrophilicity of liposomes on their interaction with plasma protein and clearance from the circulation: Studies with poly(ethylene glycol)-coated vesicles. Biochimica et Biophysica Acta (BBA) - Biomembranes 1991, 1062, 77–82. [Google Scholar] [CrossRef]
- Needham, D.; McIntosh, T.J.; Lasic, D.D. Repulsive interactions and mechanical stability of polymer-grafted lipid membranes. Biochimica et Biophysica Acta (BBA) - Biomembranes 1992, 1108, 40–48. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J Pers Med 2021, 11. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).