1. Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is a serious neurological event characterized by high mortality and significant neurological disability. Systemic inflammatory responses following hemorrhagic stroke may play an important role in mediating tissue damage both intracranially and extracranially. Biological responses to various types of invasion are intricately regulated by the immune, hormonal, and central nervous systems, and there is now considerable research interest in monitoring of cytokines as markers of the systemic response to inflammation.
In patients who have undergone surgery for SAH, secretion of corticotropin-releasing hormone from the hypothalamic paraventricular nucleus is believed to contribute to an immunosuppressive state in the postoperative period by promoting the production of adrenocorticotropic hormone and glucocorticoids. This, in turn, has been reported to lead to a decrease in the numbers of natural killer cells and T lymphocytes within the first week after surgery [
1,
2]. Moreover, several studies have found that the levels of cytokines in cerebrospinal fluid (CSF) are elevated in patients with encephalitis or traumatic brain injury [
3,
4,
5,
6,
7]. Similar findings have been reported in patients who have undergone surgery for SAH [
8,
9,
10,
11,
12]. The interleukin-6 (IL-6) level in CSF has been suggested to correlate with a poor prognosis and could serve as a useful biomarker of inflammation for prediction of clinical outcomes [
13,
14,
15].
Anti-inflammatory agents have been reported to improve the prognosis of SAH. In terms of surgery, endovascular treatment (coil embolization) has been reported to be superior to craniotomy (clipping) for aneurysmal SAH [
16,
17]. Craniotomy may be more invasive than endovascular treatment because of the need for direct manipulation of brain tissue, which could trigger production of greater amounts of cytokines. In this study, we investigated the postoperative levels of IL-6 in CSF in patients with aneurysmal SAH according to the type of surgery performed and their relationship with postoperative outcomes and cerebral vasospasm. This is the first study to highlight the clinical significance of IL-6 levels in CSF when stratified by type of surgery and the importance of selection of an appropriate surgical approach.
2. Results
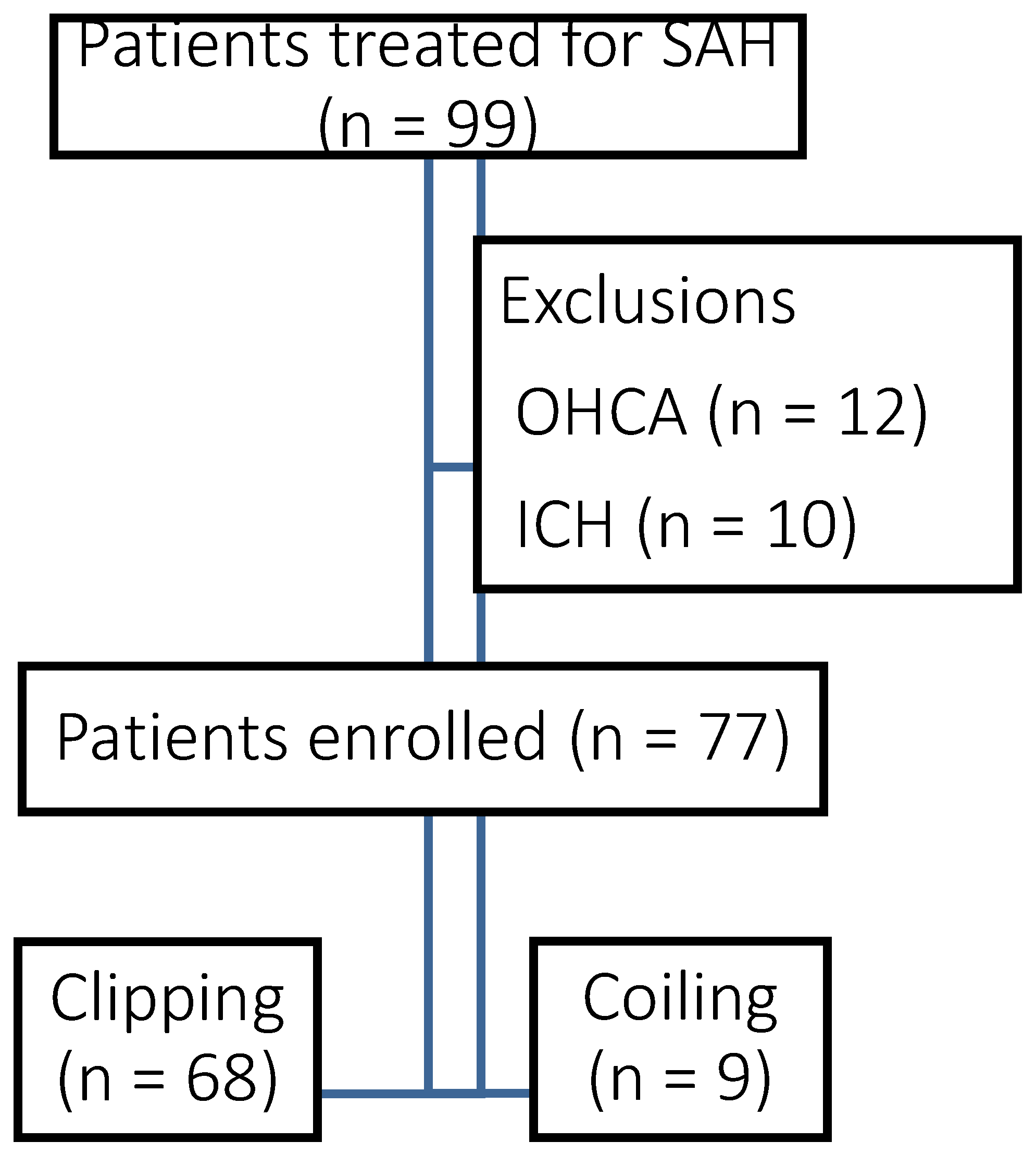
A total of 99 patients with aneurysmal SAH were admitted to our institution during the study period. After 22 exclusions (cardiopulmonary arrest, n = 12; intracerebral hematoma, n = 10), data for 77 patients were analyzed (
Figure 1). Of these, 28 patients were male and 49 were female. The median age was 65.0 years [interquartile range (IQR) 54.0, 75.3]. According to the World Federation of Neurosurgical Societies classification, 9 cases were grade I, 17 were grade II, 11 were grade III, 4 were grade IV, and 37 were grade V for severity. The ruptured aneurysms were located in the internal carotid artery in 33 cases, the anterior communicating artery in 26, the middle cerebral artery in 9, the basilar artery in 4, and the vertebral artery in 5. Craniotomy was performed in 68 cases and endovascular treatment in 9 (
Table 1).
2.1. IL-6 Levels on Postoperative Day 1
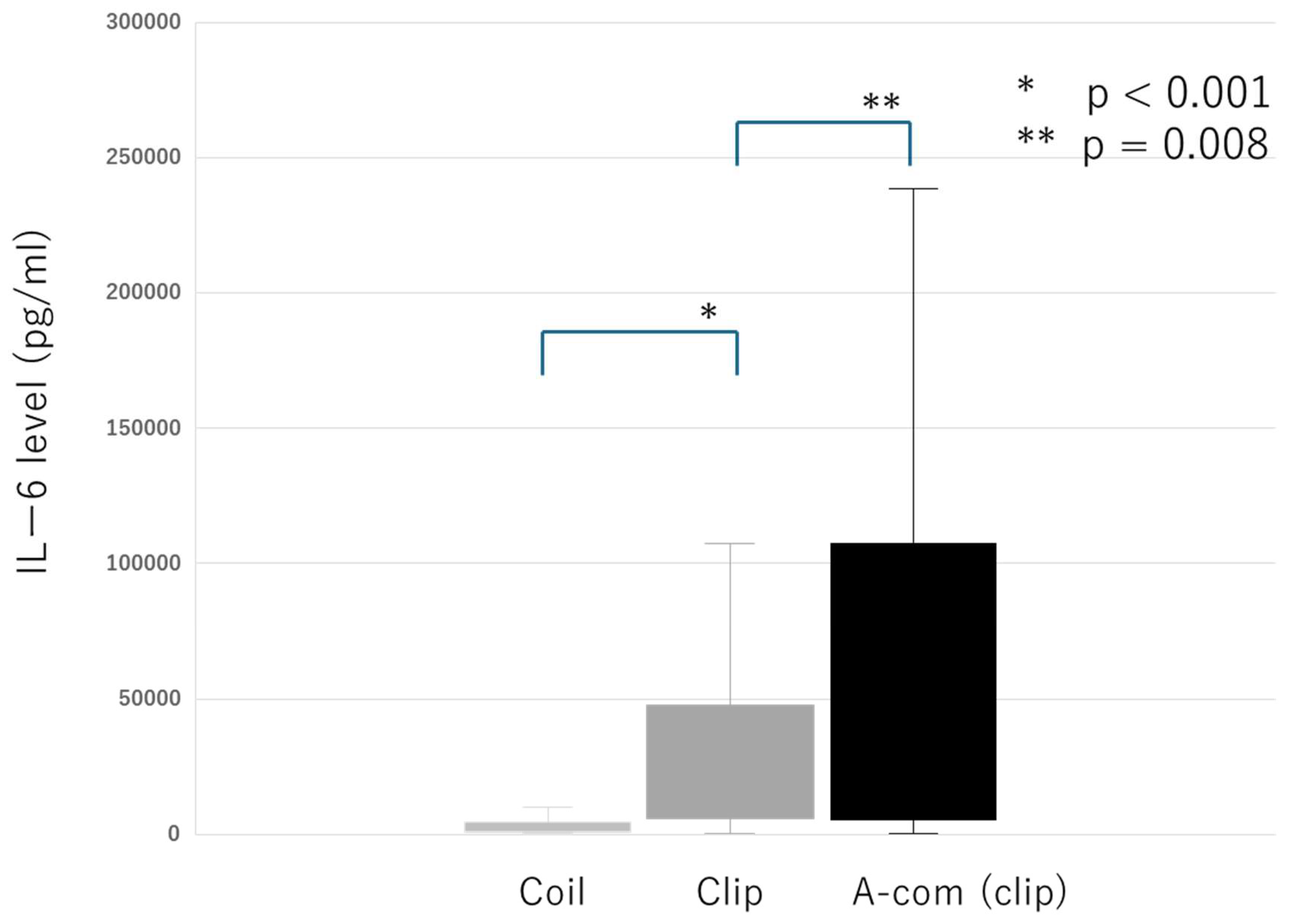
The median IL-6 level in CSF on postoperative day 1 was 10,501 pg/mL [IQR 3,037.8, 43,118.5]. IL-6 levels were significantly lower in the endovascular group than in the craniotomy group (p < 0.001). In the craniotomy group, IL-6 levels were significantly higher in patients with aneurysms in the anterior communicating artery than in those with aneurysms in the internal carotid artery or middle cerebral artery (p = 0.008) (
Figure 2). In a subset of 6 patients in whom CSF was collected during surgery, the median IL-6 level was 297 pg/mL [IQR 126, 1,148), which was higher than normal levels but significantly lower than the levels measured on postoperative day 1. This finding suggests that the increase in IL-6 levels was attributable to surgical invasiveness rather than the immediate effects of SAH itself.
2.2. Cerebral Vasospasm
Cerebral vasospasm was defined as worsening of consciousness, appearance of new focal neurological symptoms, an increase in intracranial Doppler flow velocity to more than twice the value recorded on the previous day, or stenosis of ≥50% observed on cerebral angiography. A total of 10 patients (13.0%) were observed to have cerebral vasospasm. Symptoms improved after local administration of fasudil hydrochloride and fluid loading. There were no recurrences. IL-6 levels in CSF were significantly higher in the patients who developed vasospasm than in those who did not (p = 0.003).
2.3. Outcomes
The Glasgow Outcome Scale (GOS) score for outcomes was 5 in 37 cases, 4 in 18, 3 in 9, 2 in 8, and 1 in 5. The outcome was classified as favorable in patients with a GOS score of 4 or 5 (n = 56) and as unfavorable in those with a GOS score of 1–3 (n = 21) (
Table 2). Significant correlations were found between an unfavorable outcome and a higher IL-6 level on postoperative day 1, presence of cerebral vasospasm, older age, and surgical method (
Table 3).
3. Discussion
This study demonstrated a close association of the IL-6 level in CSF measured on postoperative day 1 with postoperative outcomes and the likelihood of cerebral vasospasm in patients with aneurysmal SAH. Its findings also indicate that the type of surgical approach used and the degree of surgical invasiveness have a significant impact of the IL-6 level in the immediate postoperative period.
3.1. Production and Role of IL-6 in the Brain
IL-6 is one of the primary inflammatory cytokines associated with brain injury. Studies in animal models have demonstrated that IL-6 is produced by neurons, microglia, and astrocytes [
20,
21]. IL-6 plays a critical role in promoting neuroinflammation, inducing cerebral vasospasm, and contributing to glial scar formation, while also participating in the wound healing process [
21]. In this study, IL-6 levels in CSF were found to be elevated in all cases, confirming its role as an important marker of neuroinflammation in patients with SAH.
3.2. Association Between IL-6 and Postoperative Outcomes
Elevated IL-6 levels were significantly associated with poor clinical outcomes (GOS 1–3) and occurrence of cerebral vasospasm. Studies in animal models have found that intrathecal administration of IL-6 induces cerebral vasospasm [
22,
23], indicating involvement of IL-6 in the pathophysiology of vasospasm. In this study, IL-6 levels were higher in patients who developed vasospasm than in those who did not, suggesting that early suppression of IL-6 may be important for prevention of vasospasm and improving outcomes. However, the IL-6 level may not fully explain the occurrence of vasospasm, considering a report showing that severity of SAH and the degree of surgical invasiveness may also be contributing factors [
24]. Future studies are needed to explore the interplay of IL-6 with other inflammatory markers and clinical variables.
3.3. Impact of Surgical Invasiveness on the IL-6 Level
IL-6 levels were significantly lower in patients who received endovascular treatment than in those who underwent craniotomy, underscoring the relationship between surgical invasiveness and postoperative inflammation. In particular, IL-6 levels after craniotomy were higher when the aneurysm involved the anterior communicating artery than when it involved the internal carotid artery or middle cerebral artery. This finding suggests that factors such as increased surgical exposure and manipulation of brain tissue may exacerbate the inflammatory response. Furthermore, IL-6 levels were measured intraoperatively in a small subset of cases and found to be significantly lower than those measured postoperatively, providing further support for the role of surgical invasiveness in elevation of IL-6 levels.
3.4. Clinical Implications
The results of this study highlight the potential value of monitoring IL-6 levels in CSF as a marker of inflammation and a predictor of clinical outcomes in patients with SAH. Elevated IL-6 levels were associated with poor outcomes and occurrence of vasospasm, underscoring the need for strategies aimed at reducing IL-6 levels early in the postoperative period. Our findings also suggest that endovascular treatment, which is less invasive, is associated with lower IL-6 levels and potentially better outcomes. These results argue for preferential use of minimally invasive surgical approaches when appropriate. Targeting IL-6 using anti-inflammatory strategies may also improve postoperative outcomes. For example, artificial CSF has been reported to reduce IL-6 levels and decrease the incidence of cerebral vasospasm [
25]. Future research should focus on innovative methods for control of inflammation that may improve the prognosis and long-term outcomes in patients with SAH.
3.5. Limitations
This study has several limitations. First, it was conducted at a single institution specializing in severe cases of SAH, which may limit the generalizability of our findings. Second, variability in surgical technique among multiple surgeons may have influenced the degree of invasiveness and subsequent IL-6 levels. Third, while this study focused on IL-6 as a marker of inflammation, it did not assess other cytokines or inflammatory mediators. A more comprehensive evaluation of inflammatory responses, including multi-center studies, is needed to validate and expand upon these findings.
4. Methods
This retrospective observational study investigated the relationship between IL-6 levels in CSF, surgical invasiveness, postoperative outcomes, and cerebral vasospasm in patients with aneurysmal SAH. Patients who were admitted to our institution and underwent craniotomy (clipping) or endovascular treatment (coil embolization) within 12 h of diagnosis of aneurysmal SAH were enrolled in the study. Information on patient characteristics, including age and sex, was recorded. The severity of SAH was assessed using the World Federation of Neurosurgical Societies and Fisher classifications. The location of the ruptured aneurysm was also documented.
4.1. Collection of CSF
The methods used to collect CSF depended on the surgical method used. In the craniotomy group, a cisternal drainage tube was placed intraoperatively, and CSF was collected 24 h postoperatively after removing the internal volume of the tube. In the endovascular treatment group, a spinal drainage tube inserted preoperatively was used to collect CSF following the same protocol. In both groups, 2 mL of CSF were collected and centrifuged. The supernatant was used for measurement of the IL-6 concentration. These measurements were obtained by enzyme-linked immunosorbent assay (ELISA) at BML Inc (Tokyo, Japan).
4.2. Assessment of Cerebral Vasospasm
Cerebral vasospasm was defined as worsening of consciousness, appearance of new focal neurological symptoms, an increase in intracranial Doppler flow velocity to more than twice that measured on the previous day, or stenosis of ≥50% observed on cerebral angiography. These criteria were used to determine the presence or absence of vasospasm.
4.3. Statistical Analysis
Categorical variables are expressed as the number and percentage and were compared using the chi-squared test. Continuous variables are shown as the mean ± standard deviation or median [IQR] and analyzed using the t-test or Mann–Whitney U test as appropriate. Multiple regression analysis was used to identify factors associated with a favorable outcome (defined as a GOS score of 4 or 5) using variables that were significant in univariate analysis. Statistical analysis was performed using StatFlex Plus (ViewFlex, Inc., [Tokyo]). A p-value of < 0.05 was considered statistically significant.
4.4. Ethical Considerations
The study was conducted with the approval of the Institutional Review Board of Nippon Medical School (Approval Number: 2407244). Written informed consent was obtained from all patients upon admission. The data supporting the findings of this study are not publicly available because of privacy and ethical restrictions but can be obtained from the corresponding author upon reasonable request.
4.5. Postoperative Management Protocol
Postoperative management was standardized and included maintenance of normovolemia by fluid management, respiratory management, blood pressure control, and drainage management. Fasudil hydrochloride was administered intravenously for 14 days. No severe complications unrelated to SAH were observed during the study period.
5. Conclusion
This study found that the IL-6 level in CSF on the first postoperative day is closely associated with clinical outcomes and the occurrence of cerebral vasospasm in patients with aneurysmal SAH. In particular, higher IL-6 levels were significantly correlated with unfavorable outcomes and an increased risk of vasospasm, suggesting that IL-6 serves as a critical marker of postoperative inflammation and disease progression.
Another important finding in this study was that the IL-6 level in the immediate postoperative period was significantly influenced by the surgical method used and the extent of surgical invasiveness. lower IL-6 levels were lower after endovascular treatment than after craniotomy, indicating the potential benefit of endovascular treatment in terms of minimizing postoperative inflammation. Furthermore, in the craniotomy group, surgical approaches with greater invasiveness, such as those involving the anterior communicating artery, were associated with higher IL-6 levels. These results underscore the importance of minimizing surgical invasiveness to improve postoperative outcomes.
Monitoring IL-6 levels in CSF may serve as a valuable tool for guiding postoperative management and predicting clinical outcomes in patients with SAH. Furthermore, strategies aimed at reducing IL-6 levels, such as use of artificial CSF or anti-inflammatory therapies, may play a significant role in improving their prognosis. This study highlights the importance of selecting a minimally invasive surgical technique in patients with SAH and targeting inflammation in the immediate postoperative period. These approaches have the potential to optimize treatment strategies and improve the overall management of these patients.
Author Contributions
Hidetaka Onda: Conceptualization; Data Curation; Formal Analysis; Investigation; Methodology; Project Administration; Resources; Validation; Visualization; Writing – Original Draft Preparation; Writing – Review & Editing. Shoji Yokobori: Supervision; Writing – Review & Editing. Nodoka Miyake: Data Curation; Review & Editing. Kenta Shigeta: Data Curation; Review & Editing. Naoki Tominaga: Data Curation; Review & Editing.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Prass, K.; Meisel, C.; Höflich, C.; et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. Journal of Experimental Medicine 2003, 198, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Zierath, D.; Tanzi, P.; Shibata, D.; Becker, K.J. Cortisol is more important than metanephrines in driving changes in leukocyte counts after stroke. Journal of Stroke and Cerebrovascular Diseases 2018, 27, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J.; Saadé, N.E.; Safieh-Garabedian, B. Cytokines and neuro-immune-endocrine interactions: A role for the hypothalamic-pituitary-adrenal revolving axis. Journal of Neuroimmunology 2002, 133, 1–19. [Google Scholar] [CrossRef] [PubMed]
- 4, Besedovsky, H.O.; del Rey, A.; Klusman, I.; et al. Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. Journal of Steroid Biochemistry and Molecular Biology 1991, 40, 613–618. [CrossRef]
- Sapolsky, R.; Rivier, C.; Yamamoto, G.; et al. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 1987, 238, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Zetterling, M.; Engström, B.E.; Hallberg, L.; et al. Cortisol and adrenocorticotropic hormone dynamics in the acute phase of subarachnoid haemorrhage. British Journal of Neurosurgery 2011, 25, 684–692. [Google Scholar] [CrossRef]
- Sarrafzadeh, A.; Schlenk, F.; Meisel, A.; et al. Immunodepression after aneurysmal subarachnoid hemorrhage. Stroke 2011, 42, 53–58. [Google Scholar] [CrossRef]
- Csuka, E.; Morganti-Kossmann, M.C.; Lenzlinger, P.M.; et al. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: Relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. Journal of Neuroimmunology 1999, 101, 211–221. [Google Scholar] [CrossRef]
- Geiger, T.; Andus, T.; Klapproth, J.; et al. Induction of rat acute-phase proteins by interleukin 6 in vivo. European Journal of Immunology 1988, 18, 717–721. [Google Scholar] [CrossRef]
- Ichiyama, T.; Nishikawa, M.; Yoshitomi, T.; et al. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 in cerebrospinal fluid from children with prolonged febrile seizures: Comparison with acute encephalitis/encephalopathy. Neurology 1998, 50, 407–411. [Google Scholar] [CrossRef]
- López-Cortés, L.F.L. Interleukin 6 in cerebrospinal fluid of patients with meningitis is not a useful diagnostic marker in the differential diagnosis of meningitis. Critical Care Medicine 2000, 28, 215–219. [Google Scholar] [CrossRef]
- Matsuzono, Y.Y. Interleukin-6 in cerebrospinal fluid of patients with central nervous system infections. Acta Paediatrica 1995, 84, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Dumont, A.S.; Dumont, R.J.; Chow, M.M.; et al. Cerebral vasospasm after subarachnoid hemorrhage: Putative role of inflammation. Neurosurgery 2003, 53, 123–133; discussion 133–135.
- Hirashima, Y.; Nakamura, S.; Endo, S.; et al. Elevation of platelet activating factor, inflammatory cytokines, and coagulation factors in the internal jugular vein of patients with subarachnoid hemorrhage. Neurochemical Research 1997, 22, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, T.; Andersson, B.; Loftenius, A.; et al. Increased interleukin-6 levels in cerebrospinal fluid following subarachnoid hemorrhage. Journal of Neurosurgery 1993, 78, 562–567. [Google Scholar] [CrossRef]
- Molyneux, A.J.; Kerr, R.S.; Yu, L.M.; et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet 2002, 360, 1267–1274. [Google Scholar] [CrossRef]
- Molyneux, A.J.; Kerr, R.S.; Birks, J.; et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardized mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): Long-term follow-up. Lancet Neurology 2009, 8, 427–433. [Google Scholar] [CrossRef]
- Wu, W.; Guan, Y.; Zhao, G.; et al. Elevated IL-6 and TNF-alpha levels in cerebrospinal fluid of subarachnoid hemorrhage patients. Molecular Neurobiology 2016, 53, 3277–3285. [Google Scholar] [CrossRef]
- Azuma, H.H. Clinical significance of cytokine measurement for detection of meningitis. Journal of Pediatrics 1997, 131, 463–465. [Google Scholar] [CrossRef]
- Sawada, M.; Suzumura, A.; Marunouchi, T. Cytokine network in the central nervous system and its roles in growth and differentiation of glial and neuronal cells. International Journal of Developmental Neuroscience 1995, 13, 253–264. [Google Scholar] [CrossRef]
- Szelenyi, J. Cytokines and the central nervous system. Brain Research Bulletin 2001, 54, 329–338. [Google Scholar] [CrossRef]
- Osuka, K.; Suzuki, Y.; Tanazawa, T.; et al. Interleukin-6 and development of vasospasm after subarachnoid haemorrhage. Acta Neurochirurgica (Wien) 1998, 140, 943–951. [Google Scholar] [CrossRef]
- Peterson, J.W.; Kwun, B.D.; Hackett, J.D.; et al. The role of inflammation in experimental cerebral vasospasm. Journal of Neurosurgery 1990, 72, 767–774. [Google Scholar] [CrossRef]
- Schoch, B.; Regel, J.P.; Wichert, M.; et al. Analysis of intrathecal interleukin-6 as a potential predictive factor for vasospasm in subarachnoid hemorrhage. Neurosurgery 2007, 60, 828–836; discussion 828–836.
- Onda, H.; Kanaya, T.; Igarashi, Y.; et al. Changes in cerebrospinal fluid interleukin-6 levels after surgical treatment for subarachnoid hemorrhage. Journal of Nippon Medical School 2024, 91, 410–418. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).