1. Introduction
Neurological disorders are the leading cause of sickness and disability globally, presenting significant challenges for early diagnosis and effective management [
1]. Since 1990 to 2021, there has been an 18% increase in disability-adjusted life years (DALYs) associated with neurological diseases, which reflects the rising impact of neurological disease on global health [
2]. While age-standardized DALY rates have decreased due to advancements in care, the absolute number of people affected continues to grow due to demographic shifts and longer lifespans. Importantly, there is over 80% of neurological health loss in low- and middle-income nations, where access to therapy and health facilities remains limited. In 2021, over 3 billion people worldwide had a neurological disorder [
2], including, a wide range of diseases that impact the brain, spinal cord, and nervous system, with symptoms ranging from motor and sensory impairments to cognitive dysfunction, as well as, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, epilepsy, and stroke, as well as neurological damage caused by infections, tumors, and trauma [
2].
Early detection and classification of neurological disorders is critical for mitigating disease progression and improving patient outcomes. However, the complex and dynamic nature of the nervous system poses significant diagnostic challenges. Traditional tools such as, electroencephalograms (EEGs), MRIs, and CT scans provide valuable insights but often fall short in precision and scalability, particularly for early detection [
3,
4,
5,
6,
7]. In this context, artificial intelligence (AI) techniques, including machine learning (ML) and deep learning (DL), have emerged as transformative solutions. Leveraging computational power and data-driven algorithms, AI enables advanced analysis of neuroimaging data and delivers quantitative assessments that surpass conventional methods [
8,
9,
10,
11]. The integration of neuroscience and AI has strengthened this relationship, facilitating practical applications for detecting and classifying various neurological conditions, particularly in analyzing large datasets like biomechanical data [
12,
13].
Biomechanical data, particularly related to human motion and gait, are an essential focus area in neurological research. The brain's hierarchical coordination of movement involves the cortex, brainstem, and spinal cord, with disruptions often indicating early signs of neurological dysfunction. Advances in smart wearable technology, multi-modal sensors, and AI-driven platforms have made it possible to assess gait and balance with unprecedented accuracy [
14,
15,
16]. These tools can capture complex data on kinematics, kinetics, and spatiotemporal parameters, providing critical insights into subtle changes associated with neurological diseases. The analysis of this multimodal data requires sophisticated computational models such as, support vector machines (SVMs), Random Forests (RFs) and neural networks (NNs) [
17], which are capable of handling the non-linear dynamics and variability inherent in biomechanical data.
To better understand the role of AI in diagnosing neurological diseases through biomechanical data and gait analysis, this study employs a bibliometric analysis of the existing literature. Bibliometric analysis is a well-established scientific method that provides a comprehensive overview of a field by identifying influential publications, key researchers, prominent institutions, and emerging research trends. A review of the literature reveals that bibliometric techniques have been widely applied to explore various topics related to neurological disorders [
18,
19,
20,
21,
22,
23,
24]. Previous studies have employed these methods to examine trends and advancements in decoding the applications of DL in neuroscience, as well as the broader use of AI in this field [
25,
26]. Similarly, bibliometric analyses have investigated the contribution of AI in Neurosurgery [
27]. Additionally, the role of biomechanics in stroke neurorehabilitation has also been explored through a bibliometric approach [
28]. To date, no bibliometric study has integrated these three areas—AI, biomechanics, and neurological diseases—into a single, comprehensive analysis. Therefore, further research is required to not only clarify the intersection of these fields, but also, highlight existing research gaps to provide a foundation for further investigations.
These gaps are particularly significant given that (a) neurological diseases are increasingly diverse and prevalent; (b) AI represents a rapidly evolving field with immense potential; and (c) advancements in biomechanics provide valuable data for understanding and diagnosing these conditions. Therefore, the present study aims to examine existing international literature through a bibliometric analysis and review key topics identified using innovative bibliometric techniques to provide researchers and clinicians with a detailed understanding of the current state of AI applications in neurology, laying a strong foundation for future research and innovation.
2. Materials and Methods
2.1. Data Sources and Search Methods
The documents analyzed in this study were sourced from the Scopus database (
https://www.scopus.com/) on January 16, 2025. Scopus, recognized as one of the largest curated repositories of abstracts and citations spanning multiple disciplines [
29], is an invaluable resource for bibliometric data analysis [
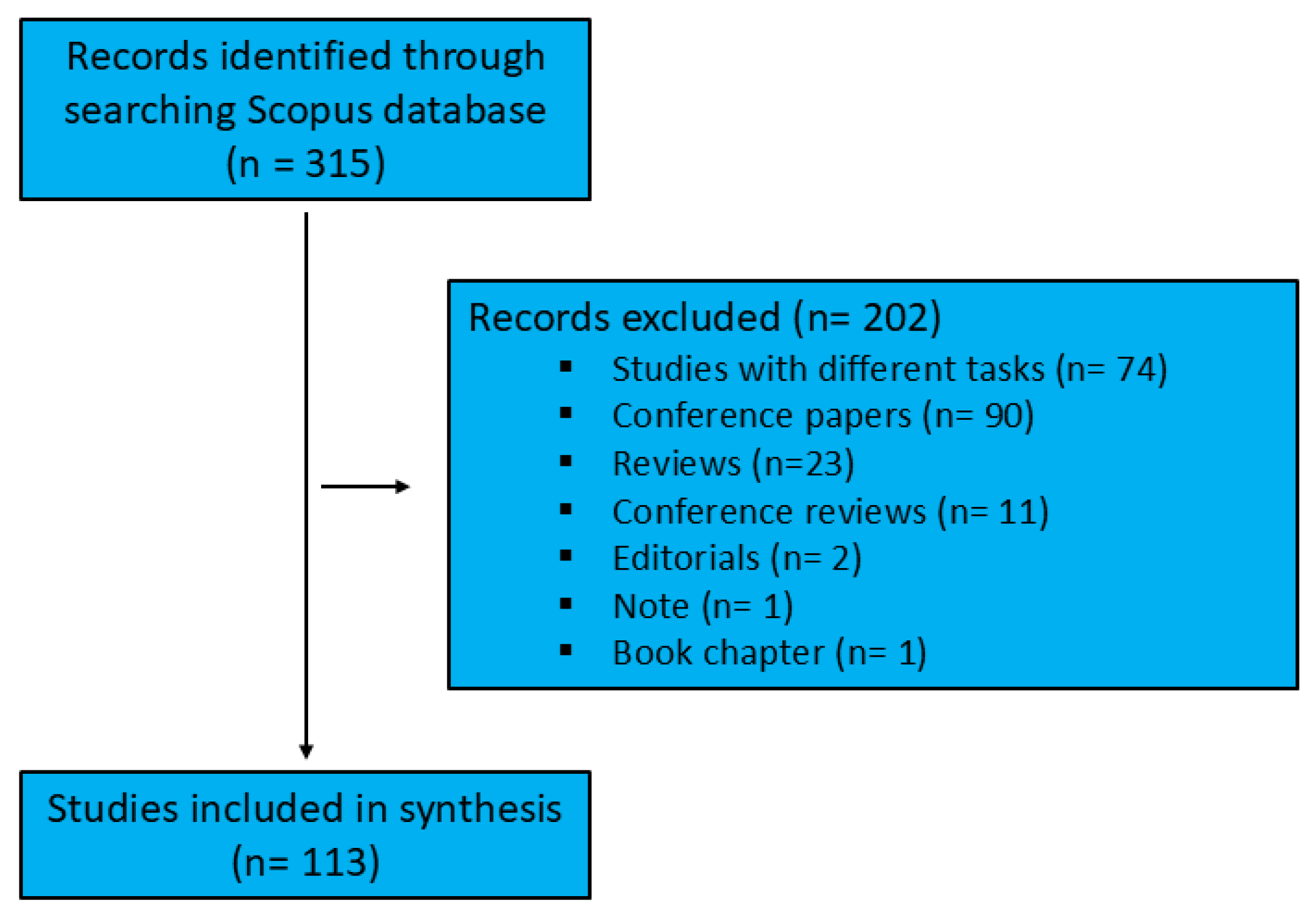
30]. The advanced search function was utilized with a search string comprising compound keywords, structured using the BOOLEAN expression: ("neurological diseases" OR parkinson* OR alzheimer* OR epilepsy OR "epileptic seizures" OR stroke OR dementia OR "idiopathic tremor" OR "multiple sclerosis") AND ("machine learning" OR "deep learning" OR "artificial intelligence") AND (diagnosis OR detection OR diagnos*) AND ("biomechanical data" OR biomechanics OR "gait analysis"). Documents were included if these terms appeared in their titles, abstracts, or keywords. The initial search yielded 315 documents. Only original, English-language articles were included, excluding reviews (or other types of articles such as opinion papers) as well as articles published in conferences, books, or other non-academic journal outlets. Accepted studies were required to specifically focus on the application of artificial intelligence in diagnosing neurological diseases. The first two authors systematically reviewed the titles and abstracts and reached a consensus on their eligibility. After reviewing the 315 records, 202 were excluded as they did not address the inclusion criteria.
Figure 1.
Workflow diagram.
Figure 1.
Workflow diagram.
2.2. Data Analysis
The CSV file exported from Scopus was processed using VOSviewer (version 1.6.20) to perform a bibliometric analysis. VOSviewer, a free software tool, facilitates the creation, visualization, and exploration of bibliometric networks, making it particularly effective for mapping the trends and the thematic structure of scientific fields [
31]. Additionally, after converting the file into an Excel (xls) format using Microsoft Excel (Office 365, Microsoft Corporation, Redmond, WA, USA), the data was imported into Microsoft Power BI (Office 365, Microsoft Corporation, Redmond, WA, USA) for visualizing the distribution of documents over time. The bibliometric analysis included both performance analysis and scientific mapping, with the latter involving clustering techniques as part of the methodology.
2.3. Performance Analysis
The performance analysis included: (a) determining the annual number of published documents, (b) ranking the authors based on their citation count, and (c) ranking the sources with the highest number of publications.
2.4. Scientific Mapping
The scientific mapping techniques applied in this study included:
(a) Co-authorship analysis: Authors, organizations, and countries were analyzed as the unit of analyses, focusing on collaborations based on their co-authored documents. The analysis using authors as the unit of analysis was limited to those with at least two documents.
(b) Bibliographic coupling: Sources were examined as the unit of study to assess the extent to which two or more sources share common references.
(c) Co-citation analysis: Sources were used as the unit of analysis to determine how frequently two or more sources are cited together in other documents. The analysis was limited to sources with at least 10 citations.
(d) Co-occurrence analysis: The unit of analysis was "author keywords," investigating how often two or more keywords appear together within the same documents. "Author keywords" is limited to terms explicitly listed by the author as "keywords." The minimum number of occurrences was set at two.
3. Results
3.1. Performance Analysis
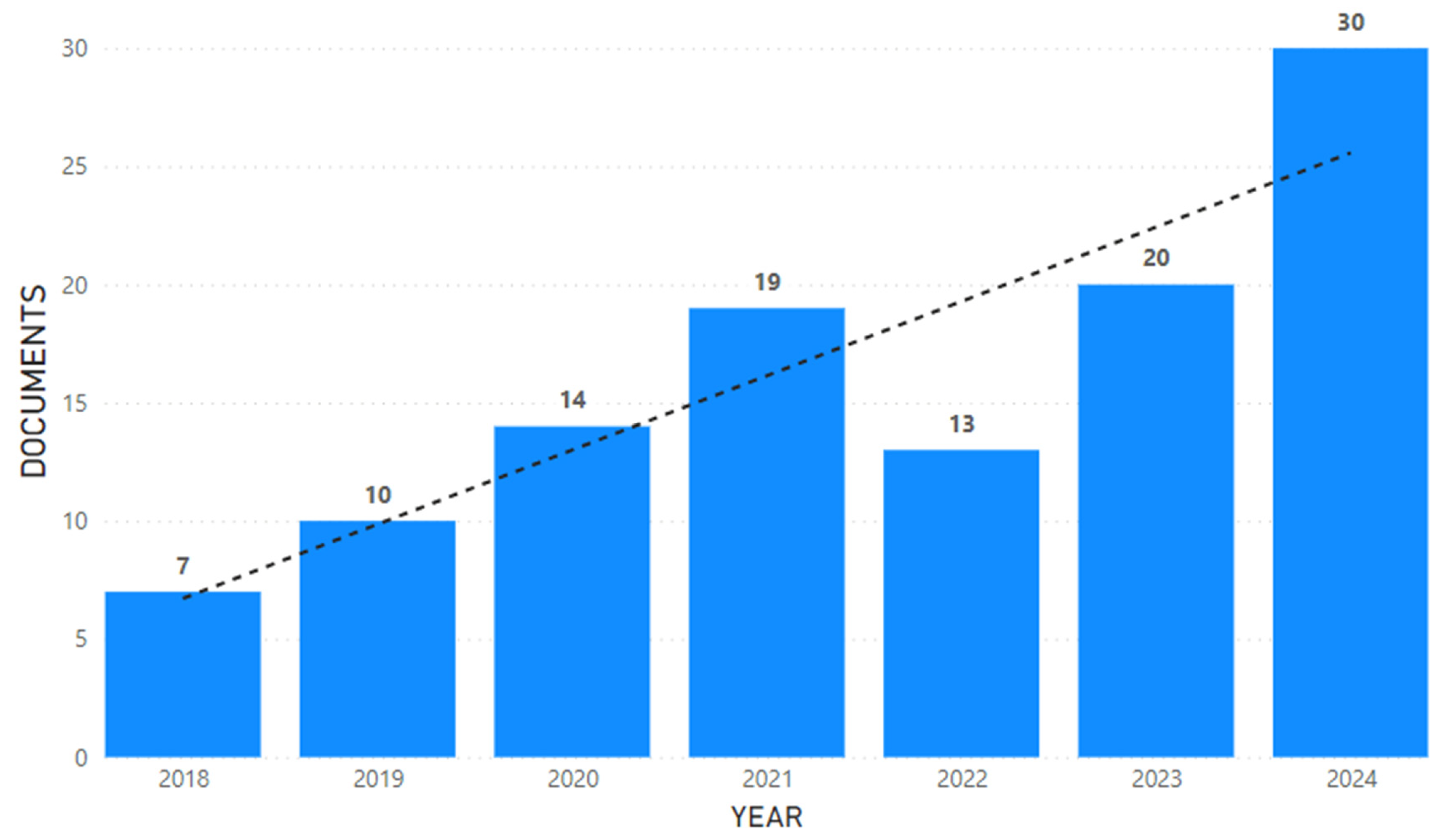
A total of 113 articles were considered suitable for inclusion in our analysis. The bar chart (
Figure 2) shows the annual number of documents published between the years 2018 and 2024. A noticeable upward trend can be observed, as depicted by the dotted trend line. There has been a steady increase in the number of documents published over the years, starting from 7 in 2018 and peaking at 30 in 2024 despite a slight dip observed in 2022.
The key contributors and their significant impact within the field are presented in
Table 1 below, showing a list of authors with a minimum of 100 citations, along with the number of documents they have contributed to. The authors Nöth, Orozco-Arroyave and Vasquez-Correa lead the rankings with 227 citations each across 2 documents. Other notable contributors include Abdulhay, Arunkumar, Narasimhan, Vellaipapan, and Venkatraman, all with 203 citations and a single document. Klucken has slightly lower citation counts (193) with two documents.
The academic sources with a minimum of two published documents and their corresponding citation counts are shown in
Table 2. Sensors journal stands out as the leading source, with 16 documents and a total of 373 citations, demonstrating its significant contribution to the field. The IEEE Transactions on Neural Systems and Rehabilitation Engineering follows with 5 documents and 62 citations. Other notable sources include the IEEE Journal of Biomedical and Health Informatics, which has 4 documents and a substantial 249 citations, and Expert Systems, which has 3 documents and 201 citations.
3.2. Science Mapping
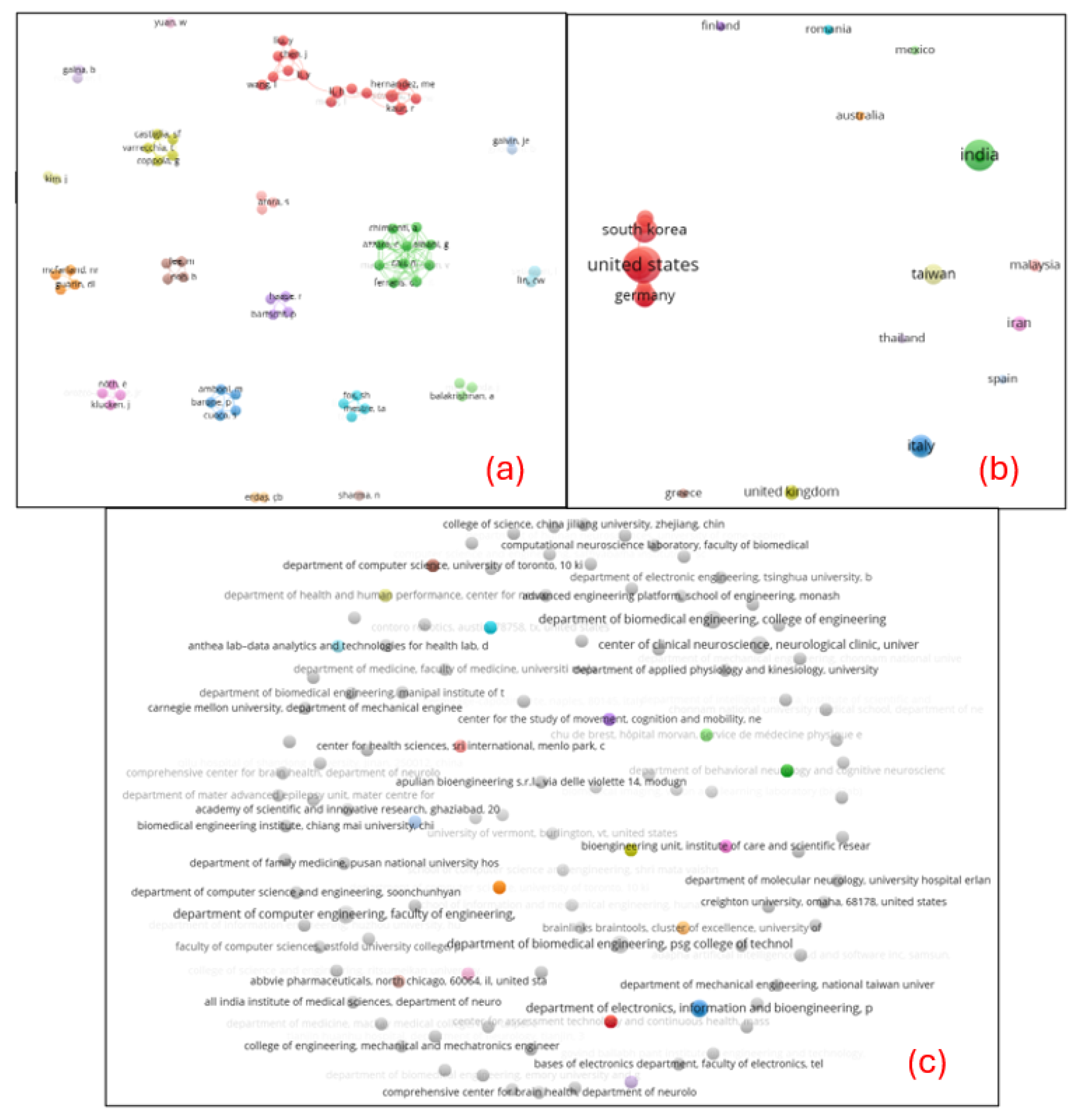
The visualization of co-authorship networks in
Figure 3 was analyzed at three levels: (a) authors, (b) countries, and (c) organizations. In panel (a), the nodes represent individual authors, while the connections between them indicate co-authorship relationships. However, the sparse connections between clusters suggest limited collaboration between researchers in this field. Similarly, in panel (b), countries are analyzed as the unit of collaboration. While the United States, South Korea, and Germany appear to play central roles, the lack of extensive interconnections among countries highlights a significant gap in international collaboration. Panel (c) focuses on organizations, showing minimal interaction between institutions.
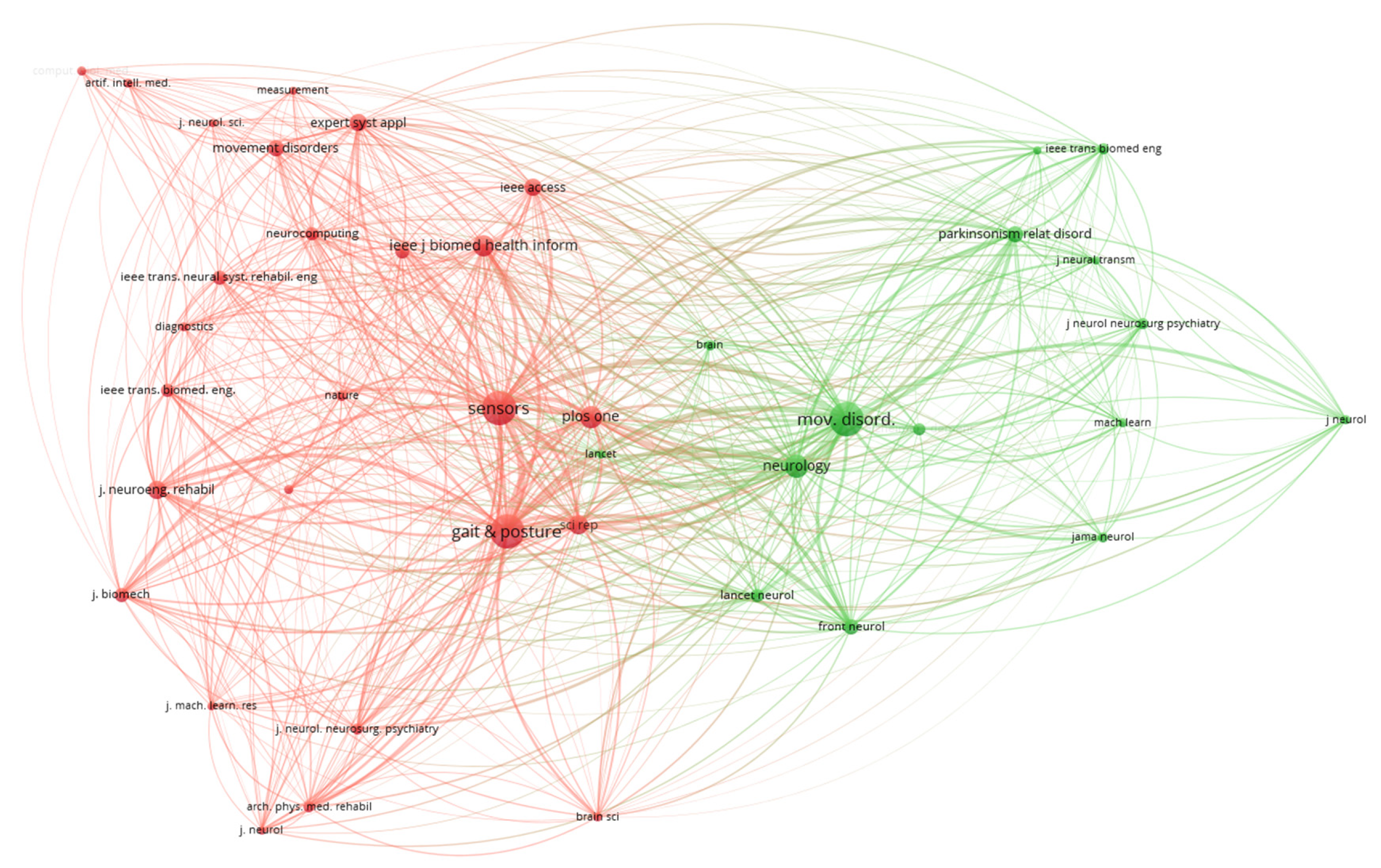
A co-citation analysis of sources in
Figure 4, shows the relationship between journals based on the frequency with which they are cited together in other documents. Each node represents a journal, while the size of the node reflects the number of times it has been cited. The connections (edges) between nodes indicate the strength of the co-citation relationship, with thicker lines representing stronger associations. The network is divided into distinct clusters, represented by different colors, which highlight thematic groupings of journals. For instance, the red cluster includes journals such as Sensors and Gait & Posture, which are heavily focused on sensor-based systems and gait analysis. The green cluster, on the other hand, revolves around journals like Movement Disorders and Neurology, which are more aligned with neurological research and disorders such as Parkinson’s disease.
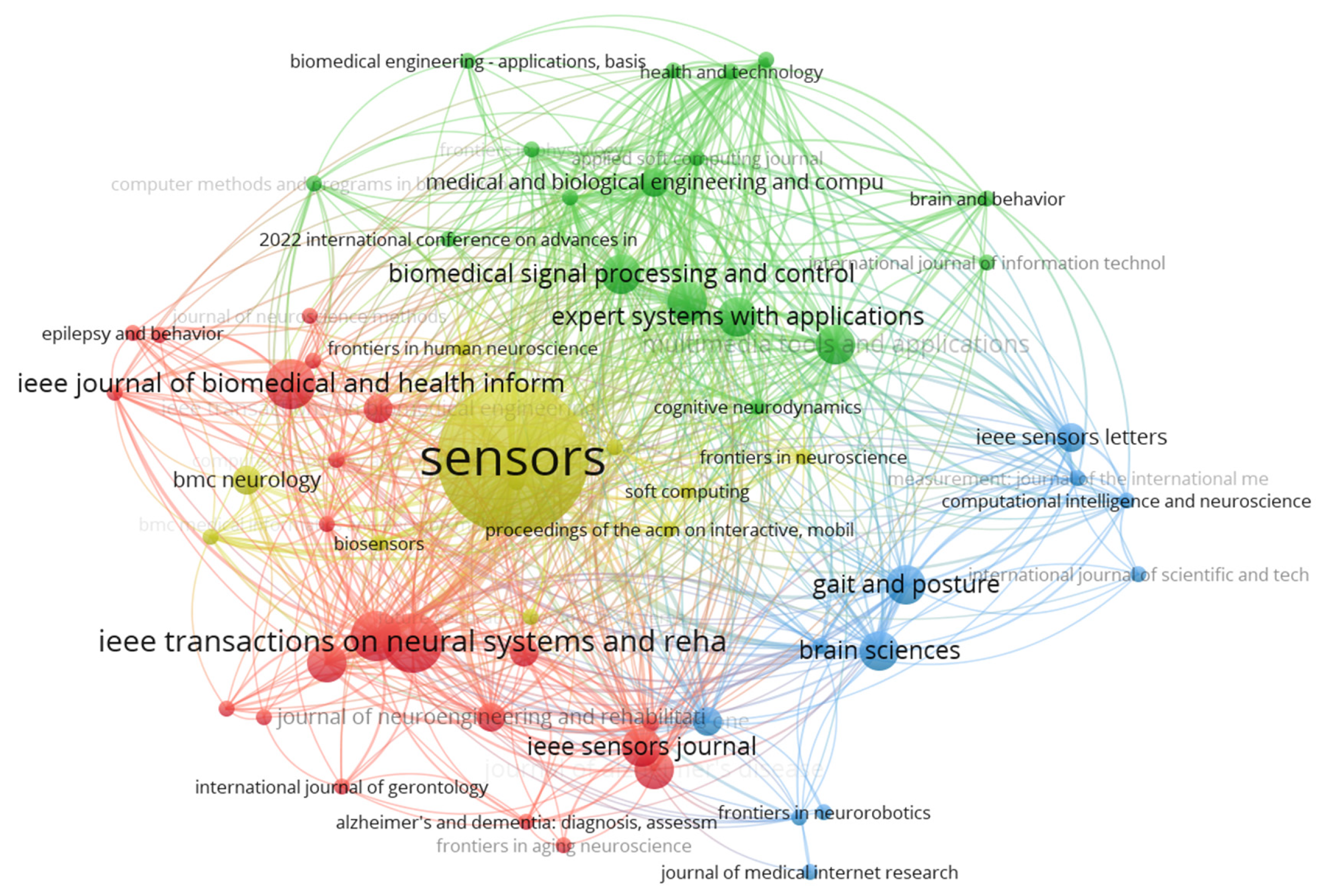
The bibliographic coupling network of journals in
Figure 5 shows where each node represents a journal and the edges (connections) indicate the extent to which the journals share common references. The network is organized into distinct clusters, represented by different colors, highlighting thematic groupings based on shared references. The yellow cluster, dominated by Sensors, is the most central and influential, reflecting its extensive coupling with journals in fields like biomedical signal processing and sensor applications. The red cluster includes journals such as the IEEE Journal of Biomedical and Health Informatics and IEEE Transactions on Neural Systems and Rehabilitation Engineering, which focus on health informatics and neural systems. The green cluster is centered around interdisciplinary journals like Expert Systems with Applications and Biomedical Signal Processing and Control, emphasizing computational and applied approaches. The blue cluster contains journals like Gait and Posture and Brain Sciences, which are more specialized in movement analysis and neuroscience.
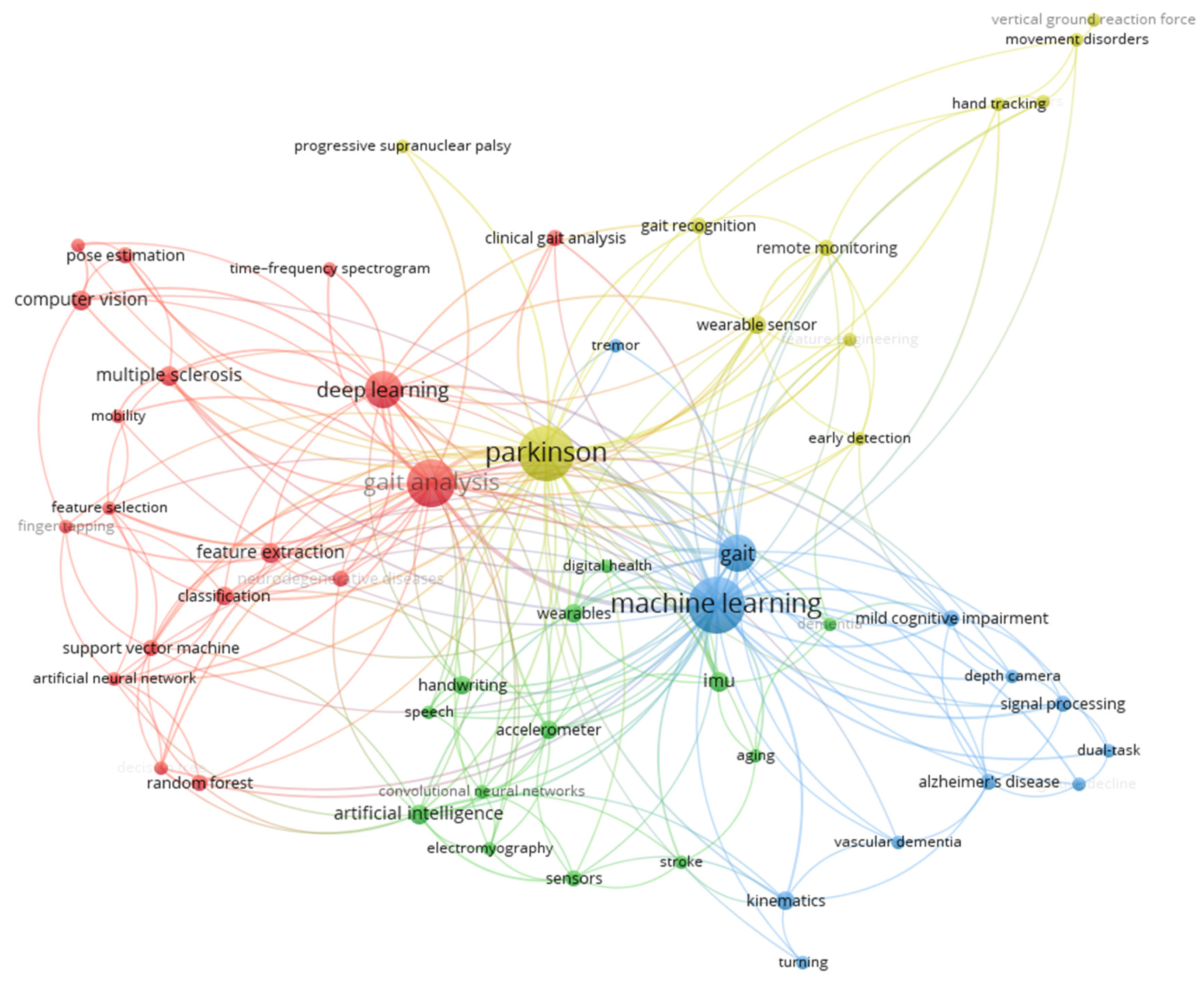
The thematic structure of the research field based on a co-occurrence analysis of keywords is shown in
Figure 6 and
Table 3. The network in
Figure 6 shows the relationships between keywords, with nodes representing individual keywords and edges indicating their co-occurrence in the same documents. The size of the nodes reflects the frequency of keyword usage, while the colors correspond to distinct clusters, representing different thematic areas.
Table 3 complements the network by categorizing the clusters and listing the keywords associated with each thematic area. Four main clusters were identified:
Machine Learning and Gait Analysis: This cluster includes keywords related to computational methods, such as "deep learning" and "support vector machine," as well as applications in "gait analysis" and "pose estimation."
Sensors and Wearable Health Technologies: Keywords in this cluster focus on sensor-based systems and wearable technologies, such as "accelerometer," "IMU," and "digital health."
Cognitive Disorders: This cluster encompasses terms related to neurological and cognitive impairments, including "Alzheimer’s disease," "cognitive decline," and "dual-task."
Neurological Disorders and Motion Recognition Technologies: Keywords in this cluster highlight technologies for the analysis and management of movement disorders, such as "Parkinson," "gait recognition," and "remote monitoring."
4. Discussion
4.1. Publications’ Trends
Our study conducted a comprehensive bibliometric analysis to investigate research trends in the field of AI in the diagnosis of neurological diseases using biomechanical data from current literature. The overall trend shows a consistent and steady growth from 2018 to 2024, with a slight dip observed in 2022, which confirms the increasing research interest and activity in the field over time.
The journals listed in
Table 2 cover a wide range of scientific fields, reflecting the interdisciplinary nature of the subject. Specifically, they represent areas such as electronics and computational engineering in journals such as, IEEE Transactions on Neural Systems and Rehabilitation Engineering, IEEE Journal of Biomedical and Health Informatics, and IEEE Sensors Journal, which focus on the use of technology and computational systems for health research and applications. In addition, journals belonging in the field of medicine, biomedical science, and neuroscience, such as Sensors, Biomedical Signal Processing and Control, Journal of Alzheimer's Disease, Journal of Neuroengineering and Rehabilitation, and Parkinsonism and Related Disorders, have also been included. Collectively, these journals emphasize the multifaceted study and technological advancement of diagnostic, monitoring, and rehabilitation approaches for human health systems and neurological disorders. The inclusion of journals like Expert Systems and Multimedia Tools and Applications highlights the growing role of intelligent systems and data analytics in modeling and addressing complex health-related problems. These multifaceted advancements strongly highlights the importance of interdisciplinary collaboration across science, technology, and medicine to tackle intricate research challenges.
The analysis of both the bibliographic coupling network and the co-citation network of sources provides an important insight into the structure and dynamics of the research field. The bibliographic coupling network highlights a high level of thematic cohesion among journals, with strong bibliographic ties indicating interconnectedness within the field. At the same time, the presence of distinct clusters points to the diversity of subfields, ranging from sensor technologies to movement analysis and computational applications. On the other hand, the co-citation network reveals that, while there is strong interconnectedness within individual clusters, cross-cluster interactions remain relatively limited. This thematic segmentation underscores the need for interdisciplinary approaches to bridge the gaps between sensor-based technologies and neurological research. Addressing this fragmentation could lead to a more integrated understanding and foster innovation by connecting related but currently isolated areas of research to breach the gap.
Another very important finding of the present study was the lack of international collaborations. This limited connectivity at all three levels (authors, countries, and organizations) of co-authorship analyses, underscores the fragmented nature of collaborative efforts in the studied field, indicating potential opportunities to foster greater cooperation and network development. Collaboration and networking in scientific research play a crucial role in enhancing productivity and scientific progress [
32,
33,
34]. Strengthening global partnerships and developing cross-institutional networks could lead to more comprehensive and impactful research outcomes in the field.
Our study identifies several critical research gaps in the application of AI for the diagnosis of neurological diseases. One major limitation is the lack of robust external validation for AI models, which constrains the generalization with diverse populations and clinical settings. Furthermore, the explainability and interpretability of AI systems, particularly deep learning models, remain significantly challenging. Often functioning as "black boxes," these systems make it difficult for clinicians to understand or trust their predictions, emphasizing the need for explainable AI frameworks that enhance clinical acceptance. Another notable issue is the reliance on small or homogeneous datasets, which fail to capture the variability present in broader patient populations. Larger, multicenter datasets are essential to ensure the reliability and applicability of AI models across different settings. Additionally, the thematic segmentation observed in bibliometric networks highlights the fragmented nature of research efforts, underscoring the importance of interdisciplinary collaboration among experts in AI, biomechanics, and neuroscience. Enhanced collaboration across these fields could drive innovation by integrating complementary expertise. Lastly, there is a pressing need for studies that assess the performance of AI systems across diverse populations, including variations in age, ethnicity, and socioeconomic backgrounds. Addressing these gaps will not only improve the accuracy and reliability of AI diagnostic tools but also promote equity and inclusivity in their application. Coordinated efforts from interdisciplinary research teams, leveraging advanced computational techniques and fostering collaboration across institutions and countries. By overcoming these limitations, the field can move toward more accurate, explainable, and widely applicable AI systems for the diagnosis and management of neurological disorders.
4.2. Thematic Areas
The co-occurrence analysis of author keywords revealed four clusters, each corresponding to a distinct thematic area.
4.2.1. Machine Learning and Gait Analysis
The specific focus is on the development and application of ML techniques for analyzing and diagnosing neurological disorders using gait data, with particular emphasis on conditions such as Parkinson's disease, multiple sclerosis, and other neurodegenerative diseases. Common themes across these studies include the use of gait data and ML techniques to detect motor abnormalities, the enhancement of diagnosis through DL algorithms, and the high accuracy achieved in classifying patients from healthy controls. Specifically, the studies employ a variety of approaches for data collection and analysis, such as motion sensors, vGRF analysis, EMG, and 3D gait kinematics [
35,
36,
37,
38,
39,
40]. Furthermore, the included studies often employed ML, including techniques such as RFs, decision trees, SVMs, and DL to classify or assess neurological diseases based on gait characteristics.
The application of specialized tools and methods for detecting early motor changes is also a recurring theme in the studies, enabling early diagnosis. Additionally, the personalization of approaches is crucial for enhancing accuracy and improving clinical monitoring. The findings indicate that the combined use of these techniques can significantly improve diagnostic procedures and support the monitoring of therapeutic outcomes in patients [
36,
37,
41,
42,
43]. Ultimately, the studies highlight the versatility of modern ML algorithms and data analysis methods in identifying motor disorders, offering new opportunities for more accurate and earlier diagnosis of neurodegenerative diseases.
Overall, these studies demonstrate the importance of combining gait analysis with ML and DL techniques for the accurate diagnosis and monitoring of neurological and non-neurological disorders. These technologies not only provide new possibilities for early diagnosis and patient monitoring, but also, allow for a deeper understanding of motor problems associated with various diseases. Looking ahead, advancements in AI-powered gait analysis hold the potential to revolutionize personalized healthcare.
4.2.2. Sensors and Wearable Health Technologies
AI-powered sensors and wearable devices are revolutionizing health monitoring, enabling real-time data analysis for applications ranging from handwriting, movement analysis and speech tracking to stroke recovery, and digital health management. Utilising technologies such as, inertial measurement units (IMU) and sensors, can enable the creation of personalized, continuous monitoring systems that process large-scale of data efficiently. The integration of AI into wearables represents a significant step forward in preventive care and long-term health management.
A common thread across these studies is the need for enhanced accuracy in AI algorithms, particularly, applications for elderly patients and those with neurological conditions such as, Parkinson’s [
44,
45,
46,
47]. Another critical focus is developing devices that can seamlessly and effectively integrate into patients’ daily lives, promoting independence and improving quality of life. While significant progress has been made in detecting motor abnormalities, these systems must be fine-tuned for widespread clinical use.
In conclusion, wearable technologies powered by AI are aimed to transform healthcare by offering new possibilities for early diagnosis and continuous monitoring of diseases like Parkinsons. As research advances, these innovations will enable clinicians to provide more personalized, effective treatments while improving patient outcomes and quality of life.
4.2.3. Cognitive Disorders
The use of AI to understand and diagnose cognitive disorders is a vital focus of this cluster. Researchers are particularly interested in aging-related neurological conditions, such as Alzheimer’s disease, mild cognitive impairment (MCI) and vascular dementia. AI is being employed to analyze data from tools like depth cameras and sensors, which help assess behavior, cognition and motor abilities. Techniques such as AI-driven dual-task performance analysis and tremor pattern detection have shown promise in identifying early signs of cognitive decline [
48,
49,
50,
51,
52,
53]. This research highlights the potential of AI to revolutionize early diagnosis, clinical monitoring, and intervention strategies for cognitive disorders.
A common thread across these studies is the integration of AI, ML, and motion analysis with other behavioral data to enable comprehensive assessments of cognitive health. The combination of these technologies may allow researchers to provide an early and a more accurate diagnosis, enabling a timely intervention and a better management of cognitive disorders. This holistic approach has the potential to significantly improve the quality of life for patients and reduce the burden on caregivers.
Overall, this cluster underscores the importance of leveraging AI to address the growing challenges of cognitive decline. By offering tools for earlydiagnosis and more personalized care, these innovations are opening new pathways for clinical intervention to improve cognitive health outcomes.
4.2.4. Neurological Disorders and Motion Recognition Technologies
This thematic area integrates motion recognition technologies and AI to study neurological disorders like Parkinson’s disease, progressive supranuclear palsy, and other movement disorders. Researchers are leveraging motion analysis for early detection, using tools such as remote monitoring, vertical ground reaction force data and hand tracking. These technologies, often paired with wearable sensors powered by AI, allow for continuous tracking of symptoms such as tremors and gait abnormalities. By improving remote monitoring systems, these studies pave the way for scalable and efficient management of chronic neurological conditions.
Beyond Parkinson’s disease, the prediction of global cognitive function decline using ML offers a valuable tool for identifying risk in older adults. By analyzing gait and physical fitness parameters, researchers can enable earlier interventions that improve the quality of life for the elderly [
54,
55,
56,
57]. Additionally, combining dual-task gait evaluations with ML may provide more comprehensive cognitive assessments to enhance diagnostic accuracy for mild cognitive impairment, as well as, create opportunities for early intervention.
A recurring theme has been identified across these studies, which is the integration of cutting-edge technologies, such as motion analysis and ML, to enable precise and early diagnosis of neurological and cognitive decline. By continuing to develop and refine these tools, researchers are driving advancements in personalized care, offering hope for better disease management and improved patient outcomes.
4.3. Limitations
Although valuable insights have been identified in research trends and thematic areas in the field of AI for the diagnosis of neurological diseases using biomechanical data, the present study has certain limitations (1) the analysis was based solely on data sourced from the Scopus database, which, while comprehensive, may exclude relevant articles indexed in other databases such as PubMed or Web of Science; (2) the focus on English-language articles might have led to the exclusion of significant contributions published in other languages; (3) the exclusion of articles from conferences and books might have overlooked some important contributions to the field that have not yet been published in academic journals; and (4) the bibliometric approach inherently emphasizes quantitative metrics, which may not fully capture the qualitative impact of specific studies, although a brief qualitative synthesis of the four main thematic areas was conducted in our study.
5. Conclusions
The present study provides a strong foundation for the development of robust and effective AI-drive healthcare solutions, which is crucial for future research and the need for interdisciplinary collaboration amongst researchers. The bibliometric analysis using AI in diagnosing and managing neurological disorders, breaches the gap of current trends and research patterns. The analysis of 113 studies published between the years 2018 and 2024, identified key thematic areas—machine learning and gait analysis, sensors and wearable health technologies, cognitive disorders, and motion recognition technologies—that reflect the diverse and interdisciplinary nature of AI applications in neurology. The analysis reveals a clear trend of increasing research interest over the years, underscoring the growing recognition of AI’s transformative potential in this field. Furthermore, the present findings highlight how the convergence of disciplines such as neurology, data science, and occupational therapy can be utilized in an effort to foster collaboration to tackle complex neurological conditions.
Future research should develop further AI capabilities by creating standardized datasets to improve reproducibility, exploring underrepresented neurological conditions to broaden the scope of applications, and developing explainable AI (XAI) systems that clinicians can trust and effectively use. Collaborative efforts among researchers, clinicians, occupational therapists, and institutions will be essential to designing impactful studies and driving innovation. Additionally, future investigations could progress the analysis with emerging trends beyond 2024, evaluating the clinical implementation of AI tools, and exploring interdisciplinary approaches to bridge gaps between research and practice.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chandra, V.; Pandav, R.; Laxminarayan, R.; Tanner, C.; Manyam, B.; Rajkumar, S.; Silberberg, D.; Brayne, C.; Chow, J.; Herman, S. Neurological disorders. Disease control priorities 2006, 21. [Google Scholar]
- Steinmetz, J.D.; Seeher, K.M.; Schiess, N.; Nichols, E.; Cao, B.; Servili, C.; Cavallera, V.; Cousin, E.; Hagins, H.; Moberg, M.E. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet Neurology 2024, 23, 344–381. [Google Scholar] [CrossRef] [PubMed]
- Mistur, R.; Mosconi, L.; De Santi, S.; Guzman, M.; Li, Y.; Tsui, W.; de Leon, M.J. Current challenges for the early detection of Alzheimer's disease: brain imaging and CSF studies. Journal of clinical neurology 2009, 5, 153–166. [Google Scholar] [CrossRef]
- Koenig, M.; Klotz, E.; Luka, B.; Venderink, D.J.; Spittler, J.F.; Heuser, L. Perfusion CT of the brain: diagnostic approach for early detection of ischemic stroke. Radiology 1998, 209, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Siuly, S.; Zhang, Y. Medical big data: neurological diseases diagnosis through medical data analysis. Data Science and Engineering 2016, 1, 54–64. [Google Scholar] [CrossRef]
- Al-Qazzaz, N.K.; Ali, S.H.B.M.; Ahmad, S.A.; Chellappan, K.; Islam, M.S.; Escudero, J. Role of EEG as biomarker in the early detection and classification of dementia. The Scientific World Journal 2014, 2014, 906038. [Google Scholar] [CrossRef]
- McEvoy, L.K.; Brewer, J.B. Quantitative structural MRI for early detection of Alzheimer’s disease. Expert review of neurotherapeutics 2010, 10, 1675–1688. [Google Scholar] [CrossRef]
- Jha, K.; Kumar, A. Role of Artificial Intelligence in Detecting Neurological Disorders. International Research Journal on Advanced Engineering Hub (IRJAEH) 2024, 2, 73–79. [Google Scholar] [CrossRef]
- Paul, S.; Bhattacharya, P.; Bit, A. Early detection of neurological disorders using machine learning systems; IGI Global: 2019.
- Singh, K.R.; Dash, S. Early detection of neurological diseases using machine learning and deep learning techniques: A review. Artificial Intelligence for Neurological Disorders 2023, 1–24. [Google Scholar] [CrossRef]
- Vandana, J.; Nirali, N. A review of EEG signal analysis for diagnosis of neurological disorders using machine learning. Journal of Biomedical Photonics & Engineering 2021, 7, 40201. [Google Scholar]
- Khan, P.; Kader, M.F.; Islam, S.R.; Rahman, A.B.; Kamal, M.S.; Toha, M.U.; Kwak, K.-S. Machine learning and deep learning approaches for brain disease diagnosis: principles and recent advances. Ieee Access 2021, 9, 37622–37655. [Google Scholar] [CrossRef]
- Kalani, M.; Anjankar, A. Revolutionizing Neurology: The Role of Artificial Intelligence in Advancing Diagnosis and Treatment. Cureus 2024, 16, e61706. [Google Scholar] [CrossRef]
- Celik, Y.; Aslan, M.F.; Sabanci, K.; Stuart, S.; Woo, W.L.; Godfrey, A. Improving inertial sensor-based activity recognition in neurological populations. Sensors 2022, 22, 9891. [Google Scholar] [CrossRef]
- Vilas-Boas, M.D.C.; Rocha, A.P.; Cardoso, M.N.; Fernandes, J.M.; Coelho, T.; Cunha, J.P.S. Supporting the assessment of hereditary transthyretin amyloidosis patients based on 3-D gait analysis and machine learning. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2021, 29, 1350–1362. [Google Scholar] [CrossRef]
- Babu, A.; Ranpariya, S.; Sinha, D.K.; Mandal, D. Deep learning enabled perceptive wearable sensor: an interactive gadget for tracking movement disorder. Advanced Materials Technologies 2023, 8, 2300046. [Google Scholar] [CrossRef]
- Billones, C.D.; Demetria, O.J.L.D.; Hostallero, D.E.D.; Naval, P.C. DemNet: a convolutional neural network for the detection of Alzheimer's disease and mild cognitive impairment. In Proceedings of the 2016 IEEE region 10 conference (TENCON); 2016; pp. 3724–3727. [Google Scholar]
- Wilson, M.; Sampson, M.; Barrowman, N.; Doja, A. Bibliometric analysis of neurology articles published in general medicine journals. JAMA network open 2021, 4, e215840–e215840. [Google Scholar] [CrossRef]
- Facciorusso, S.; Spina, S.; Reebye, R.; Turolla, A.; Calabrò, R.S.; Fiore, P.; Santamato, A. Sensor-based rehabilitation in neurological diseases: a bibliometric analysis of research trends. Brain Sciences 2023, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.-A.; Kozlowski, D.; Segal, J.; Messer, M.; Ocay, D.D.; Saari, T.; Ferland, C.E.; Larivière, V. Trends in brain research: A bibliometric analysis. Canadian Journal of Neurological Sciences 2023, 1–11. [Google Scholar] [CrossRef]
- Xu, F.; Dai, Z.; Ye, Y.; Hu, P.; Cheng, H. Bibliometric and visualized analysis of the application of artificial intelligence in stroke. Frontiers in Neuroscience 2024, 18, 1411538. [Google Scholar] [CrossRef]
- An, X.; He, J.; Bi, B.; Wu, G.; Xu, J.; Yu, W.; Ren, Z. The application of artificial intelligence in diagnosis of Alzheimer’s disease: a bibliometric analysis. Frontiers in Neurology 2024, 15, 1510729. [Google Scholar] [CrossRef]
- Wu, C.-C.; Su, C.-H.; Islam, M.M.; Liao, M.-H. Artificial Intelligence in dementia: A bibliometric study. Diagnostics 2023, 13, 2109. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Xie, H.; Tao, X.; Wang, F.L.; Xie, N.; Hao, T. A bibliometric and visual analysis of artificial intelligence technologies-enhanced brain MRI research. Multimedia Tools and Applications 2021, 80, 17335–17363. [Google Scholar] [CrossRef]
- Dener, M.; Tekin, U. A Bibliometric Analysis of Studies on Artificial Intelligence in Neuroscience. Frontiers in Neurology 2025, 16, 1474484. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, Z. Decoding the application of deep learning in neuroscience: a bibliometric analysis. Frontiers in Computational Neuroscience 2024, 18, 1402689. [Google Scholar] [CrossRef] [PubMed]
- El-Hajj, V.G.; Gharios, M.; Edström, E.; Elmi-Terander, A. Artificial intelligence in neurosurgery: a bibliometric analysis. World Neurosurgery 2023, 171, 152–158.e154. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Plakias, S.; Karakitsiou, G.; Nikova, A.; Christidi, F.; Kokkotis, C.; Giarmatzis, G.; Tsakni, G.; Katsouri, I.-G.; Dimitrios, S. Mapping the Landscape of Biomechanics Research in Stroke Neurorehabilitation: A Bibliometric Perspective. Biomechanics 2024, 4, 664–684. [Google Scholar] [CrossRef]
- Pranckutė, R. Web of Science (WoS) and Scopus: The titans of bibliographic information in today’s academic world. Publications 2021, 9, 12. [Google Scholar] [CrossRef]
- Baas, J.; Schotten, M.; Plume, A.; Côté, G.; Karimi, R. Scopus as a curated, high-quality bibliometric data source for academic research in quantitative science studies. Quantitative science studies 2020, 1, 377–386. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Visualizing bibliometric networks. In Measuring scholarly impact: Methods and practice; Springer: 2014; pp. 285-320.
- Plakias, S.; Tsatalas, T.; Mina, M.A.; Kokkotis, C.; Kellis, E.; Giakas, G. A Bibliometric Analysis of Soccer Biomechanics. Applied Sciences 2024, 14, 6430. [Google Scholar] [CrossRef]
- Xiao, X.; Prompanyo, M. Influence of Scientific Collaboration Network on Academic Performance. International Journal of Business and Management 2023, 16, 1–68. [Google Scholar] [CrossRef]
- Adelowo, C.M.; Ilevbare, O.E.; Orewole, M.O. Collaboration, networking and research productivity in Nigeria’s research institutes: Empirical evidence. International Journal of Business Reflections 2023, 3. [Google Scholar] [CrossRef]
- Negi, P.C.; Pandey, S.; Sharma, S.; Sharma, N. Scalogram-Based Gait Abnormalities Classification Using Deep Convolutional Networks for Neurological and Non-Neurological Disorders. Journal of Medical and Biological Engineering 2024, 1–15. [Google Scholar] [CrossRef]
- Rangel, E.; Martínez, F. Parkinsonian gait modelling from an anomaly deep representation. Multimedia Tools and Applications 2024, 1–19. [Google Scholar] [CrossRef]
- Setiawan, F.; Lin, C.-W. Implementation of a deep learning algorithm based on vertical ground reaction force time–frequency features for the detection and severity classification of Parkinson’s disease. Sensors 2021, 21, 5207. [Google Scholar] [CrossRef]
- Erdaş, Ç.B.; Sümer, E. CNN-Based Neurodegenerative Disease Classification Using QR-Represented Gait Data. Brain and Behavior 2024, 14, e70100. [Google Scholar] [CrossRef] [PubMed]
- Guarín, D.L.; Wong, J.K.; McFarland, N.R.; Ramirez-Zamora, A.; Vaillancourt, D.E. What the trained eye cannot see: Quantitative kinematics and machine learning detect movement deficits in early-stage Parkinson's disease from videos. Parkinsonism & Related Disorders 2024, 127, 107104. [Google Scholar] [CrossRef]
- Muñoz-Mata, B.G.; Dorantes-Méndez, G.; Piña-Ramírez, O. Classification of Parkinson’s disease severity using gait stance signals in a spatiotemporal deep learning classifier. Medical & Biological Engineering & Computing 2024, 1–14. [Google Scholar] [CrossRef]
- Begum, S.V.; Rani, M.P. Classification of gait dynamics in neurodegenerative disease patients using machine learning techniques. International Journal of Scientific and Technology Research 2020, 9, 6250–6254. [Google Scholar]
- Yin, W.; Zhu, W.; Gao, H.; Niu, X.; Shen, C.; Fan, X.; Wang, C. Gait analysis in the early stage of Parkinson’s disease with a machine learning approach. Frontiers in Neurology 2024, 15, 1472956. [Google Scholar] [CrossRef]
- Apostolidis, K.; Kokkotis, C.; Karakasis, E.; Karampina, E.; Moustakidis, S.; Menychtas, D.; Giarmatzis, G.; Tsiptsios, D.; Vadikolias, K.; Aggelousis, N. Innovative visualization approach for biomechanical time series in stroke diagnosis using explainable machine learning methods: A proof-of-concept study. Information 2023, 14, 559. [Google Scholar] [CrossRef]
- Guarín, D.L.; Wong, J.K.; McFarland, N.R.; Ramirez-Zamora, A. Characterizing disease progression in Parkinson’s disease from videos of the finger tapping test. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2024. [Google Scholar] [CrossRef]
- Johnson, S.; Kantartjis, M.; Severson, J.; Dorsey, R.; Adams, J.L.; Kangarloo, T.; Kostrzebski, M.A.; Best, A.; Merickel, M.; Amato, D. Wearable Sensor-Based Assessments for Remotely Screening Early-Stage Parkinson’s Disease. Sensors 2024, 24, 5637. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Dadlani, A.; Lee, H.; Kim, K. Automated prescreening of mild cognitive impairment using shank-mounted inertial sensors based gait biomarkers. IEEE Access 2022, 10, 15835–15844. [Google Scholar] [CrossRef]
- Ileșan, R.R.; Cordoș, C.-G.; Mihăilă, L.-I.; Fleșar, R.; Popescu, A.-S.; Perju-Dumbravă, L.; Faragó, P. Proof of concept in artificial-intelligence-based wearable gait monitoring for Parkinson’s disease management optimization. Biosensors 2022, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Obuchi, S.P.; Kojima, M.; Suzuki, H.; Garbalosa, J.C.; Imamura, K.; Ihara, K.; Hirano, H.; Sasai, H.; Fujiwara, Y.; Kawai, H. Artificial intelligence detection of cognitive impairment in older adults during walking. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring 2024, 16, e70012. [Google Scholar] [CrossRef]
- Trabassi, D.; Serrao, M.; Varrecchia, T.; Ranavolo, A.; Coppola, G.; De Icco, R.; Tassorelli, C.; Castiglia, S.F. Machine learning approach to support the detection of Parkinson’s disease in IMU-based Gait analysis. Sensors 2022, 22, 3700. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Shinkawa, K.; Kobayashi, M.; Caggiano, V.; Nemoto, M.; Nemoto, K.; Arai, T. Combining multimodal behavioral data of gait, speech, and drawing for classification of Alzheimer’s disease and mild cognitive impairment. Journal of Alzheimer's Disease 2021, 84, 315–327. [Google Scholar] [CrossRef]
- Hao, C.; Zhang, X.; An, J.; Bao, W.; Yang, F.; Chen, J.; Hou, S.; Wang, Z.; Du, S.; Zhao, Y. An effective screening model for subjective cognitive decline in community-dwelling older adults based on gait analysis and eye tracking. Frontiers in Aging Neuroscience 2024, 16, 1444375. [Google Scholar] [CrossRef]
- Chen, P.-H.; Lien, C.-W.; Wu, W.-C.; Lee, L.-S.; Shaw, J.-S. Artificial Intelligence of Neuropsychological Tests for the Prediction and Verification of Decline in Gait Parameters in Patients with Mild Cognitive Impairment. International Journal of Gerontology 2020, 14. [Google Scholar] [CrossRef]
- Noh, B.; Yoon, H.; Youm, C.; Kim, S.; Lee, M.; Park, H.; Kim, B.; Choi, H.; Noh, Y. Prediction of decline in global cognitive function using machine learning with feature ranking of gait and physical fitness outcomes in older adults. International Journal of Environmental Research and Public Health 2021, 18, 11347. [Google Scholar] [CrossRef]
- Davoudi, A.; Dion, C.; Amini, S.; Tighe, P.J.; Price, C.C.; Libon, D.J.; Rashidi, P. Classifying non-dementia and Alzheimer’s disease/vascular dementia patients using kinematic, time-based, and visuospatial parameters: the digital clock drawing test. Journal of Alzheimer's Disease 2021, 82, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Sarbaz, Y.; Abedi, B. Presenting a new decision support system for screening parkinson’s disease patients using symlet wavelet. Biomedical Engineering: Applications, Basis and Communications 2019, 31, 1950026. [Google Scholar] [CrossRef]
- Recenti, M.; Gargiulo, P.; Chang, M.; Ko, S.B.; Kim, T.J.; Ko, S.U. Predicting stroke, neurological and movement disorders using single and dual-task gait in Korean older population. Gait & Posture 2023, 105, 92–98. [Google Scholar] [CrossRef]
- Fraiwan, L.; Hassanin, O. Computer-aided identification of degenerative neuromuscular diseases based on gait dynamics and ensemble decision tree classifiers. Plos one 2021, 16, e0252380. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).