1. Introduction
Obesity, recognized as a disease in the US by the American Heart Association in 2013, is a growing concern for many countries in the world [
1]. In 2018, one third of all adults in the US were considered obese [
2] and the rates are projected to increase to around 50% by 2030 [
3]. Obese adults and children are at higher risk of developing type 2 diabetes, cardiovascular disease, and several cancers [
4]. With obesity, the imbalance between energy uptake (overeating) and expenditure (reduced activity), causes increased lipid storage in the adipose tissues [
5] and lipid accumulation in non-adipose tissues [
6]. Obesity-induced mitochondrial dysfunction, inflammation, and oxidative stress disrupt metabolism and the functions of other organs [
4,
7,
8,
9,
10]. These smaller disruptions that occur at a cellular and tissue level can impact other organs and tissues on a functional level and lead to comorbidities such as infertility [
11], cardiovascular dysfunction [
12], and the development of non-alcoholic fatty liver disease [
13]. While adipose tissue dysfunction negatively impacts peripheral organ function, the cellular and molecular mechanisms that underlie this association are not well understood. We use the model organism
Drosophila melanogaster to uncover how the adipose tissue communicates to other organs.
Drosophila melanogaster is a robust model system that is widely used to study inter-organ communication [
14]. Many biological pathways are conserved between humans and flies, with around 85% of human-disease causing genes having homologs in
Drosophila [
15,
16]. Additionally, the genetic toolkit of
Drosophila allows tissue- and cell type-specific manipulation of gene expression [
17]. The
Drosophila fat body, composed of adipocytes and hepatocyte-like oenocytes, plays similar energy storage and endocrine roles as the mammalian adipose tissue [
18]. In addition to being used as a model for diet- and genetic-associated obesity [
19,
20], several
Drosophila studies have begun to elucidate the mechanisms employed by the fat body to communicate to other tissues [
21]. Not surprisingly,
Drosophila adipose tissue serves as a central nutrient-sensing depot that relays information about dietary input to the highly nutrient-responsive ovary.
The
Drosophila fat body employs nutrient-sensing pathways, nuclear hormone receptors, nutrient transport proteins, and metabolic enzymes to remotely control the diet-dependent process of oogenesis [
22,
23,
24,
25,
26,
27,
28,
29]. The
Drosophila ovary is made up of 16 to 20 ovarioles, each one being an individual egg-producing unit containing the progressive stages of oocyte development [
30]. The apical most part of the ovariole, the germarium, contains germline stem cells (GSCs) whose undifferentiated state is maintained by adherence to and signaling from cap cells (CCs) [
31]. GSCs divide asymmetrically to self-renew and generate differentiated cystoblasts that divide four times synchronously with incomplete cytokinesis to form a 16-cell germline cyst. A single-cell layer of epithelial follicle cells surrounds each 16-cell cyst that buds from the germarium to form an individual egg chamber. One of the cells in the cyst becomes the oocyte while the remaining 15 cells become nurse cells that support oocyte development. Each ovariole contains six to eight developing egg chambers [
31], with the most developed egg chamber, or mature oocyte, at the posterior end ready for ovulation and fertilization. Previous studies show that GSC maintenance, germline cyst survival, progression through vitellogenesis, and ovulation are regulated by nutrient-sensing pathways, including mechanistic Target of Rapamycin (mTOR), the amino acid response pathway (AAR), insulin/insulin-like growth factor (IIS), and Ras/MAPK signaling [
22,
24,
25]. However, the fat body-derived factors that mediate adipose tissue control of oogenesis are unknown.
In humans, the adipose tissue secretes adipokines, such as leptin, tumor necrosis factors and interleukins to carry out endocrine control of multiple aspects of physiology, such as controlling nutritional intake, insulin sensitivity, and moderating inflammatory responses [
32,
33,
34]. During development in
Drosophila, the fat body secretes a variety of adipokines that control overall growth and developmental timing, similar to human adipocyte signaling [
21,
35,
36]. The nutrient-sensitive neuropeptides, CCHamide-1 (
CCHa1) and CCHamide-2 (
CCHa2), are produced in the gut and fat body [
37].
CCHa1 interacts with the anterior dorsal neuron and the pigment dispersing factors in the brain to regulate sleep cycles [
38] and stabilize circadian behavioral rhythms [
39].
CCHa2 binds to its receptor in the brain (
CCHa2R) to promote
Drosophila insulin-like peptide (dILP) production [
40], with mutations in
CCHa2 and
CCHa2R resulting in larval growth defects [
40].
Drosophila tumor necrosis factor, eiger (
egr), is released from the fat body in response to starvation [
41]. During starvation,
egr binds to its receptor Grindlewald, expressed in the brain, to suppress
dILP expression [
41] and to Wengen, expressed in the gut enterocytes to restrict lipid catabolism and maintain tissue homeostasis [
42]. Growth-blocking peptides (
Gbp) regulate immune responses [
43] and stimulate
dILP production by acting on insulin producing cells (IPCs) in the brain. Knockdown of
Gbps in the larval fat body led to smaller sized adults [
43]. Unpaired 2 (
upd2) is secreted from the fat body in response to dietary fats and sugars [
44]. Similar to human leptin,
Drosophila upd2 is upregulated with elevated fat stores and is downregulated during reduced nutritional conditions [
45,
46].
Upd2 regulates how much insulin is released into the circulation from the fly IPCs [
47]. Given their role in controlling growth during larval development, we proposed that these adipokines may also mediate fat body control of oogenesis during adulthood. Here we show that the adult fat body expresses
CCHa1,
CCHa2,
egr,
Gbp3, and
upd2. Adipocyte-specific knockdown of these adipokines leads to a general increase in triglyceride storage. We find that none of these adipokines are required for proper GSC maintenance. However, our evidence indicates that
egr,
upd2, and
CCHa1 are important for germline survival while
Gbp3 promotes ovulation. This work identifies fat-derived factors that modulate specific steps of oogenesis, thus filling in the knowledge gap of how the adipose tissue relays information to other organs.
2. Materials and Methods
2.1. Drosophila Strains and Culture Conditions
Fly lines used for this study were obtained from the Bloomington
Drosophila Stock Center (BDSC). The following transgenic fly lines were used for these series of experiments: RFP-RNAi (#31417),
CCHa1-RNAi (#57562),
CCHa2-RNAi (#57183),
egr-RNAi (#55276 and #58993),
upd2-RNAi (#33949 and #33988), and
Gbp3-RNAi (#64108). We used the driver line
tubPGal80ts;Lsp2(3.1)Gal4/TM6B for temperature sensitive adipocyte specific knockdown [
22] as well as the w
1118 line to stimulate oogenesis with our selected progeny. Canton S and Oregon R fly lines were used to determine adipokine presence in adult fat. Extended stocks were kept at room temperature (20-25°C) on a molasses medium (Archon Scientific) and flipped weekly.
2.2. Adipocyte Specific Manipulation of Gene Expression
Crosses were set up in triplicate, with each bottle containing 20 to 30 virgin adult female tubPGal80ts;3.1Lsp2-Gal4/TM6B flies and 10 adult male UAS-transgene-RNAi flies. UAS RFP-RNAi was used as a control in each cross. Flies were kept on a molasses medium diet supplemented with wet yeast. Crosses were kept in an 18°C incubator and flipped every 4 days until progeny emerged. Virgin adult female progeny with both UAS-target-RNAi and tubPGal80ts;Lsp2(3.1)Gal4 were isolated using the balancers as a guide. w1118 male adult flies were added to target female progeny to stimulate oogenesis and kept at 18°C for four days to clear larval fat before moving to 29°C incubator for 10 days for adipokine knockdown in the adipocytes.
2.3. Ovary Immunostaining and Fluorescence Microscopy
After 10 days of transgene expression, ovaries from adult target female flies were dissected in PBS. Ovaries were fixed in 5.3% paraformaldehyde (PFA) (Electron Microscopy Sciences) in PBS for 13 minutes. The ovaries were rinsed twice in 0.5% Triton-X (VWR Life Sciences) in PBS (PBT) and washed three times on a nutator for 15 minutes. Samples were placed in a blocking solution (5% Bovine Serum Albumin, 5% Normal Goat Serum, and 0.5% Trition-X in PBS) and incubated for at least 24 hours. After blocking, primary antibodies mouse alpha spectrin (2 µg/ml, Developmental Studies Hybridoma Bank (DHSB)), mouse anti-Lamin C (2 µg/ml DSHB), and rabbit anti-cleaved Dcp-1 (1:250, Cell Signaling Technology) were diluted in blocking buffer and added to the samples. After an overnight incubation, the primary antibody solution was removed, and the samples were washed three times for 10 minutes each in PBT. A secondary antibody solution containing Alexa-Fluor anti-mouse 488, Alexa-Fluor anti-rabbit 568 and blocking solution were added to each sample (1:250 each) and incubated for two hours shielded from light. Once the secondary antibody solution was removed the samples were washed 3 times in PBT for 15 minutes each protected from the light. After the last wash, all the PBT was removed and the samples were stored in Vectashield with 4’,6-diamidino-2-phenylindole (DAPI). Ovaries were mounted onto glass slides with glass coverslips before analyzing on a confocal microscope.
2.4. Adipocyte Immunostaining and Fluorescence Microscopy
Abdominal carcasses were collected from adult female target progeny after 10 days of transgene activation. The gut, ovaries, and malpighian tubes were removed from the carcasses, leaving only the fat body attached to the carcass. Insect dissection pins (Austerlitz) were used to anchor the corners of the carcasses to the bottom of a Sylgard (DOW Chemical) coated twelve well tissue culture dish. The carcasses were fixed for 20 minutes in 5.3% PFA in PBS. At room temperature, samples were rinsed twice and washed three times for 15 minutes in 0.1% Tween-20 in PBS (PBT). Abdominal carcasses were incubated overnight in blocking solution (5% NGS, 5% BSA, 0.1% PBT in PBS). The carcasses were then incubated with 3 µg/ml mouse anti-alpha spectrin (DSHB) diluted in blocking solution overnight. Samples were then washed three times for 15 minutes in 0.1% PBT before a 2-hour incubation period protected from light with anti-mouse Alexa-Fluor 568 diluted in blocking solution (1:250). To visualize lipid droplets, samples were washed three times in 0.1% PBT before a 30-minute incubation with BODIPY 505/510 (25 ng/ml) at room temperature and protected from light. Samples were then washed with 0.1% PBT and abdominal carcasses were stored in Vectashield containing DAPI. Fat body tissues were removed from the abdominal carcass onto slides prior to imaging.
2.5. Ovarian Analysis
All ovarian analysis was done using a Zeiss LSM 800 confocal microscope using ZEN 2.6 software. Using 63x magnification, cap cells (CC) were counted using nuclear morphology and Lamin C staining. Germline stem cells (GSC) were counted using alpha spectrin labeling of the fusome. Dcp-1+ germaria were counted based on the presence of Dcp-1 in any of the cysts present in the germaria. GSC, CC, and Dcp-1+ germaria were counted at 0 and 10 days of transgene expression. At least 3 biological replicates were analyzed with 50 to 150 germaria counted per timepoint, per sample in each replicate. Rates of GSC loss were statistically verified using two-way ANOVA with interaction (GraphPad Prism 8). Shifts in rates of Dcp-1+ germaria from the control were analyzed using a student’s t-test for statistical significance between the control and transgene samples. A 20x magnification was used to analyze the presence of dying vitellogenic follicles in the ovary samples. The total number of complete ovarioles were counted and the total number of ovarioles that contained Dcp-1 positive staining or pyknotic nuclei at or after stage 8 of vitellogenesis was recorded. The percentages of dying vitellogenic follicles (DVF) in each transgene group were tabulated and analyzed using a student’s T test against the control group.
2.6. Blocked Ovulation Analysis
15 to 20 adipokine knockdown progeny after 4 days of incubation in 18°C were placed into vials with male w1118 flies in molasses media supplemented with wet yeast. Flies were then placed in a 29°C incubator and flipped daily. After the tenth day, the ovaries were dissected from the female progeny and analyzed for blocked ovulation. An ovary was considered blocked if it had more than one stage 14 egg chamber present in any of the ovarioles. A percentage was made using the number of blocked ovaries against the total number of ovaries counted. A Student’s t-test was used to determine if there were statistically significant changes between the transgene samples and the control.
2.7. Measurement of Adipocyte and Lipid Droplet Size
Using the confocal microscope, z-stack images were taken from fat body samples to capture multiple images at 1 µm depth for adipocyte size and lipid droplet size analysis. Using ZEN Blue lite 2.8 software and ImageJ, measurements were taken using a stylus for the largest areas present in the fat body according to the alpha spectrin staining. Around 10-15 measurements were taken per fat body for a total of around 50-150 measurements per sample analyzed. The measurements were averaged, and a Student’s T test was used to determine if there were any statistical differences between the control group and the adipokine knockdown groups. ImageJ was also used to determine lipid droplet size for each of the fat bodies analyzed. The lipid droplets were made visible by the BODIPY staining. Each lipid droplet per sample was measured by automated segmentation using the threshold selection method in ImageJ. The background of each image was subtracted, and the threshold was determined based on a setting that resembled the edges of the lipid droplets. The total measurements were averaged in each biological replicate, and a Student’s T test was done to determine if the distribution between the control group and the adipokine knockdown groups were statistically significant.
2.8. Adipocyte Bradford and Triglyceride Assays
After 10 days of transgene expression, the abdominal carcasses of control and transgene female progeny were dissected. Carcasses were placed in a 1.5 ml microcentrifuge tube and submerged in a triglyceride lysis buffer (140 mM NaCl, 50 mM Tris-HCl, 0.1% Triton-X in water with protease inhibitor cocktail [Research Products International] added). A pestle was used to grind carcasses in lysis solution. Samples were then centrifuged, and the supernatant was removed and placed into a clean, labeled microcentrifuge tube. The Bradford assay and triglyceride assay was done according to the VWR Bradford Method Protein Assay kit (M173-KIT) and Stanbio Liquicolor Triglycerides Kit (STANBIO 2100-225). Measurements were taken in triplicate using SoftMaxPro 6.4 software on a Spectramax i3 Plate Reader. For each biological replicate, all values were converted to µg/ml. Triglyceride to Protein ratios were made by dividing the amount of triglycerides by the amount of protein. Fold change was calculated by dividing the triglyceride/protein ratio of each transgenic sample to the ratio of its RFP-RNAi control for that biological replicate. A Student’s T test was used to determine if the fold changes from the transgenic groups were statistically significant from the control groups.
2.9. RNA Isolation, RT-PCR, and qPCR
After 10 days of transgene expression at 29°C, abdominal carcasses were dissected, in PBS, the ovary, gut and malpighian tubes removed from the carcass. Carcasses with fat bodies attached were stored in RNA Shield (Zymo Research). RNA was isolated from each sample using the Quick RNAi Mini-Prep kit (Zymo Research). 100 ng of RNA was used to synthesize cDNA using the Verso cDNA synthesis Kit (ThermoFisher Scientific). RNA and cDNA quantity and A260/A280 quality was measured using Smartdrop L spectrophotometer (Accuris Instruments). Primers were derived from previously published RT-PCR and qPCR work and FlyPrimerBank (
Table S1). Canton S, IV, and Oregon R adult fat body cDNA samples were used for RT-PCR quantification of adipokine presence in adult fat. RT-PCR quantification was done using Econotaq PLUS GREEN (Lucigen) on an iCycler Thermocycler (Bio-Rad). Alpha tubulin primers were used as a control. Knockdown efficiency was quantified using SYBR Green reagents (PowerTrack SYBR Green Master Mix – Applied Biosciences) and a Quantstudio 3 Real-Time PCR System (Applied Biosystems, Thermofisher Scientific) Relative quantification of at least three biological replicates was done using the comparative Ct method. A Student’s T-test was used to determine the statistical significance between the knockdown samples to the control samples.
4. Discussion
A substantial body of work provides evidence that the fat body relays nutritional information to the ovary [
22,
23,
24,
25,
26,
27,
28]. While the fat body secretes many factors [
53], the identity of fat body-derived factors that communicate to the ovary are unknown. We took clues from studies of larval development that have identified several adipokines shown to regulate overall growth and postulated that
CCHa1,
CCHa2,
egr,
Gbp3, and
upd2 might mediate fat-to-ovary communication observed in adult females. Using the
Gal80ts/Gal4/UAS gene expression system to knockdown each adipokine in adult adipocytes, we find that all these adipokines, excluding
CCHa2, have distinct roles in controlling specific nutrient-sensitive stages of oogenesis. None of the adipokines are required for GSC maintenance (
Figure 3) and
CCHa2 did not play a role in any of the stages of oogenesis examined. However,
egr and
upd2 regulate survival of early germline cysts, and along with
CCHa1 regulate survival of vitellogenic egg chambers (
Figure 4). Lastly, we find the adipocyte-derived
Gbp3 specifically controls ovulation (
Figure 5). This work continues to advance understanding of how the fat body remotely controls oogenesis, by uncovering adipokines that regulate several aspects of oogenesis.
4.1. Adipocyte to Ovary Communication Is Maintained by Multiple Pathways, with No Single Adipokine Controlling Ovarian Homeostasis
We have observed from earlier work that the ovary is very sensitive to nutritional changes. Depending on the diet fed to flies, the amount of egg production varies. High sugar diets cause
Drosophila to lay less eggs over time and increase the number of dying vitellogenic follicles [
54]. The changes in diet are facilitated by nutrient signaling pathways, such as insulin and insulin like growth factor signaling, amino acid sensing, and TOR signaling [
55]. Knockdown of components of these pathways such as the insulin receptor (InR) [
24] and the Ras/MAPK pathway components [
25] led to significant increases in cell death in the germaria.
egr,
upd2,
CCHa2, are adipokines that communicate with their own respective receptors in the brain to control the amount of dILP secretion into the hemolymph [
40,
41,
43,
45]. Based on our data, no one adipokine is responsible for maintaining ovary stem cell homeostasis, but a reduction in the amount present in the fat body through genetic manipulation can exacerbate cell death in the ovary (
Figure 4). There are multiple signaling pathways that interact with the IPCs in the
Drosophila brain to regulate metabolism and energy storage. Each target knockdown sample only targeted one adipokine, and the presence of other nutrient sensing adipokines may compensate for the lack of one. A
Drosophila model that removes two or more of our targeted adipokines from the fat would give more information as to the compensatory nature of these multiple nutrient sensing pathways. Another genetic system of transgene regulation such as CrispR/Cas9 [
56] may need to be used to completely remove a specific adipokine from the fat body, as knockout experiments may highlight which adipokines are essential to ovarian regulation or if a particular gene is part of a sequence of interactions needed to relay nutrient status.
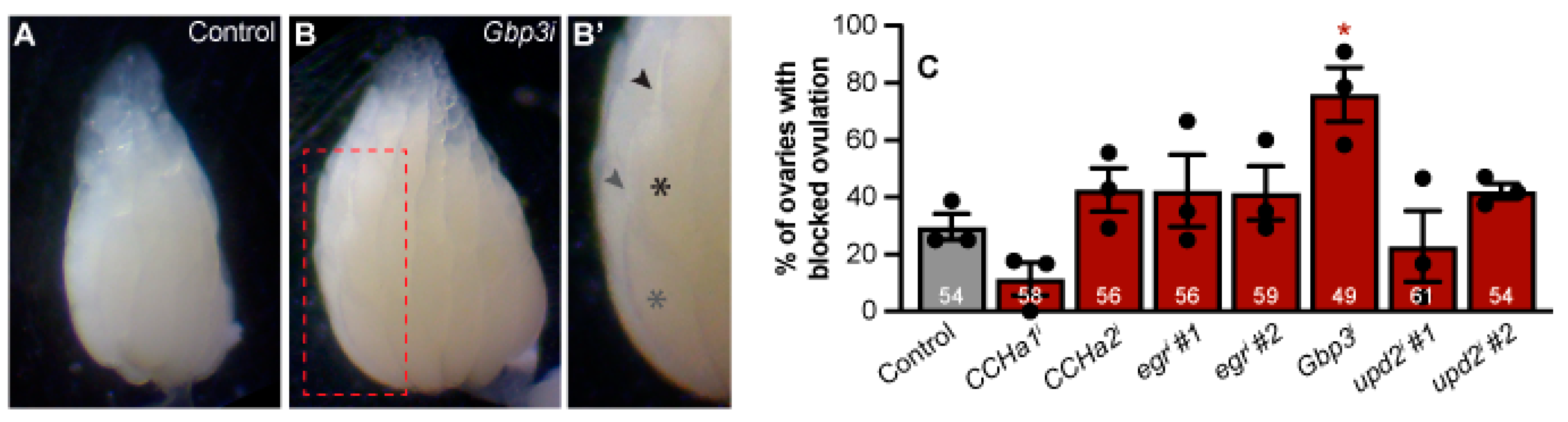
4.2. Gbp3 May Have a Distinct Role for Ovulation Outside of Stem Cell Maintenance
Gbp3, a homolog of
Gbp1, has not been intensively studied.
Gbp3 knockdown in larval fat resulted in smaller body sizes by weight in adult male and female flies [
43]. We observed an increase in adipocyte size and lipid content in
Gbp3 adipocyte knockdown female flies, but that may not account for size reductions in the other parts of the fly’s physiology under adipocyte knockdown. When comparing the rates of blocked ovulation of the adipokine knockdown targets,
Gbp3 was the most drastic and statistically significant (
Figure 5C). Physiological size assessments as well as tests into the signaling networks that regulate proper ovulation will need to be done to determine the cause of the blocked ovulation. The increased blocked ovulation may indicate that
Gbp3 levels may influence ovulation on a neuronal or physiological level distinct from overall metabolic status.
Gbp1 and
Gbp2 will also need to be investigated to determine if the same patterns of blocked ovulation are present or if
Gbp3 is unique in this aspect.
4.3. Partial Knockdown of Target Adipokines from the Fat May Not Be Enough to Influence Ovarian Homeostasis
The knockdown of the nutrient signaling adipokines from the fat led to an increase in triglyceride levels, but did not cause any significant changes to the morphology of the adipocyte. On average, all adipokine target knockdowns led to increases in triglyceride/protein ratio when compared to the control (
Figure 2D). For some targets like
upd2, this is unusual, as
upd2 is secreted by the fat body in response to dietary fat and sugar.
upd2 activates JAK/STAT signaling in GABAergic neurons, to stop inhibition of the IPCs, which then secrete dILPs to promote growth and fat storage [
44,
45,
46].
upd2 reduction in the fat would slow IPC dILP production, which should result in smaller flies with less triglyceride levels [
44]. Our fly model was a temperature sensitive, RNAi mediated knockdown, instead of a homozygous deletion mutant [
44], so residual
upd2 expression and additional unperturbed nutrient signaling pathways may stabilize body size.
Nutrient sensing is vital for maintaining growth and energy storage in both humans and in Drosophila. Understanding how adipocytes signal nutrient status to other tissues can improve clinical research and spur developments and treatments for obesity and its associated comorbidities. Through this work we examined how reductions of key signaling adipokines in the fat can alter the maintenance and physiology of the ovary. Not one single adipokine studied was crucial for maintaining ovarian homeostasis, but the reduction of them led to increased cell death on average. Future studies should be directed at determining if there are compensatory mechanisms in nutrient signaling. We also observed that Gbp3 adipocyte specific knockdown may destabilize egg laying processes and block ovulation. Investigating Gbp1 and Gbp2 adipocyte specific knockdown’s effect on the ovary may give more insight as to how those adipokines modulate ovarian processes outside of nutrient status.
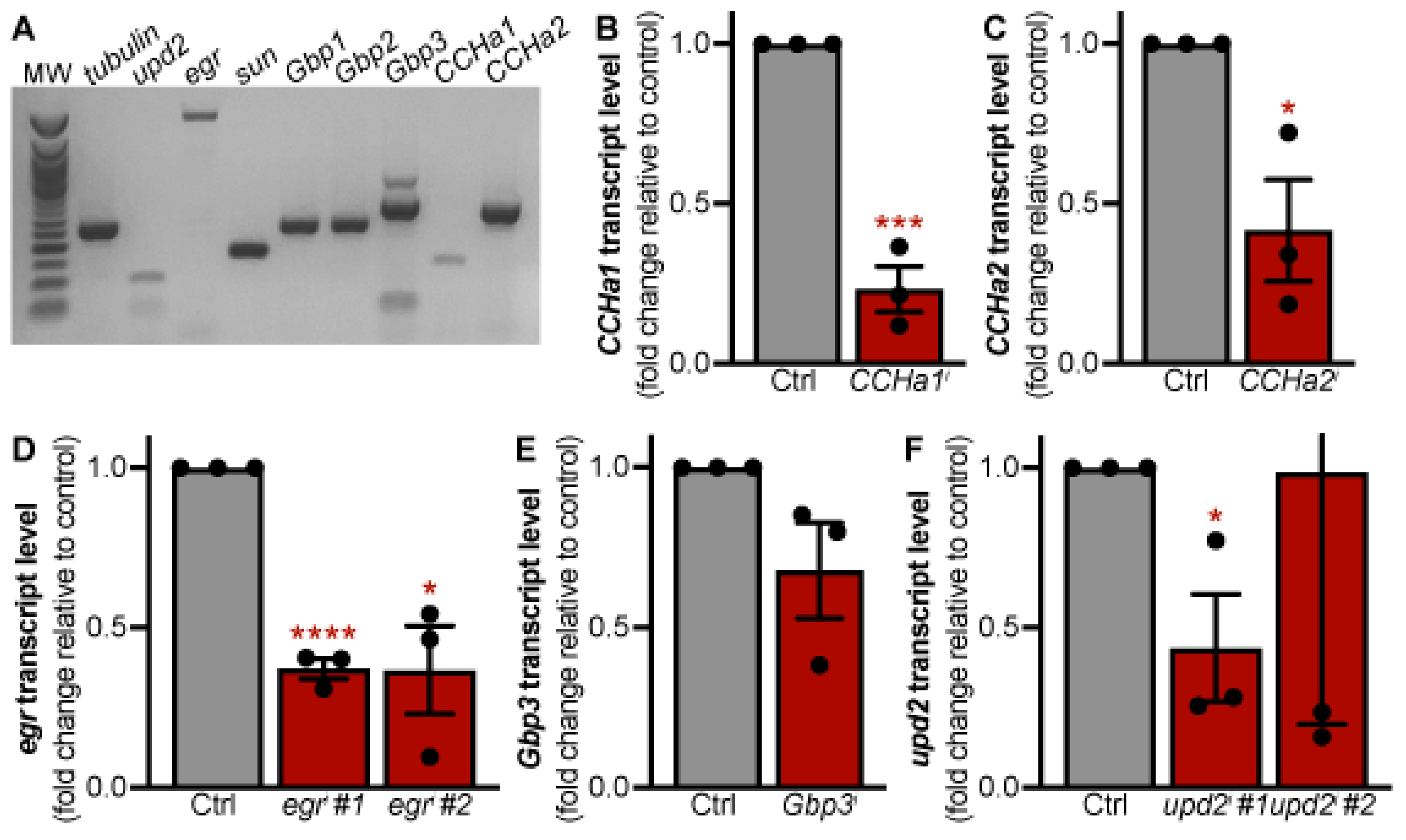
Figure 1.
RNAi-mediated knockdown of adipokines expressed in adult adipocytes. (A) RT-PCR analysis of larval adipokine expression in adult female fat bodies (MW = molecular weight ladder; tubulin used as a loading control). (B-F) qRT-PCR analysis of mRNA expression in fat body tissue from females with RNAi-mediated knockdown of CCHa1 (B), CCHa2 (C), egr (D), Gbp3 (E), and upd2 (F) compared to an RFP-RNAi control (Ctrl). Data shown as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 Student’s, two-tailed t-test.
Figure 1.
RNAi-mediated knockdown of adipokines expressed in adult adipocytes. (A) RT-PCR analysis of larval adipokine expression in adult female fat bodies (MW = molecular weight ladder; tubulin used as a loading control). (B-F) qRT-PCR analysis of mRNA expression in fat body tissue from females with RNAi-mediated knockdown of CCHa1 (B), CCHa2 (C), egr (D), Gbp3 (E), and upd2 (F) compared to an RFP-RNAi control (Ctrl). Data shown as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 Student’s, two-tailed t-test.
Figure 2.
CCHa1, CCHa2, egr, Gbp3, and upd2 control lipid storage but not adipocyte size. (A) Representative image of adipocytes from females labeled with alpha spectrin (magenta, cell membranes), BODIPY (yellow, lipid droplets), and DAPI (cyan, nuclei). (B) The percentage of adipocytes within small (100-400 bin), medium (500-1000), and large (greater than 1100) size bins measured in arbitrary units. Total number of adipocytes measured per sample indicated at the bottom of each bar. Error bars represent the SEM across biological replicates for each bin. No statistical significance observed between the controls and target knockdown sample bins as assessed via ordinary one-way ANOVA with multiple comparisons (each knockdown condition compared to control). (C) The percentage of lipid droplets within very small (less than 10), small (10-20), medium (30-50), large (60-90), and very large (greater than 100) size bins measured in arbitrary units. Error bars represent the SEM across biological replicates for each bin. No statistical significance observed between the controls and target knockdown sample bins as assessed via ordinary one-way ANOVA with multiple comparisons (each knockdown condition compared to control). (D) Fold change triglyceride to protein ratio relative to control for each adipokine knockdown from five biological replicates (individual data points). Data represented as mean ± SEM. *p < 0.05, **p < 0.01, Student’s two-tailed t-test.
Figure 2.
CCHa1, CCHa2, egr, Gbp3, and upd2 control lipid storage but not adipocyte size. (A) Representative image of adipocytes from females labeled with alpha spectrin (magenta, cell membranes), BODIPY (yellow, lipid droplets), and DAPI (cyan, nuclei). (B) The percentage of adipocytes within small (100-400 bin), medium (500-1000), and large (greater than 1100) size bins measured in arbitrary units. Total number of adipocytes measured per sample indicated at the bottom of each bar. Error bars represent the SEM across biological replicates for each bin. No statistical significance observed between the controls and target knockdown sample bins as assessed via ordinary one-way ANOVA with multiple comparisons (each knockdown condition compared to control). (C) The percentage of lipid droplets within very small (less than 10), small (10-20), medium (30-50), large (60-90), and very large (greater than 100) size bins measured in arbitrary units. Error bars represent the SEM across biological replicates for each bin. No statistical significance observed between the controls and target knockdown sample bins as assessed via ordinary one-way ANOVA with multiple comparisons (each knockdown condition compared to control). (D) Fold change triglyceride to protein ratio relative to control for each adipokine knockdown from five biological replicates (individual data points). Data represented as mean ± SEM. *p < 0.05, **p < 0.01, Student’s two-tailed t-test.
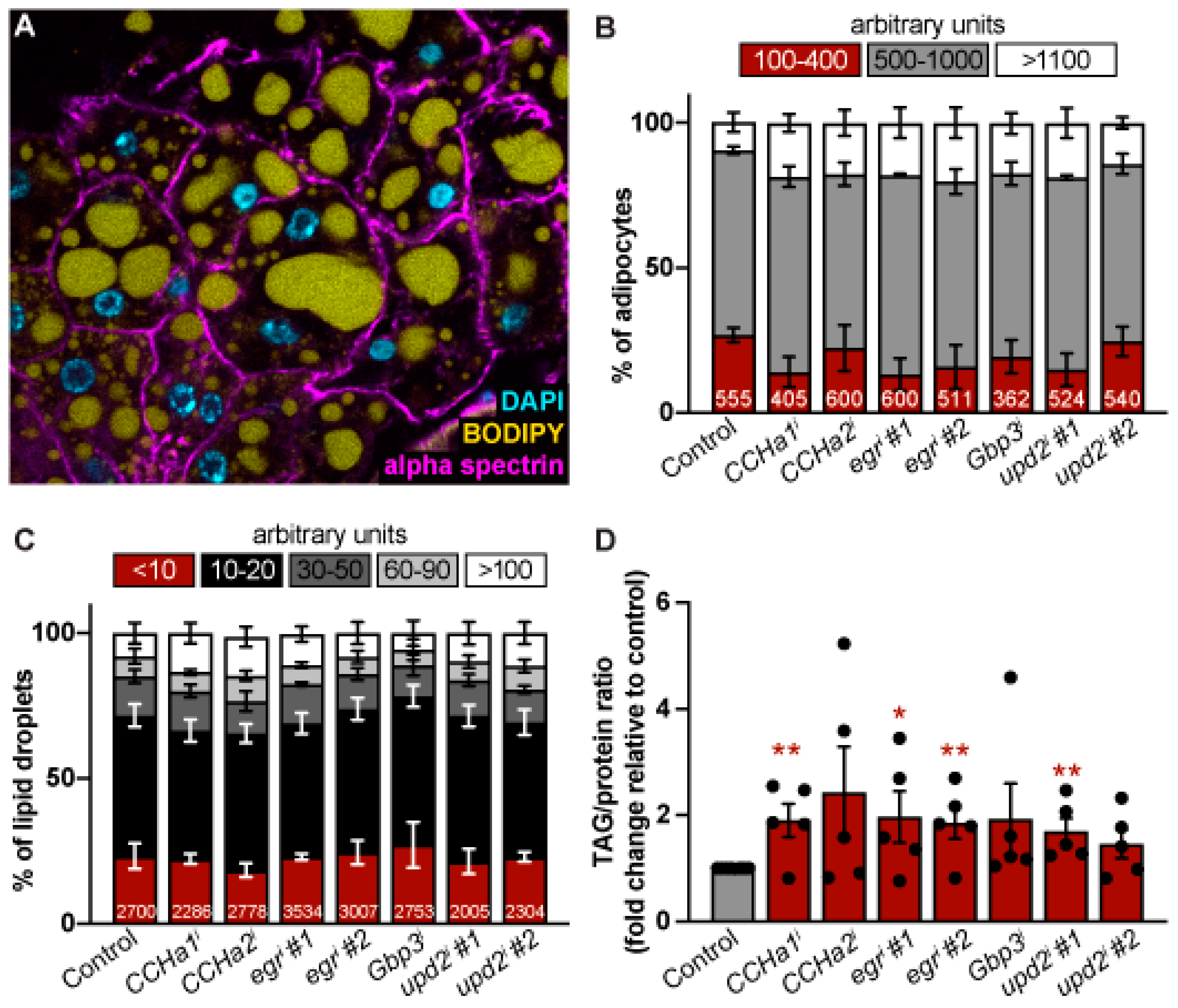
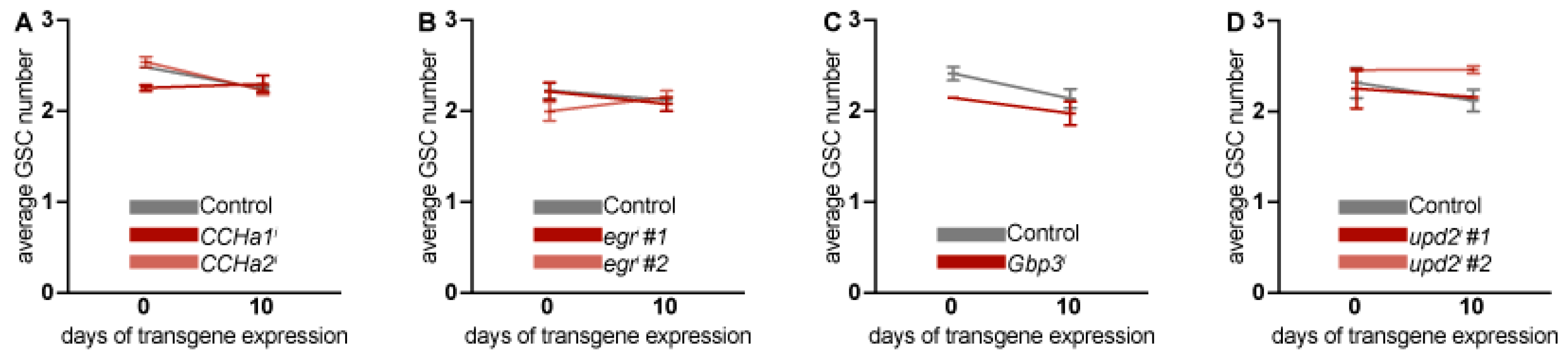
Figure 3.
Adult adipocyte-specific knockdown of
CCHa1,
CCHa2,
egr,
Gbp3, and
upd2 does not affect ovarian GSC number. The average number of GSCs were counted in ovaries from the control group and from adipocyte-specific RNAi groups
CCHa1i and
CCHa2i (A),
egri (B),
Gbp3i (C), and
upd2i (D). Data represented as mean ± SEM for two to seven biological replicates. The total number of germaria counted for 0 and 10 days of transgene expression are given in
Table S7. No statistically significant differences in GSC number were observed using two-way ANOVA with interaction.
Figure 3.
Adult adipocyte-specific knockdown of
CCHa1,
CCHa2,
egr,
Gbp3, and
upd2 does not affect ovarian GSC number. The average number of GSCs were counted in ovaries from the control group and from adipocyte-specific RNAi groups
CCHa1i and
CCHa2i (A),
egri (B),
Gbp3i (C), and
upd2i (D). Data represented as mean ± SEM for two to seven biological replicates. The total number of germaria counted for 0 and 10 days of transgene expression are given in
Table S7. No statistically significant differences in GSC number were observed using two-way ANOVA with interaction.
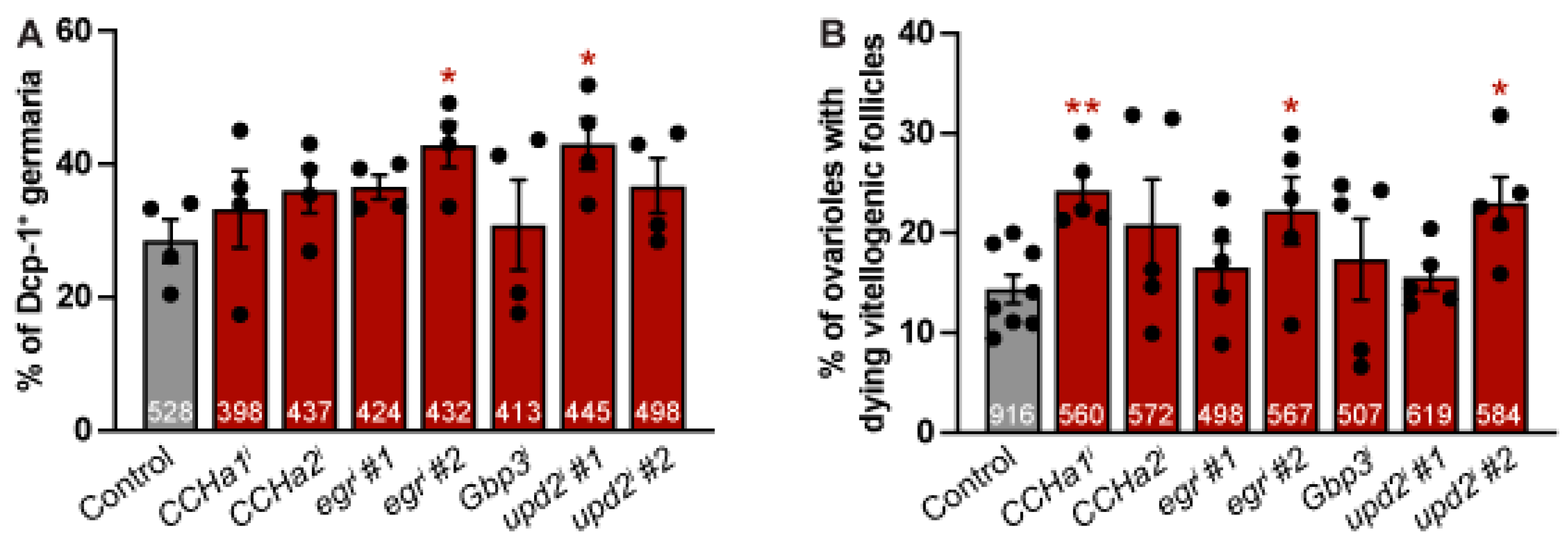
Figure 4.
Adipocyte-derived CCHa1, egr, and upd2 promote germline survival at nutritional checkpoints. (A) Percentage of germaria with Dcp-1 immunoreactivity in the germline after 10 days of adipokine knockdown. The total number of germaria analyzed over four independent trials indicated at the bottom of each bar. Data shown as mean ± SEM. *p < 0.05, Student’s two-tailed t-test (B) Percentage of ovarioles with dying vitellogenic follicles based on Dcp-1 positive immunoreactivity after 10 days of adipokine knockdown. The number of ovarioles examined over five independent trials are shown at the bottom of each bar. Data shown as mean ± SEM. *p < 0.05, **p < 0.01, Student’s two-tailed t-test.
Figure 4.
Adipocyte-derived CCHa1, egr, and upd2 promote germline survival at nutritional checkpoints. (A) Percentage of germaria with Dcp-1 immunoreactivity in the germline after 10 days of adipokine knockdown. The total number of germaria analyzed over four independent trials indicated at the bottom of each bar. Data shown as mean ± SEM. *p < 0.05, Student’s two-tailed t-test (B) Percentage of ovarioles with dying vitellogenic follicles based on Dcp-1 positive immunoreactivity after 10 days of adipokine knockdown. The number of ovarioles examined over five independent trials are shown at the bottom of each bar. Data shown as mean ± SEM. *p < 0.05, **p < 0.01, Student’s two-tailed t-test.
Figure 5.
Gbp3 in adipocytes promotes ovulation. (A-B’) Representative stereomicroscope images of ovaries from controls (A) and Gbp3 knockdown (B) females. (B’) Region highlighted by the dotted outline in B blown up, labeling two oocytes (asterisks) in one ovariole and their corresponding dorsal appendages (arrowheads). (C) Percentage of ovaries showing blocked ovulation. The total number of ovarioles counted over three biological replicates indicated inside each bar. Data shown as mean ± SEM. *p < 0.05, Student’s two-tailed t-test.
Figure 5.
Gbp3 in adipocytes promotes ovulation. (A-B’) Representative stereomicroscope images of ovaries from controls (A) and Gbp3 knockdown (B) females. (B’) Region highlighted by the dotted outline in B blown up, labeling two oocytes (asterisks) in one ovariole and their corresponding dorsal appendages (arrowheads). (C) Percentage of ovaries showing blocked ovulation. The total number of ovarioles counted over three biological replicates indicated inside each bar. Data shown as mean ± SEM. *p < 0.05, Student’s two-tailed t-test.