1. Introduction
ZEBRA batteries (Na-NiCl
2 solid electrolyte batteries, SEBs) have commercial applications in e.g., energy storage, energy back up and transportation [
1], due to their low costs and recyclability, long lifetime and high safety [
2]. In commercial ZEBRA batteries, Ni electrode and beta’’-alumina solid electrolyte (BASE) have a > 70% share of the cell material costs [
3]. By using abundant and low-cost Zn to replace Ni, the next-generation ZEBRA (Na-ZnCl
2 SEB) battery could have much lower costs [
4]. Na-ZnCl
2 all liquid batteries (ALBs) replace (in addition to Zn replacement) the BASE electrolyte of the Na-ZnCl
2 SEB with a molten salt electrolyte for even lower costs, longer lifetime and higher safety [
5]. Compared to the commercial ZEBRA cells (operating at around 300°C), the Na-ZnCl
2 ALB cells do not use a BASE primary electrolyte to reduce cell costs and make the scale-up easier [
5,
6]. It has a higher operating temperature of around 600°C to operate in all liquid state for better performance, e.g., to increase the possibility of charging and discharging at high current density [
5,
6].
In the commercial ZEBRA battery with a BASE primary electrolyte, AlCl
3-NaCl-NiCl
2 is used as molten salt electrolyte, which consists of a secondary electrolyte (NaAlCl
4) with the melting temperature of about 150°C and partly active electrode components (NaCl and NiCl
2) [
3]. In our previous work an adapted ZEBRA battery (called here
next-gen ZEBRA) has been intensively studied. The difference of the next-gen ZEBRA compared to ZEBRA is that Zn replaces Ni, which has the electrochemical reaction of ZnCl
2 + Na ↔ 2NaCl + Zn (about 2 V at around 300°C) during charging/discharging [
7,
8]. Sieuw, et al. (2024) investigated the influence of precursor morphology and Zn cathode processing on performance and cycle life of the next-gen ZEBRA cell at 300°C [
7]. Moreover, the dis-/charge cycling performance of Na-ZnCl
2 cells was correlated with the ternary ZnCl
2-NaCl-AlCl
3 phase diagram, and mass transport through the secondary NaAlCl
4 electrolyte was identified as an important contribution to the cell resistance [
7]. These insights enable the optimization of the design of the battery cell, e.g., to have tailored cathode microstructures. Kumar, et al. (2023) studied the AlCl
3-NaCl-ZnCl
2 salt electrolyte of the next-gen ZEBRA (Na-ZnCl
2) battery in depth [
8]. Experimental and modeling methods were used to examine its binary and ternary phase diagrams and vapor pressures theoretically via FactSage simulation and experimentally via Differential Scanning Calorimetry (DSC) and a melting point apparatus (OptiMelt
TM) [
8]. These results have been successfully correlated with the cell performance and could be used in future cell improvement [
7].
This simulation-assisted method based on thermodynamic simulation via FactSage
TM and thermal analysis via DSC and OptiMelt
TM has also been successfully applied for selection of a molten salt electrolyte for liquid metal batteries (LMBs) , which are another group of batteries using liquid metals and molten alts [
9,
10]. A low-melting-point NaCl-LiCl-KCl molten salt electrolyte was selected for a Na-based LMB (Na-LMB) based on the phase diagram and other experimental data [
9]. The Na-LMB test cell with the selected molten salt electrolyte shows an energy efficiency of about 80% and a Coulombic efficiency of >97% [
10]. Moreover, the Levelized Cost of Storage (LCOS) of the Na-LMB system with the selected NaCl-LiCl-KCl molten salt electrolyte was estimated to be 0.027~0.029 USD/kWh for scaled application conditions, based on a 1MW/5MWh demo energy storage plant made of 100 Ah cells [
10].
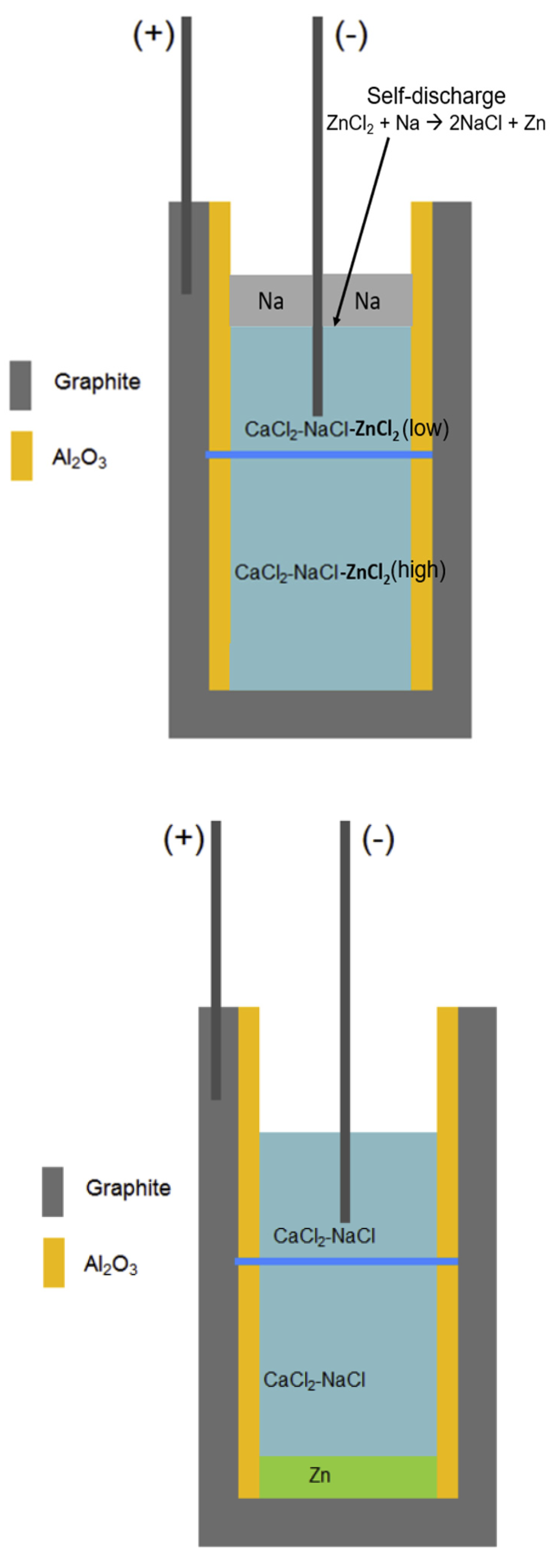
Different from the ZEBRA battery, the AlCl
3 salt cannot be used in the molten salt electrolyte of
Na-ZnCl2 ALB, since it does not use a solid ceramic electrolyte to separate the Na electrode and molten salt electrolyte, and thus the molten salt electrolyte has a direct contact with the Na metal anode (see
Figure 1 right). To avoid spontaneous reactions of the salt with Na which lead to co-reduction and safety issue, the chloride salts like CaCl
2, BaCl
2, KCl, SrCl
2, LiCl, whose electromotive forces (EMFs) are higher than Na/NaCl [
11] should be preferred. Moreover, another reason for not using AlCl
3 is the safety issue caused by its very high vapor pressure (>>10 atm) at the operating temperature (around 600°C) of the ALB [
8]. The above-mentioned chloride salts have low vapor pressures, and will therefore also be a preferred choice for the molten salt electrolyte for Na-ZnCl
2 ALB. Moreover, to have a large operation range, the molten salt electrolyte needs to have a low melting temperature, since the formed NaCl during discharging increases the melting temperature of the salt mixture electrolyte fast. Xu, et al. (2016) [
5] and Xu, et al. (2017) [
6] used a eutectic NaCl-CaCl
2 (50:50 mol%) electrolyte in their ALB cells (see
Figure 1). CaCl
2 has the EMF of 3.462 V, higher than but close to that of NaCl (3.424 V) [
11]. The co-reduction of Ca in Na electrode was observed during charging [
5,
6,
12]. This could lead to lower CaCl
2 content in the molten salt electrolyte compared to the ideal charging (without co-reduction). The molten salt electrolyte with lower CaCl
2 content than the eutectic mixture has an increased melting temperature, as shown in the NaCl-CaCl
2 phase diagram [
13]. The NaCl-CaCl
2 (50:50 mol%) electrolyte used in the ALB cell of Xu, et al. (2016) [
5] has a eutectic melting temperature of about 500°C [
5,
13]. The increased melting temperature could have significantly negative effects on the battery performance, e.g., a reduced charge/discharge range. The cell testing shows that the discharge flat voltage of the ALB cell at 560°C is in the range of about 1.4 V to 1.8 V, and the cycle coulombic efficiency achieved is about 90% at low discharge current densities (below 40 mA/cm
2) [
5]. In the ALB cell as shown in
Figure 1, a porous ceramic diaphragm (blue line in
Figure 1) is used to separate the molten salt electrolyte into two compartments and thus slow the Zn
2+ transport down from bottom to top in order to reduce the self-discharge rate. The self-discharge of this ALB is mainly caused by reaction of Zn
2+ with Na metal [
5,
6]. Thus, the solubility of Na metal in the molten salt electrolyte has a significant the effect on self-discharge. Although using the diaphragm, the self-discharge rate is still high. The open circuit voltage is about 1.83 V in the fully charged state. After leaving the cell at open circuit for 9 h, the open circuit voltage value drops to 1.69 V, while it decreases to 1.6 V after 10 h [
5]. This means the charged cell is almost fully discharged after 10 h due to self-discharge. Overall, the molten salt electrolyte is a key part of the ALB and could significantly affect its storage performance, operation safety and economics. Besides some references on ALB with a NaCl-CaCl
2 molten salt electrolyte, to our best knowledge, an in-depth study on the molten salt electrolyte for ALB has not been done and there is no available literature showing the methods and results on such molten salt ALB electrolyte selection.
To improve the cell design and fabrication of the Na-ZnCl
2 ALB, recently some techniques like neutron imaging [
14] and cell modeling [
15] were developed and used. Sarma et al. (2024) [
14] used in situ dynamic neutron radiography to develop a reusable, hermetically sealed, high temperature and sufficiently corrosion resistant cell, in order to enable long-term cycling of the Na-Zn battery in a realistic environment. The design as well as various approaches for assembling and filling the cell were also presented in their study [
14]. Godinez-Brizuela et al. (2023) [
15] developed a continuous multiphase model for liquid metal - molten salt batteries including LMBs and the ALB batteries studies in this work. It used the Na-ZnCl
2 ALB with the NaCl-CaCl
2 electrolyte as the model system. The simulation model can simulate the changes in electrode and electrolyte volume during charging and discharging, and resolve the spatial variations in the chemistry of the electrolyte that accompany the interfacial reactions. It was found that volume change and species redistribution were important in predicting the maximum theoretical capacity of the cell when neglecting other transport mechanisms. Weber et al. (2024) performed a risk assessment for the Na-ZnCl
2 ALB according to ISO 12100 to minimize the risks involved in the battery fabrication and operation [
16]. Hazard identification and risk evaluation were systematically addressed, including a thorough literature review, theoretical calculations and selected experiments. Cell overpressure was found to be one of the main risks. Overpressure might be caused either by mistakes in battery production (humidity) or operation (over-charge/discharge). In terms of cell housing, the feedthrough was identified as the weakest component. Its failure might lead to the release of hazardous aerosols to the environment. In this context, the candidate electrolyte components LiCl and BaCl
2 were identified as dangerous salt components [
16].
In this work, the promising molten salt electrolytes for the Na-ZnCl
2 ALB cell containing NaCl, CaCl
2, BaCl
2, SrCl
2 and/or KCl are selected based on the data such as EMFs of single salts, phase diagrams of salt mixtures and material costs, which are available in literature or obtained from simulation and experiments in this work. Since LiCl has high material cost [
17] and the ALB uses large amount of salt electrolyte, LiCl is not considered for the molten salt electrolyte here. In this work, the promising molten salt electrolytes mentioned above were in-depth investigated with thermodynamic simulation via FactSage
TM and thermal analysis via DSC. Moreover, the solubilities of Na and Zn electrodes in the selected promising molten salt electrolytes were measured with an analysis method based on salt titrations. Overall, this paper presents these simulated and measured data of the key thermal properties of salt systems for Na-ZnCl
2 ALBs. These properties can be also used for the battery design, optimization and operation. The presented simulation and experimental methods for molten salt electrolyte selection used in this work could also be implemented with other salt-based electrolyte batteries.
2. Materials and Methods
This section describes the methods used to select the promising molten salt electrolytes in ALB based on their various characteristics such as phase diagrams for battery improvement. Chloride salt mixtures are promising molten salt electrolytes for molten salt batteries due to their high thermal and electrochemical stabilities, and low costs [
5,
9,
18]. As shown in
Figure 1 left, NaCl in the molten salt electrolyte acts as a source of Na forming negative electrode during charging. However, NaCl has a melting temperature of > 800°C [
17]. Adding other chloride salts, the melting temperature can be significantly reduced due to the eutectic effect, for example eutectic NaCl-MgCl
2-KCl has a melting temperature of <400°C [
17]. Thus, in this work NaCl was investigated in combination with other chloride salts with low costs and high EMFs (i.e., CaCl
2, BaCl
2, SrCl
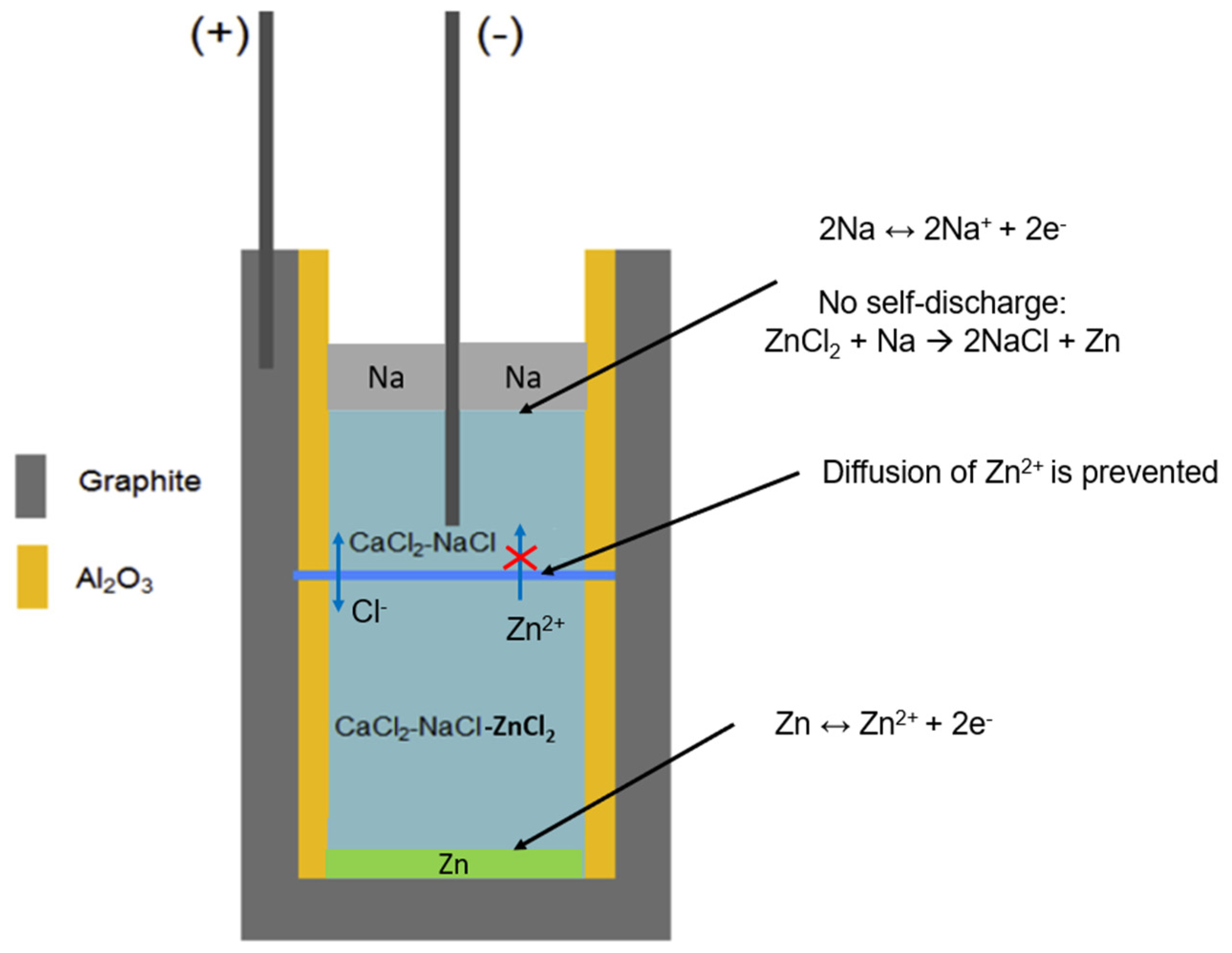
2 and KCl) in binary and ternary systems, in order to select promising molten salt electrolytes based on the key factors like melting temperature, elctctrode solubilities (self-discharge), material costs and EMFs (co-reduction issue of the Na electrode). In this work, it is assumed in the selection of molten salt electrolytes that the porous ceramic diaphragm in the ideal ALB cell (see
Figure 2) can perfectly prevent the diffusion of Zn
2+ from bottom to top, i.e., prevent the main self-discharge reaction at the interface of Na electrode with Zn
2+ in the salt as shown in
Figure 1 left. Moreover, in charge/discharge of the ALB cell, the molten salt electolyte above the diaphragm has a higher melting temperature than that below the diaphragm, due to higher content of NaCl and lower contect of ZnCl
2.
Thus for simplicity, the molten salt electolyte selection in this work is based on the molten salt electolyte above the diaphragm of the ideal ALB cell, which does not contain ZnCl2. In this work, firstly, the simulations via FactSage were performed to obtain the information like melting temperature (phase diagrams) and melting behavior for selection of the promising molten salt electrolytes. Secondly, the selected salt mixtures were prepared in moisture-free inert environment inside a glovebox and used in DSC experiments to evaluate the FactSage simulation results. After that, the experiments of the solubilities of the electrode materials (i.e., Na and Zn) in the selected salt mixtures were performed in an oven inside the glovebox. The metal solubilities were measured with a titration method developed by our DLR group previously [
9].
2.1. FactSageTM Thermodynamic Simulation
FactSage
® is a thermochemical database system with the modules of information, database, calculation and manipulation [
19]. Based on the minimum Gibbs free energy and CalPhaD (Calculation of Phase Diagrams), the calculation modules contain the main function modules of FactSage, including Reaction, Predom, EpH, Equilib, Phase Diagram, and Optisage. FactSage gives access to databases of thermodynamic data for thousands of compounds as well as to evaluated and optimized databases for hundreds of liquid and solid metals, liquid and solid oxides, molten and solid salts, aqueous solutions [
19].
This work utilizes Version 8.1 of FactSage. The Phase Diagram module and the database FTsalt were used to obtain the phase diagrams of selected binary/ternary salts of NaCl, CaCl2, BaCl2, SrCl2 and KCl. Moreover, the Equilib module was selected to plot ΔCp (J/K/mol) vs T (°C). The plots were compared with the DSC experiments performed to confirm the melting temperature and melting behavior.
2.2. Sample Preparation for DSC and Solubility Analysis
For Differential Scanning Calorimetry (DSC), anhydrous salts with a purity of 99.99% were used to prepare the salt mixtures. Due to the hygroscopicity of chloride salts (particularly CaCl2, BaCl2, SrCl2), the storage, weighing, mixing and sample preparation of the salts were carried out in a glovebox (GS Glovebox System Technik GmbH, Glovebox Mega 2, O2 < 0.5 ppm, H2O < 1 ppm) swept with ultra-high-purity argon gas (Ar 5.0, purity > 99.999%). All salt mixtures (~1 g) for thermal analysis were grinded in a ceramic mortar for at least 30 minutes by hand, so that they were mixed well with acceptable small salt particle size for thermal analysis with DSC.
For solubility analysis of Na and Zn electrode metals in the molten salts, anhydrous salts with a purity of 99% were used to prepare the salt mixtures. Same as the salt mixture samples for DSC, their storage, weighing, mixing and sample preparation were carried out in the glovebox swept with ultra-high-purity argon gas. Moreover, the Na and Zn metals with a purity of 99% were used in the measurements.
2.3. Differential Scanning Calorimetry (DSC) for Thermal Analysis
DSC is a powerful thermo-analytical technique to measure the melting temperature and phase change enthalpy of the tested sample such as a salt [
20]. Normally in a DSC measurement the temperature of the sample is increased linearly and compared to a empty reference crucible (see
Figure 3b). A sample of known mass (e.g. about 20 mg salt mixture in this work) is placed in a sample crucible and then heated and cooled at a constant rate (e.g. 10 K/min) under the sweeping of Ar 5.0 at 100 mL/min. The behavior of the heat flow inside the sample can reveal the thermal information about the melt, for example, glass transitions, phase changes, curing and melting. This method has been successfully used by many research groups including our group to measure e.g., the melting temperatures of the salt mixtures for thermal energy storage [
21] or as of salt electrolytes in molten salt batteries like liquid metal batteries [
9,
10] and metal-chloride batteries [
8].
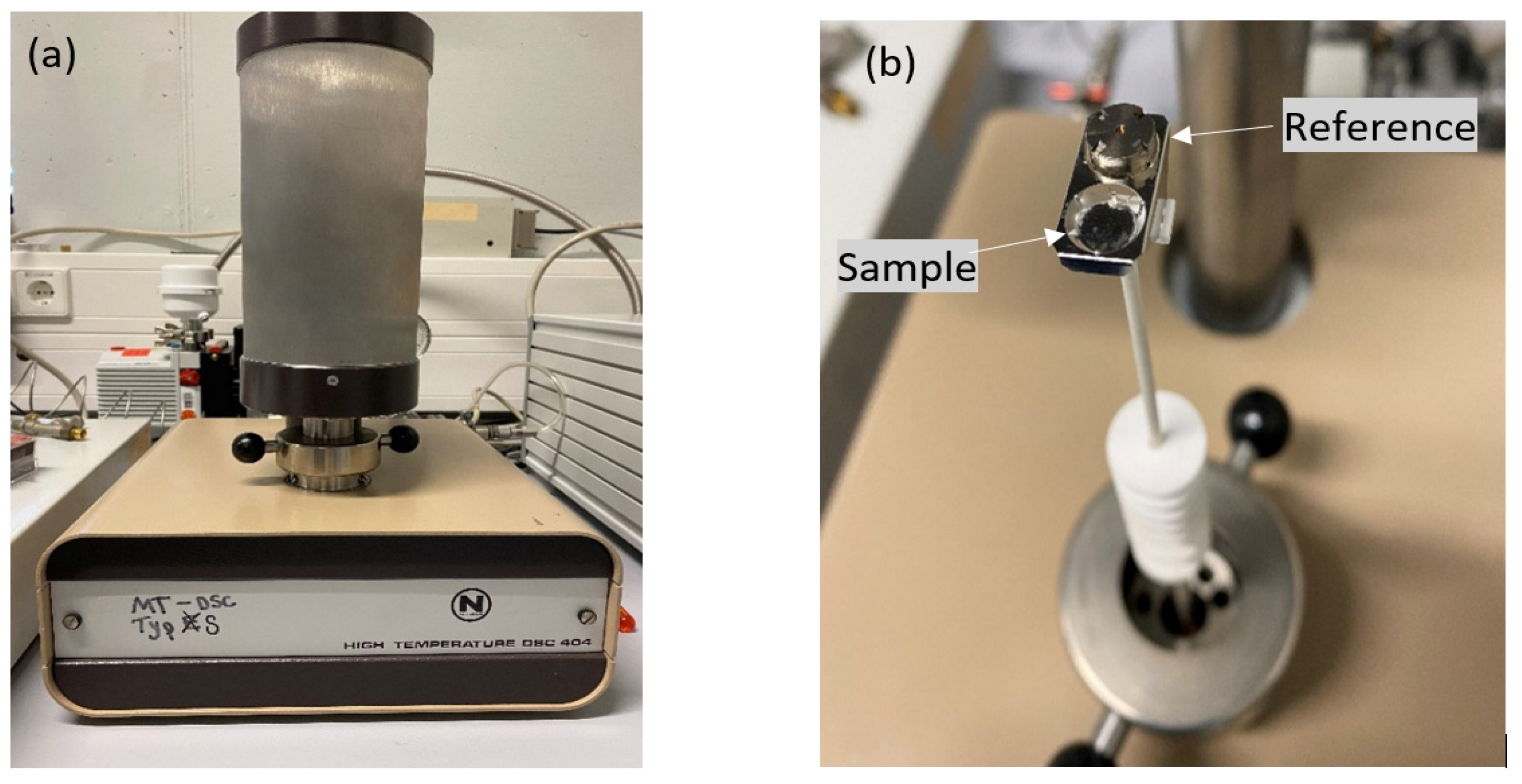
A high Temperature DSC 404
® (Netzsch) oven (see
Figure 3a) was used to study the melting process of the selected salts. The holder inside the oven with two slots for the placement of crucibles is shown in
Figure 3b. In this work, gold-plated stainless steel (SS) and Ni-based 2.4816 alloy crucibles from Netzsch were used in closed state with a maximum operation temperature of about 600°C (gold-platted stainless steel) and 650°C (Ni-based). Such high temperatures are required for this work since the salt electrolyte should be liquid at the ALB working temperature of around 600°C. Before testing the desired samples by DSC, a temperature calibration was carried out to ensure accurate readings of the instrument. Standard reference materials with known melting points were used to perform temperature calibration. Additionally, several baseline measurements were carried out before each sample measurement to ensure that the DSC instrument records a stable and accurate baseline. This involves running an empty pan with a reference pan so that it records a flat or near-zero heat flow signal. Inside the glovebox, a tool kit was used to seal the crucibles tightly (with or without salt sample). Subsequently, the crucibles were washed to rinse off any salt contaminants from the outer surface and dried before placing it in the DSC device. In every DSC measurement, 3-4 cycles were repeated for every salt sample in this work to get reliable results.
2.4. Solubility Measurement and Salt-Metal Post-Analysis
To measure the solubilities of Na and Zn electrode metals in the selected molten salt electrolytes (NaCl-CaCl
2-BaCl
2 37-45-18 mol%, NaCl-SrCl
2-KCl 30.0-26.5-43.5 mol%), the electrode metal was in direct contact with the electrolyte at the ALB’s operating temperature (600°C) and the solubility was measured versus the time of contact until saturation was reached. The tests were performed in an Ar inert environment (H
2O and O
2 < 1 ppm) inside the glovebox, due to the salt mixture’s strong hygroscopicity. As shown in
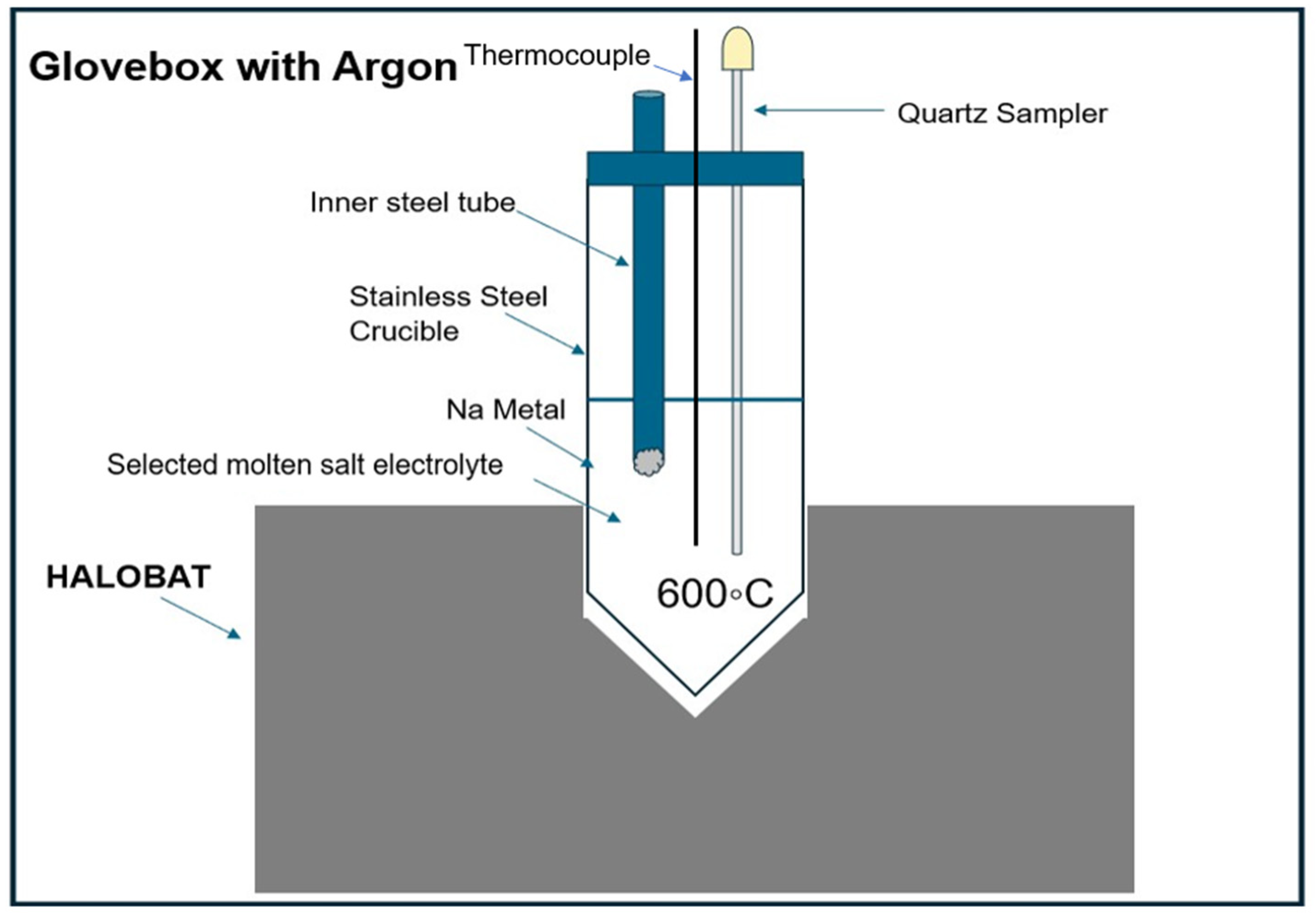
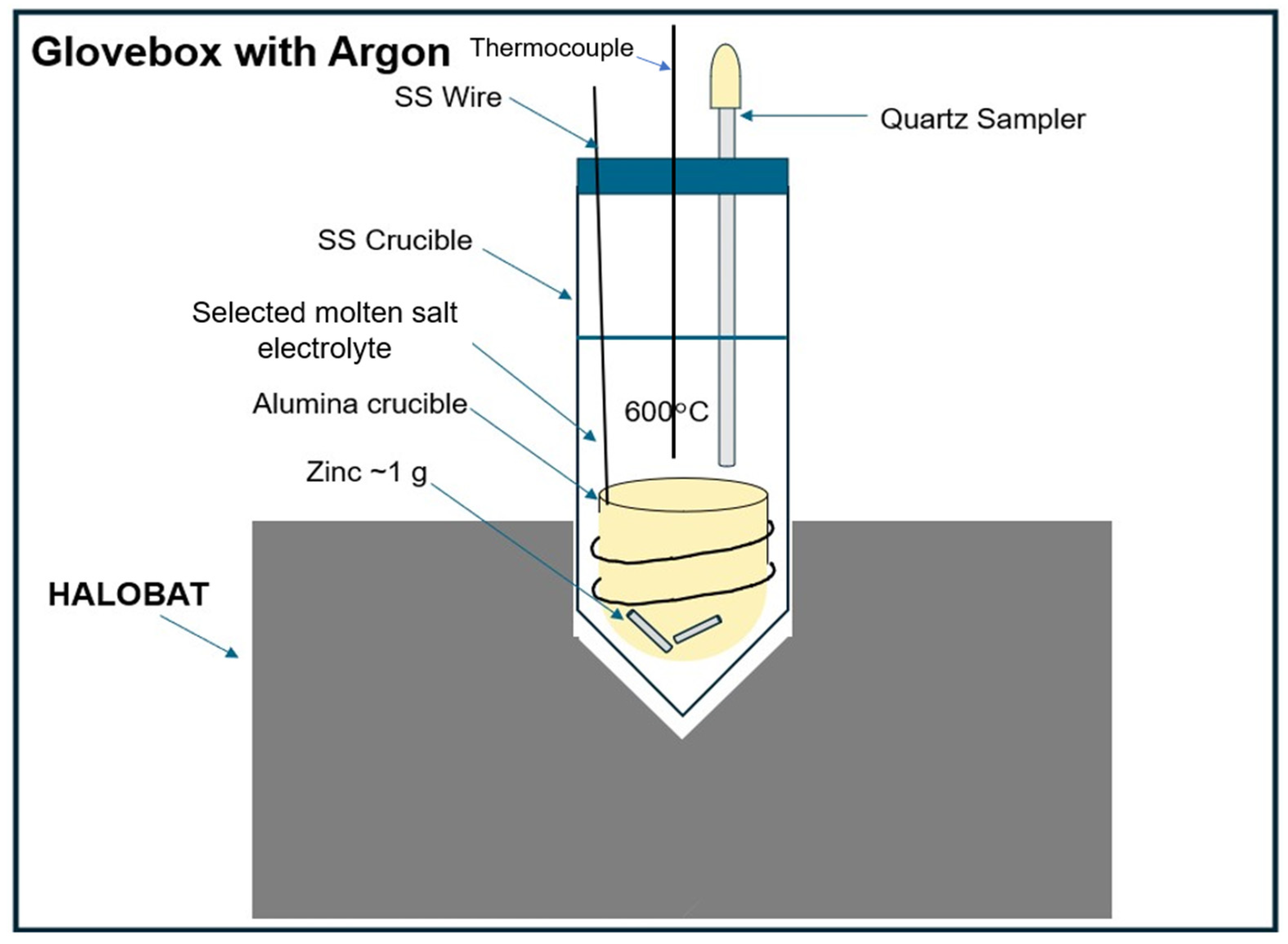
Figure 4, a specialized set-up (named HaloBat) was prepared for the tests. This experimental set-up consists of 1) an oven, 2) a stainless steel (SS) crucible containing the molten salt, and 3) a thermocouple to control the salt temperature with a temperature regulator. A quartz sampler as shown in
Figure 4 (right) was used to collect salt samples. The amount of dissolved electrode metal in the electrolyte salt mixture was then analysed with titration by a commercial automatic titrator (Titrando,
Metrohm, Germany).
2.4.1. Na Solubility Measurement
The Na solubilities of two selected molten salt electrolytes (NaCl-CaCl
2-BaCl
2 37-45-18 mol%, NaCl-SrCl
2-KCl 30.0-26.5-43.5 mol%) at 600°C were studied. Anhydrous salts with a purity of 99% were used for mixture preparation in the glovebox. The mole percent mixture ratio was converted to weight percent and the balance was used to prepare 60 g of the required salt mixture inside the glovebox. The salt mixture was put into the SS crucible and heated to 600°C by the HaloBat. As shown in
Figure 4, about 1 g Na metal was weighted and put into the inner steel tube, after the salt mixture in the SS crucible was completely molten. The added Na metal was melted immediately and floated on the molten salt due to its lower density. After adding the Na metal, salt samples (of 2-4 g) were taken after every half to one hour to determine the change of the concentration of dissolved Na, which were measured with direct titration by the automatic titrator Titrando. The Na concentration steadily increases with time until a constant value (i.e., the solubility) is obtained for the point with maximum amount of Na that can be dissolved in the salt.
For titration, the salt sample (1-2 g) was put in a sample beaker filled with 160 ml demineralized water. Afterwards, the beaker was attached to the automatic titrator and the impeller was used for completely dissolving the salt in the water to prepare the titration solution. During salt dissolution, all Na metal dissolved in the salt sample reacted with water to form sodium hydroxide according to the reaction 2Na + 2H
2O → 2NaOH + H
2. The solution was directly titrated against hydrochloric acid (HCl 0.01M) to get the equivalence point. Titration was automated by adjusting the dosing rate. At the equivalence point, the consumed volume of HCl (V
HCl) was recorded. The consumed HCl is equal to the molar amount of the NaOH in the titration solution, i.e., dissolved Na in the salt sample, according to the reaction NaOH + HCl → NaCl + H
2O. Thus, V
HCl was used to calculate the concentration of dissolved Na with the following equation:
where
CNa represents the concentration of dissolved Na (mol%);
VHCl is the consumed volume of HCl in L;
nsalt is the amount of the salt sample in mol.
The titration measurements on each salt sample were done twice to ensure the accuracy of the result and get the average values and measurement error bars.
2.4.2. Zn Solubility Measurement
The Zn solubilities of two selected molten salt electrolytes (NaCl-CaCl
2-BaCl
2 37-45-18 mol%, NaCl-SrCl
2-KCl 30.0-26.5-43.5 mol%) at 600°C were also studied. As for the Na solubility measurements, 60 g of the prepared salt mixture was put into the SS crucible and heated to 600°C by the HaloBat set-up. Different to the Na solubility measurements with the inner steel tube containing the floating Na, an alumina crucible was used to contain about 1 g Zn metal (see
Figure 5), since liquid Zn has a higher density than the molten salt, and good material compatibility with alumina (but not with SS). The alumina crucible with Zn was completely immersed in the molten salt after the salt is completely molten, so that the Zn metal was indirect contact with the molten salt. After that, salt samples (2-4 g) were taken after every half to one hour to determine the concentrations of dissolved Zn by back titration.
For titration, the salt sample (1-2 g) was put in the sample beaker with 160 ml demineralized water and 5 ml 0.1M HCl solution. The beaker was then attached to the automatic titrator and the impeller was used for completely dissolving the salt in the water to prepare the titration solution. During the salt dissolution, all Zn metal dissolved in the salt sample reacted with HCl to form ZnCl
2 according to the reaction Zn + 2HCl → ZnCl
2 + H
2O. The solution with excess HCl was back titrated with 0.1M NaOH to get the equivalence point. Titration was automated by adjusting the dosing rate. At the equivalence point, the consumed volume of NaOH (V
NaOH) was recorded. The molar amount of dissolved Zn (
nZn in mol) can be calculated with the following equation:
After that,
nZn in mol was used to calculate the concentration of dissolved Zn with the following equation:
where
CZn represents the concentration of dissolved Zn (mol%).
As for the Na samples, the titration Zn measurements of each salt sample were done twice to ensure the accuracy of the result and to get the average values and measurement error bars.
Figure 1.
Schematics of a Na-ZnCl
2 ALB cell with a NaCl-CaCl
2 molten salt electrolyte at a fully charged state (left) [
5] and a fully discharged state (right) with a porous ceramic diaphragm (blue line). It has the electrochemical reaction of ZnCl
2 + Na (charged) ↔ 2NaCl + Zn (discharged) during charging/discharging.
Figure 1.
Schematics of a Na-ZnCl
2 ALB cell with a NaCl-CaCl
2 molten salt electrolyte at a fully charged state (left) [
5] and a fully discharged state (right) with a porous ceramic diaphragm (blue line). It has the electrochemical reaction of ZnCl
2 + Na (charged) ↔ 2NaCl + Zn (discharged) during charging/discharging.
Figure 2.
Schematics of an ideal Na-ZnCl2 ALB cell with a NaCl-CaCl2 molten salt electrolyte and an ideal porous ceramic diaphragm (blue line), which can completely prevent the diffusion of Zn2+ from bottom to top (i.e., self-discharge).
Figure 2.
Schematics of an ideal Na-ZnCl2 ALB cell with a NaCl-CaCl2 molten salt electrolyte and an ideal porous ceramic diaphragm (blue line), which can completely prevent the diffusion of Zn2+ from bottom to top (i.e., self-discharge).
Figure 3.
(a) Netzsch DSC 404® for DSC measurements, (b) Ni-based alloy salt and reference crucibles on crucible holder of DSC.
Figure 3.
(a) Netzsch DSC 404® for DSC measurements, (b) Ni-based alloy salt and reference crucibles on crucible holder of DSC.
Figure 4.
Schematic of set-up with a Na metal holder (i.e., inner steel tube) for Na solubility measurements.
Figure 4.
Schematic of set-up with a Na metal holder (i.e., inner steel tube) for Na solubility measurements.
Figure 5.
Schematic of the set-up with a Zn metal holder (i.e., Alumina crucible) for Zn solubility measurements.
Figure 5.
Schematic of the set-up with a Zn metal holder (i.e., Alumina crucible) for Zn solubility measurements.
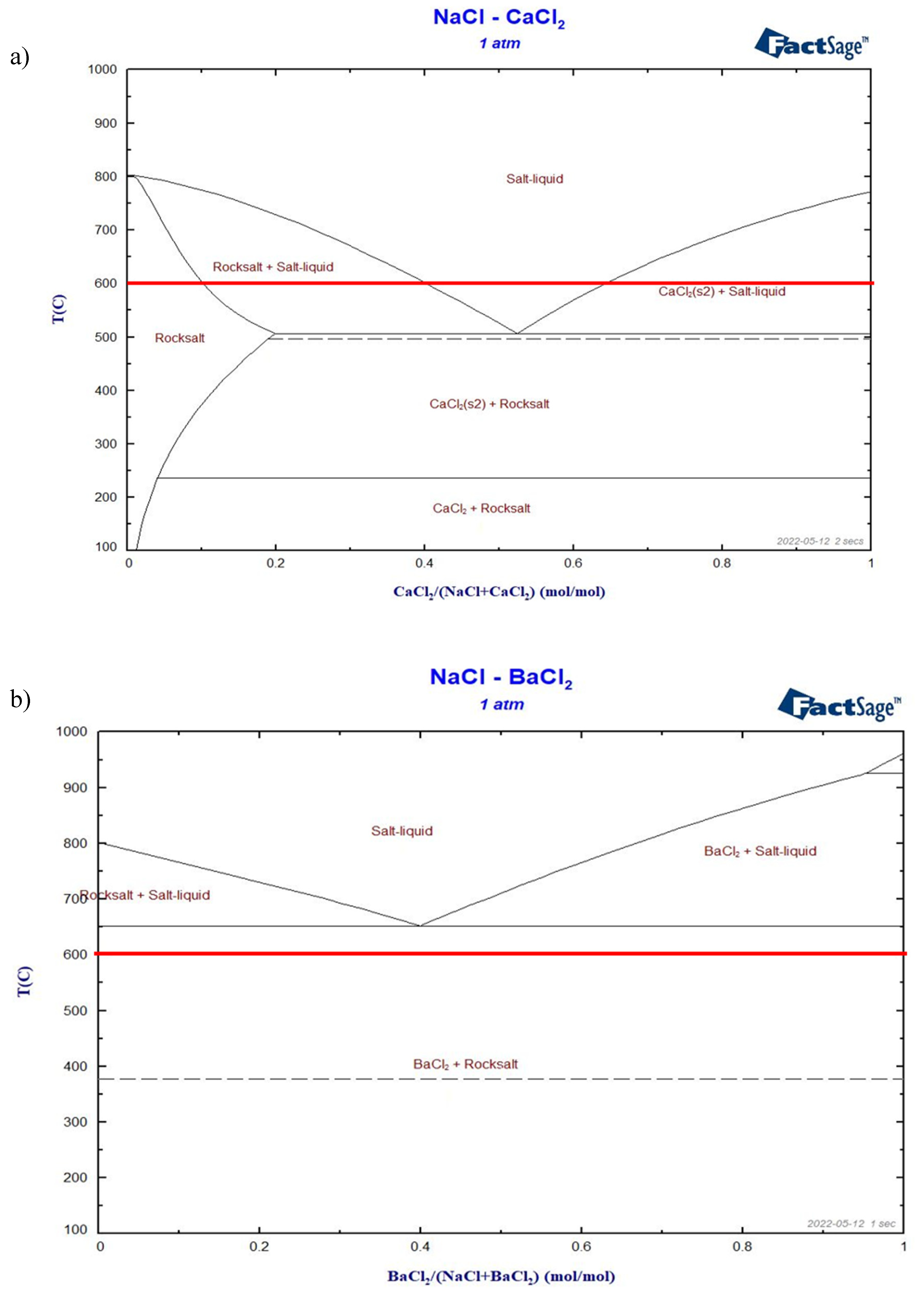
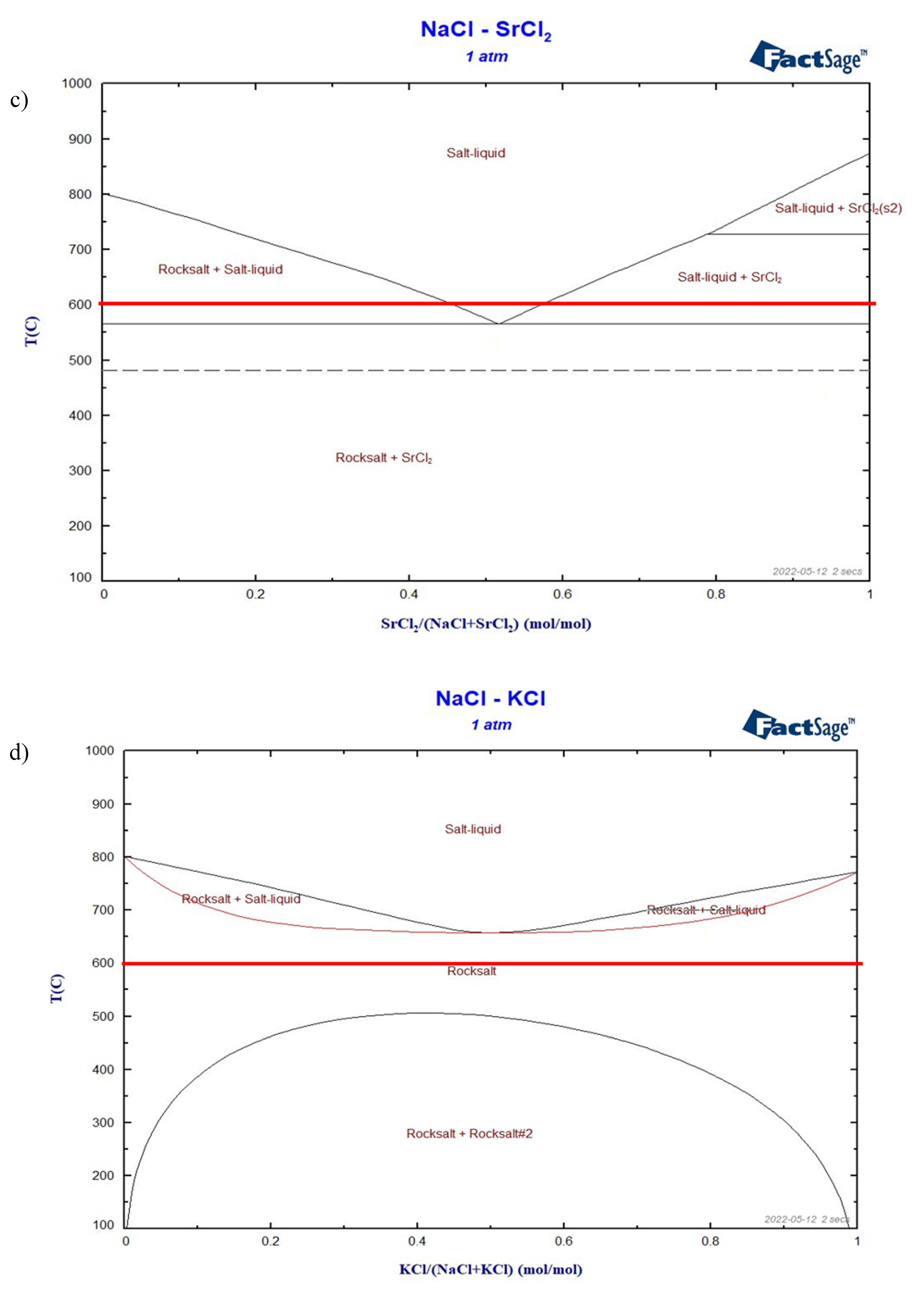
Figure 6.
Simulated phase diagram of a) NaCl-CaCl2, b) NaCl-BaCl2, c) NaCl-SrCl2, d) NaCl-KCl. The red lines show the expected ALB working temperature of 600°C.
Figure 6.
Simulated phase diagram of a) NaCl-CaCl2, b) NaCl-BaCl2, c) NaCl-SrCl2, d) NaCl-KCl. The red lines show the expected ALB working temperature of 600°C.
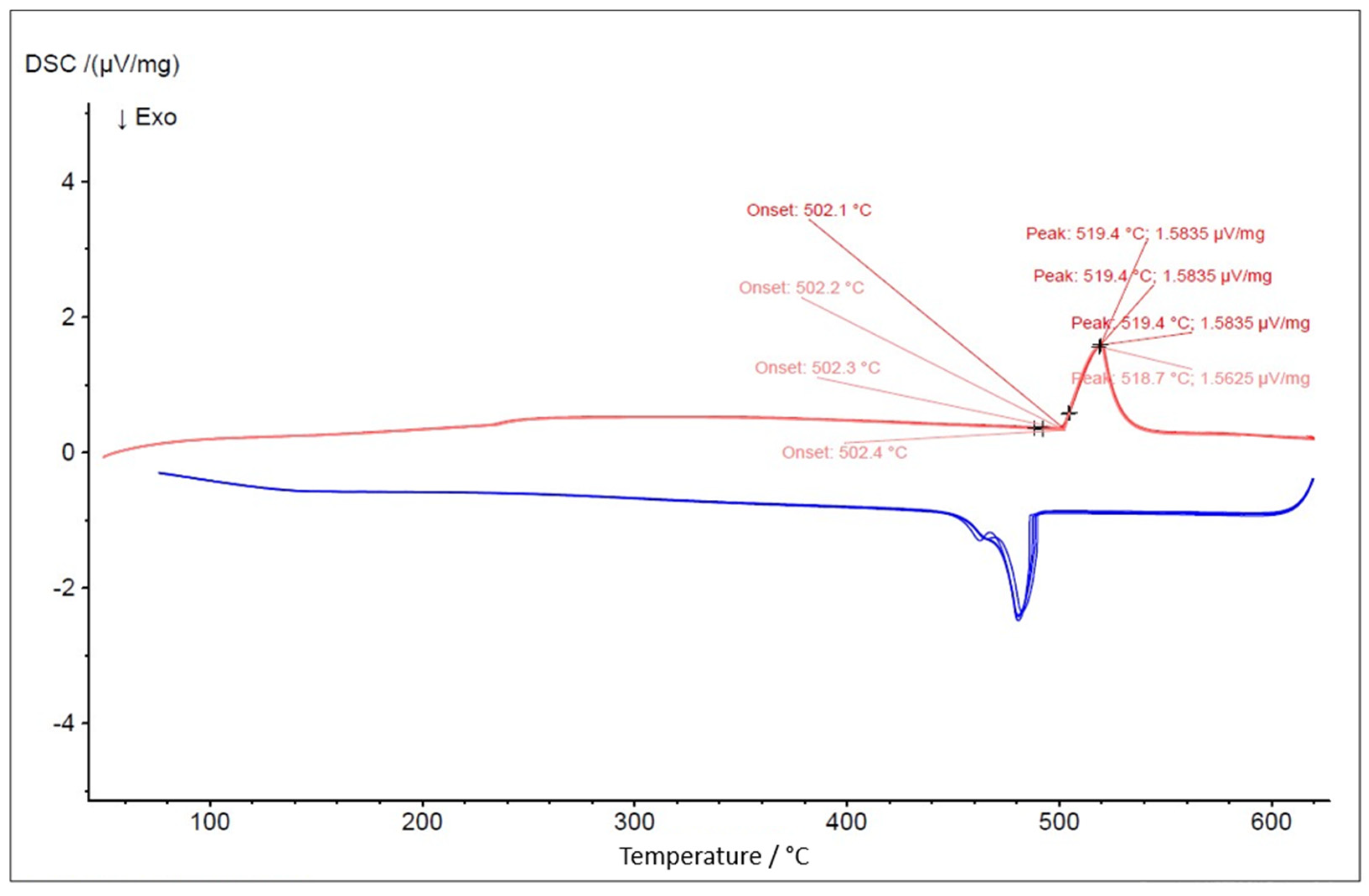
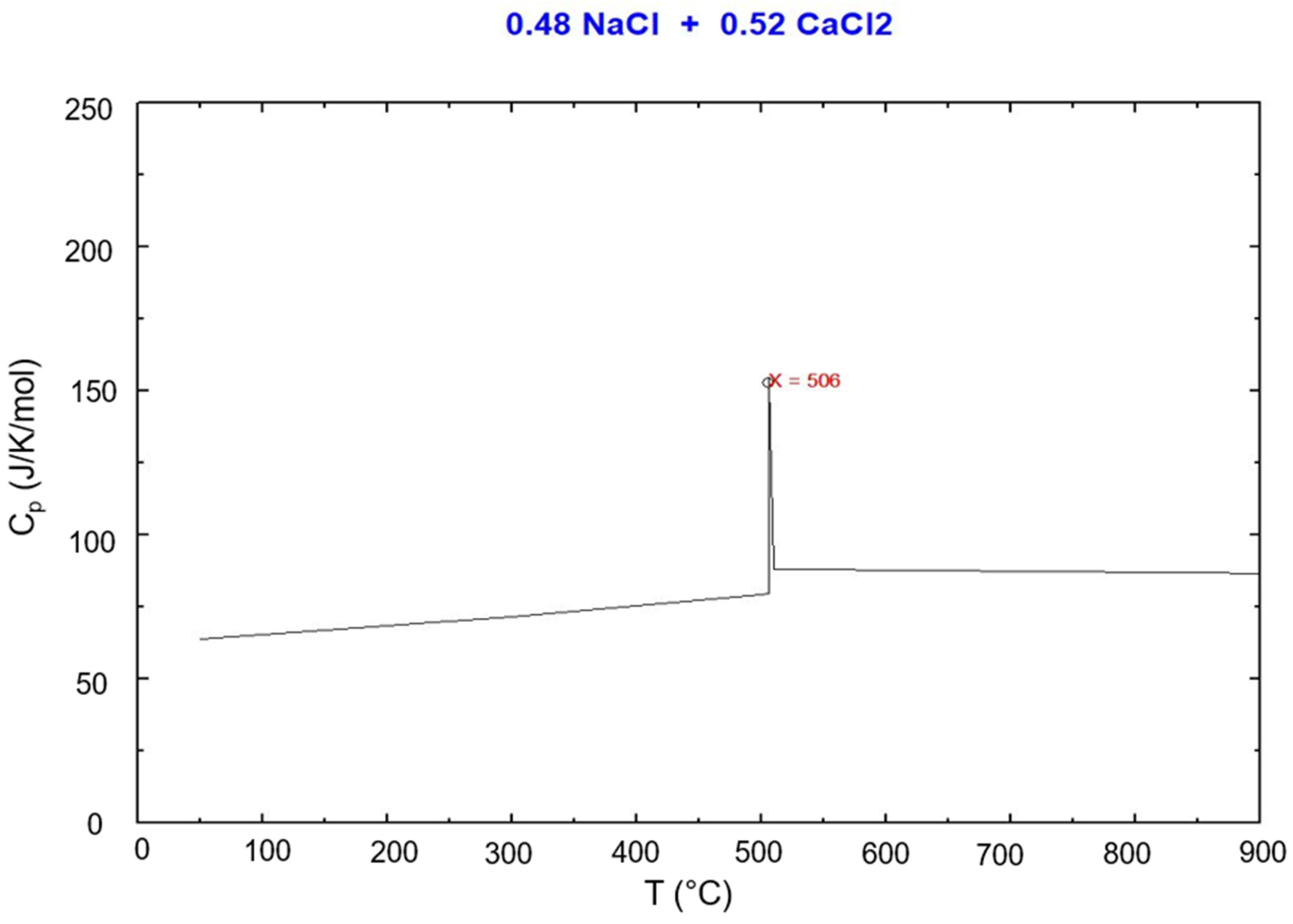
Figure 7.
DSC test result for the eutectic NaCl–CaCl2 48-52 mol% (top) and simulated Cp vs. T via FactSage (bottom).
Figure 7.
DSC test result for the eutectic NaCl–CaCl2 48-52 mol% (top) and simulated Cp vs. T via FactSage (bottom).
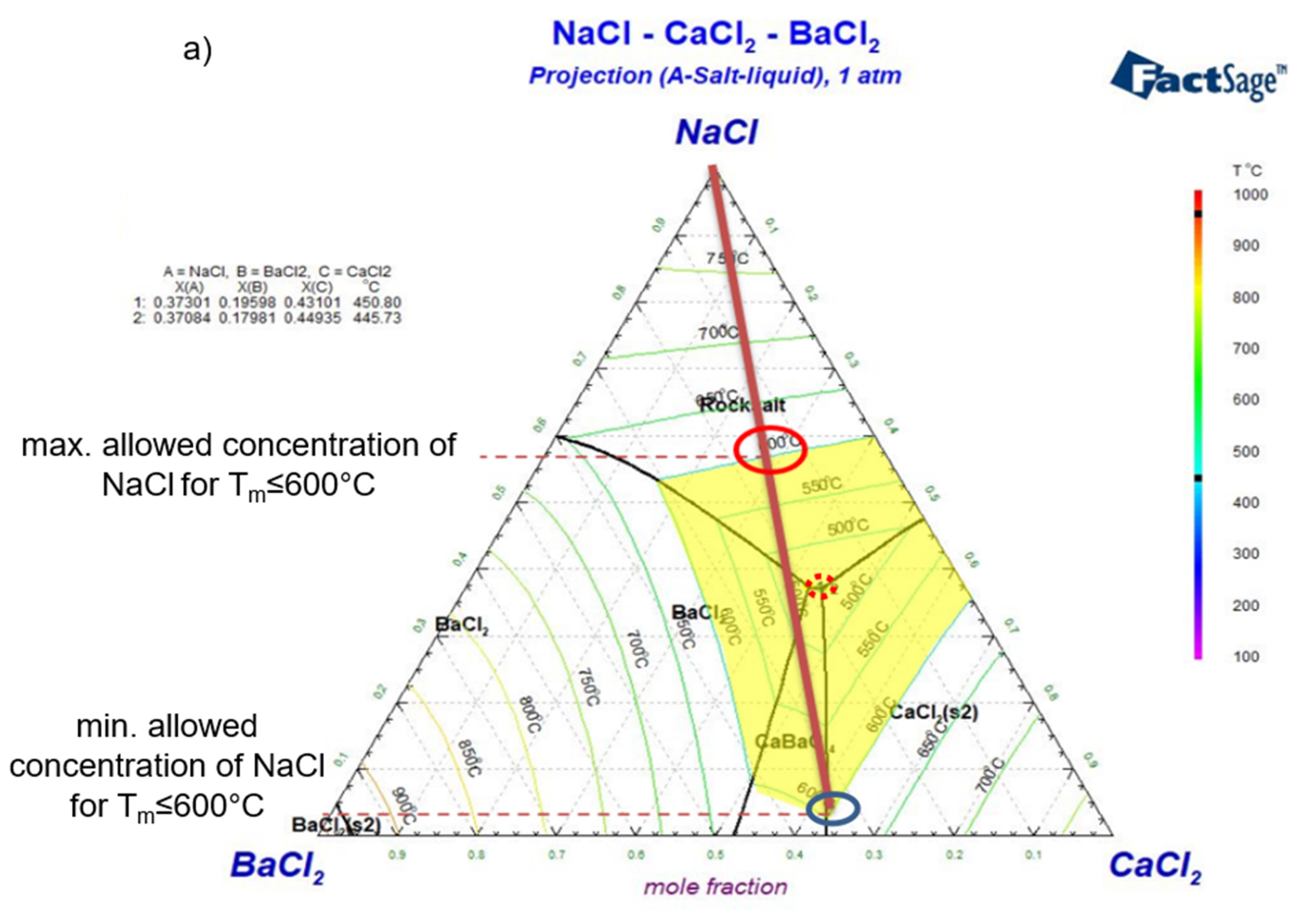
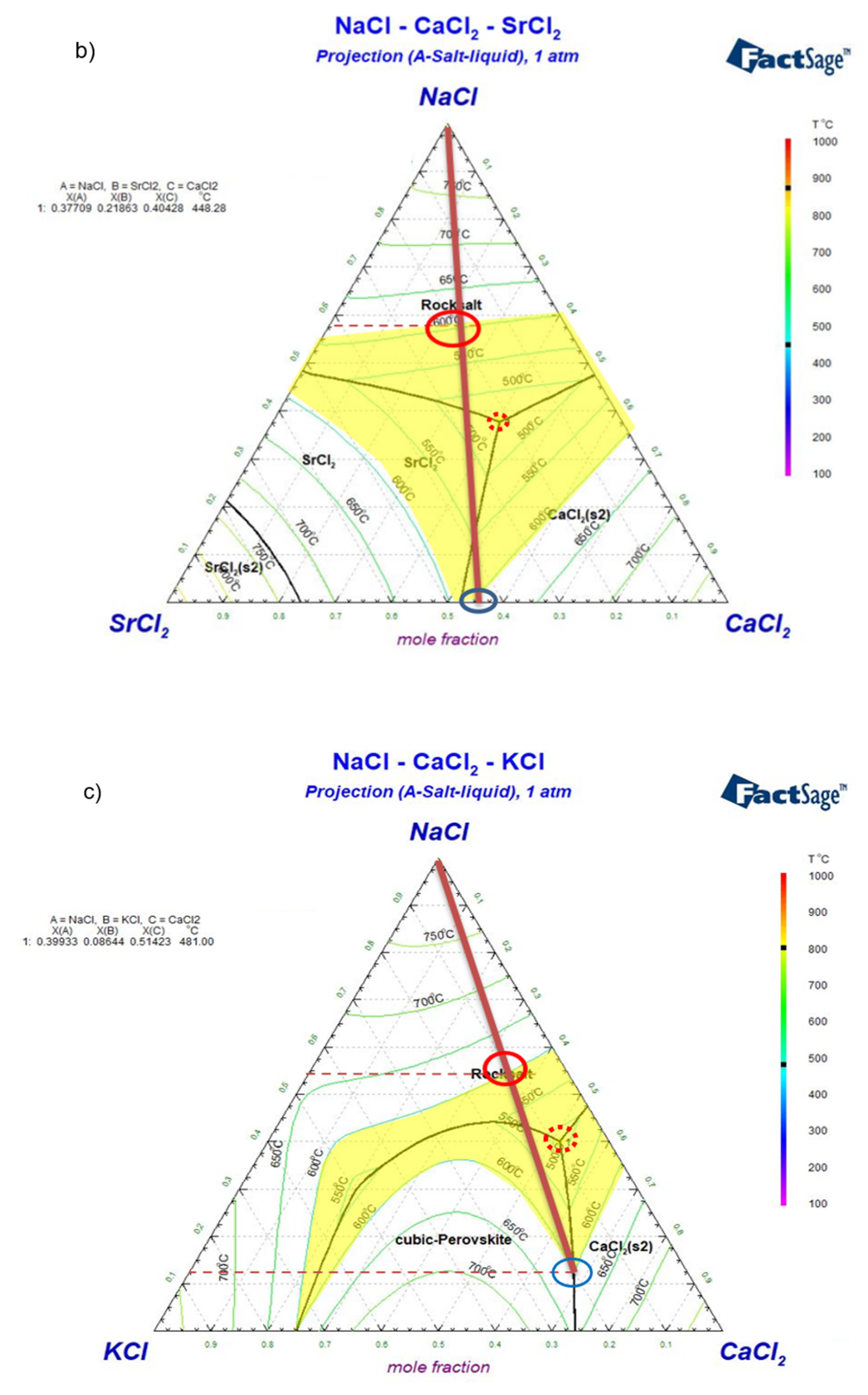
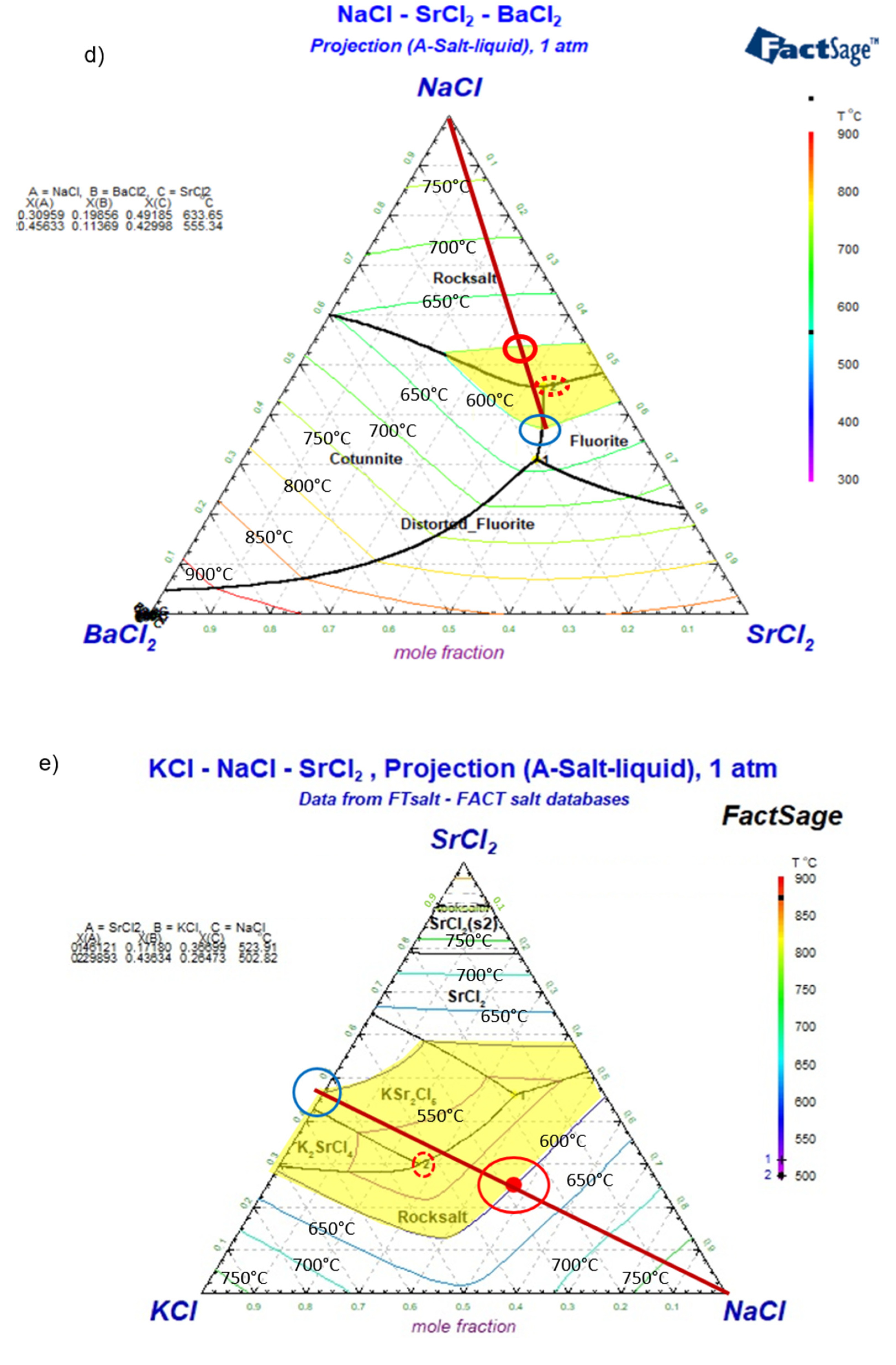
Figure 8.
Simulated ternary phase diagrams: a) NaCl-CaCl2-BaCl2, b) NaCl-CaCl2-SrCl2, c) NaCl-CaCl2-KCl, d) NaCl-SrCl2-BaCl2, e) NaCl-SrCl2-KCl. Yellow areas in the phase diagrams represent the salt composition with melting temperature not higher than 600°C. Dark red lines show the changing salt composition during charging/discharging. The minimum temperature (eutectic) composition is pointed out with a red dotted circle. The maximum and minimum allowed concentrations of NaCl for Tm≤600°C (i.e., largest potential charge/discharge ranges of the ALB cell) are pointed with red and blue solid circles in the phase diagrams.
Figure 8.
Simulated ternary phase diagrams: a) NaCl-CaCl2-BaCl2, b) NaCl-CaCl2-SrCl2, c) NaCl-CaCl2-KCl, d) NaCl-SrCl2-BaCl2, e) NaCl-SrCl2-KCl. Yellow areas in the phase diagrams represent the salt composition with melting temperature not higher than 600°C. Dark red lines show the changing salt composition during charging/discharging. The minimum temperature (eutectic) composition is pointed out with a red dotted circle. The maximum and minimum allowed concentrations of NaCl for Tm≤600°C (i.e., largest potential charge/discharge ranges of the ALB cell) are pointed with red and blue solid circles in the phase diagrams.
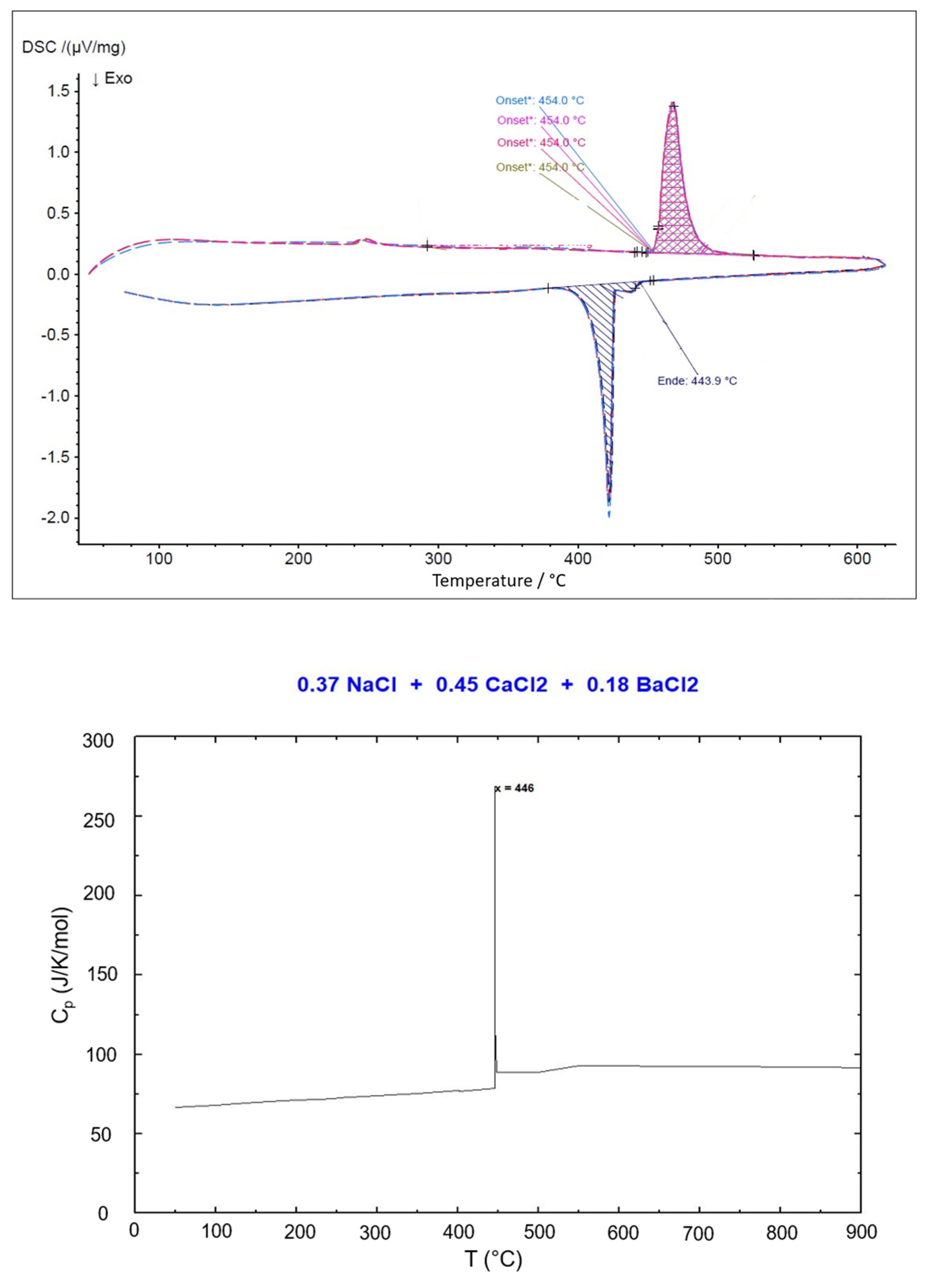
Figure 9.
DSC result (top) and simulated Cp vs. T (bottom) for the eutectic NaCl-CaCl2-BaCl2 (37.1-44.9-18.0 mol%).
Figure 9.
DSC result (top) and simulated Cp vs. T (bottom) for the eutectic NaCl-CaCl2-BaCl2 (37.1-44.9-18.0 mol%).
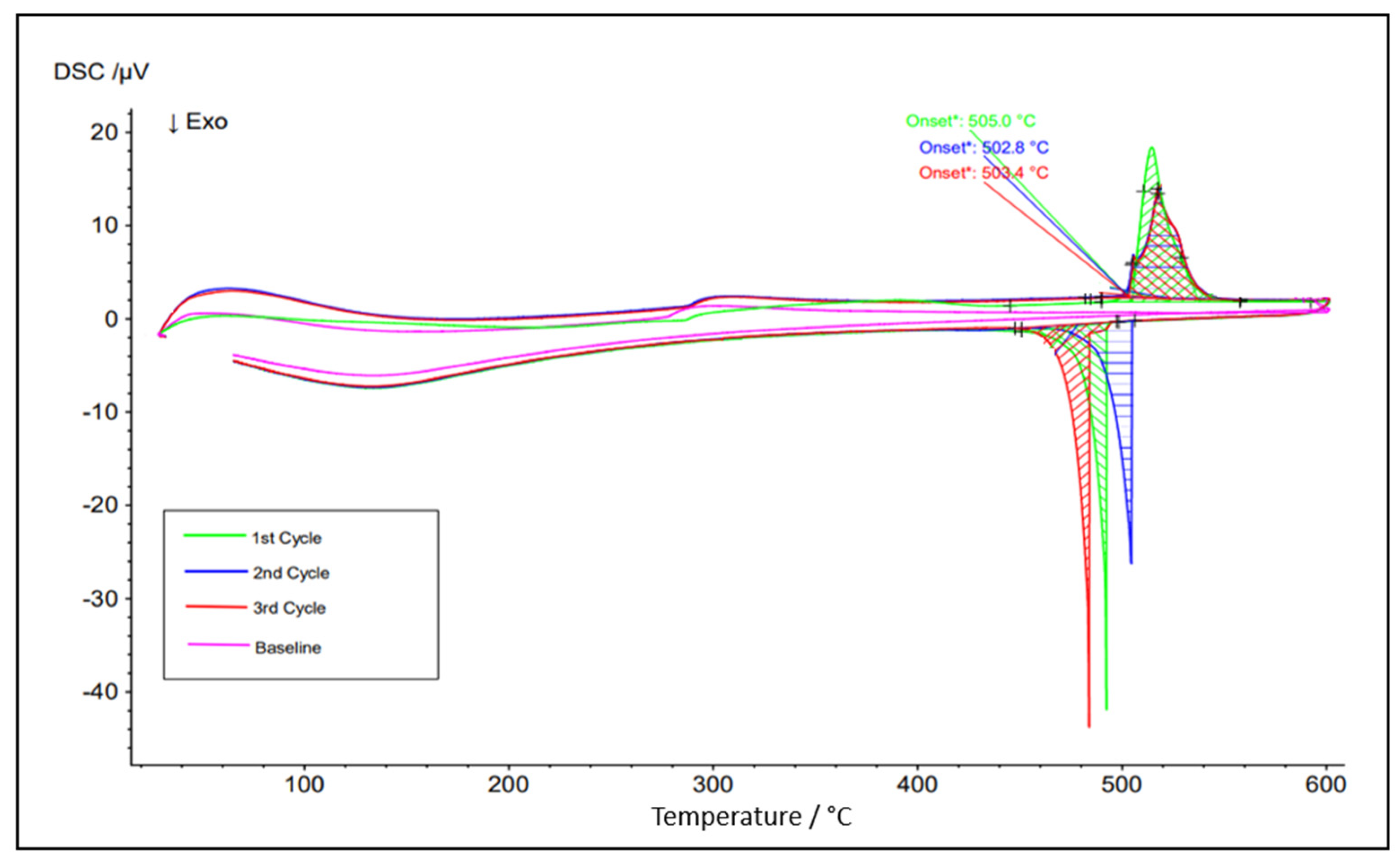
Figure 10.
DSC result for the eutectic NaCl-SrCl2-KCl (30.0-26.5-43.5 mol%).
Figure 10.
DSC result for the eutectic NaCl-SrCl2-KCl (30.0-26.5-43.5 mol%).
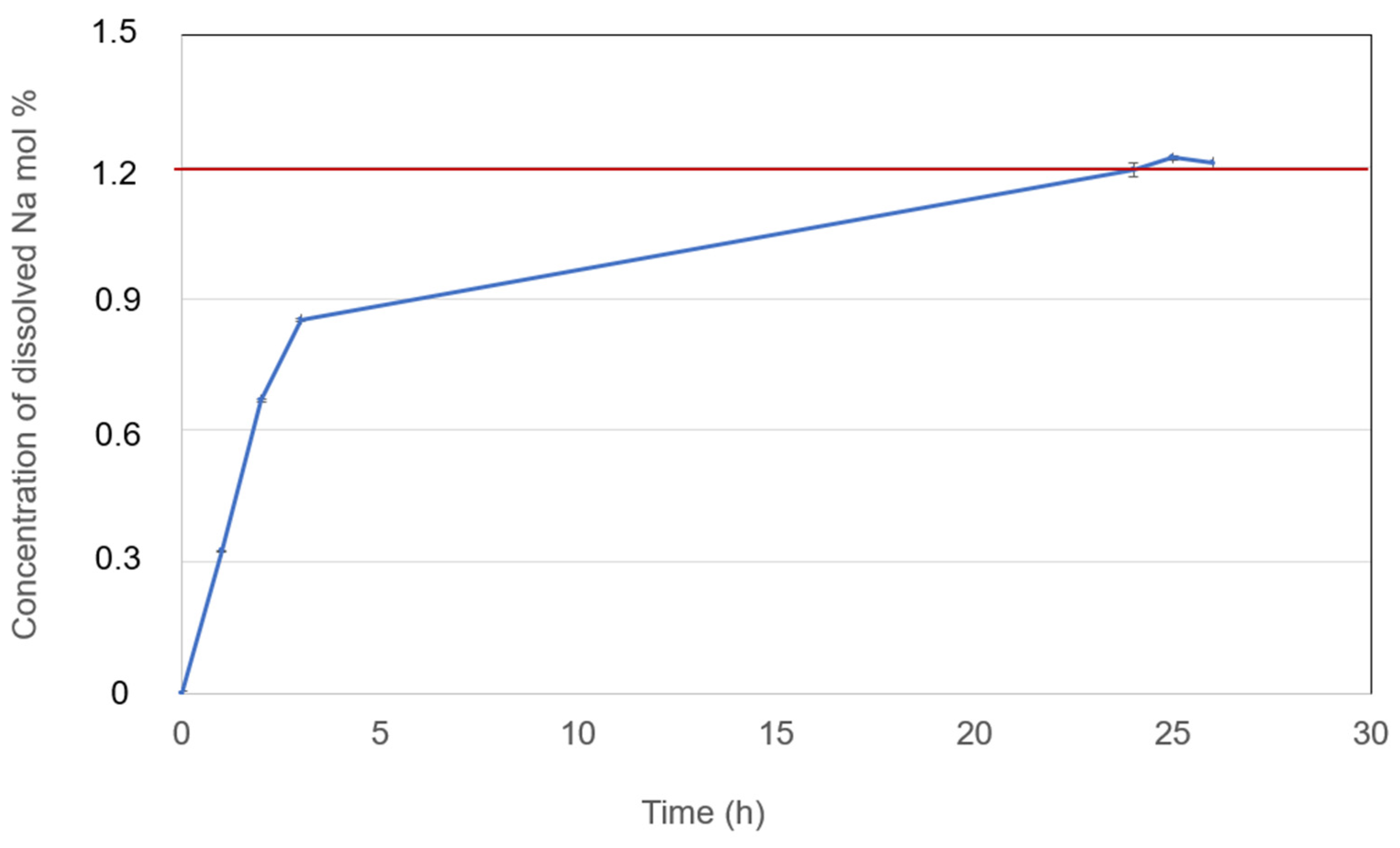
Figure 11.
Dissolved Na metal in selected NaCl-CaCl2-BaCl2 (37.1-44.9-18.0 mol%) molten salt electrolyte at 600°C with measurement error bar versus time. The measurement error bars are very small compared to the concentrations of dissolved Na.
Figure 11.
Dissolved Na metal in selected NaCl-CaCl2-BaCl2 (37.1-44.9-18.0 mol%) molten salt electrolyte at 600°C with measurement error bar versus time. The measurement error bars are very small compared to the concentrations of dissolved Na.
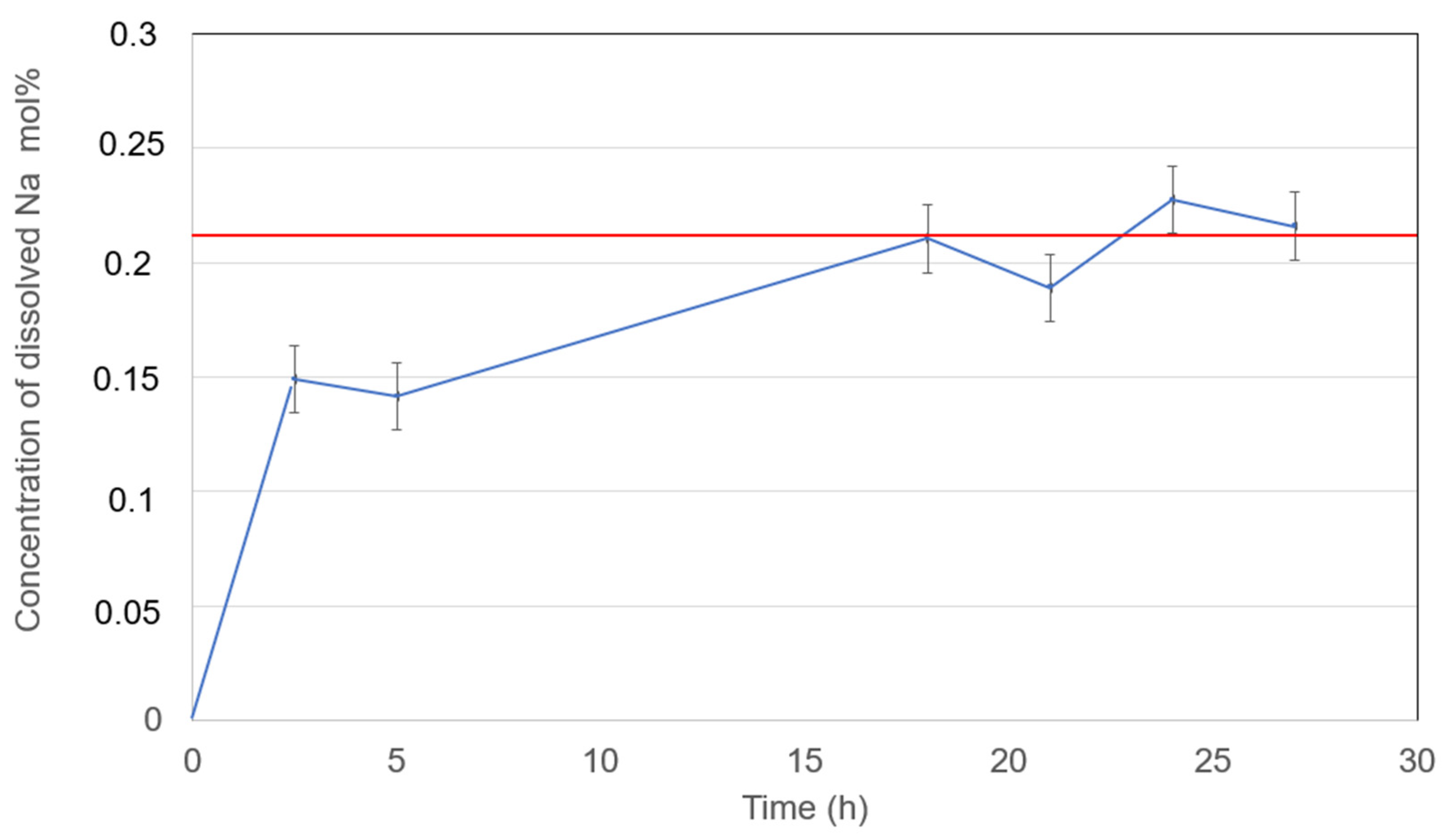
Figure 12.
Dissolved Na metal in selected NaCl-SrCl2-KCl (30.0-26.5-43.5 mol%) molten salt electrolyte at 600°C with measurement error bar versus time.
Figure 12.
Dissolved Na metal in selected NaCl-SrCl2-KCl (30.0-26.5-43.5 mol%) molten salt electrolyte at 600°C with measurement error bar versus time.
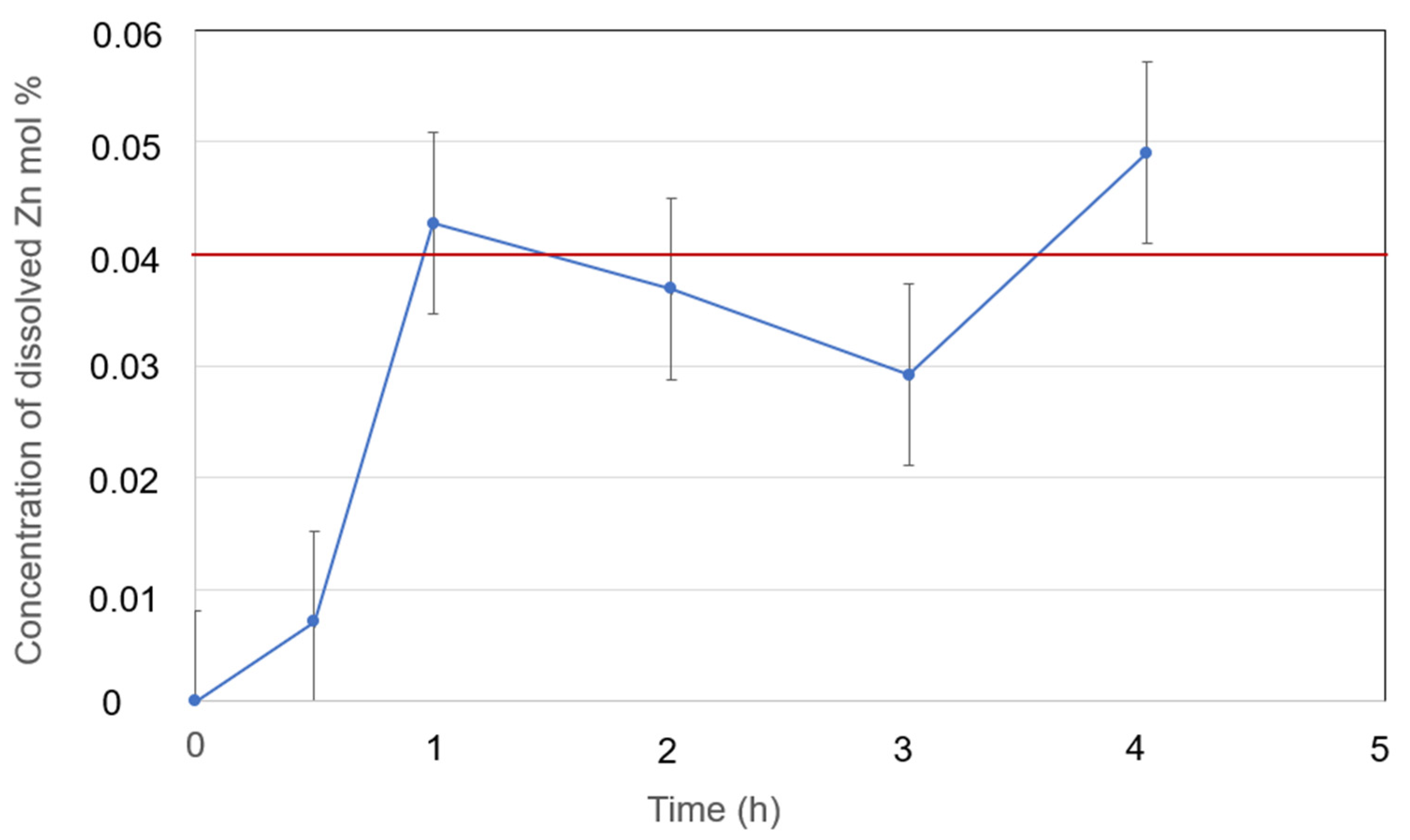
Figure 13.
Dissolved Zn metal in eutectic NaCl-CaCl2-BaCl2 (37.1-44.9-18.0 mol%) at 600°C with measurement error bar versus time.
Figure 13.
Dissolved Zn metal in eutectic NaCl-CaCl2-BaCl2 (37.1-44.9-18.0 mol%) at 600°C with measurement error bar versus time.
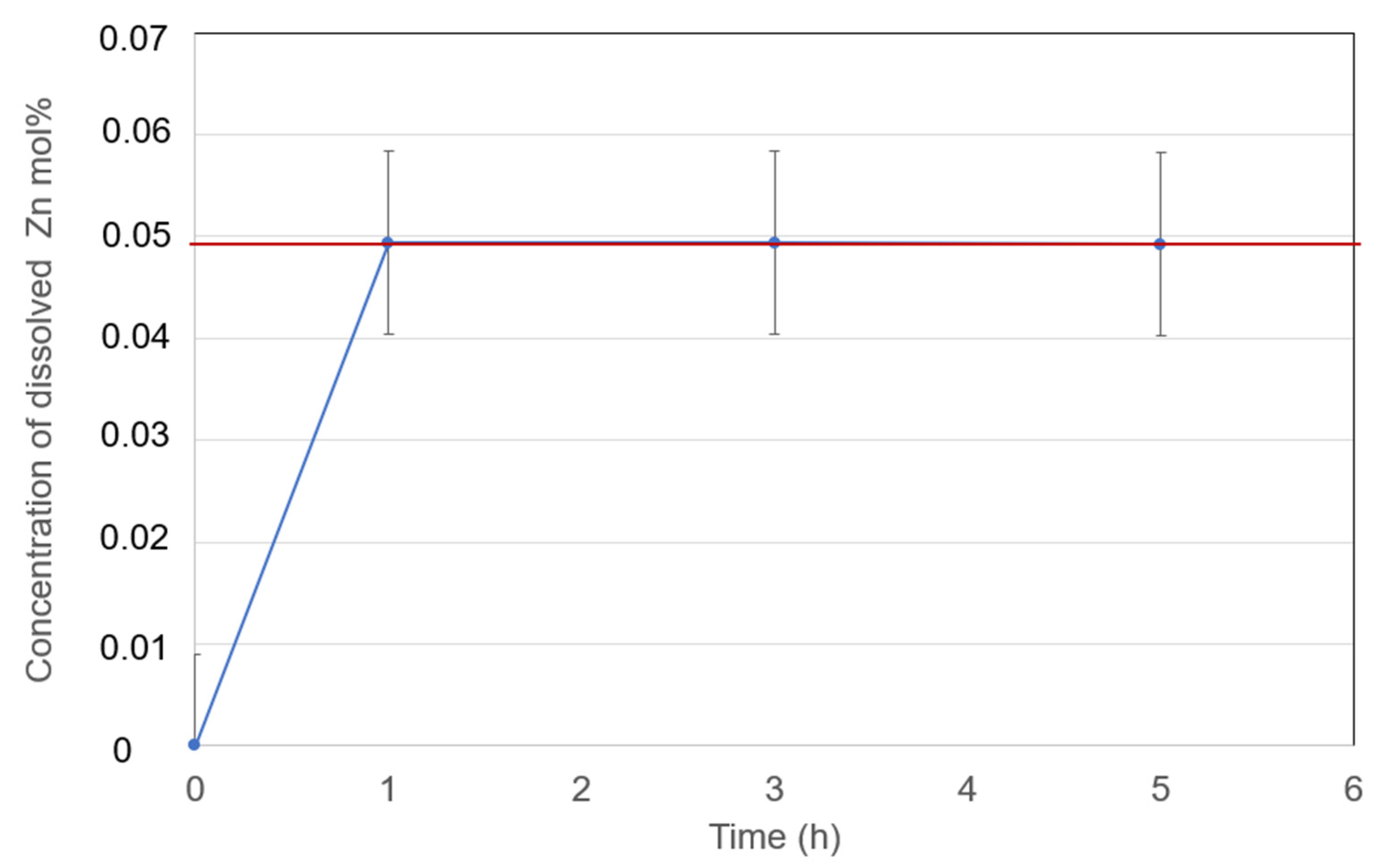
Figure 14.
Dissolved Zn metal in eutectic NaCl-SrCl2-KCl (30.0-26.5-43.5 mol%) at 600°C with measurement error bar versus time.
Figure 14.
Dissolved Zn metal in eutectic NaCl-SrCl2-KCl (30.0-26.5-43.5 mol%) at 600°C with measurement error bar versus time.
Table 1.
Comparison of simulated eutectic temperatures and salt compositions of selected binary systems with literature.
Table 1.
Comparison of simulated eutectic temperatures and salt compositions of selected binary systems with literature.
| Binary system |
Eutectic temperature (°C) |
Eutectic composition (NaCl mol %) |
Soruce |
| NaCl-CaCl2
|
506 |
48 % |
This work |
| NaCl-CaCl2
|
508 |
50 % |
[22] |
| NaCl-CaCl2
|
500 |
48 % |
[23] |
| NaCl-BaCl2
|
650 |
60 % |
This work |
| NaCl-BaCl2
|
654 |
61 % |
[24] |
| NaCl-SrCl2
|
560 |
48 % |
This work |
| NaCl-SrCl2
|
560 |
48 % |
[25] |
| NaCl-KCl |
660 |
50 % |
This work |
| NaCl-KCl |
685 |
50 % |
[23] |
Table 2.
Simulated eutectic temperatures and salt compositions of selected ternary systems.
Table 2.
Simulated eutectic temperatures and salt compositions of selected ternary systems.
| Ternary systems |
Min. melting temperature (°C) |
Eutectic composition (mol %) |
Soruce |
| NaCl-CaCl2-BaCl2
|
446 |
37.1-44.9-18.0 |
This work, FactSage |
| NaCl-CaCl2-BaCl2
|
454 |
37.1-44.9-18.0 |
This work, DSC |
| NaCl-CaCl2-SrCl2
|
448 |
37.7-40.4-21.9 |
This work, FactSage |
| NaCl-CaCl2-SrCl2
|
471 |
41.9-40.6-18.1 |
[25] |
| NaCl-CaCl2-KCl |
481 |
40.0-51.4-8.6 |
This work, FactSage |
| NaCl-CaCl2-KCl |
504 |
42-52-6 |
[24] |
| NaCl-SrCl2-BaCl2
|
555 |
45.6-43.0-11.4 |
This work, FactSage |
| NaCl-SrCl2-KCl |
503 |
30.0-26.5-43.5 |
This work, FactSage |
| NaCl-SrCl2-KCl |
503-505 |
30.0-26.5-43.5 |
This work, DSC |
Table 3.
Comparison of theoretical (EMFs of metal chlorides at 600°C [
11] and their large-scale costs [
17].
Table 3.
Comparison of theoretical (EMFs of metal chlorides at 600°C [
11] and their large-scale costs [
17].
| Metal chlorides |
EMF (V) at 600 °C |
Cost (USD/kg) |
| BaCl2
|
3.728 |
~ 0.5 |
| KCl |
3.658 |
~ 0.4 |
| SrCl2
|
3.612 |
~ 1* |
| CaCl2
|
3.462 |
~ 0.3 |
| NaCl |
3.424 |
~ 0.06 |
Table 4.
Comparison of Na solubilities in molten chlorides obtianed in this work and available in literature.
Table 4.
Comparison of Na solubilities in molten chlorides obtianed in this work and available in literature.
| Molten chlorides |
Na solubility (mol.%) |
Source |
| NaCl-CaCl2-BaCl2 (37.1-44.9-18.0 mol%) |
1.2 (600°C) |
This work |
| NaCl-SrCl2-KCl (30.0-26.5-43.5 mol%) |
0.22 (600°C) |
This work |
| NaCl-CaCl2 (eutectic) |
~3.3 (600°C) |
[26] |
| NaCl |
2.1 (795°C) |
[18] |
| LiCl-NaCl-KCl (59-5-36 mol%) |
0.09 (450°C), 0.15 (560°C), 0.18 (600°C) |
[9] |
Table 5.
Comparison of Zn solubilities in molten salts obtianed in this work and available in literature, and Mg solubilities in molten salts available in literature.
Table 5.
Comparison of Zn solubilities in molten salts obtianed in this work and available in literature, and Mg solubilities in molten salts available in literature.
| Metal in molten salts |
Metal solubility (mol.%) |
Source |
| Zn in NaCl-CaCl2-BaCl2 (37.1-44.9-18.0 mol%) |
0.04 (600°C) |
This work |
| Zn in NaCl-SrCl2-KCl (30.0-26.5-43.5 mol%) |
0.05 (600°C) |
This work |
| Zn in ZnCl2
|
0.187 (498°C), 0.61 (600°C) |
[27] |
| Zn in ZnI2
|
0.28 (498°C), 0.87 (600°C) |
[27] |
| Mg in MgCl2
|
0.20-1.2 (714-900°C), 0.07 (600°C) |
[18] |