Submitted:
23 January 2025
Posted:
26 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experiment Design
2.2.1. Forest Structure
2.2.2. Leaf Litter Production
2.2.3. Root Production
2.2.4. Climatic Data and Interstitial Salinity
2.3. Statistical Analysis
3. Results
3.1. Structure
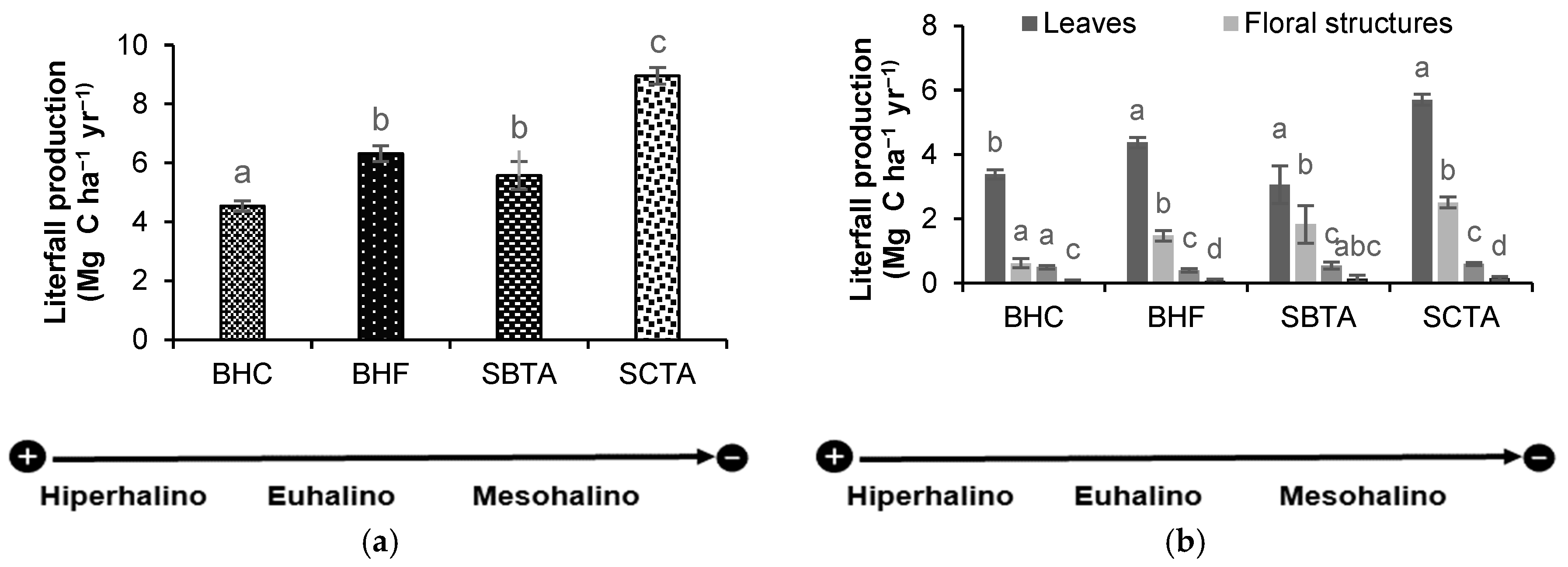
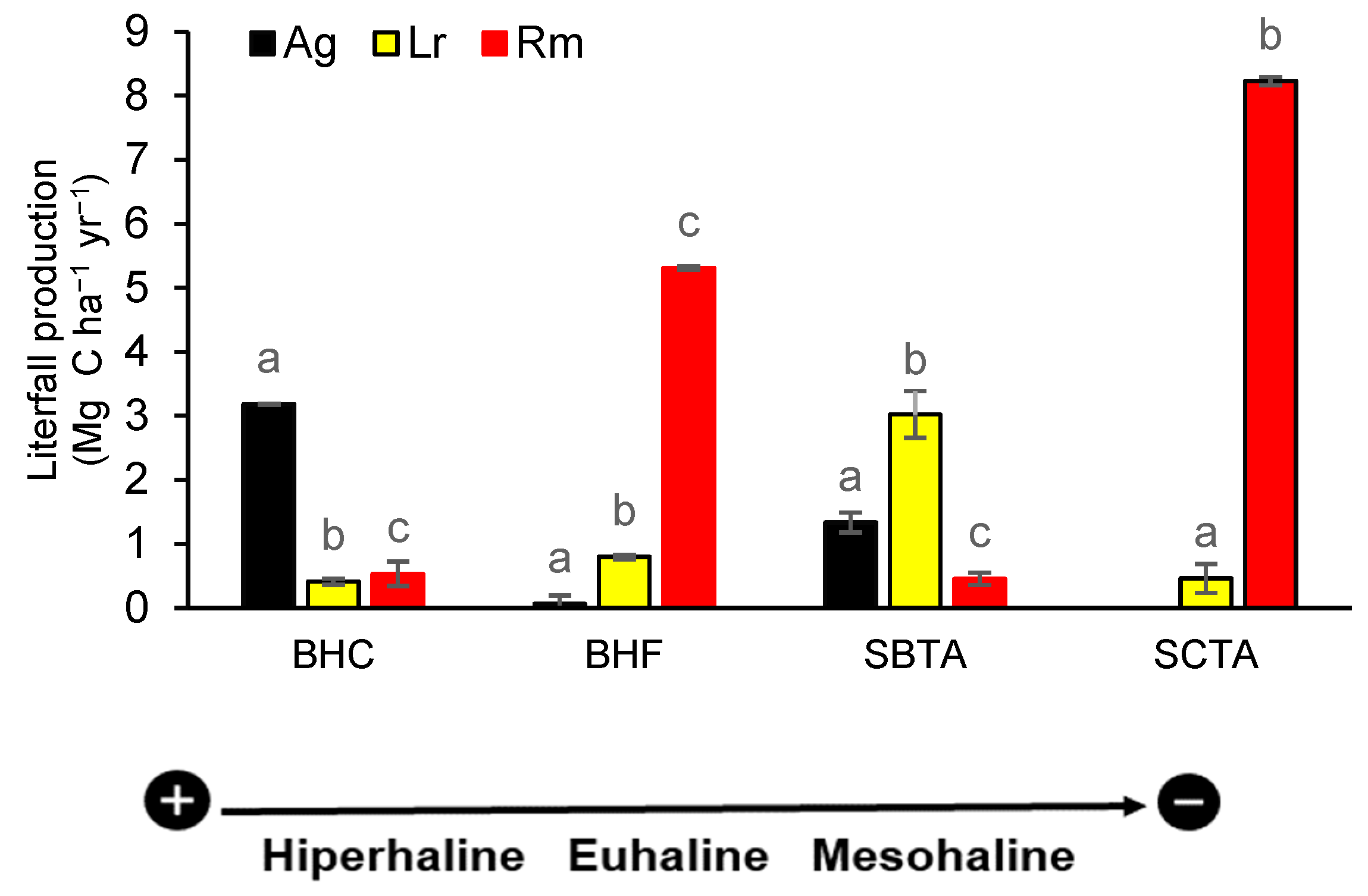
3.2. Carbon Content of Litterfall Production in Mangrove Forests with Different Salinity Gradients
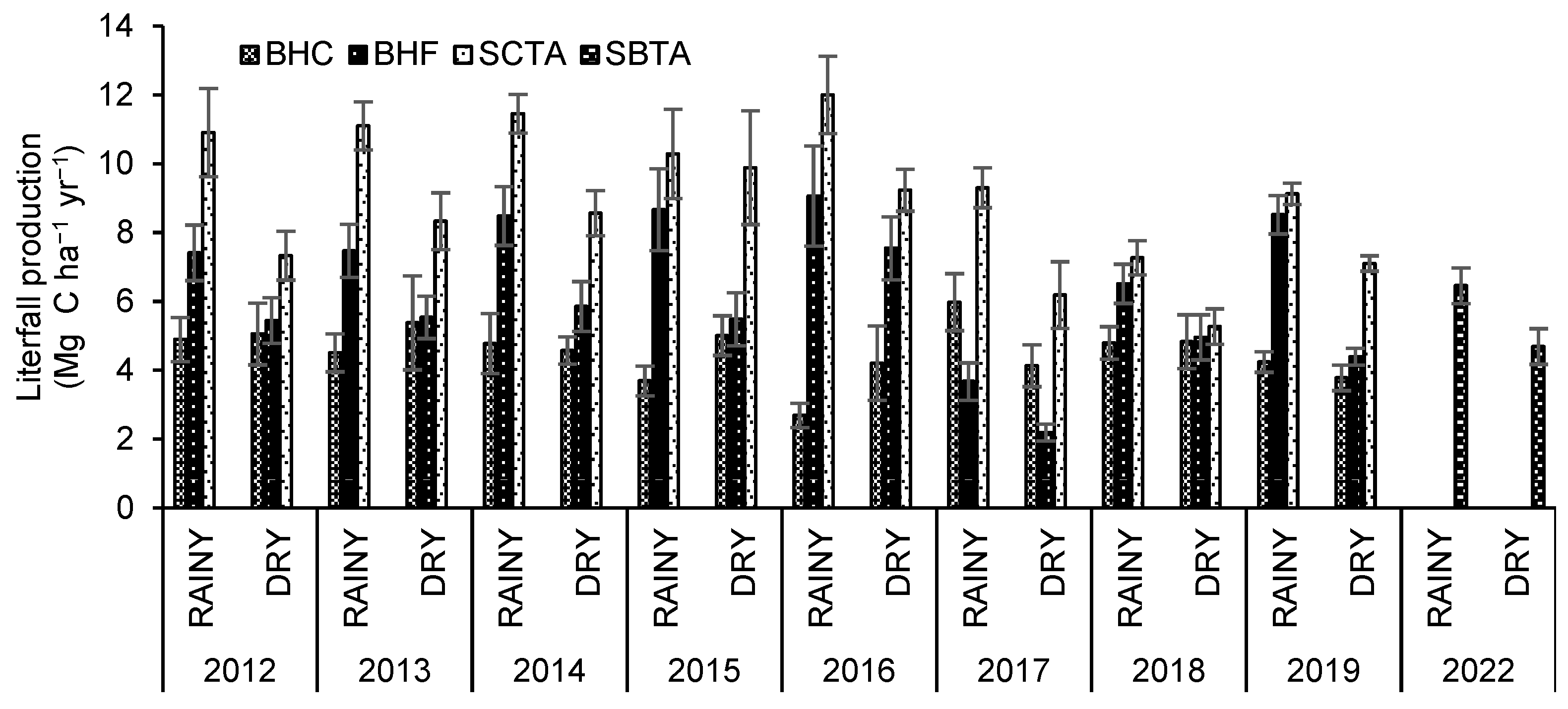
3.3. Carbon Content in Multitemporal Litterfall Production
3.4. Carbon Content During Root Production in Forests with Different Salinity Gradients
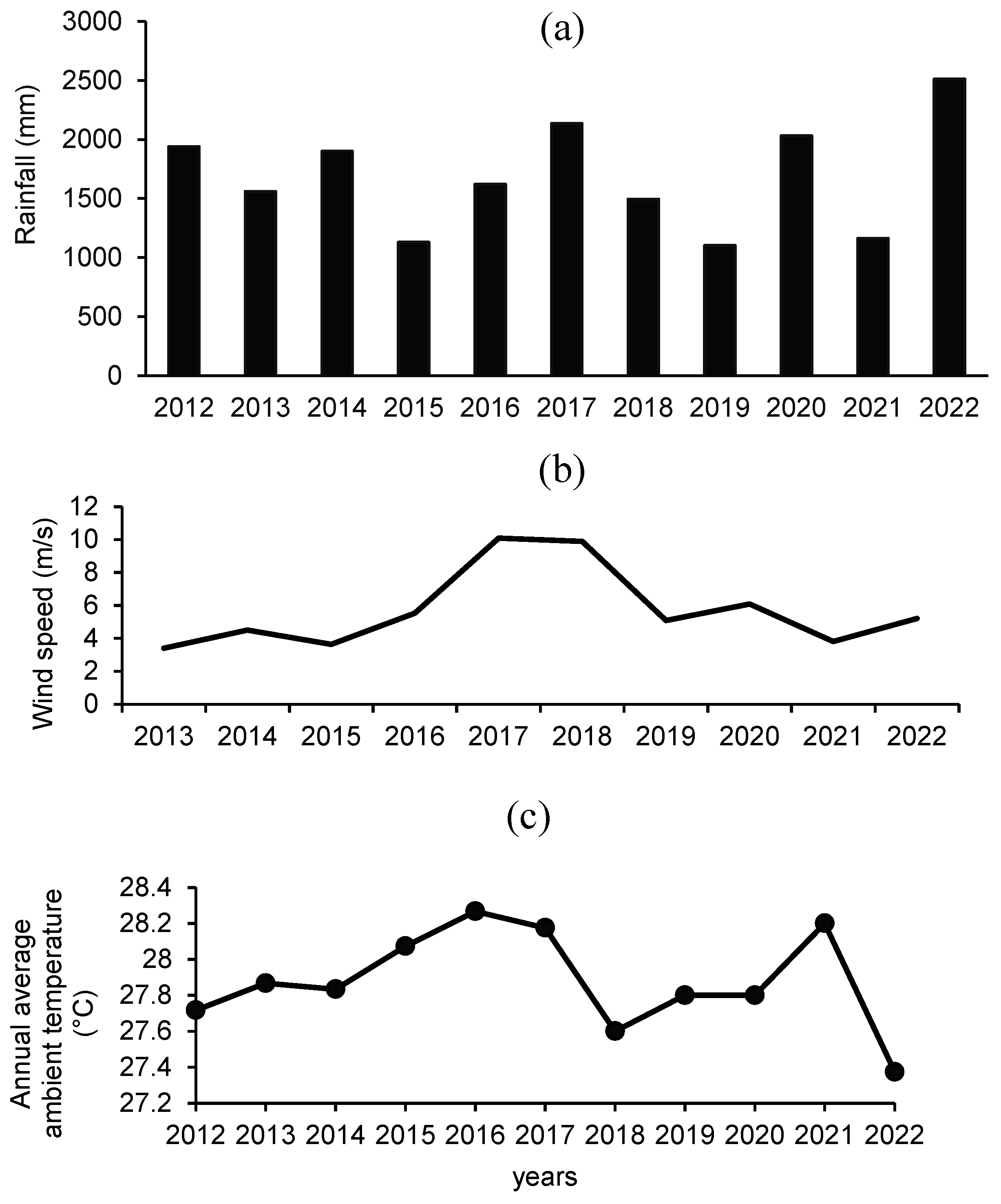
3.5. Interstitial Salinity and Climatic Variables
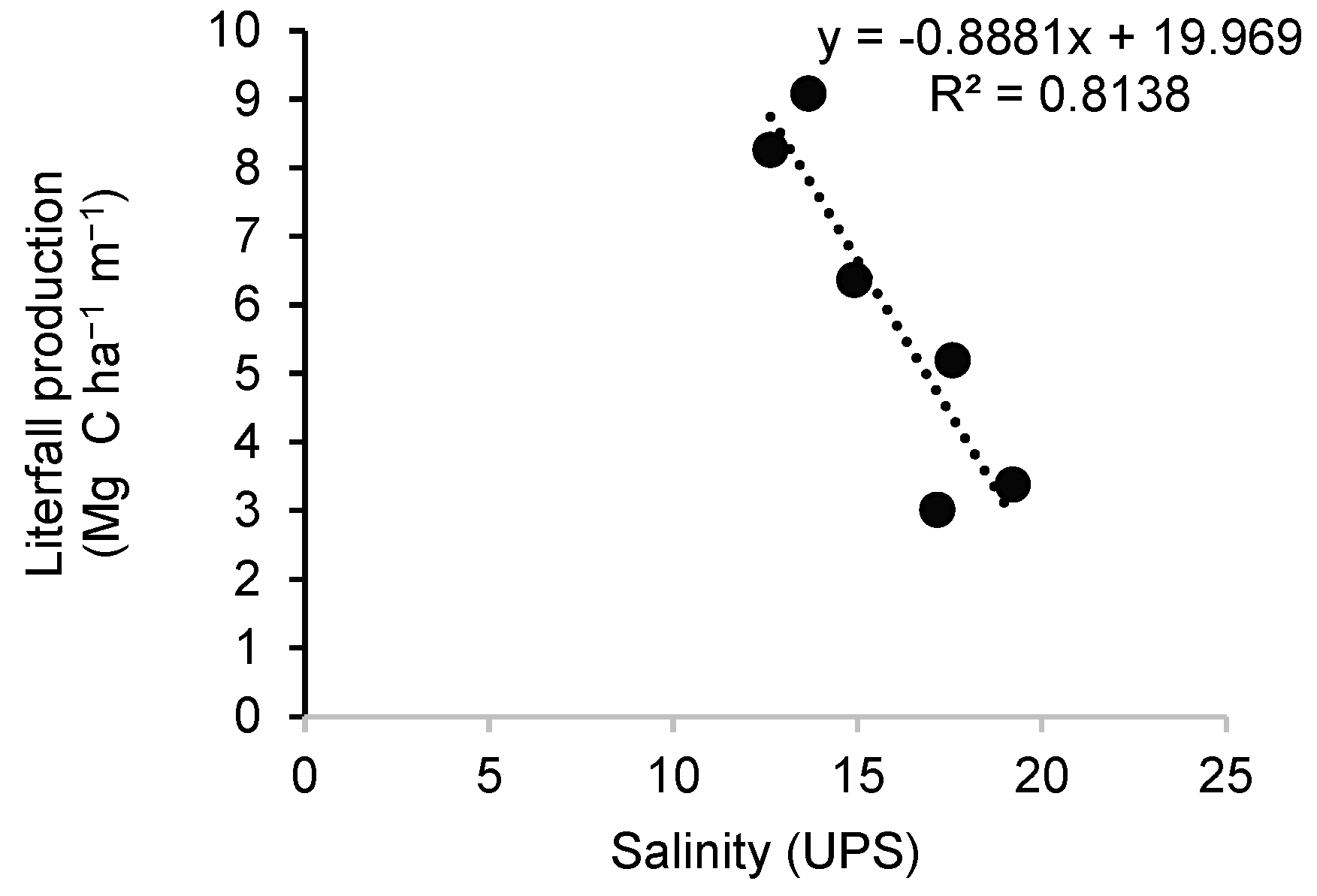
3.6. Relationships of Climatic Variables and Interstitial Salinity to Litterfall Production
4. Discussion
4.1. Litter Production
4.1.1. Variation of Carbon Contents in Multitemporal and Multiannual Litterfall Production
4.1.2. Deltaic and Karstic Mangrove Analysis
4.2. Root Production
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
| Country | Geomorphic setting | Mangrove ecotype | Litterfall production (Mg C ha-1y-1) | Salinity (PSU) | Autor |
|---|---|---|---|---|---|
| Colombia | Terrigenous | River | 10 | Riascos y Blanco- Libreros (2019) | |
| Colombia | Carbonate | Inland | 9.74 | 9.63 ±6.26 | Present studio |
| Brazil | Terrigenous | River | 7.9 | Nordhaus et al. (2006) | |
| Mexico | Terrigenous | River | 7.9 | Barreiro- Güemes (1999) | |
| Brazil | Terrigenous | River | 7.0 | Bernini y Rezende (2010) | |
| Puerto rico | Terrigenous | River | 6.9 | Pool et al. (1975) | |
| US | Terrigenous | River | 6.9 | Sell (1977) | |
| Mexico | Terrigenous | River | 6.8 | Flores Verdugo et al. (1990) | |
| Colombia | Terrigenous | River | 6.6 | Mullen y Hernández (1978) | |
| Mexico | Terrigenous | River | 6.6 | Utrera-López y Moreno-Casasola (2008) | |
| Colombia | Carbonate | Fringe | 6.38 | 37.47 ± 5.76 | Present studio |
| Colombia | Carbonate | Inland | 6.12 | 11.54 ± 7.46 | Present studio |
| Mexico | Terrigenous | River | 6.1 | Coronado-Molina et al. (2012) | |
| Mexico | Terrigenous | River | 5.9 | Agraz et al. (2011) | |
| Mexico | Carbonate | Fringe | 4.7 | < 20 | Camacho et al. (2021) |
| Colombia | Carbonate | Basin | 4.68 | 62.36 ±10.54 | Present studio |
| US | Carbonate | Fringe | 4.4 | Coronado-Molina et al. (2012) | |
| Mexico | Carbonate | River | 4.4 | Herrera Silveira et al. (2016) | |
| Mexico | Carbonate | Fringe | 4.3 | Herrera Silveira et al. (2016) | |
| Mexico | Carbonate | Fringe | 4 | > 40 | Camacho et a. (2021) |
| Colombia | Terrigenous | River | 4.0 | Romero et al. (2000) | |
| US | Terrigenous | River | 3.9 | Castañeda-Moya et al. (2013) | |
| Mexico | Carbonate | Basin | 3.6 | Herrera Silveira et al. (2016) | |
| Brazil | Terrigenous | River | 3.6 | Fernández et al. (2007) | |
| Ecuador | Terrigenous | River | 3.1 | Twilley et al. (1997) | |
| US | Carbonate | Basin | 3.1 | Coronado-Molina et al. (2012) | |
| Mexico | Carbonate | Inland | 3.0 | Adame et al (2013) | |
| Mexico | Terrigenous | Fringe | 2.6 | 25.1 | Torres et al. (2023) |
| Mexico | Carbonate | Basin | 2.5 | Coronado-Molina et al. (2012) | |
| US | Carbonate | Dwarf | 1.2 | Coronado-Molina et al. (2012) | |
| Mexico | Carbonate | Dwarf | 0.55 | Herrera Silveira et al. (2016) |
| Country | Geomorphic setting | Mangrove ecotype | Roots production (Mg C ha-1 y-1) | Depth measurement(cm) | Salinity (PSU) | Autor | |
|---|---|---|---|---|---|---|---|
| China | Terrigenous | River | 11.74 | 100 | 40.9 | Xiong et al., 2016 | |
| China | Terrigenous | River | 8.05 | 100 | 26.5 | Xiong et al., 2016 | |
| China | Terrigenous | River | 7.30 | 100 | 27.9 | Xiong et al., 2016 | |
| Malasia | Terrigenous | 4.95 | 50 | 3.3 | Muhammad Nor et al., 2019 | ||
| China | Terrigenous | River | 4.12 | 100 | 16.2 | Xiong et al., 2016 | |
| US | Terrigenous | 3.11 | 30 | McKee et al.,2011 | |||
| US | Terrigenous | 2.87 | 30 | 30 | Mckee y Faulkner., 2000 | ||
| Mexico | Terrigenous | 2.75 | 35 | 32.3 | Xiong et al., 2016 | ||
| US | Carbonate | 2.74 | 90 | Castañeda-Moya et al.,2011 | |||
| Belize | Carbonate | Fringe | 2.56 | 30 | McKee et al.,2011 | ||
| US | Terrigenous | 2.54 | 30 | McKee et al.,2011 | |||
| US | Carbonate | 2.53 | 90 | 27 | Castañeda-Moya et al.,2011 | ||
| Australia | Terrigenous | River | 2.36 | 20 | Lovelock et al.,2015 | ||
| Australia | Terrigenous | River | 2.25 | 30 | Hayes et al.,2017 | ||
| China | Terrigenous | River | 2.25 | 100 | 15.10 | Xiong et al., 2016 | |
| Mexico | Terrigenous | 2.22 | 35 | 43.1 | Torres et al., 2019 | ||
| US | Terrigenous | River | 2.20 | 30 | 28 | Mckee y Faulkner., 2000 | |
| Kenya | Terrigenous | 2.12 | 40 | Lang'at et al., 2012 | |||
| Belize | Carbonate | Fringe | 2.05 | 30 | McKee et al., 2007 | ||
| China | Terrigenous | River | 1.99 | 60 | 4.6 | Zhang et al., 2021 | |
| Thailand | Terrigenous | River | 1.95 | 30 | Poungparn et al., 2016 | ||
| US | Terrigenous | 1.94 | 30 | 23 | Giraldo, 2005 | ||
| US | Carbonate | 1.91 | 90 | Castañeda-Moya et al.,2011 | |||
| US | Terrigenous | 1.91 | 30 | 43 | Giraldo, 2005 | ||
| US | Carbonate | 1.88 | 90 | 32.7 | Castañeda-Moya et al.,2011 | ||
| US | Carbonate | 1.84 | 90 | Castañeda-Moya et al.,2011 | |||
| US | Carbonate | 1.82 | 90 | 20.8 | Castañeda-Moya et al.,2011 | ||
| US | Terrigenous | 1.79 | 30 | McKee et al.,2011 | |||
| Thailand | Terrigenous | River | 1.78 | 30 | Poungparn et al., 2016 | ||
| Mexico | Terrigenous | 1.72 | 35 | 46.1 | Torres et al., 2019 | ||
| US | Carbonate | 1.63 | 90 | Castañeda-Moya et al.,2011 | |||
| US | Terrigenous | 1.63 | 30 | 34 | Mckee y Faulkner., 2000 | ||
| Mexico | Carbonate | Fringe- Basin | 1.61 | 30 | 42 | Perez-Ceballos et al., 2018 | |
| Australia | Terrigenous | River | 1.59 | 30 | Hayes et al.,2017 | ||
| Mexico | Terrigenous | 1.54 | 35 | 46.4 | Torres et al., 2019 | ||
| Belize | Carbonate | Basin | 1.54 | 30 | McKee et al., 2007 | ||
| US | Terrigenous | 1.47 | 30 | 24 | Giraldo, 2005 | ||
| US | Terrigenous | 1.46 | 30 | 33 | Giraldo, 2005 | ||
| Thailand | Terrigenous | River | 1.45 | 30 | Poungparn et al., 2016 | ||
| US | Terrigenous | 1.43 | 30 | 45 | Mckee y Faulkner., 2000 | ||
| US | Terrigenous | 1.35 | 30 | 24.5 | Perry & Mendelssohn., 2009 | ||
| US | Terrigenous | 1.35 | 30 | 31 | Giraldo, 2005 | ||
| Kenya | Terrigenous | 1.32 | 40 | Lang'at et al., 2012 | |||
| Japan | Terrigenous | 1.32 | 40 | Kihara et al., 2022 | |||
| Colombia | Carbonate | Fringe | 1.30 | 45 | 37.4 | Presente estudio | |
| Honduras | Carbonate | Basin | 1.30 | 30 | Cahoon et al., 2003 | ||
| Australia | Terrigenous | River | 1.26 | 30 | 6.2 | Hayes et al., 2019 | |
| Kenya | Terrigenous | 1.26 | 40 | Lang'at et al., 2012 | |||
| US | Carbonate | 1.26 | 90 | 20.2 | Castañeda-Moya et al.,2011 | ||
| Kenya | Terrigenous | 1.25 | 40 | Lang'at et al., 2012 | |||
| Honduras | Carbonate | Fringe | 1.21 | 30 | Cahoon et al., 2003 | ||
| US | Terrigenous | 1.21 | 30 | Giraldo, 2005 | |||
| Colombia | Carbonate | Inland | 1.19 | 45 | 11.5 | Presente estudio | |
| Mexico | Terrigenous | 1.10 | 35 | 70.6 | Torres et al., 2019 | ||
| Mexico | Terrigenous | 1.07 | 35 | 38.6 | Torres et al., 2019 | ||
| Belize | Carbonate | Basin | 1.05 | 30 | McKee et al.,2011 | ||
| Kenya | Terrigenous | 0.97 | 40 | Lang'at et al., 2012 | |||
| Mexico | Carbonate | Fringe- basin | 0.91 | 30 | 48 | Pérez-Ceballos et al., 2018 | |
| US | Terrigenous | 0.84 | 30 | 30 | Giraldo, 2005 | ||
| US | Terrigenous | River | 0.81 | 30 | 23 | Radabaugh et al., 2021 | |
| Mexico | Carbonate | 0.79 | 35 | 50.2 | Adame et al., 2014 | ||
| Mexico | Carbonate | Fringe- basin | 0.77 | 30 | 56 | Pérez-Ceballos et al., 2018 | |
| US | Terrigenous | 0.75 | 30 | 33 | Giraldo, 2005 | ||
| China | Terrigenous | River | 0.75 | 60 | 7.3 | Zhang et al., 2021 | |
| China | Terrigenous | River | 0.65 | 100 | 14.04 | He et al., 2021 | |
| China | Terrigenous | River | 0.63 | 100 | He et al., 2021 | ||
| Australia | Terrigenous | River | 0.60 | 30 | 33.3 | Hayes et al., 2019 | |
| US | Terrigenous | 0.59 | 30 | McKee et al.,2011 | |||
| China | Terrigenous | River | 0.56 | 100 | 29.67 | He et al., 2021 | |
| Australia | Terrigenous | River | 0.51 | 20 | Lovelock et al.,2015 | ||
| Kenya | Terrigenous | 0.48 | 40 | Lang'at et al., 2012 | |||
| Micronesia | Carbonate | 0.46 | 45 | 18 | Cormier et al., 2015 | ||
| Mexico | Carbonate | 0.44 | 35 | 26.9 | Adame et al., 2014 | ||
| US | Terrigenous | 0.44 | 30 | McKee et al.,2011 | |||
| Kenya | Terrigenous | 0.42 | 40 | Lang'at et al., 2012 | |||
| US | Terrigenous | 0.42 | 30 | 25 | Radabaugh et al., 2021 | ||
| Colombia | Carbonate | Inland | 0.41 | 45 | 9.7 | Presente estudio | |
| Mexico | Carbonate | 0.41 | 35 | 37.1 | Adame et al., 2014 | ||
| Micronesia | Carbonate | 0.39 | 45 | 17.1 | Cormier et al., 2015 | ||
| Micronesia | Carbonate | 0.37 | 45 | 10.7 | Cormier et al., 2015 | ||
| Micronesia | Carbonate | 0.36 | 45 | 15.1 | Cormier et al., 2015 | ||
| China | Terrigenous | River | 0.35 | 60 | 4.7 | Zhang et al., 2021 | |
| Belize | Carbonate | Inland | 0.32 | 30 | McKee et al.,2011 | ||
| Belize | Carbonate | Inland | 0.32 | 30 | McKee et al., 2007 | ||
| Mexico | Terrigenous | 0.31 | 45 | 12.08 | Ochoa-Gomez et al., 2019 | ||
| Australia | Terrigenous | River | 0.29 | 30 | 34.8 | Hayes et al., 2019 | |
| Mexico | Terrigenous | 0.27 | 45 | Ochoa-Gomez et al., 2019 | |||
| Micronesia | Carbonate | 0.25 | 45 | 19.8 | Cormier et al., 2015 | ||
| Colombia | Carbonate | Basin | 0.24 | 45 | 62.3 | Presente estudio | |
| Mexico | Terrigenous | 0.22 | 45 | Ochoa-Gomez et al., 2019 | |||
| Micronesia | Carbonate | 0.18 | 45 | 34.2 | Cormier et al., 2015 |
References
- Bouillon, S. Storage beneath Mangroves. Nat Geosci 2011, 4, 282–283. [Google Scholar] [CrossRef]
- Duke, N.C.; Meynecke, J.-O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A World Without Mangroves? Science (1979) 2007, 317, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Palacios Peñaranda, M.L.; Cantera Kintz, J.R.; Peña Salamanca, E.J. Carbon Stocks in Mangrove Forests of the Colombian Pacific. Estuar Coast Shelf Sci 2019, 227, 106299. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Adame, M.F.; Arifanti, V.B.; Schile-Beers, L.M.; Bernardino, A.F.; Bhomia, R.K.; Donato, D.C.; Feller, I.C.; Ferreira, T.O.; Jesus Garcia, M. del C.; et al. Total Ecosystem Carbon Stocks of Mangroves across Broad Global Environmental and Physical Gradients. Ecol Monogr 2020, 90. [Google Scholar] [CrossRef]
- Taillardat, P.; Friess, D.A.; Lupascu, M. Mangrove Blue Carbon Strategies for Climate Change Mitigation Are Most Effective at the National Scale. Biol Lett 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, J.A.; Mancera Pineda, J.E.; Melgarejo, L.M.; Medina Calderón, J.H. Functional Traits of Leaves and Forest Structure of Neotropical Mangroves under Different Salinity and Nitrogen Regimes. Flora: Morphology, Distribution, Functional Ecology of Plants 2018, 239, 52–61. [Google Scholar] [CrossRef]
- Herrera Silveira, J.A.; Camacho Rico, A.; Pech, E.; Pech, M.; Ramírez Ramírez, J.; Teutli Hernández, C. Dinámica Del Carbono (Almacenes y Flujos) En Manglares De. Terra Latinoamamericana 2016, 34, 61–72. [Google Scholar]
- Sitoe, A.; Mandlate, L.; Guedes, B. Biomass and Carbon Stocks of Sofala Bay Mangrove Forests. Forests 2014, 5, 1967–1981. [Google Scholar] [CrossRef]
- Agraz-Hernández, C.M.; Chan-Keb, C.A.; Chávez-Barrera, J.; Osti-Sáenz, J.; Expósito-Díaz, G.; Alonso-Campos, V.A.; Muñiz-Salazar, R.; Ruiz-Fernández, A.C.; Pérez-Bernal, L.H.; Sánchez-Cabeza, J.A.; et al. Carbon Stocks in a Mangrove Ecosystem in Northern Mexico: Environmental Changes for 35 Years. Rev Mex Biodivers 2020, 91. [Google Scholar] [CrossRef]
- Bouillon, S.; Borges, A. V.; Castañeda-Moya, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.J.; et al. Mangrove Production and Carbon Sinks: A Revision of Global Budget Estimates. Global Biogeochem Cycles 2008, 22. [Google Scholar] [CrossRef]
- Rovai, A.S.; Twilley, R.R.; Worthington, T.A.; Riul, P. Brazilian Mangroves: Blue Carbon Hotspots of National and Global Relevance to Natural Climate Solutions. Frontiers in Forests and Global Change 2022, 4. [Google Scholar] [CrossRef]
- Twilley, R.R.; Castañeda-Moya, E.; Rovai, A. Productivity and Carbon Dynamics in Mangrove Wetlands. In Mangrove Ecosystems: A Global Biogeographic Perspective; Rivera-Monroy, V., Lee, S., Kristensen, E., Twilley, R., Eds.; Springer, Cham, 2017.
- Medina-Calderón, J.H.; Mancera-Pineda, J.E.; Castañeda-Moya, E.; Rivera-Monroy, V.H. Hydroperiod and Salinity Interactions Control Mangrove Root Dynamics in a Karstic Oceanic Island in the Caribbean Sea (San Andres, Colombia). Front Mar Sci 2021, 7. [Google Scholar] [CrossRef]
- Twilley, R.R.; Rovai, A.S.; Riul, P. Coastal Morphology Explains Global Blue Carbon Distributions. Front Ecol Environ 2018, 16, 503–508. [Google Scholar] [CrossRef]
- Mckee, K.L.; Cahoon, D.R.; Feller, I.C. Caribbean Mangroves Adjust to Rising Sea Level through Biotic Controls on Change in Soil Elevation. Global Ecology and Biogeography 2007, 16, 545–556. [Google Scholar] [CrossRef]
- Day, J.W.; Coronado-Molina, C.; Vera-Herrera, F.R.; Twilley, R.; Rivera-Monroy, V.H.; Alvarez-Guillen, H.; Day, R.; Conner, W. Aquatic Botany A 7 Year Record of Above-Ground Net Primary Production in a Southeastern Mexican Mangrove Forest; 1996; Vol. 55;
- Torres-Fernández del Campo, J.; Olvera-Vargas, M.; Figueroa-Rangel, B.L.; Cuevas-Guzmán, R.; Iñiguez-Dávalos, L.I. Patterns of Spatial Diversity and Structure of Mangrove Vegetation in Pacific West-Central Mexico. Wetlands 2018, 38, 919–931. [Google Scholar] [CrossRef]
- Restrepo, J.D.; Kjerfve, B. The Pacific and Caribbean Rivers of Colombia: Water Discharge, Sediment Transport and Dissolved Loads. In Environmental Geochemistry in Tropical and Subtropical Environments; Springer Berlin Heidelberg, 2004; pp. 169–187.
- Riascos, J.M.; Blanco-Libreros, J.F. Pervasively High Mangrove Productivity in a Major Tropical Delta throughout an ENSO Cycle (Southern Caribbean, Colombia). Estuar Coast Shelf Sci 2019, 227. [Google Scholar] [CrossRef]
- Pérez-Ceballos, R.; Rivera-Rosales, K.; Zaldívar-Jiménez, A.; Canales-delgadillo, J.; Brito-Pérez, R.; Del Ángel, L.A.; Merino-Ibarra, M. Efecto de La Restauración Hidrológica Sobre La Productividad de Raíces Subterráneas En Los Manglares de Laguna de Términos, México. Bot Sci 2018, 96, 569–581. [Google Scholar] [CrossRef]
- Muhammad-Nor, S.M.; Huxham, M.; Salmon, Y.; Duddy, S.J.; Mazars-Simon, A.; Mencuccini, M.; Meir, P.; Jackson, G. Exceptionally High Mangrove Root Production Rates in the Kelantan Delta, Malaysia; An Experimental and Comparative Study. For Ecol Manage 2019, 444, 214–224. [Google Scholar] [CrossRef]
- Arnaud, M.; Morris, P.J.; Baird, A.J.; Dang, H.; Nguyen, T.T. Fine Root Production in a Chronosequence of Mature Reforested Mangroves. New Phytologist 2021, 232, 1591–1602. [Google Scholar] [CrossRef]
- Castañeda-Moya, E.; Twilley, R.R.; Rivera-Monroy, V.H. Allocation of Biomass and Net Primary Productivity of Mangrove Forests along Environmental Gradients in the Florida Coastal Everglades, USA. For Ecol Manage 2013, 307, 226–241. [Google Scholar] [CrossRef]
- Tamooh, F.; Huxham, M.; Karachi, M.; Mencuccini, M.; Kairo, J.G.; Kirui, B. Below-Ground Root Yield and Distribution in Natural and Replanted Mangrove Forests at Gazi Bay, Kenya. For Ecol Manage 2008, 256, 1290–1297. [Google Scholar] [CrossRef]
- Castañeda-Moya, E.; Twilley, R.R.; Rivera-Monroy, V.H.; Marx, B.D.; Coronado-Molina, C.; Ewe, S.M.L. Patterns of Root Dynamics in Mangrove Forests Along Environmental Gradients in the Florida Coastal Everglades, USA. Ecosystems 2011, 14, 1178–1195. [Google Scholar] [CrossRef]
- Sánchez-Núñez, D.A.; Mancera-Pineda, J.E. Flowering Patterns in Three Neotropical Mangrove Species: Evidence from a Caribbean Island. Aquat Bot 2011, 94, 177–182. [Google Scholar] [CrossRef]
- Gavio, B.; Palmer-Cantillo, S.; Mancera, J.E. Historical Analysis (2000-2005) of the Coastal Water Quality in San Andrés Island, SeaFlower Biosphere Reserve, Caribbean Colombia. Mar Pollut Bull 2010, 60. [Google Scholar] [CrossRef] [PubMed]
- Vargas, G. Geología y Aspectos Geográficos de La Isla de San Andrés, Colombia. Geología Colombiana 2004, 29, 71–87. [Google Scholar]
- 2017.
- CORALINA; INVEMAR Atlas de La Reserva de Biósfera Seaflower. Archipiélago de San, Providencia y Santa Catalina. Instituto de Investigaciones y Costeras “José Benito Vives De Andréis” -INVEMAR- Y Para El Desarrollo Sostenible Del Archipiélago de San, Providencia y Santa Catalina -CORALINA; Gómez López, D.I., Segura Quintero, C., Sierra Correa, P.C., Garay Tinoco, J., Eds.; Serie de Publicaciones Especiales de INVEMAR # 28: Santa Marta, 2012. [Google Scholar]
- Medina Calderon, J.H. 2016.
- Urrego, L.E.; Polanía, J.; Buitrago, M.F.; Cuartas, L.F.; Lema, A. DISTRIBUTION OF MANGROVES ALONG ENVIRONMENTAL GRADIENTS ON SAN ANDRES ISLAND (COLOMBIAN CARIBBEAN); 2009; Vol. 85;
- Carriker, M.R. Ecology of Estuarine Benthic Invertebrates: A Perspective. Estuaries 1967, 83, 442–487. [Google Scholar]
- 2014.
- Mckee, K.L.; Mendelssohn, I.A.; Hester, M.W. Reexamination of Pore Water Sulfide Concentrations and Redox Potentials Near the Aerial Roots of Rhizophora Mangle and Avicennia Germinans; 1988; Vol. 75;
- Adame, M.F.; Fry, B. Source and Stability of Soil Carbon in Mangrove and Freshwater Wetlands of the Mexican Pacific Coast. Wetl Ecol Manag 2016, 24, 129–137. [Google Scholar] [CrossRef]
- Coronado-Molina, C.; Alvarez-Guillen, H.; Day, J.W.; Reyes, E.; Perez, B.C.; Vera-Herrera, F.; Twilley, R. Litterfall Dynamics in Carbonate and Deltaic Mangrove Ecosystems in the Gulf of Mexico. Wetl Ecol Manag 2012, 20, 123–136. [Google Scholar] [CrossRef]
- Ribeiro, R. de A.; Rovai, A.S.; Twilley, R.R.; Castañeda-Moya, E. Spatial Variability of Mangrove Primary Productivity in the Neotropics. Ecosphere 2019, 10. [Google Scholar] [CrossRef]
- Agraz Hernández, C.M.; García Zaragoza, C.; Iriarte-Vivar, S.; Flores-Verdugo, F.J.; Moreno Casasola, P. Forest Structure, Productivity and Species Phenology of Mangroves in the La Mancha Lagoon in the Atlantic Coast of Mexico. Wetl Ecol Manag 2011, 19, 273–293. [Google Scholar] [CrossRef]
- Torres, J.R.; Sánchez-Mejía, Z.M.; Arreola-Lizárraga, J.A.; Galindo-Félix, J.I.; Mascareño-Grijalva, J.J.; Rodríguez-Pérez, G. Environmental Factors Controlling Structure, Litter Productivity, and Phenology of Mangroves in Arid Region of the Gulf of California. Acta Oecologica 2022, 117, 103861. [Google Scholar] [CrossRef]
- Lopes, D.M.S.; Tognella, M.M.P.; Falqueto, A.R.; Soares, M.L.G. Salinity Variation Effects on Photosynthetic Responses of the Mangrove Species Rhizophora Mangle l. Growing in Natural Habitats. Photosynthetica 2019, 57, 1142–1155. [Google Scholar] [CrossRef]
- Bompy, F.; Lequeue, G.; Imbert, D.; Dulormne, M. Increasing Fluctuations of Soil Salinity Affect Seedling Growth Performances and Physiology in Three Neotropical Mangrove Species. Plant Soil 2014, 380, 399–413. [Google Scholar] [CrossRef]
- Jithesh, M.N.; Prashanth, S.R.; Sivaprakash, K.R.; Parida, A. Monitoring Expression Profiles of Antioxidant Genes to Salinity, Iron, Oxidative, Light and Hyperosmotic Stresses in the Highly Salt Tolerant Grey Mangrove, Avicennia Marina (Forsk.) Vierh. by MRNA Analysis. Plant Cell Rep 2006, 25, 865–876. [Google Scholar] [CrossRef]
- Takemura, T.; Hanagata, N.; Sugihara, K.; Baba, S.; Karube, I.; Dubinsky, Z. Physiological and Biochemical Responses to Salt Stress in the Mangrove, Bruguiera Gymnorrhiza. Aquat Bot 2000, 68, 15–28. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. The Value of Wetlands: Importance of Scale and Landscape Setting. Ecological Economics 2000, 35, 25–33. [Google Scholar] [CrossRef]
- Naidoo, G.; Tuffers, A. V.; von Willert, D.J. Changes in Gas Exchange and Chlorophyll Fluorescence Characteristics of Two Mangroves and a Mangrove Associate in Response to Salinity in the Natural Environment. Trees 2002, 16, 140–146. [Google Scholar] [CrossRef]
- Goussi, R.; Manaa, A.; Derbali, W.; Cantamessa, S.; Abdelly, C.; Barbato, R. Comparative Analysis of Salt Stress, Duration and Intensity, on the Chloroplast Ultrastructure and Photosynthetic Apparatus in Thellungiella Salsuginea. J Photochem Photobiol B 2018, 183, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.R.; Barba, E.; Choix, F.J. Production and Biomass of Mangrove Roots in Relation to Hydroperiod and Physico-Chemical Properties of Sediment and Water in the Mecoacan Lagoon, Gulf of Mexico. Wetl Ecol Manag 2019, 27, 427–442. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B.; Sanada, Y.; Mohanty, P. Effects of Salinity on Biochemical Components of the Mangrove, Aegiceras Corniculatum. Aquat Bot 2004, 80, 77–87. [Google Scholar] [CrossRef]
- Zlatev, Z.S.; Yordanov, I.T. EFFECTS OF SOIL DROUGHT ON PHOTOSYNTHESIS AND CHLOROPHYLL FLUORESCENCE IN BEAN PLANTS; 2004; Vol. 30;
- Quadros, A.F.; Nordhaus, I.; Reuter, H.; Zimmer, M. Modelling of Mangrove Annual Leaf Litterfall with Emphasis on the Role of Vegetation Structure. Estuar Coast Shelf Sci 2019, 218, 292–299. [Google Scholar] [CrossRef]
- Moreno, E.; .Guerrero, A.; Gutiérrez, M.D.C.; Ortiz, C.A.; Palma, D.J. Los Manglares de Tabasco, Una Reserva Natural de Carbono. Madera y Bosques 2002, 8, 115–128. [Google Scholar] [CrossRef]
- Yáñez-Arancibia, A.; Twilley, R.R.; Lara-Domínguez, A.L. Los Ecosistemas de Manglar Frente al Cambio Climático Global; 1998; Vol. 4;
- McKee, K.L. Seedling Recruitment Patterns in a Belizean Mangrove Forest: Effects of Establishment Ability and Physico-Chemical Factors. Oecologia 1995, 101, 448–460. [Google Scholar] [CrossRef]
- Sierra-Rozo, O.; Ernesto, J.; Pineda, M.; Santos-Martínez, A.; Co, J.E. ISLA, CARIBE COLOMBIANO*. 2009.
- Núñez-Ravelo, F.; Ugas-Pérez, M.; Calderón-Castellanos, R.; Rivas-Meriño, F. Cuantificación Del Carbono Orgánico y Materia Orgánica En Suelos No Rizosféricos o Cubiertos Por Avicennia Germinans (L.) y Conocarpus Erectus (L.) Emplazados En Boca de Uchire, Laguna de Unare, Estado de Anzoátegui, Venezuela. Revista Geográfica de América Central 2021, 1, 371–398. [Google Scholar] [CrossRef]
- García- Hansen, I.; Gaviria- Chiquazuque, J.F.; Prada- Triana, M.C.; Alvarez-León, R. Producción de Hojarasca de Los Manglares de La Isla de San Andrés, Caribe Colombiano. Revista Biología Tropical 2002, 50, 273–291. [Google Scholar]
- Pezeshki, S.R.; DeLaune, R.D.; Meeder, J.F. Carbon Assimilation and Biomass Partitioning in Avicennia Germinans and Rhizophora Mangle Seedlings in Response to Soil Redox Conditions. Environ Exp Bot 1997, 37, 161–171. [Google Scholar] [CrossRef]
- Krauss, K.W.; Twilley, R.R.; Doyle, T.W.; Gardiner, E.S. Leaf Gas Exchange Characteristics of Three Neotropical Mangrove Species in Response to Varying Hydroperiod. Tree Physiol 2006, 26, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.E.; Fahey, T.J.; Martinez, P. Spatial Patterns of Biomass and Aboveground Net Primary Productivity in a Mangrove Ecosystem in the Dominican Republic. Ecosystems 2003, 6, 384–398. [Google Scholar] [CrossRef]
- Chen, G.C.; Tam, N.F.Y.; Ye, Y. Summer Fluxes of Atmospheric Greenhouse Gases N2O, CH4 and CO2 from Mangrove Soil in South China. Science of The Total Environment 2010, 408, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Agraz Hernández, C.M.; Chan Keb, C.A.; Iriarte-Vivar, S.; Posada Venegas, G.; Serratos, B.V.; Osti Sáenz, J. Phenological Variation of Rhizophora Mangle and Ground Water Chemistry Associated to Changes of the Precipitation Variación Fenológica de Rhizophora Mangle y Química Del Agua Intersticial Asociada a Cambios de La Precipitación; 2015; Vol. 25;
- Lovelock, C.E.; Feller, I.C. Photosynthetic Performance and Resource Utilization of Two Mangrove Species Coexisting in a Hypersaline Scrub Forest. Oecologia 2003, 134, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Poveda, G.; Mesa, Ó.J. Las Fases Extremas Del Fenómeno ENSO (El Niño y La Niña) y Su Influencia Sobre La Hidrología de Colombia. Ingenieria Hidráulica en México 1996, 9, 21–37. [Google Scholar]
- Robles Sanchéz, A. ; Mancera Pineda José Ernesto; Marquínez Casas Xavier; Medina Calderón Jairo Humberto Influence of Edaphic Salinity on Leaf Morphoanatomical Functional Traits on Juvenile and Adult Trees of Red Mangrove (Rhizophora Mangle): Implications with Relation to Climate Change. Forests 2021, 12, 1586. [Google Scholar] [CrossRef]
- Adame, M.F.; Kauffman, J.B.; Medina, I.; Gamboa, J.N.; Torres, O.; Caamal, J.P.; Reza, M.; Herrera-Silveira, J.A. Carbon Stocks of Tropical Coastal Wetlands within the Karstic Landscape of the Mexican Caribbean. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Torres V., J. R.; Barba-Macías, E.; Sánchez, A.J. Tres Años de Producción de Hojarasca Del Manglar y Su Relación Con Las Condiciones Ambientales En La Laguna Mecoacán, Golfo de México. Ecosistemas 2023, 32, 2368. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Mayalagu, R. 2008.
- Rovai, A.S.; Riul, P.; Twilley, R.R.; Castañeda-Moya, E.; Rivera-Monroy, V.H.; Williams, A.A.; Simard, M.; Cifuentes-Jara, M.; Lewis, R.R.; Crooks, S.; et al. Scaling Mangrove Aboveground Biomass from Site-level to Continental-scale. Global Ecology and Biogeography 2016, 25, 286–298. [Google Scholar] [CrossRef]
- Huxham, M.; Whitlock, D.; Githaiga, M.; Dencer-Brown, A. Carbon in the Coastal Seascape: How Interactions Between Mangrove Forests, Seagrass Meadows and Tidal Marshes Influence Carbon Storage. Current Forestry Reports 2018, 4, 101–110. [Google Scholar] [CrossRef]
- Twilley, R.R. Properties of Mangrove Ecosystems Related to the Energy Signature of Coastal Environments. In Maximum power: the ideas and applications of H. T. Odum; Twilley Robert, R, Ed.; University Press of Colorado: Denver, Colorado, USA, 1995; pp. 43–62. [Google Scholar]
- Saintilan, N.; Khan, N.S.; Ashe, E.; Kelleway, J.J.; Rogers, K.; Woodroffe, C.D.; Horton, B.P. Thresholds of Mangrove Survival under Rapid Sea Level Rise. Science (1979) 2020, 368, 1118–1121. [Google Scholar] [CrossRef]
- Rajaniemi, T.K.; Allison, V.J. Abiotic Conditions and Plant Cover Differentially Affect Microbial Biomass and Community Composition on Dune Gradients. Soil Biol Biochem 2009, 41, 102–109. [Google Scholar] [CrossRef]
- Adame, M.F.; Teutli, C.; Santini, N.S.; Caamal, J.P.; Zaldívar-Jiménez, A.; Herńndez, R.; Herrera-Silveira, J.A. Root Biomass and Production of Mangroves Surrounding a Karstic Oligotrophic Coastal Lagoon. Wetlands 2014, 34, 479–488. [Google Scholar] [CrossRef]
- Meera, S.P.; Bhattacharyya, M.; Kumar, A. Dynamics of Mangrove Functional Traits under Osmotic and Oxidative Stresses. Plant Growth Regul 2023, 101, 285–306. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Stanton, D.E.; Schmitz, N.; Farquhar, G.D.; Ball, M.C. Growth Responses of the Mangrove Avicennia Marina to Salinity: Development and Function of Shoot Hydraulic Systems Require Saline Conditions. Ann Bot 2015, 115, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Vovides, A.G.; Berger, U.; Grueters, U.; Guevara, R.; Pommerening, A.; Lara-Domínguez, A.L.; López-Portillo, J. Change in Drivers of Mangrove Crown Displacement along a Salinity Stress Gradient. Funct Ecol 2018, 32, 2753–2765. [Google Scholar] [CrossRef]
- Pi, N.; Tam, N.F.Y.; Wu, Y.; Wong, M.H. Root Anatomy and Spatial Pattern of Radial Oxygen Loss of Eight True Mangrove Species. Aquat Bot 2009, 90, 222–230. [Google Scholar] [CrossRef]
- López Rodríguez, A.; Cecilia, Á.; Correa, S.; Cristina, P.; Ortiz, H.; Guzmán, M.; Zapata, L. 2009.
- Ahmed, S.; Sarker, S.K.; Friess, D.A.; Kamruzzaman, Md.; Jacobs, M.; Islam, Md.A.; Alam, Md.A.; Suvo, M.J.; Sani, Md.N.H.; Dey, T.; et al. Salinity Reduces Site Quality and Mangrove Forest Functions. From Monitoring to Understanding. Science of The Total Environment 2022, 853, 158662. [Google Scholar] [CrossRef]
- Arnaud, M.; Krause, S.; Norby, R.J.; Dang, T.H.; Acil, N.; Kettridge, N.; Gauci, V.; Ullah, S. Global Mangrove Root Production, Its Controls and Roles in the Blue Carbon Budget of Mangroves. Glob Chang Biol 2023, 29, 3256–3270. [Google Scholar] [CrossRef]
- Krauss, K.W.; McKee, K.L.; Lovelock, C.E.; Cahoon, D.R.; Saintilan, N.; Reef, R.; Chen, L. How Mangrove Forests Adjust to Rising Sea Level. New Phytologist 2014, 202, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Salmo, S.G.; Lovelock, C.; Duke, N.C. Vegetation and Soil Characteristics as Indicators of Restoration Trajectories in Restored Mangroves. Hydrobiologia 2013, 720, 1–18. [Google Scholar] [CrossRef]
- McKee, K.L.; Krauss, K.W.; Cahoon, D.R. Does Geomorphology Determine Vulnerability of Mangrove Coasts to Sea-Level Rise? In Dynamic Sedimentary Environments of Mangrove Coasts; Elsevier, 2021; pp. 255–272.
- Breithaupt, J.L.; Steinmuller, H.E. Refining the Global Estimate of Mangrove Carbon Burial Rates Using Sedimentary and Geomorphic Settings. Geophys Res Lett 2022, 49. [Google Scholar] [CrossRef]
| Forest | Salinity (PSU) | Species | Density In ha-1 |
DBH (≥2.5 cm) |
Basal area (m2 ha-1) |
IVI |
|---|---|---|---|---|---|---|
| BHC | 62.4± 10.4 | Ag | 2242 ± 32.4 | 9.1 ± 0.3 | 19.8 ± 0.5 | 221 |
| Lr | 125 ± 55.9 | 10.8 ± 1.1 | 1.3 ± 0.08 | 37 | ||
| Rm | 183 ± 67.7 | 7.1 ±0.5 | 0.8 ± 0.04 | 42 | ||
| Total | 2550 ± 71.7 a | 9.1 ± 0.3 b | 21.9 ± 0.5 a | 300 | ||
| BHF | 37.5± 5.8 | Ag | 215 ± 0.5 | 8.8 ± 0.8 | 1.7 ± 0.2 | 26 |
| Lr | 920± 1.7 | 8.1 ± 0.3 | 6.3 ± 0.3 | 104 | ||
| Rm | 2520 ± 2.3 | 6.8 ± 0.1 | 10.9 ± 0.2 | 170 | ||
| Total | 3655 ± 43.7 ab | 7.3 ± 0.1b | 18.9 ± 0.4 a | 300 | ||
| SBTA | 11.5 ± 7.5 | Ag | 356 ± 0.5 | 14.8 ± 1.1 | 9.8 ± 1.2 | 133 |
| Lr | 188 ± 0.8 | 27.6 ± 2 | 13.2 ± 2 | 99 | ||
| Rm | 194 ± 1.1 | 13.04 ± 1.5 | 4.2 ± 0.8 | 68 | ||
| Total | 738 ± 8.8 b | 18.5 ± 0.73 b | 27.3 ± 0.4 b | 300 | ||
| SCTA | 9.6± 6.3 | Lr | 75±13 | 34.1 ± 1.4 | 9.6 ± 2.6 | 61 |
| Rm | 313 ± 24 | 28.5 ± 0.7 | 28.2 ± 0.1 | 182 | ||
| Other spp. | 16± 9 | 7.2 ± 0.4 | 58 | |||
| Total | 404± 26 ab | 23.3 ± 8.2b | 37.8 ± 2.5 b | 300 | ||
| Df | 3 | 3 | 3 | |||
| P | 0.01073 | 0.006131 | 0.05688 | |||
| X2 | 11.192 | 12.4 | 7.5267 | |||
| Size of roots | ||||
|---|---|---|---|---|
| Forest | < 2 mm Mg C ha-1y-1 |
2 - 5 mm Mg C ha-1y-1 |
> 5 mm Mg C ha-1y-1 |
Total Mg C ha-1y-1 |
| BHC | 0.08 ±0.06 | 0.09 ± 0.07 | 0.07 ± 0.10 | 0.24 ± 0.20 ab |
| BHF | 0.48 ± 0.30 | 0.28 ± 0.10 | 0.54 ± 0.34 | 1.30 ± 0.5 a |
| SBTA | 0.41 ± 0.08 | 0.24 ± 0.07 | 0.54 ± 0.36 | 1.19 ± 0.46 ab |
| SCTA | 0.16 ± 0.03 | 0.10 ± 0.02 | 0.16 ± 0.06 | 0.41± 0.08 a |
| P | 0.0007887 | 0.0002478 | 0.02384 | 0.005174 |
| Df | 3 | 3 | 3 | 3 |
| X2 | 16.77 | 19.21 | 9.45 | 12.76 |
| Forest | PSU anual | PSU by period | |
|---|---|---|---|
| Rainy | Dry | ||
| BHC | 62.3 ±10.5 a | 60.7± 0.9 a | 62.5± 1.0 a |
| BHF | 37.4 ± 5.7 b | 36.6± 0.4 b | 39.0± 0.3 b |
| SBTA | 11.5± 7.4 c | 9.4± 0.6 c | 17.0± 0.8d |
| SCTA | 9.6± 6.2 d | 9.5± 0.4 c | 9.8 ± 0.5 c |
| Production Forest (Mg C Ha-1y-1) |
Ws Prom m/s |
P Mm |
T °C |
|||
|---|---|---|---|---|---|---|
| R | P | R | P | r | p | |
| BHC | -0.73 | 0.0394* | 0.16 | 0.6963 | 0.10 | 0.8186 |
| BHF | 0.46 | 0.2508 | 0.12 | 0.7820 | -0.36 | 0.3794 |
| SCTA | 0.38 | 0.3489 | 0.43 | 0.2916 | -0.23 | 0.5800 |
| Production Forest (Mg C Ha-1m-1) |
Salinity PSU |
Ws Prom m/s |
P Mm |
T °C |
||||
|---|---|---|---|---|---|---|---|---|
| R | P | R | P | R | P | r | P | |
| SBTA | -0.21 | 0.5024 | -0.21 | 0.5070 | 0.45 | 0.1464 | 0.22 | 0.4820 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





