Introduction
For years, developed and developing countries have had to rely on burning of fossils such as coal, oil and gas to produce energy that powers up industries, vehicles and systems. Subsequently, weather patterns have changed as hotter temperatures are experienced, leading to severe heat strokes and diseases, severe storms, increased droughts and a rising ocean level [
1]. There have been studies looking into green energy to minimise or even reverse most of these deleterious effects [
2]. Green energy, also known as renewable or low-carbon energy, is the energy obtained from the direct environment and has a way of restoring itself [
3]. Biomass is an example of a renewable source of energy that involves matter from the tissues of animals and plants [
4]. Its application also stretches far beyond the provision of energy but also reduces pollution. This is because many of these wastes contribute to heaps of debris that may cause harm to residents and destabilise ecosystems where these wastes exist [
5]. Biogas is a natural gas obtained from the anaerobic digestion of wastes by anaerobic microorganisms [
6]. The resulting gas is a fuel consisting mainly of methane (CH
4) and subsequently, other minor gases such as hydrogen sulphide (H
2S), carbon dioxide (CO
2), water, and impurities in trace amounts. The chain of reaction for the process of biogas production involves four stages; hydrolysis, acidogenesis, acetogenesis, and methanogenesis [
7]. The production of biogas is heavily dependent on various production process parameters. These parameters are necessary for optimal production of biogas and they are the core of this study. These include temperature, C to N ratio, substrate-to-co-substrate ratio, pH, among others [
8].
The optimal carbon-to-nitrogen ratio for biogas production is between 20 - 30:1 [
9] above and below which stunts microbial activity. When the ratio is too low, it can lead to an accumulation of ammonia in the digester and this can limit biogas production [
10] Similarly, when it is too high, it indicates too much carbon in the process and this slows down the digestion and lowers biogas yield [
10]. The pH for anaerobic digestion has an optimal range within which biogas production is effective, this range is found between 6.5 and 7.2 [
11]. Temperature is also an important parameter to consider when operating a biogas plant. The microbes responsible for methane production operate within a range called the mesophilic range (35-40 °C) and within this range, methanogens are active, causing higher biogas yield [
12].
A study [
13] indicates that anaerobic digestion is an efficient and environmentally friendly way of generating energy. Against this backdrop, this study aims to optimise these identified process parameters of biogas production through biochemical and microbial analyses using cow dung, cow rumen and cassava water. It also reports the microbial investigation done on microorganisms associated with waste substrates used in the biodigester.
Materials and Methods
Biodigester Setup
For the study, a 20 L water dispenser bottle was used as the biodigester. The biodigester was bored at the side of the neck for the feed inlet and at the bottom for the sludge outlet. The original opening for the dispenser bottle was reconstructed with a hose connected through it to a tyre tube for gas collection.
Plate 1.
The digester set up.
Plate 1.
The digester set up.
Biodigester Feedstock
The feedstock used for the final setup was cow dung, cassava water, and cow rumen as inoculum. All substrates were sourced locally and collected fresh, early in the morning. The feedstock was properly mashed to reduce lumps and increase surface area. The mixing ratio of the substrates was based on an individual Carbon-to-Nitrogen ratio of the substrates using Equation 1 [
14].
The mixing ratio of substrate-to-inoculum was based on a recommended ratio of 1:1 [
13]
After mixing, the pH of the slurry was taken using a benchtop pH metre and adjusted to 6.8 using NaOH as an alkali pretreatment method [
15]. After feeding, the digester was agitated daily to ensure proper mixing and prevent the formation of scum and dead zones in the digester. To optimise the temperature, the biodigester was placed in an open field and covered with a black polyethylene bag to keep the heat in and prevent light-activated reactions that may cause the growth of algae. Daily readings of the ambient and digester temperatures were done using an infrared thermometer.
Chemical Optimisation
It is established that process inhibition is related to the particular characteristics of the substrate to be anaerobically digested, including the pH, process temperature (mesophilic or thermophilic), type of the seed sludge (inoculum), the reactor configuration, and the concentrations of ammonium and ammonia [
12]. This can be overcome using chemical optimisation [
16]. Literature [
12,
17,
18] shows that the optimal carbon-to-nitrogen ratio of uninhibited anaerobic digestion is 25 - 30:1, which forms the basis for calculating the masses of each substrate used as shown in
Table 1. Each substrate was then measured according to these calculated values as seen in
Table 2. A weighing scale was used to measure solid substrates and a graduated cylinder was used to weigh the liquid substrates. The substrates were all collected fresh; cow dung and cow rumen were collected from an abattoir located at Odo Eran in thick polythene bags to avoid spillage, and cassava water was collected from a local fufu shop at AP, Ile-Ife in two 10 litres kegs. Each mass of solid feedstock was weighed using a weighing balance and liquid feedstock was measured using graduated cylinders. The substrates were then mixed based on C/N ratios obtained from the literature [
9,
19] (see
Table 1).
Equations 2 and 3 were used to calculate for a total solids of 10% for the substrates. The process involved drying samples in the oven at a temperature of 105°C until a constant weight of the samples was observed [
20], at the Separation Laboratory, Chemical Engineering Department, Obafemi Awolowo University, Ile-Ife, Osun State. After the samples were dried to a constant weight, the new weights were applied to equation (3) to calculate the substrate-to-water ratio of which a ratio of 1:2 was obtained.
where;
W1 = weight of crucible + weight of dry substrate,
W2 = weight of crucible
W3 = weight of crucible + weight of wet substrate
Where = actual moisture content
= desired moisture content
Another pre-treatment method applied was adding NaOH solution to the mixture as a buffer. Before adding the buffer, the pH for the digester was 4.3. This highly acidic medium does not favour biogas production, hence the need for pretreatment. After buffering the pH, the digester pH became 7.4. The substrates were fed into the digester at once and the digester manually agitated frequently.
Microbial Analysis
The samples were collected aseptically from different locations in Ile-Ife and processed in the Department of Microbiology laboratory. The substrates investigated were cow dung, cow rumen contents, and cassava water. One [
1] g of each substrate was inoculated into 9 mL of sterile peptone water as a pre-enrichment medium and incubated at 37
oC for 24 h. Following incubation 1 mL from the 24 h ‘old’ pre-enrichment medium was serially diluted to a 5-fold dilution and plated on a sterile plate using a pour plate method [
21]. Isolation of bacterial from the digestates was carried out by taking 1 g of the digestate aseptically into 9 mL of sterile peptone water and incubated in an anaerobic jar at 37
oC for 24 h, after which it was serially diluted to a 5-fold dilution and plated on a sterile plate using pour plate method Different agar was used which include Nutrient Agar (NA), Eosin Methylene Blue agar (EMB) and Man Ragosa Sharpe agar (MRS). The plates were incubated at 37
oC for 24 h. Isolates were streaked for the pure colony and identified using biochemical characterisation [
22]. The total heterotrophic bacteria count was also estimated. The biochemical tests [
23] carried out include catalase, lactose, glucose, sucrose, citrate, indole, H
2S production, gas, methyl red, Voges-Proskauer, and motility tests. They were carried out to identify and characterise the organisms detected in the substrates responsible for producing the biogas.
Results
This section presents the results of the chemical optimisation of the process parameters in biogas production as well as the microbial analysis of the substrates.
Chemical Optimisation
The Carbon-to-Nitrogen ratio was obtained from the literature (Cow dung (24), Cassava water (28.8)), and using Equation 1, the optimal ratio was calculated to be 26.4:1 when the substrates were mixed in a 1:1:1 ratio.
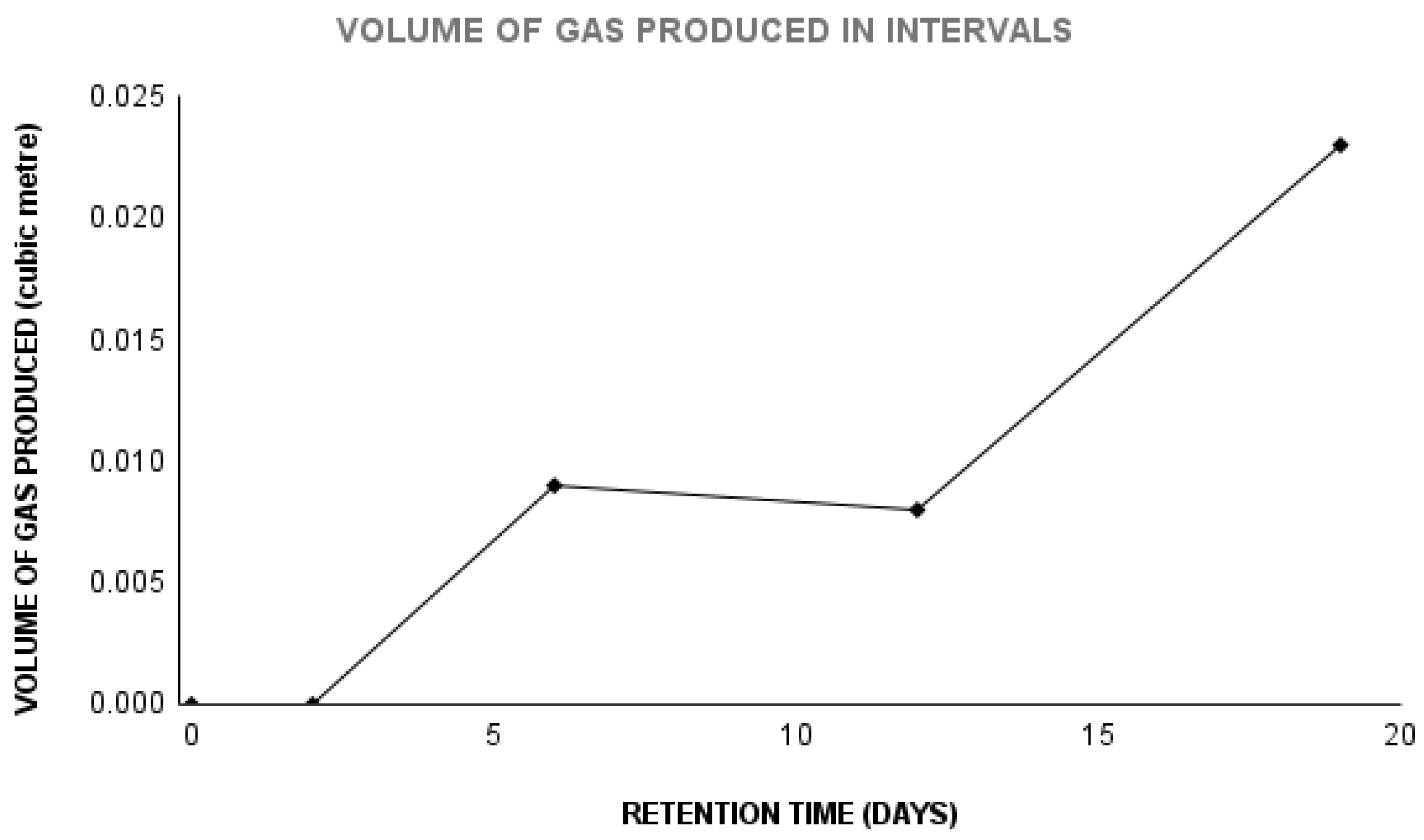
A cumulative of 39.13 L of biogas was produced after a 19-day digester period as seen in Figure 3. Gas production started 24 h, similar to what was reported in previous studies [
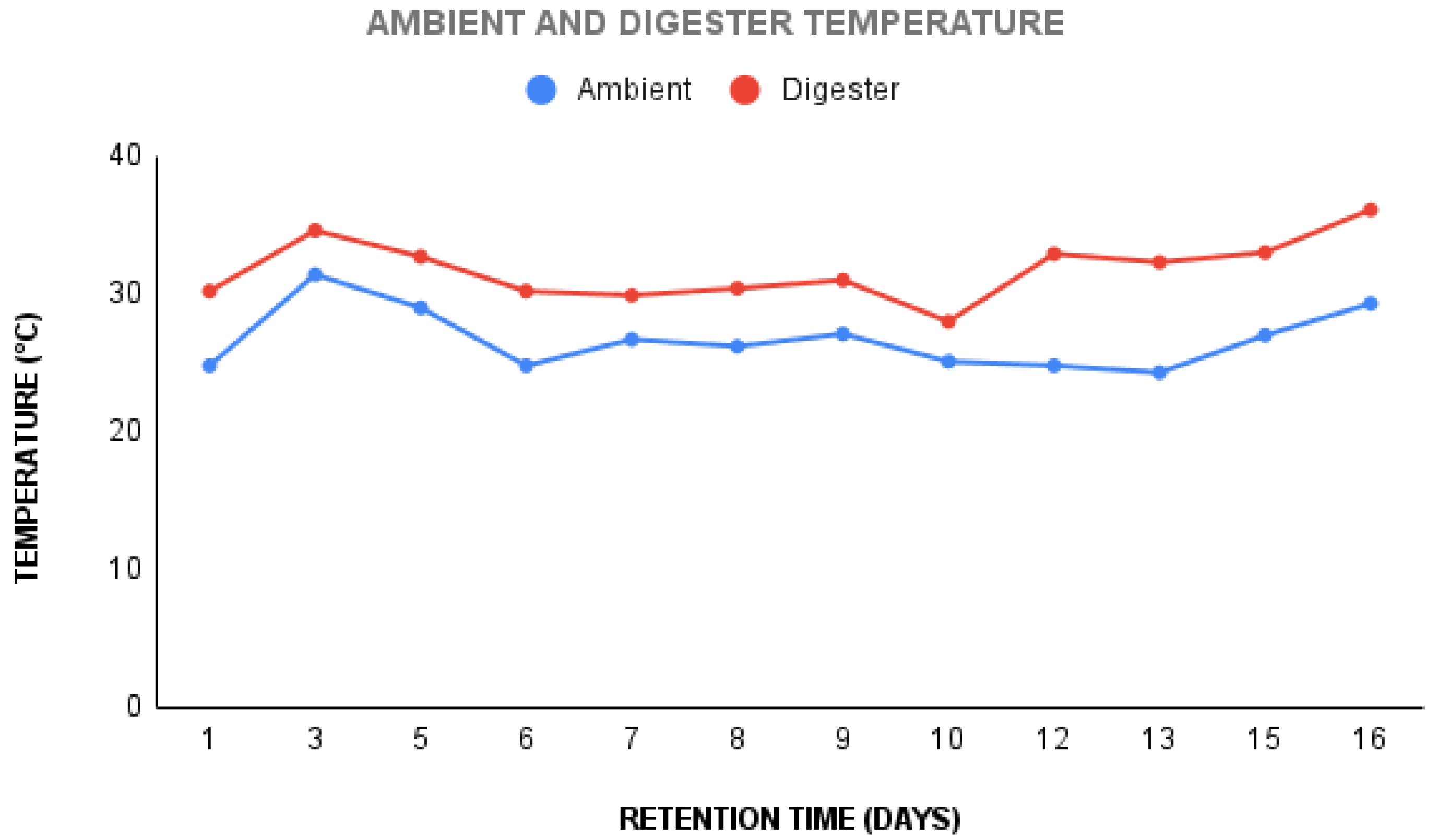
13] and on the fourth day of the hydraulic retention period, the tube had inflated as seen in Figure 2. After a hydraulic retention time of 19 days, a decline in the gas being produced was observed. We deduce that this could have been caused by the drop in temperature during the frequent rainy days as reflected in Figure 2 and possible leakages around the digester. The ambient temperature fell between a range of 24.3 °C to 31.8 °C and averaged 26 °C, while the digester temperature fell between 28 °C and 36.1 °C and averaged 31.6°C. The pH of the slurry was taken after 19 days and was found to be 6.24. The results from the biochemical characterisation of microbes obtained from the digestates can be found in Table 3.
Plate 2.
Inflated tube after 4 days.
Plate 2.
Inflated tube after 4 days.
Table 1.
Percentage of carbon and nitrogen content found in each feed stock (substrate) and their carbon-to-nitrogen ratio.
Table 1.
Percentage of carbon and nitrogen content found in each feed stock (substrate) and their carbon-to-nitrogen ratio.
| Feedstock |
Carbon Content |
Nitrogen Content |
C/N |
Cassava Water
Cow Manure |
28.8
24 |
1
1 |
28.8
24 |
Table 2.
Mixing ratios of various feedstock for optimal production of biogas using different masses for Digester.
Table 2.
Mixing ratios of various feedstock for optimal production of biogas using different masses for Digester.
| Feedstock |
Mass (kg) |
%C |
M * %C |
%N |
M * %N |
Cassava Water
Cow Manure
Cow Rumen
Water |
4.46
4.46
4.46
1.62 |
28.8
24
–
– |
1.285
1.070
–
– |
1
1
–
– |
0.046
0.046
–
– |
| Total |
15 |
|
2.355 |
|
0.0892 |
Figure 1.
Graph of the volume of gas produced.
Figure 1.
Graph of the volume of gas produced.
Figure 2.
Ambient and digester temperature.
Figure 2.
Ambient and digester temperature.
Microbial Analysis
The microbial analysis becomes significant as anaerobic digestion is highly selective in terms of the type of feedstock being utilised, operating temperature, and methods of pre-treatment [
24].
The laboratory results of the microbial analysis showed potential microbes such as
Bacillus sp.
Escherichia coli,
Pseudomonas sp.,
Proteus sp.,
Klebsiella sp.,
Lactobacillus sp., and Staphylococcus aureus, are involved in the digestion of organic matter present in the substrates [
25]. This agrees with other authors who isolated
Escherichia coli from cow dung [
26],
Staphylococcus sp, and
Escherichia coli from cow dung [
27]. The total heterotrophic bacteria count ranged from 1.6 x10
5 to 8.7 x10
5 Cfu/g and cow dung had the highest bacteria count in
Table 3. The high presence of bacteria in the cow dung could be attributable to its fecal origin. The lower bacteria count presented by cassava water could have resulted from being acidic which selects for the growth of acidophiles; these are bacteria that can grow in acidic medium. These consortiums of microorganisms suggest that they are involved in a couple of synergistic reactions leading to biogas production. The pH of the digestate could be attributed to the slight variation in their bacterial community. Availability of nutrients and pH determine the types of organisms in a micro-community. The methanogenic bacteria isolated from the digestates are
Pseudomonas sp,
Bacillus sp,
Proteus sp., Saccharomyces sp, and
Lactobacillus sp. This agrees with the work of [
14] who isolated similar microbes in an anaerobic biodigester. These bacteria were isolated from both digestate fermented glucose anaerobically with carbon dioxide shown in the biochemical test (
Table 4). This agrees with [
28] which reported that facultative bacteria can convert glucose into lactate, succinate, acetate, and ethanol with carbon dioxide which are intermediate products of biogas production. Isolates from both digestates utilise citrate as shown in the results from the biochemical tests. This also indicates that these microbes can convert citrate into oxaloacetate and acetate which can be further converted to pyruvate and carbon dioxide [
29].
Conclusion and Recommendation
The experiment showed that using chemical optimisation principles makes it possible to adjust some process parameters such as the pH and Carbon-to-Nitrogen ratio of the substrates for improved biogas yield. This is because using physical and chemical pretreatment methods such as adding a buffer to increase the pH and reducing the substrate sizes, also aided and sped up the process, causing gas to be produced a day after feeding the digester. While placing the digester where there was direct interaction with sunlight was the best option to promote microbial activity in the digester, the temperature fluctuations as seen in Figure 4 caused an early reduction in the volume of gas. After the retention time, a slightly reduced initial pH of 6.8 to 6.24 was noticed, indicating that pH might have had a minimal effect on reducing the gas produced as time went on. Also, the biochemical analysis of the microbes using tests such as the indole, lactase, etc., and microbial analysis done on the substrates was an indication that biogas production was on the right path from the beginning as identified microbes involved in the first stage of anaerobic digestion - hydrolysis were present. The isolation of microbes from the digestates revealed their character as bacteria that produce intermediate products and subsequent gases needed for biogas formation.
It is recommended that the retention time be longer as the microbial analysis on the digestate shows it was still very active when dislodging the biodigester and more biogas could have been produced. For better results, it is also recommended that the carbon-to-nitrogen ratio for each substrate be obtained from laboratory analysis rather than from literature review as substrates may vary from region to region.
Author Contributions
“Conceptualization, A. S. Momodu; methodology, A. Adedire, A. C. Babajide; validation, R. O. Adesina, E. O. Kalu. and F. A. Ayofe; formal analysis, A. Adedire, A. C. Babajide; investigation, A. Adedire, A. C. Babajide; resources, A. Adedire, A. C. Babajide, A. S. Momodu, E. F. Aransiola; data curation, A. Adedire, A. C. Babajide, F. A. Buhari.; writing—original draft preparation, A. Adedire, A. C. Babajide, A. S. Momodu; writing—A. S. Momodu, R. O. Adesina, E. O. Kalu; visualization, A. C. Babajide; supervision, A. S. Momodu and E. F. Aransiola; project administration, A. S. Momodu; funding acquisition, A. S. Momodu.
Funding
This research was funded by Tertiary Education Funds (TETFund) with Ref No: TETF/DR&D/CE/UNIV/ILE-IFE/IBR/2023/VOL1/023 and “The APC is not funded.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Health Research Ethics Committee of Obafemi Awolowo University (protocol code HREC No: IPH/OAU/12/2602 and date of approval 7th August 2024).”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Clarke B, Otto F, Stuart-Smith R, Harrington L. Extreme weather impacts of climate change: an attribution perspective. Environmental Research: Climate. 2022 Sep 1;1(1):012001. [CrossRef]
- Ang TZ, Salem M, Kamarol M, Das HS, Nazari MA, Prabaharan N. A comprehensive study of renewable energy sources: Classifications, challenges and suggestions. Vol. 43, Energy Strategy Reviews. Elsevier Ltd; 2022. [CrossRef]
- Sehgal K. Current State and Future Prospects of Global Biogas Industry. In 2018. p. 449–72. [CrossRef]
- Ben-Iwo J, Manovic V, Longhurst P. Biomass resources and biofuels potential for the production of transportation fuels in Nigeria. Vol. 63, Renewable and Sustainable Energy Reviews. Elsevier Ltd; 2016. p. 172–92. [CrossRef]
- Environmental Impact of Indiscriminate Waste Disposal “A Case study of Nigerian Air force Base Kaduna” [Internet]. Available from: www.ijeas.org.
- Jameel MK, Mustafa MA, Ahmed HS, Mohammed A jassim, Ghazy H, Shakir MN, et al. Biogas: Production, properties, applications, economic and challenges: A review. Vol. 7, Results in Chemistry. Elsevier B.V.; 2024. [CrossRef]
- Witantri RG, Purwoko T, Sunarto, Mahajoeno E. Bioethanol Production by Utilizing Cassava Peels Waste Through Enzymatic and Microbiological Hydrolysis. In: IOP Conference Series: Earth and Environmental Science. Institute of Physics Publishing; 2017. [CrossRef]
- Nsair A, Cinar SO, Alassali A, Qdais HA, Kuchta K. Operational Parameters of Biogas Plants: A Review and Evaluation Study. Vol. 13, Energies. MDPI AG; 2020. [CrossRef]
- Orhorhoro OW, Orhorhoro EK, Ebunilo PO. Analysis of the Effect of Carbon/Nitrogen (C/N) Ratio on the Performance of Biogas Yields For Non-Uniform Multiple Feed Stock Availability and Composition in Nigeria [Internet]. Vol. 3, IJISET-International Journal of Innovative Science, Engineering & Technology. 2016. Available from: www.ijiset.com.
- 10. Orhan Yenigün, Burak Demirel, Ammonia inhibition in anaerobic digestion: A review, Process Biochemistry, Volume 48, Issues 5–6, 2013, Pages 901-911, ISSN 1359-5113, . [CrossRef]
- Sunny SM, Joseph K. REVIEW ON FACTORS AFFECTING BIOGAS PRODUCTION [Internet]. Vol. 5, International Journal For Technological Research In Engineering. 2018. Available from: www.ijtre.com.
- Yenigün, O., & Demirel, B. (2013). Ammonia inhibition in anaerobic digestion: A review. Process Biochemistry, 48(5-6), 901-911. [CrossRef]
- Fagbenle EO, Olukanni DO. Production and purification of biogas from cassava peel using cow dung as inoculum. In: IOP Conference Series: Earth and Environmental Science. IOP Publishing Ltd; 2022. [CrossRef]
- Dougherty, M. 1999. Field guide to on-farm composting, NRAES-114. Natural Resource, Agriculture, and Engineering Service. Ithaca, N.Y.
- Shukla A, Kumar D, Girdhar M, Kumar A, Goyal A, Malik T, et al. Strategies of pretreatment of feedstocks for optimized bioethanol production: distinct and integrated approaches. Vol. 16, Biotechnology for Biofuels and Bioproducts. BioMed Central Ltd; 2023. [CrossRef]
- Issah, A.A., Kabera, T. and Kemausuor, F., 2020. Biogas optimisation processes and effluent quality: A review. Biomass and bioenergy, 133, p.105449. [CrossRef]
- Zeshan, K., & Visvanathan, C. (2012). The effect of C/N ratio and ammonia-N accumulation in a cellulose-rich anaerobic system. Bioresource Technology, 113, 294-301. [CrossRef]
- Wang, X., Yang, G., Feng, Y., Ren, G. and Han, X., 2012. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresource technology, 120, pp.78-83. [CrossRef]
- Dewilda Y, Aziz R, Handayani RA. The effect of additional vegetables and fruits waste on the quality of compost of cassava chip industry solid waste on Takakura composter. IOP Conf. Ser.: Mater. Sci. Eng.. 2019;602(1):012060. [CrossRef]
- US Environmental Protection Agency. METHOD 200.7. Trace Elements in Water, Solids, and Biosolids by Inductively Coupled Plasma-Atomic Emission Spectrometry. 2001. EPA-821-R-01-010.
- Babu G, Pradhan P. Isolation and identification of methanogenic bacteria from cow dung [Internet]. Article in International Journal of Current Research. 2012. Available from: https://www.researchgate.net/publication/286192096.
- Ilesanmi OI, Adekunle AE, Omolaiye JA, Olorode EM, Ogunkanmi AL. Isolation, optimization and molecular characterization of lipase producing bacteria from contaminated soil. Sci Afr. 2020 Jul 1;8. [CrossRef]
- Chauhan, A., Jindal, T., Chauhan, A. and Jindal, T., 2020. Biochemical and molecular methods for bacterial identification. Microbiological methods for environment, food and pharmaceutical analysis, pp.425-468. [CrossRef]
- Ngo T, Ball AS, Shahsavari E. The Current Status, Potential Benefits and Future Prospects of the Australian Biogas Sector. J Sustain Bioenergy Syst. 2021;11(01):14–32. [CrossRef]
- Shah TA, Lee CC, Orts WJ, Tabassum R. Biological pretreatment of rice straw by ligninolytic Bacillus sp. strains for enhancing biogas production. Environ Prog Sustain Energy. 2019 May 1;38(3). [CrossRef]
- Ya’aba, Y. & Ramalan, A. Isolation, Identification and Characterization of Some Bacteria Associated with Biogas Production from Cow Dung. Equity Journal of Science and Technology, 2020 7(2): 91 – 99.
- International Journal of Advances in Pharmacy, Biology and Chemistry Research Article [Internet]. Available from: www.ijapbc.com.
- Duport C, Zigha A, Rosenfeld E, Schmitt P. Control of enterotoxin gene expression in Bacillus cereus F4430/73 involves the redox-sensitive ResDE signal transduction system. J Bacteriol. 2006 Sep;188(18):6640–51. [CrossRef]
- Samosir, G. R., Nainggolan, E. A. Kinda, M.M and Anwar, D. (2023) Isolation and Identification of Biogas -producing Methanogenic Bacteria A. Lelono et al. (Eds.): ICOLIB 2021, ABSR 27, pp. 444–450, . [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).