Submitted:
29 January 2025
Posted:
31 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Overview of GnIH and Its Impact on the Endocrine System
2.1. GnIH and Its Receptors
2.2. Regulation of the Endocrine System by GnIH
2.2.1. Role of GnIH on the Reproductive Axis
2.2.2. Involvement of GnIH in the Stress Axis
2.2.3. Involvement of GnIH in the thyroid axis
3. Metabolic Regulation by Hypothalamic GnIH
3.1. Effect of Central GnIH Administration on Feeding Behavior
3.2. Interaction with the Melanocortin System
3.3. Hypothlamic GnIH Regulation by Peripheral Hormones, Leptin and Ghrelin
4. Metabolic Regulation by Peripheral GnIH
4.1. Effect of Peripheral GnIH Administration
4.2. Adipose Tissue
4.3. Pancreas
4.4. GI Tract
4.5. Liver and Skeletal Muscle
5. Nutritional Status and GnIH: Obesity and Fasting
6. Conclusions
Author Contributions
Funding
Institutional Review Bord Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsutsui, K.; Saigoh, E.; Ukena, K.; Teranishi, H.; Fujisawa, Y.; Kikuchi, M.; Ishii, S.; Sharp, P.J. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun 2000, 275, 661-667. [CrossRef]
- Satake, H.; Hisada, M.; Kawada, T.; Minakata, H.; Ukena, K.; Tsutsui, K. Characterization of a cDNA encoding a novel avian hypothalamic neuropeptide exerting an inhibitory effect on gonadotropin release. Biochem J 2001, 354, 379-385. [CrossRef]
- Ikemoto, T.; Park, M.K. Chicken RFamide-related peptide (GnIH) and two distinct receptor subtypes: identification, molecular characterization, and evolutionary considerations. J Reprod Dev 2005, 51, 359-377. [CrossRef]
- Osugi, T.; Ukena, K.; Bentley, G.E.; O'Brien, S.; Moore, I.T.; Wingfield, J.C.; Tsutsui, K. Gonadotropin-inhibitory hormone in Gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. J Endocrinol 2004, 182, 33-42.
- Ubuka, T.; Kim, S.; Huang, Y.C.; Reid, J.; Jiang, J.; Osugi, T.; Chowdhury, V.S.; Tsutsui, K.; Bentley, G.E. Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in European starling brain. Endocrinology 2008, 149, 268-278.
- Tobari, Y.; Iijima, N.; Tsunekawa, K.; Osugi, T.; Okanoya, K.; Tsutsui, K.; Ozawa, H. Identification of gonadotropin-inhibitory hormone in the zebra finch (Taeniopygia guttata): Peptide isolation, cDNA cloning and brain distribution. Peptides 2010, 31, 816-826. [CrossRef]
- Tsutsui, K. A new key neurohormone controlling reproduction, gonadotropin-inhibitory hormone (GnIH): Biosynthesis, mode of action and functional significance. Prog Neurobiol 2009, 88, 76-88. [CrossRef]
- Tsutsui, K.; Bentley, G.E.; Bedecarrats, G.; Osugi, T.; Ubuka, T.; Kriegsfeld, L.J. Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Front Neuroendocrinol 2010, 31, 284-295. [CrossRef]
- Tsutsui, K.; Ubuka, T.; Bentley, G.E.; Kriegsfeld, L.J. Review: regulatory mechanisms of gonadotropin-inhibitory hormone (GnIH) synthesis and release in photoperiodic animals. Front Neurosci 2013, 7, 60. [CrossRef]
- Hinuma, S.; Shintani, Y.; Fukusumi, S.; Iijima, N.; Matsumoto, Y.; Hosoya, M.; Fujii, R.; Watanabe, T.; Kikuchi, K.; Terao, Y.; et al. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol 2000, 2, 703-708.
- Bonini, J.A.; Jones, K.A.; Adham, N.; Forray, C.; Artymyshyn, R.; Durkin, M.M.; Smith, K.E.; Tamm, J.A.; Boteju, L.W.; Lakhlani, P.P.; et al. Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J Biol Chem 2000, 275, 39324-39331. [CrossRef]
- Yin, H.; Ukena, K.; Ubuka, T.; Tsutsui, K. A novel G protein-coupled receptor for gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica): identification, expression and binding activity. J Endocrinol 2005, 184, 257-266.
- Mollereau, C.; Mazarguil, H.; Marcus, D.; Quelven, I.; Kotani, M.; Lannoy, V.; Dumont, Y.; Quirion, R.; Detheux, M.; Parmentier, M.; et al. Pharmacological characterization of human NPFF(1) and NPFF(2) receptors expressed in CHO cells by using NPY Y(1) receptor antagonists. Eur J Pharmacol 2002, 451, 245-256. [CrossRef]
- Gouarderes, C.; Mazarguil, H.; Mollereau, C.; Chartrel, N.; Leprince, J.; Vaudry, H.; Zajac, J.M. Functional differences between NPFF1 and NPFF2 receptor coupling: high intrinsic activities of RFamide-related peptides on stimulation of [35S]GTPgammaS binding. Neuropharmacology 2007, 52, 376-386.
- Ubuka, T.; Son, Y.L.; Bentley, G.E.; Millar, R.P.; Tsutsui, K. Gonadotropin-inhibitory hormone (GnIH), GnIH receptor and cell signaling. Gen Comp Endocrinol 2013, 190, 10-17.
- Oishi, H.; Klausen, C.; Bentley, G.E.; Osugi, T.; Tsutsui, K.; Gilks, C.B.; Yano, T.; Leung, P.C. The human gonadotropin-inhibitory hormone ortholog RFamide-related peptide-3 suppresses gonadotropin-induced progesterone production in human granulosa cells. Endocrinology 2012, 153, 3435-3445. [CrossRef]
- Son, Y.L.; Ubuka, T.; Millar, R.P.; Kanasaki, H.; Tsutsui, K. Gonadotropin-inhibitory hormone inhibits GnRH-induced gonadotropin subunit gene transcriptions by inhibiting AC/cAMP/PKA-dependent ERK pathway in LbetaT2 cells. Endocrinology 2012, 153, 2332-2343.
- Son, Y.L.; Ubuka, T.; Soga, T.; Yamamoto, K.; Bentley, G.E.; Tsutsui, K. Inhibitory action of gonadotropin-inhibitory hormone on the signaling pathways induced by kisspeptin and vasoactive intestinal polypeptide in GnRH neuronal cell line, GT1-7. Faseb J 2016, 30, 2198-2210.
- Ubuka, T.; Morgan, K.; Pawson, A.J.; Osugi, T.; Chowdhury, V.S.; Minakata, H.; Tsutsui, K.; Millar, R.P.; Bentley, G.E. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS One 2009, 4, e8400. [CrossRef]
- Ubuka, T.; Inoue, K.; Fukuda, Y.; Mizuno, T.; Ukena, K.; Kriegsfeld, L.J.; Tsutsui, K. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology 2012, 153, 373-385.
- Yano, T.; Iijima, N.; Kakihara, K.; Hinuma, S.; Tanaka, M.; Ibata, Y. Localization and neuronal response of RFamide related peptides in the rat central nervous system. Brain Res 2003, 982, 156-167. [CrossRef]
- Johnson, M.A.; Tsutsui, K.; Fraley, G.S. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav 2007, 51, 171-180.
- Ukena, K.; Ubuka, T.; Tsutsui, K. Distribution of a novel avian gonadotropin-inhibitory hormone in the quail brain. Cell Tissue Res 2003, 312, 73-79.
- Ubuka, T.; Ueno, M.; Ukena, K.; Tsutsui, K. Developmental changes in gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica) hypothalamo-hypophysial system. J Endocrinol 2003, 178, 311-318. [CrossRef]
- Kriegsfeld, L.J.; Mei, D.F.; Bentley, G.E.; Ubuka, T.; Mason, A.O.; Inoue, K.; Ukena, K.; Tsutsui, K.; Silver, R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A 2006, 103, 2410-2415.
- Legagneux, K.; Bernard-Franchi, G.; Poncet, F.; La Roche, A.; Colard, C.; Fellmann, D.; Pralong, F.; Risold, P.Y. Distribution and genesis of the RFRP-producing neurons in the rat brain: comparison with melanin-concentrating hormone- and hypocretin-containing neurons. Neuropeptides 2009, 43, 13-19.
- Revel, F.G.; Saboureau, M.; Pevet, P.; Simonneaux, V.; Mikkelsen, J.D. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology 2008, 149, 902-912. [CrossRef]
- Ukena, K.; Tsutsui, K. Distribution of novel RFamide-related peptide-like immunoreactivity in the mouse central nervous system. Neurosci Lett 2001, 300, 153-156.
- Mason, A.O.; Duffy, S.; Zhao, S.; Ubuka, T.; Bentley, G.E.; Tsutsui, K.; Silver, R.; Kriegsfeld, L.J. Photoperiod and reproductive condition are associated with changes in RFamide-related peptide (RFRP) expression in Syrian hamsters (Mesocricetus auratus). J Biol Rhythms 2010, 25, 176-185. [CrossRef]
- Tsutsui, K.; Bentley, G.E.; Kriegsfeld, L.J.; Osugi, T.; Seong, J.Y.; Vaudry, H. Discovery and evolutionary history of gonadotrophin-inhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction. J Neuroendocrinol 2010, 22, 716-727.
- Tsutsui, K.; Ubuka, T.; Bentley, G.E.; Kriegsfeld, L.J. Gonadotropin-inhibitory hormone (GnIH): discovery, progress and prospect. Gen Comp Endocrinol 2012, 177, 305-314. [CrossRef]
- Tsutsui, K.; Ubuka, T.; Son, Y.L.; Bentley, G.E.; Kriegsfeld, L.J. Contribution of GnIH Research to the Progress of Reproductive Neuroendocrinology. Front Endocrinol (Lausanne) 2015, 6, 179. [CrossRef]
- Kriegsfeld, L.J.; Ubuka, T.; Bentley, G.E.; Tsutsui, K. Seasonal control of gonadotropin-inhibitory hormone (GnIH) in birds and mammals. Front Neuroendocrinol 2015, 37, 65-75.
- Smith, J.T.; Coolen, L.M.; Kriegsfeld, L.J.; Sari, I.P.; Jaafarzadehshirazi, M.R.; Maltby, M.; Bateman, K.; Goodman, R.L.; Tilbrook, A.J.; Ubuka, T.; et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 2008, 149, 5770-5782.
- Kriegsfeld, L.J.; Gibson, E.M.; Williams, W.P., 3rd; Zhao, S.; Mason, A.O.; Bentley, G.E.; Tsutsui, K. The roles of RFamide-related peptide-3 in mammalian reproductive function and behaviour. J Neuroendocrinol 2010, 22, 692-700.
- Rizwan, M.Z.; Poling, M.C.; Corr, M.; Cornes, P.A.; Augustine, R.A.; Quennell, J.H.; Kauffman, A.S.; Anderson, G.M. RFamide-related peptide-3 receptor gene expression in GnRH and kisspeptin neurons and GnRH-dependent mechanism of action. Endocrinology 2012, 153, 3770-3779. [CrossRef]
- Bentley, G.E.; Perfito, N.; Ukena, K.; Tsutsui, K.; Wingfield, J.C. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. J Neuroendocrinol 2003, 15, 794-802.
- Ducret, E.; Anderson, G.M.; Herbison, A.E. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology 2009, 150, 2799-2804.
- Wu, M.; Dumalska, I.; Morozova, E.; van den Pol, A.N.; Alreja, M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol 2009, 587, 1401-1411. [CrossRef]
- Poling, M.C.; Quennell, J.H.; Anderson, G.M.; Kauffman, A.S. Kisspeptin neurones do not directly signal to RFRP-3 neurones but RFRP-3 may directly modulate a subset of hypothalamic kisspeptin cells in mice. J Neuroendocrinol 2013, 25, 876-886.
- Maddineni, S.; Ocon-Grove, O.M.; Krzysik-Walker, S.M.; Hendricks, G.L., 3rd; Proudman, J.A.; Ramachandran, R. Gonadotrophin-inhibitory hormone receptor expression in the chicken pituitary gland: potential influence of sexual maturation and ovarian steroids. J Neuroendocrinol 2008, 20, 1078-1088.
- Clarke, I.J.; Sari, I.P.; Qi, Y.; Smith, J.T.; Parkington, H.C.; Ubuka, T.; Iqbal, J.; Li, Q.; Tilbrook, A.; Morgan, K.; et al. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology 2008, 149, 5811-5821. [CrossRef]
- Ubuka, T.; Lai, H.; Kitani, M.; Suzuuchi, A.; Pham, V.; Cadigan, P.A.; Wang, A.; Chowdhury, V.S.; Tsutsui, K.; Bentley, G.E. Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. J Comp Neurol 2009, 517, 841-855.
- Murakami, M.; Matsuzaki, T.; Iwasa, T.; Yasui, T.; Irahara, M.; Osugi, T.; Tsutsui, K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol 2008, 199, 105-112.
- Gibson, E.M.; Humber, S.A.; Jain, S.; Williams, W.P., 3rd; Zhao, S.; Bentley, G.E.; Tsutsui, K.; Kriegsfeld, L.J. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology 2008, 149, 4958-4969.
- Kadokawa, H.; Shibata, M.; Tanaka, Y.; Kojima, T.; Matsumoto, K.; Oshima, K.; Yamamoto, N. Bovine C-terminal octapeptide of RFamide-related peptide-3 suppresses luteinizing hormone (LH) secretion from the pituitary as well as pulsatile LH secretion in bovines. Domest Anim Endocrinol 2009, 36, 219-224.
- Sari, I.P.; Rao, A.; Smith, J.T.; Tilbrook, A.J.; Clarke, I.J. Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology 2009, 150, 5549-5556.
- Bentley, G.E.; Ubuka, T.; McGuire, N.L.; Chowdhury, V.S.; Morita, Y.; Yano, T.; Hasunuma, I.; Binns, M.; Wingfield, J.C.; Tsutsui, K. Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system. Gen Comp Endocrinol 2008, 156, 34-43. [CrossRef]
- McGuire, N.L.; Bentley, G.E. A functional neuropeptide system in vertebrate gonads: Gonadotropin-inhibitory hormone and its receptor in testes of field-caught house sparrow (Passer domesticus). Gen Comp Endocrinol 2010, 166, 565-572. [CrossRef]
- Zhao, S.; Zhu, E.; Yang, C.; Bentley, G.E.; Tsutsui, K.; Kriegsfeld, L.J. RFamide-related peptide and messenger ribonucleic acid expression in mammalian testis: association with the spermatogenic cycle. Endocrinology 2010, 151, 617-627. [CrossRef]
- Qi, Y.; Oldfield, B.J.; Clarke, I.J. Projections of RFamide-related peptide-3 neurones in the ovine hypothalamus, with special reference to regions regulating energy balance and reproduction. J Neuroendocrinol 2009, 21, 690-697.
- Kirby, E.D.; Geraghty, A.C.; Ubuka, T.; Bentley, G.E.; Kaufer, D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci U S A 2009, 106, 11324-11329. [CrossRef]
- Son, Y.L.; Ubuka, T.; Narihiro, M.; Fukuda, Y.; Hasunuma, I.; Yamamoto, K.; Belsham, D.D.; Tsutsui, K. Molecular basis for the activation of gonadotropin-inhibitory hormone gene transcription by corticosterone. Endocrinology 2014, 155, 1817-1826. [CrossRef]
- Geraghty, A.C.; Muroy, S.E.; Zhao, S.; Bentley, G.E.; Kriegsfeld, L.J.; Kaufer, D. Knockdown of hypothalamic RFRP3 prevents chronic stress-induced infertility and embryo resorption. Elife 2015, 4. [CrossRef]
- Moustafa, A. Changes in nitric oxide, carbon monoxide, hydrogen sulfide and male reproductive hormones in response to chronic restraint stress in rats. Free radical biology & medicine 2021, 162, 353-366. [CrossRef]
- Yang, J.A.; Song, C.I.; Hughes, J.K.; Kreisman, M.J.; Parra, R.A.; Haisenleder, D.J.; Kauffman, A.S.; Breen, K.M. Acute Psychosocial Stress Inhibits LH Pulsatility and Kiss1 Neuronal Activation in Female Mice. Endocrinology 2017, 158, 3716-3723. [CrossRef]
- Yang, J.A.; Hughes, J.K.; Parra, R.A.; Volk, K.M.; Kauffman, A.S. Stress rapidly suppresses in vivo LH pulses and increases activation of RFRP-3 neurons in male mice. J Endocrinol 2018, 239, 339-350. [CrossRef]
- Mamgain, A.; Sawyer, I.L.; Timajo, D.A.M.; Rizwan, M.Z.; Evans, M.C.; Ancel, C.M.; Inglis, M.A.; Anderson, G.M. RFamide-Related Peptide Neurons Modulate Reproductive Function and Stress Responses. J Neurosci 2021, 41, 474-488. [CrossRef]
- Calisi, R.M.; Rizzo, N.O.; Bentley, G.E. Seasonal differences in hypothalamic EGR-1 and GnIH expression following capture-handling stress in house sparrows (Passer domesticus). Gen Comp Endocrinol 2008, 157, 283-287.
- León, S.; García-Galiano, D.; Ruiz-Pino, F.; Barroso, A.; Manfredi-Lozano, M.; Romero-Ruiz, A.; Roa, J.; Vázquez, M.J.; Gaytan, F.; Blomenrohr, M.; et al. Physiological roles of gonadotropin-inhibitory hormone signaling in the control of mammalian reproductive axis: studies in the NPFF1 receptor null mouse. Endocrinology 2014, 155, 2953-2965. [CrossRef]
- Simonin, F.; Schmitt, M.; Laulin, J.-P.; Laboureyras, E.; Jhamandas, J.H.; MacTavish, D.; Matifas, A.; Mollereau, C.; Laurent, P.; Parmentier, M.; et al. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proceedings of the National Academy of Sciences of the United States of America 2006, 103, 466-471. [CrossRef]
- Batool, A.; Naz, R.; Wazir, M.; Azam, A.; Ullah, R.; Wahab, F.; Shahab, M. Acute fasting-induced repression of the hypothalamic-pituitary-gonadal axis is reversed by RF-9 administration in the adult male macaque. Horm Metab Res 2014, 46, 927-832. [CrossRef]
- Schneider, J.E.; Benton, N.A.; Russo, K.A.; Klingerman, C.M.; Williams, W.P., 3rd; Simberlund, J.; Abdulhay, A.; Brozek, J.M.; Kriegsfeld, L.J. RFamide-related Peptide-3 and the Trade-off between Reproductive and Ingestive Behavior. Integrative and comparative biology 2017, 57, 1225-1239. [CrossRef]
- Chowdhury, V.S.; Tomonaga, S.; Nishimura, S.; Tabata, S.; Cockrem, J.F.; Tsutsui, K.; Furuse, M. Hypothalamic gonadotropin-inhibitory hormone precursor mRNA is increased during depressed food intake in heat-exposed chicks. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2012, 162, 227-233. [CrossRef]
- Bahry, M.A.; Yang, H.; Tran, P.V.; Do, P.H.; Han, G.; Eltahan, H.M.; Chowdhury, V.S.; Furuse, M. Reduction in voluntary food intake, but not fasting, stimulates hypothalamic gonadotropin-inhibitory hormone precursor mRNA expression in chicks under heat stress. Neuropeptides 2018, 71, 90-96. [CrossRef]
- Son, Y.L.; Ubuka, T.; Tsutsui, K. Regulation of stress response on the hypothalamic-pituitary-gonadal axis via gonadotropin-inhibitory hormone. Front Neuroendocrinol 2022, 64, 100953. [CrossRef]
- McGuire, N.L.; Koh, A.; Bentley, G.E. The direct response of the gonads to cues of stress in a temperate songbird species is season-dependent. PeerJ 2013, 1, e139. [CrossRef]
- Clarke, I.J.; Bartolini, D.; Conductier, G.; Henry, B.A. Stress Increases Gonadotropin Inhibitory Hormone Cell Activity and Input to GnRH Cells in Ewes. Endocrinology 2016, 157, 4339-4350. [CrossRef]
- Samson, W.K.; Keown, C.; Samson, C.K.; Samson, H.W.; Lane, B.; Baker, J.R.; Taylor, M.M. Prolactin-releasing peptide and its homolog RFRP-1 act in hypothalamus but not in anterior pituitary gland to stimulate stress hormone secretion. Endocrine 2003, 20, 59-66. [CrossRef]
- Kim, J.S.; Brownjohn, P.W.; Dyer, B.S.; Beltramo, M.; Walker, C.S.; Hay, D.L.; Painter, G.F.; Tyndall, J.D.; Anderson, G.M. Anxiogenic and Stressor Effects of the Hypothalamic Neuropeptide RFRP-3 Are Overcome by the NPFFR Antagonist GJ14. Endocrinology 2015, 156, 4152-4162. [CrossRef]
- Prevot, V.; Croix, D.; Bouret, S.; Dutoit, S.; Tramu, G.; Stefano, G.B.; Beauvillain, J.C. Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience 1999, 94, 809-819. [CrossRef]
- Yamamura, T.; Hirunagi, K.; Ebihara, S.; Yoshimura, T. Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in Japanese quail. Endocrinology 2004, 145, 4264-4267. [CrossRef]
- Yamamura, T.; Yasuo, S.; Hirunagi, K.; Ebihara, S.; Yoshimura, T. T(3) implantation mimics photoperiodically reduced encasement of nerve terminals by glial processes in the median eminence of Japanese quail. Cell Tissue Res 2006, 324, 175-179. [CrossRef]
- Kiyohara, M.; Son, Y.L.; Tsutsui, K. Involvement of gonadotropin-inhibitory hormone in pubertal disorders induced by thyroid status. Sci Rep 2017, 7, 1042. [CrossRef]
- Tachibana, T.; Sato, M.; Takahashi, H.; Ukena, K.; Tsutsui, K.; Furuse, M. Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks. Brain Res 2005, 1050, 94-100. [CrossRef]
- Clarke, I.J.; Smith, J.T.; Henry, B.A.; Oldfield, B.J.; Stefanidis, A.; Millar, R.P.; Sari, I.P.; Chng, K.; Fabre-Nys, C.; Caraty, A.; et al. Gonadotropin-inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology 2012, 95, 305-316.
- Fraley, G.S.; Coombs, E.; Gerometta, E.; Colton, S.; Sharp, P.J.; Li, Q.; Clarke, I.J. Distribution and sequence of gonadotropin-inhibitory hormone and its potential role as a molecular link between feeding and reproductive systems in the Pekin duck (Anas platyrhynchos domestica). Gen Comp Endocrinol 2013, 184, 103-110. [CrossRef]
- McConn, B.; Wang, G.; Yi, J.; Gilbert, E.R.; Osugi, T.; Ubuka, T.; Tsutsui, K.; Chowdhury, V.S.; Furuse, M.; Cline, M.A. Gonadotropin-inhibitory hormone-stimulation of food intake is mediated by hypothalamic effects in chicks. Neuropeptides 2014, 48, 327-334. [CrossRef]
- Moriwaki, S.; Narimatsu, Y.; Fukumura, K.; Iwakoshi-Ukena, E.; Furumitsu, M.; Ukena, K. Effects of Chronic Intracerebroventricular Infusion of RFamide-Related Peptide-3 on Energy Metabolism in Male Mice. Int J Mol Sci 2020, 21. [CrossRef]
- Cazarez-Marquez, F.; Milesi, S.; Laran-Chich, M.P.; Klosen, P.; Kalsbeek, A.; Simonneaux, V. Kisspeptin and RFRP3 modulate body mass in Phodopus sungorus via two different neuroendocrine pathways. J Neuroendocrinol 2019, 31, e12710. [CrossRef]
- Cázarez-Márquez, F.; Laran-Chich, M.P.; Klosen, P.; Kalsbeek, A.; Simonneaux, V. RFRP3 increases food intake in a sex-dependent manner in the seasonal hamster Phodopus sungorus. J Neuroendocrinol 2020, 32, e12845. [CrossRef]
- Cázarez-Márquez, F.; Eliveld, J.; Ritsema, W.; Foppen, E.; Bossenbroek, Y.; Pelizzari, S.; Simonneaux, V.; Kalsbeek, A. Role of central kisspeptin and RFRP-3 in energy metabolism in the male Wistar rat. J Neuroendocrinol 2021, 33, e12973. [CrossRef]
- Kovacs, A.; Laszlo, K.; Galosi, R.; Toth, K.; Ollmann, T.; Peczely, L.; Lenard, L. Microinjection of RFRP-1 in the central nucleus of amygdala decreases food intake in the rat. Brain Res Bull 2012, 88, 589-595. [CrossRef]
- Liu, Q.; Guan, X.M.; Martin, W.J.; McDonald, T.P.; Clements, M.K.; Jiang, Q.; Zeng, Z.; Jacobson, M.; Williams, D.L., Jr.; Yu, H.; et al. Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J Biol Chem 2001, 276, 36961-36969. [CrossRef]
- Jacobi, J.S.; Coleman, H.A.; Enriori, P.J.; Parkington, H.C.; Li, Q.; Pereira, A.; Cowley, M.A.; Clarke, I.J. Paradoxical effect of gonadotrophin-inhibiting hormone to negatively regulate neuropeptide Y neurones in mouse arcuate nucleus. J Neuroendocrinol 2013, 25, 1308-1317. [CrossRef]
- Fu, L.Y.; van den Pol, A.N. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci 2010, 30, 10205-10219. [CrossRef]
- Leon, S.; Velasco, I.; Vázquez, M.J.; Barroso, A.; Beiroa, D.; Heras, V.; Ruiz-Pino, F.; Manfredi-Lozano, M.; Romero-Ruiz, A.; Sanchez-Garrido, M.A.; et al. Sex-Biased Physiological Roles of NPFF1R, the Canonical Receptor of RFRP-3, in Food Intake and Metabolic Homeostasis Revealed by its Congenital Ablation in mice. Metabolism: clinical and experimental 2018, 87, 87-97. [CrossRef]
- Poling, M.C.; Shieh, M.P.; Munaganuru, N.; Luo, E.; Kauffman, A.S. Examination of the influence of leptin and acute metabolic challenge on RFRP-3 neurons of mice in development and adulthood. Neuroendocrinology 2014, 100, 317-333. [CrossRef]
- Rizwan, M.Z.; Harbid, A.A.; Inglis, M.A.; Quennell, J.H.; Anderson, G.M. Evidence that hypothalamic RFamide related peptide-3 neurones are not leptin-responsive in mice and rats. J Neuroendocrinol 2014, 26, 247-257. [CrossRef]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschop, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649-661. [CrossRef]
- Lu, S.; Guan, J.L.; Wang, Q.P.; Uehara, K.; Yamada, S.; Goto, N.; Date, Y.; Nakazato, M.; Kojima, M.; Kangawa, K.; et al. Immunocytochemical observation of ghrelin-containing neurons in the rat arcuate nucleus. Neurosci Lett 2002, 321, 157-160. [CrossRef]
- Kageyama, H.; Kitamura, Y.; Hosono, T.; Kintaka, Y.; Seki, M.; Takenoya, F.; Hori, Y.; Nonaka, N.; Arata, S.; Shioda, S. Visualization of ghrelin-producing neurons in the hypothalamic arcuate nucleus using ghrelin-EGFP transgenic mice. Regulatory peptides 2008, 145, 116-121. [CrossRef]
- Celik, O.; Celik, N.; Aydin, S.; Aygun, B.K.; Haberal, E.T.; Kuloglu, T.; Ulas, M.; Aktun, L.H.; Acet, M.; Celik, S. Ghrelin action on GnRH neurons and pituitary gonadotropes might be mediated by GnIH-GPR147 system. Hormone molecular biology and clinical investigation 2016, 25, 121-128. [CrossRef]
- Singh, P.; Anjum, S.; Srivastava, R.K.; Tsutsui, K.; Krishna, A. Central and peripheral neuropeptide RFRP-3: A bridge linking reproduction, nutrition, and stress response. Front Neuroendocrinol 2022, 65, 100979. [CrossRef]
- van Harmelen, V.; Dicker, A.; Sjolin, E.; Blomqvist, L.; Wiren, M.; Hoffstedt, J.; Ryden, M.; Arner, P. Effects of pain controlling neuropeptides on human fat cell lipolysis. Int J Obes (Lond) 2010, 34, 1333-1340. [CrossRef]
- Huo, K.; Li, X.; Hu, W.; Song, X.; Zhang, D.; Zhang, X.; Chen, X.; Yuan, J.; Zuo, J.; Wang, X. RFRP-3, the Mammalian Ortholog of GnIH, Is a Novel Modulator Involved in Food Intake and Glucose Homeostasis. Front Endocrinol (Lausanne) 2020, 11, 194. [CrossRef]
- Lefrere, I.; De Coppet, P.; Camelin, J.C.; Le Lay, S.; Mercier, N.; Elshourbagy, N.; Bril, A.; Berrebi-Bertrand, I.; Feve, B.; Krief, S. Neuropeptide AF and FF modulation of adipocyte metabolism. Primary insights from functional genomics and effects on beta-adrenergic responsiveness. J Biol Chem 2002, 277, 39169-39178. [CrossRef]
- Wang, X.; Li, X.; Hu, C. Distribution of gonadotropin-inhibitory hormone (GnIH) in male Luchuan piglets. Gene expression patterns : GEP 2018, 28, 42-53. [CrossRef]
- Zhang, W.; Wang, L.; Yu, X.; Jia, A.; Ming, J.; Ji, Q. RFamide-related peptide-3 promotes alpha TC1 clone 6 cell survival likely via GPR147. Peptides 2018, 107, 39-44. [CrossRef]
- Li, X.; Su, J.; Lei, Z.; Zhao, Y.; Jin, M.; Fang, R.; Zheng, L.; Jiao, Y. Gonadotropin-inhibitory hormone (GnIH) and its receptor in the female pig: cDNA cloning, expression in tissues and expression pattern in the reproductive axis during the estrous cycle. Peptides 2012, 36, 176-185. [CrossRef]
- Gospodarska, E.; Kozak, L.P.; Jaroslawska, J. Isolation and identification of endogenous RFamide-related peptides 1 and 3 in the mouse hypothalamus. J Neuroendocrinol 2019, 31, e12668. [CrossRef]
- Anjum, S.; Krishna, A.; Tsutsui, K. Possible Role of GnIH as a Mediator between Adiposity and Impaired Testicular Function. Front Endocrinol (Lausanne) 2016, 7, 6. [CrossRef]
- Luo, R.; Chen, L.; Song, X.; Zhang, X.; Xu, W.; Han, D.; Zuo, J.; Hu, W.; Shi, Y.; Cao, Y.; et al. Possible Role of GnIH as a Novel Link between Hyperphagia-Induced Obesity-Related Metabolic Derangements and Hypogonadism in Male Mice. Int J Mol Sci 2022, 23. [CrossRef]
- Chen, L.; Zhang, X.; Song, X.; Han, D.; Han, K.; Xu, W.; Luo, R.; Cao, Y.; Shi, Y.; Liu, C.; et al. Peripheral Gonadotropin-Inhibitory Hormone (GnIH) Acting as a Novel Modulator Involved in Hyperphagia-Induced Obesity and Associated Disorders of Metabolism in an In Vivo Female Piglet Model. Int J Mol Sci 2022, 23. [CrossRef]
- Xu, C.; Han, D.; Song, X.; Zhang, X.; Liu, C.; Zhang, J.; Shen, B.; Li, Z.; Ma, R.; Li, Y.; et al. The possibly role of GnIH in stress and gut dysfunction in chicken. Poultry science 2024, 103, 103757. [CrossRef]
- De Jong, K.A.; Siddig, S.; Pfeifer, A.; Nikolaev, V.O. The role of compartmentalized beta-AR/cAMP signaling in the regulation of lipolysis in white and brown adipocytes. The FEBS journal 2025, 292, 261-271. [CrossRef]
- Son, Y.L.; Ubuka, T.; Millar, R.P.; Kanasaki, H.; Tsutsui, K. Gonadotropin-inhibitory hormone inhibits GnRH-induced gonadotropin subunit gene transcriptions by inhibiting AC/cAMP/PKA-dependent ERK pathway in LβT2 cells. Endocrinology 2012, 153, 2332-2343. [CrossRef]
- Granata, R.; Settanni, F.; Trovato, L.; Gallo, D.; Gesmundo, I.; Nano, R.; Gallo, M.P.; Bergandi, L.; Volante, M.; Alloatti, G.; et al. RFamide peptides 43RFa and 26RFa both promote survival of pancreatic beta-cells and human pancreatic islets but exert opposite effects on insulin secretion. Diabetes 2014, 63, 2380-2393. [CrossRef]
- Benton, N.A.; Russo, K.A.; Brozek, J.M.; Andrews, R.J.; Kim, V.J.; Kriegsfeld, L.J.; Schneider, J.E. Food restriction-induced changes in motivation differ with stages of the estrous cycle and are closely linked to RFamide-related peptide-3 but not kisspeptin in Syrian hamsters. Physiol Behav 2018, 190, 43-60. [CrossRef]
- Klingerman, C.M.; Williams, W.P., 3rd; Simberlund, J.; Brahme, N.; Prasad, A.; Schneider, J.E.; Kriegsfeld, L.J. Food Restriction-Induced Changes in Gonadotropin-Inhibiting Hormone Cells are Associated with Changes in Sexual Motivation and Food Hoarding, but not Sexual Performance and Food Intake. Front Endocrinol (Lausanne) 2011, 2, 101. [CrossRef]
- Lynn, S.E.; Perfito, N.; Guardado, D.; Bentley, G.E. Food, stress, and circulating testosterone: Cue integration by the testes, not the brain, in male zebra finches (Taeniopygia guttata). Gen Comp Endocrinol 2015, 215, 1-9. [CrossRef]
- Tang, F.; Hsieh, A.C.; Lee, C.P.; Baconshone, J. Interaction of cold and starvation in the regulation of plasma corticosterone levels in the male rat. Horm Metab Res 1984, 16, 445-448. [CrossRef]
- Pirke, K.M.; Spyra, B. Catecholamine turnover in the brain and the regulation of luteinizing hormone and corticosterone in starved male rats. Acta Endocrinol (Copenh) 1982, 100, 168-176. [CrossRef]
- Palmblad, J.; Levi, L.; Burger, A.; Melander, A.; Westgren, U.; von Schenck, H.; Skude, G. Effects of total energy withdrawal (fasting) on thelevels of growth hormone, thyrotropin, cortisol, adrenaline, noradrenaline, T4, T3, and rT3 in healthy males. Acta Med Scand 1977, 201, 15-22. [CrossRef]
| Animal models | Injection | Metabolic effects | Ref |
|---|---|---|---|
| Male domestic chicken chick | Single ICV (0.3, 0.9, or 2.6 nmol) | ・Increased food intake at 1 and 2 h | [75] 2005 |
| Single ICV of anti-GnIH antiserum | ・Reduction in food deprivation-induced feeding ・No effect on ad libitum feeding |
||
| Male domestic chicken chick | Single ICV (0.9, 2.6, or 7.8 nmol) | ・Increased food intake at 0.5 to 2 h ・No effect on water intake ・Increased feeding pecks at 5 min to 30 min |
[78] 2014 |
| Adult male Pekin duck | Single ICV (100 ng) | ・Increased food intake at 2 h | [77] 2013 |
| Adult male mouse | Single ICV (25, 50, or 100 ng) | ・Increased food intake at 0.5, 1, 2 h | [76] 2012 |
| Adult male mouse | ICV (6 nmol/day) for 13 days | ・Increased food intake, BW, BAT and liver mass ・No effects on WATs and muscle mass ・Decreased O2/CO2 metabolism, energy expenditure, and core body temperature1 ・No effects on locomotor activity |
[79] 2020 |
| SD-adapted male hamster | ICV (8.25 pmol/h) for 5 weeks | ・Increased BW and food intake ・Increased circulating insulin and leptin ・No effects on hypothalamic metabolic genes |
[80] 2019 |
| SD-adapted female hamster | ・No effects on BW, food intake, circulating insulin/leptin, and hypothalamic metabolic genes | ||
| Male hamster | Single ICV (0.5 or 1.5 µg) | ・No effect on food intake regardless of LD or SD | [81] 2020 |
| Female hamster | ・Increased food intake in both LD (0.5 µg) and SD (1.5 µg), and NPY expression at 3 h | ||
| Adult male rat | Single ICV (100 or 500 ng) | ・Increased food intake at 2 h ・No effect on BW at 24 h |
[22] 2007 |
| Adult male rat | ICV (1 µg/h) for 5 days | ・Increased food and water intake ・No effects on whole-body energy expenditure and BAT thermogenesis |
[76] 2012 |
| Adult male rat | Single ICV (50 or 250 pmol) | ・No effect on food intake | [82] 2021 |
| Adult male rat | Intraamygdaloid microinjection of RFRP-1 (37.8 pmol) | ・Decreased liquid food intake over 1 h ・No effect on locomotor activity |
[83] 2012 |
| RF9 (41.4 pmol) + RFRP-1 | ・Prevents RFRP-1 effect on food intake | ||
| Ovariectomized adult female sheep | ICV (40 µg/h) for 4 h | ・Increased food intake at 2, 4 h ・No effects on thermogenesis of muscle and visceral WAT |
[76] 2012 |
| Adult male cynomolgus macaque monkey | ICV (3 µg/kg/h) for 9 days | ・Increased food intake | [76] 2012 |
| Tissue | Subject | Expression of GnIH or GnIH-Rs | Method | Ref | |
|---|---|---|---|---|---|
| Adipose tissue | Human | Tissue | GPR147/GPR74 | RT-PCR | [95] |
| Mature adipocyte | GPR147/GPR74 | Western | |||
| Rat | Tissue | GPR147 | RT-PCR, Western | [96] | |
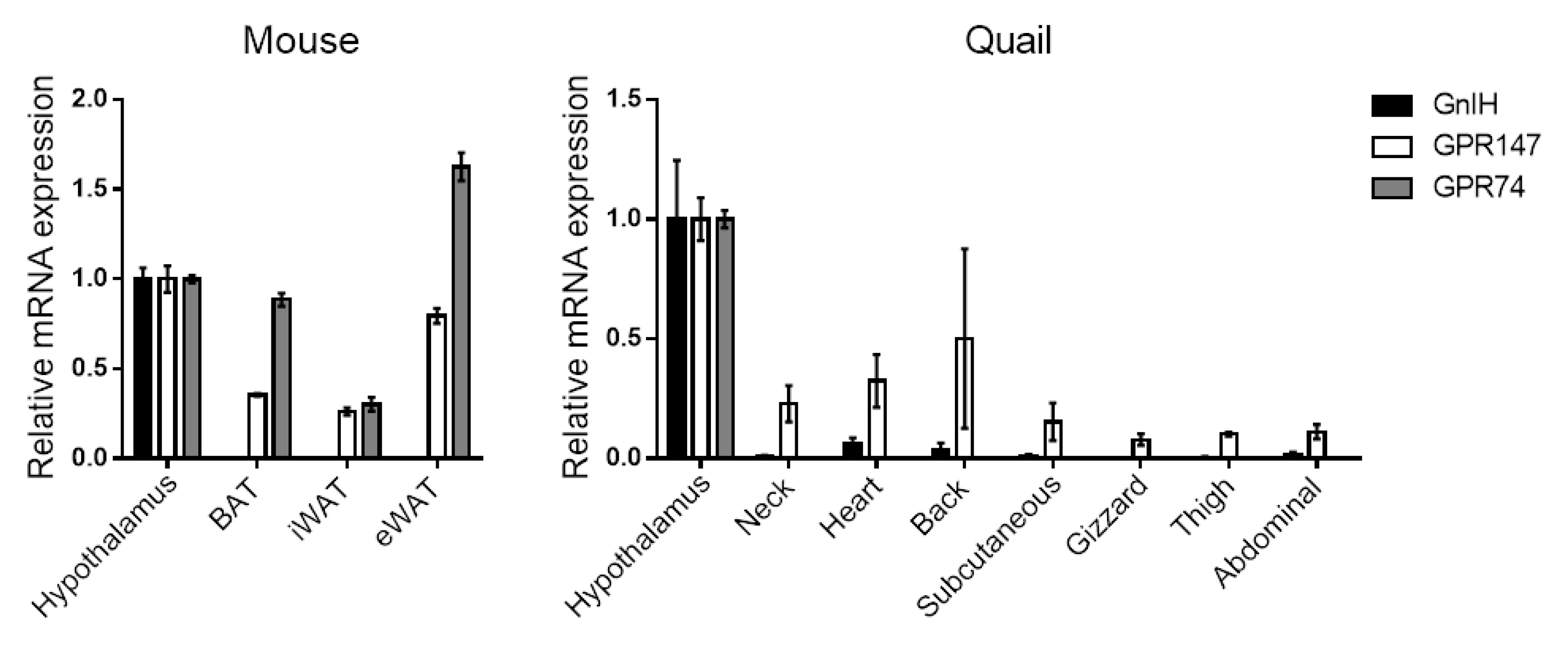
| Mouse | Tissue | GPR147/GPR74 | RT-PCR | Figure 1 | |
| Japanese quail | Tissue | GPR147 | RT-PCR | Figure 1 | |
| Mouse 3T3-L11 | Mature adipocyte2 | GPR147/GPR74 | RT-PCR | [97] | |
| Pancreas | Piglet | Islet | GnIH | Immunostaining | [98] |
| Rat | Tissue | GnIH and GPR147 | RT-PCR, Western, Immunostaining |
[96] | |
| Mouse | Islet | GPR147 | Immunostaining | [99] | |
| Mouse αTC11 | α-cells of islet | GPR147 | RT-PCR, Immunostaining | [99] | |
| GI tract | Piglet | Esophagus, stomach, small and large intestine | GnIH | Immunostaining | [98] |
| Female pig | Intestine | GPR147 | RT-PCR | [100] | |
| Mouse | Stomach, ileum, and colon | GnIH and GPR147 | RT-PCR | [101] | |
| Liver | Rat | Tissue | GPR147 | RT-PCR, Western | [96] |
| Skeletal muscle | Rat | Tissue | GPR147 | RT-PCR, Western | [96] |
| Animal models | IP injection of GnIH | Metabolic effects | Ref. |
|---|---|---|---|
| Female domestic chicken | 30 nmol × twice/day for 14 days | ・Disrupts the physical and chemical barriers of the intestine ・Increased intestinal inflammation |
[105] 2024 |
| Male mouse | 20 ng, 200 ng, or 2 µg/day for 8 days | ・Increased food intake ・Increased BW ・Increased WAT mass |
[102] 2016 |
| Male mouse | 20 µg × twice/day for 21 days | ・Increased food intake and BW ・Increased liver and eWAT mass ・Decreased testis mass ・Glucose intolerance and insulin resistance |
[103] 2022 |
| Rat (male and female mixed population) | 1 or 10 µg × twice/day for 14 days | ・Increased food intake during photophase ・Increased meal frequency ・Increased BW ・Glucose intolerance and insulin resistance ・Increased inflammation in liver, skeletal muscle, or WAT |
[96] 2020 |
| Female piglet | 0.1 or 1 mg × twice/day for 14 days | ・Increased food intake and BW ・Increased organ mass in pancreas, pgWAT, iWAT and liver ・Glucose intolerance ・Altered gene expression in liver, pgWAT, and iWAT related to lipid and glucose-metabolism |
[104] 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





