Submitted:
22 January 2025
Posted:
23 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
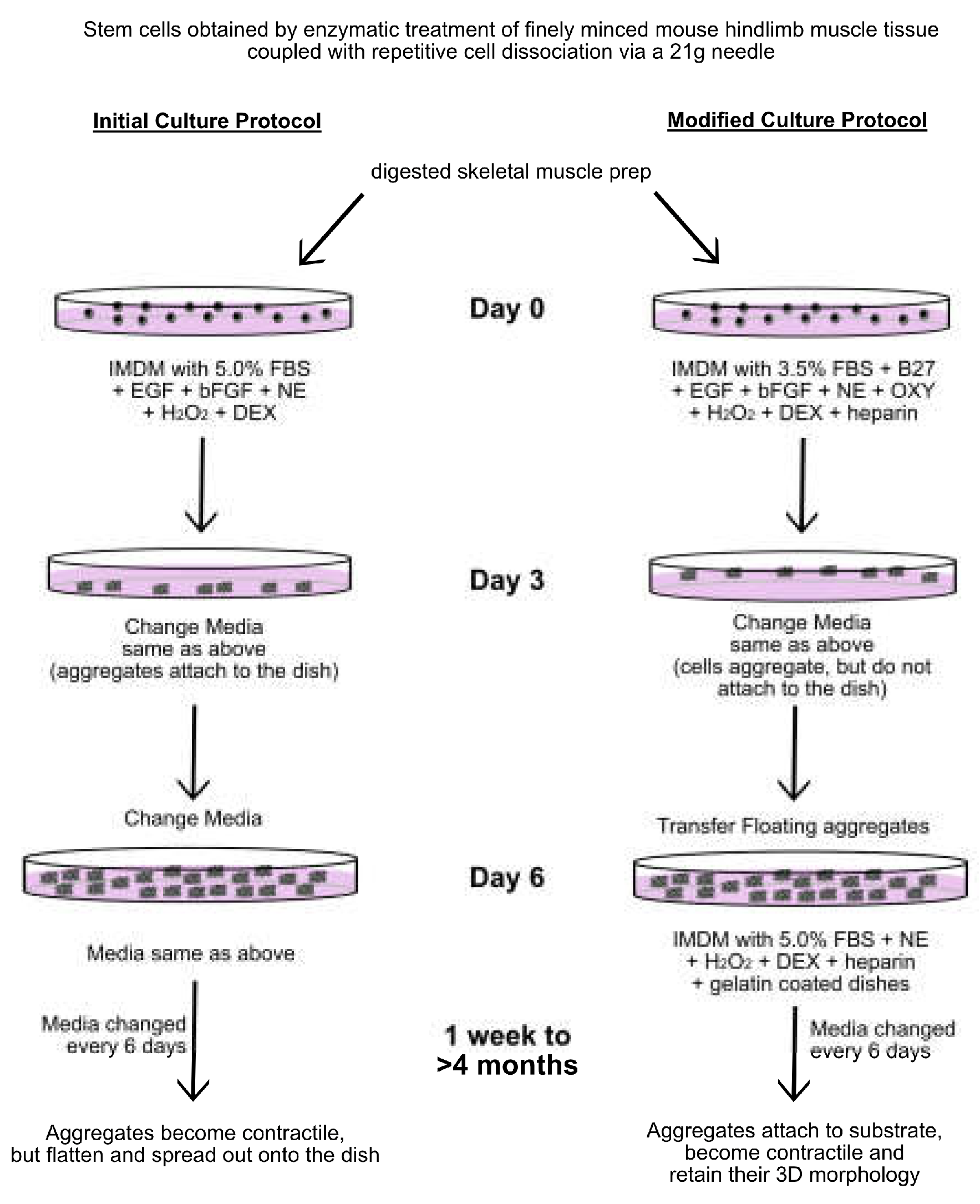
2.1. Stem Cell Isolation
2.2. Skeletal Muscle Differentiation
2.3. Analysis of the Initial Non-Adherent Cell Population
2.4. Cardiac Myosphere Differentiation
2.5. Long-Term Myosphere Cultures
2.6. Immunocytochemistry of Myospheres
2.7. Contractility and Live Imaging Analysis of Cardiac Myospheres
3. Results
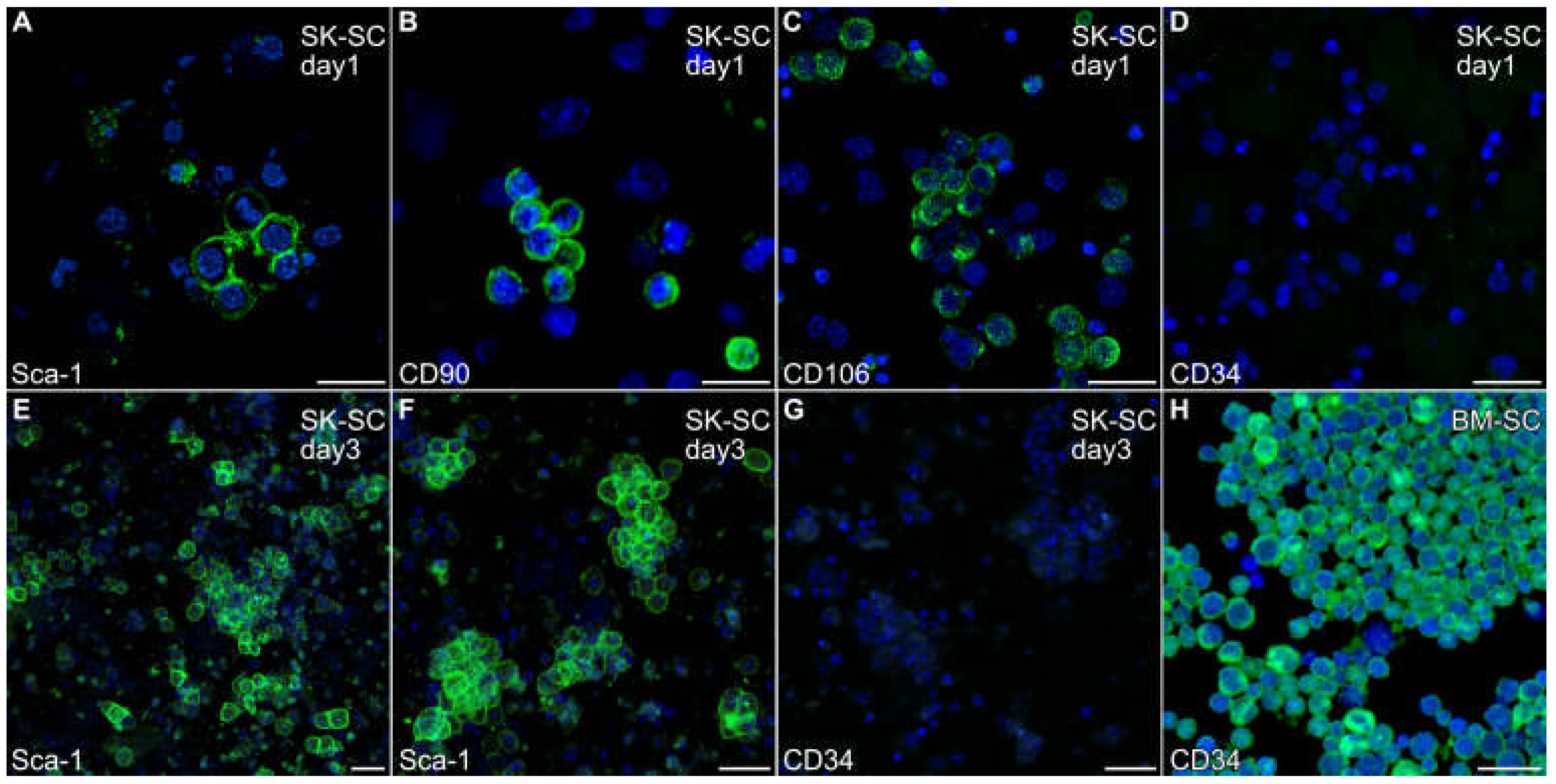
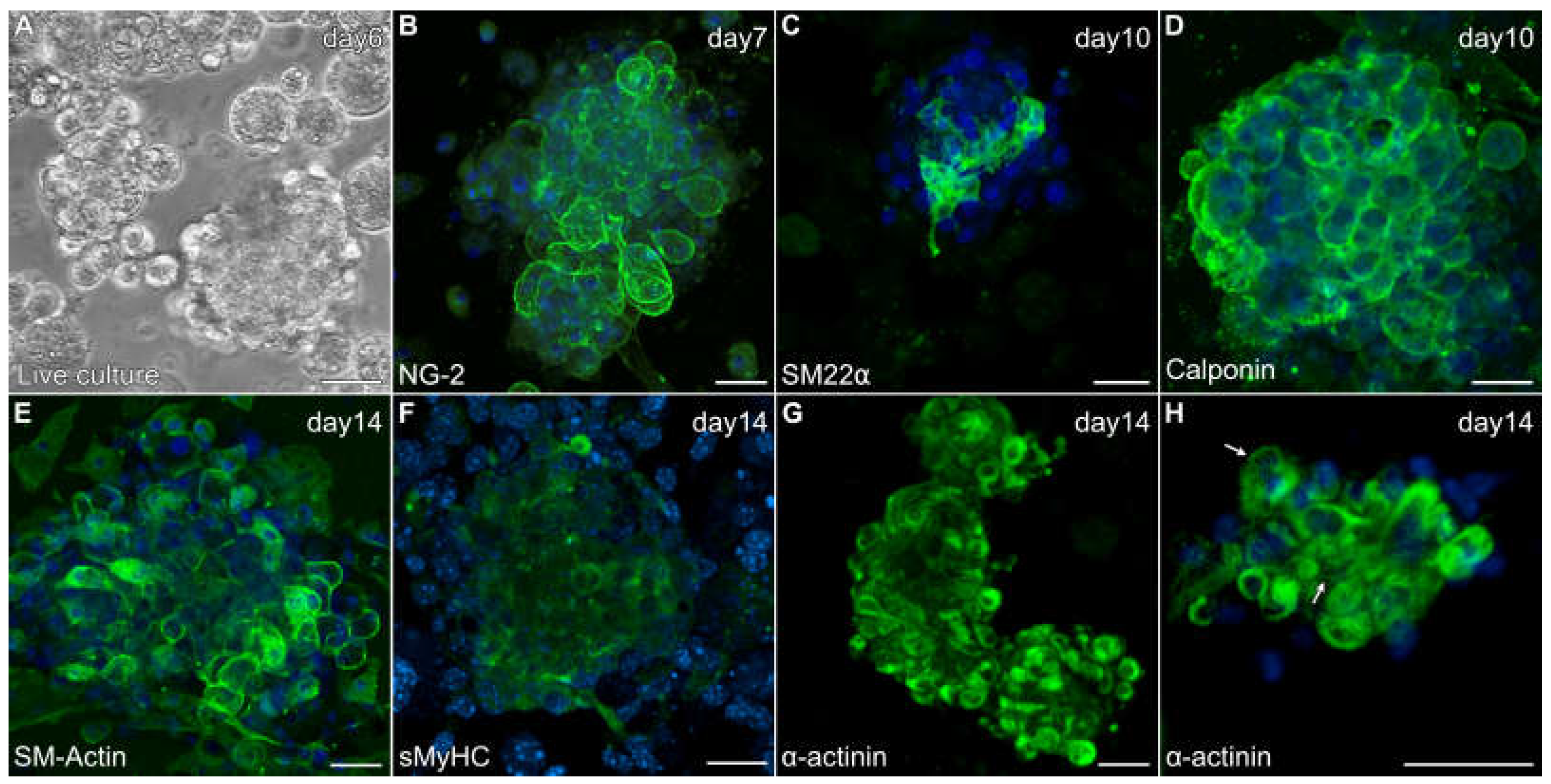
3.1. Characterization of Skeletal Muscle Stem Cells
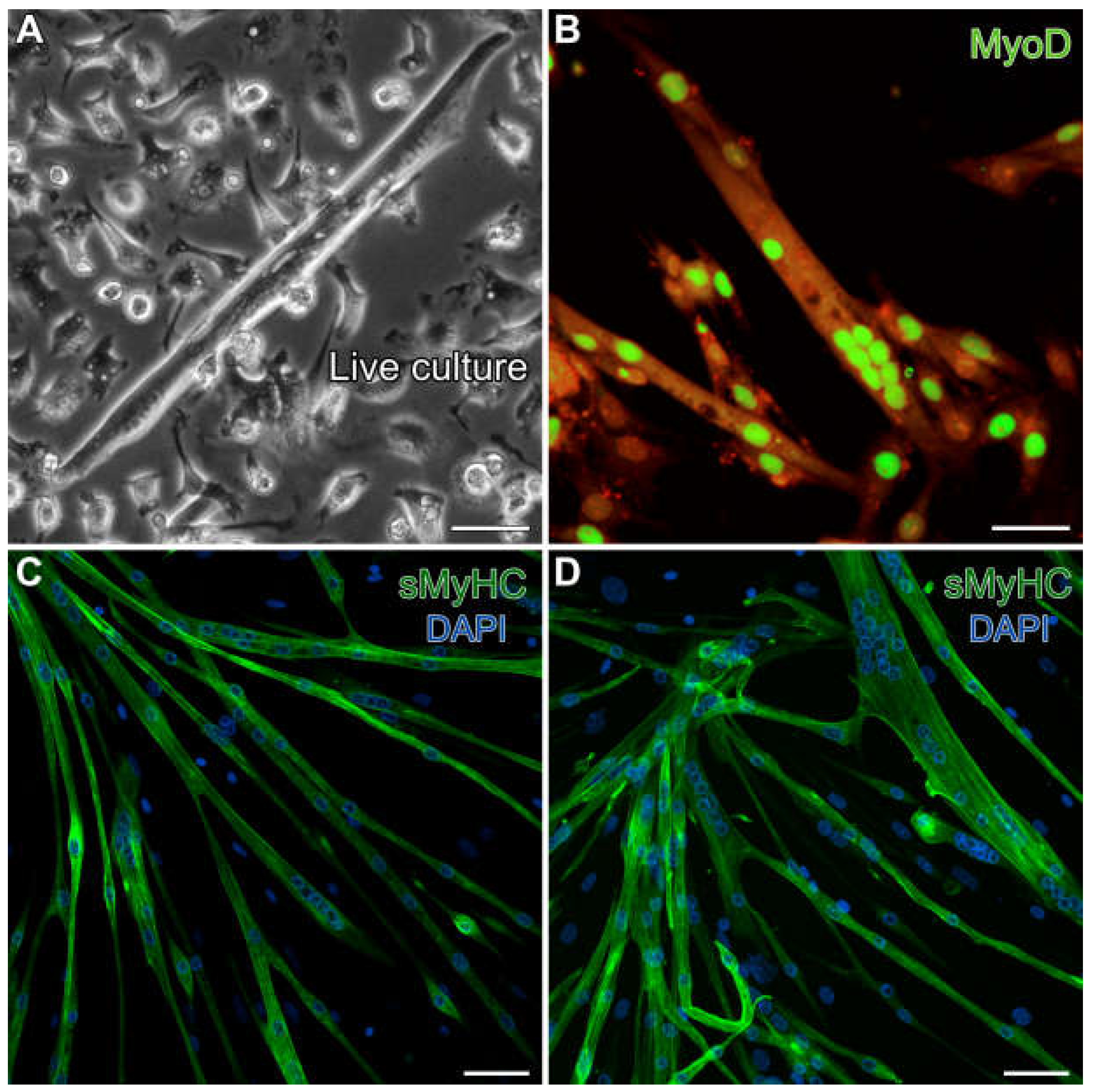
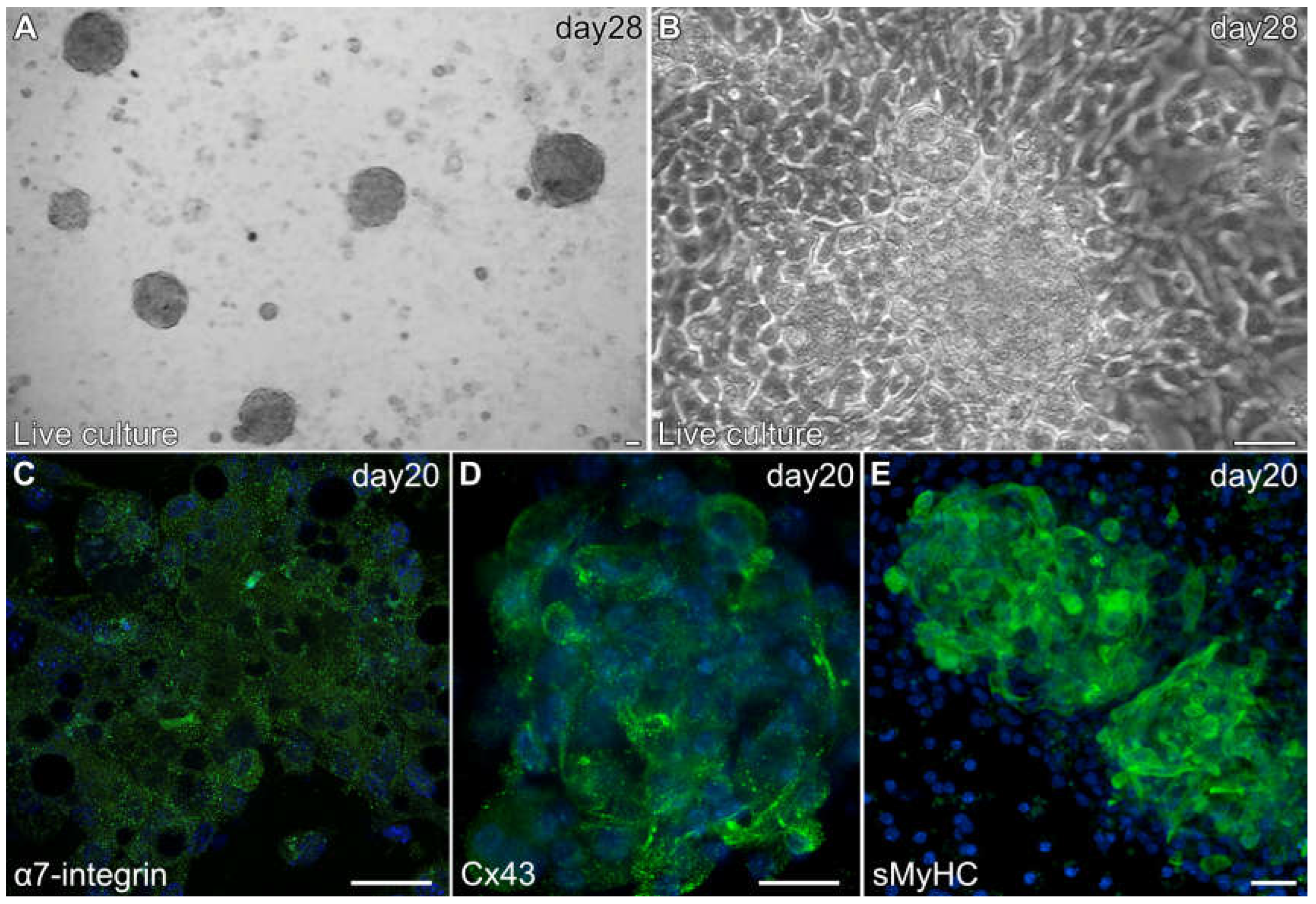
3.2. Aggregation of Skeletal-Muscle Stem Cells and Cardiac Differentiation
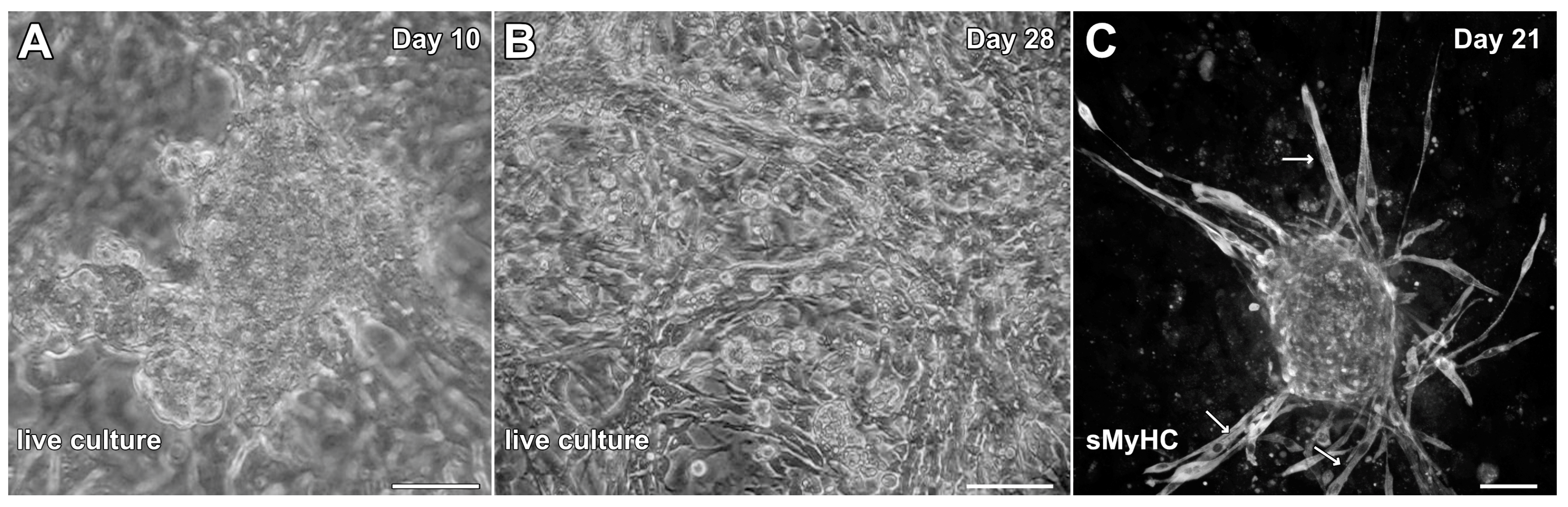
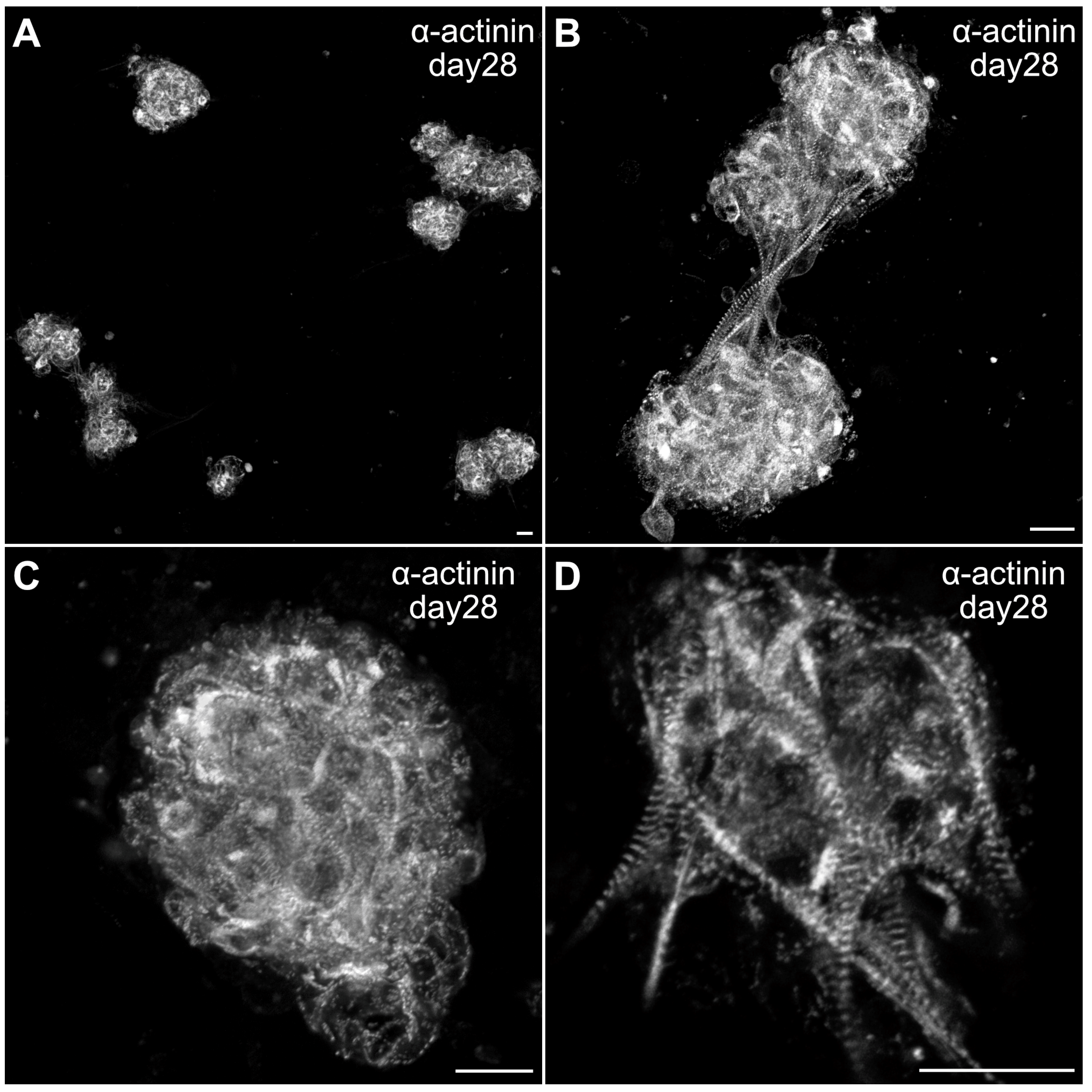
3.3. Formation of Contractile Cardiac Tissue Without Skeletal-Muscle Contamination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Redkar, A.; Montgomery, M.; Litvin, J. Fate map of early avian cardiac progenitor cells. Development 2001, 128, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Stalsberg, H.; DeHaan, R.L. The precardiac areas and formation of the tubular heart in the chick embryo. Dev Biol 1969, 19, 128–159. [Google Scholar] [CrossRef] [PubMed]
- Camp, E.; Dietrich, S.; Münsterberg, A. Fate mapping identifies the origin of SHF/AHF progenitors in the chick primitive streak. PLoS One 2012, 7, e51948. [Google Scholar] [CrossRef]
- Mjaatvedt, C.; Nakaoka, T.; Moreno-Rodriguez, R.; Norris, R.; Kern, M.; Eisenberg, C.; Turner, D.; Markwald, R. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol 2001, 238, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Ordahl, C.P.; Douarin, N.M.L. Two myogenic lineages within the developing somite. Development 1992, 114, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Piatkowska, A.M.; Evans, S.E.; Stern, C.D. Cellular aspects of somite formation in vertebrates. Cells Dev 2021, 168, 203732. [Google Scholar] [CrossRef]
- Adams, R.J.; Schwartz, A. Comparative mechanisms for contraction of cardiac and skeletal muscle. Chest 1980, 78, 123–139. [Google Scholar] [CrossRef]
- Bottinelli, R.; Reggiani, C. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol 2000, 73, 195–262. [Google Scholar] [CrossRef]
- Ríos, E.; Figueroa, L.; Manno, C.; Kraeva, N.; Riazi, S. The couplonopathies: A comparative approach to a class of diseases of skeletal and cardiac muscle. J Gen Physiol 2015, 145, 459–474. [Google Scholar] [CrossRef]
- Akazawa, H.; Komuro, I. Cardiac transcription factor Csx/Nkx2-5: Its role in cardiac development and diseases. Pharmacology & Therapeutics 2005, 107, 252–268. [Google Scholar] [CrossRef]
- Cao, C.; Li, L.; Zhang, Q.; Li, H.; Wang, Z.; Wang, A.; Liu, J. Nkx2. 5: a crucial regulator of cardiac development, regeneration and diseases. Front Cardiovasc Med 2023, 10, 1270951. [Google Scholar] [CrossRef] [PubMed]
- Pownall, M.E.; Gustafsson, M.K.; Emerson Jr, C.P. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu Rev Cell Dev Biol 2002, 18, 747–783. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Miano, J.M.; Cserjesi, P.; Olson, E.N. SM22α, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res 1996, 78, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Sugi, Y.; Lough, J. Onset of expression and regional deposition of alpha-smooth and sarcomeric actin during avian heart development. Dev Dyn 1992, 193, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Ya, J.; Markman, M.W.; Wagenaar, G.T.; Blommaart, P.J.B.; Moorman, A.F.; Lamers, W.H. Expression of the smooth-muscle proteins α-smooth-muscle actin and calponin, and of the intermediate filament protein desmin are parameters of cardiomyocyte maturation in the prenatal rat heart. Anat Rec 1997, 249, 495–505. [Google Scholar] [CrossRef]
- Ordahl, C.P. The skeletal and cardiac α-action genes are coexpressed in early embryonic striated muscle. Dev Biol 1986, 117, 488–492. [Google Scholar] [CrossRef]
- Clause, K.C.; Tchao, J.; Powell, M.C.; Liu, L.J.; Huard, J.; Keller, B.B.; Tobita, K. Developing cardiac and skeletal muscle share fast-skeletal myosin heavy chain and cardiac troponin-I expression. PLoS One 2012, 7, e40725. [Google Scholar] [CrossRef]
- Cuda, G.; Fananapazir, L.; Zhu, W.; Sellers, J.; Epstein, N. Skeletal muscle expression and abnormal function of beta-myosin in hypertrophic cardiomyopathy. J Clin Invest 1993, 91, 2861–2865. [Google Scholar] [CrossRef]
- McNally, E.M.; Golbus, J.R.; Puckelwartz, M.J. Genetic mutations and mechanisms in dilated cardiomyopathy. J Clin Invest 2013, 123, 19–26. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, J.; Liu, Q.; Cai, J.; Zheng, Y.; Zhang, Y.; Yu, D.; Liu, H.; Zhang, Z. IGF1 Knockdown Hinders Myocardial Development through Energy Metabolism Dysfunction Caused by ROS-Dependent FOXO Activation in the Chicken Heart. Oxid Med Cell Longev 2019, 2019, 7838754. [Google Scholar] [CrossRef]
- Takebayashi-Suzuki, K.; Pauliks, L.B.; Eltsefon, Y.; Mikawa, T. Purkinje fibers of the avian heart express a myogenic transcription factor program distinct from cardiac and skeletal muscle. Dev Biol 2001, 234, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Haider, H.K.; Ye, L.; Ashraf, M. Skeletal muscle derived stem cells for myocardial repair. In Frontiers in Cardiovascular Drug Discovery; 2010; Volume 1, pp. 288-306.
- Hassan, N.; Tchao, J.; Tobita, K. Concise review: skeletal muscle stem cells and cardiac lineage: potential for heart repair. Stem Cells Trans Med 2014, 3, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P. Skeletal myoblasts and cardiac repair. J Mol Cell Cardiol 2008, 45, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Pedrotty, D.M.; Koh, J.; Davis, B.H.; Taylor, D.A.; Wolf, P.; Niklason, L.E. Engineering skeletal myoblasts: roles of three-dimensional culture and electrical stimulation. Am J Physiol Heart Circ Physiol 2005, 288, H1620–H1626. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H.; Poppa, V.; Murry, C.E. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol 2002, 34, 241–249. [Google Scholar] [CrossRef]
- Rikhtegar, R.; Pezeshkian, M.; Dolati, S.; Safaie, N.; Rad, A.A.; Mahdipour, M.; Nouri, M.; Jodati, A.R.; Yousefi, M. Stem cells as therapy for heart disease: iPSCs, ESCs, CSCs, and skeletal myoblasts. Biomed Pharmacother 2019, 109, 304–313. [Google Scholar] [CrossRef]
- Rubart, M.; Soonpaa, M.H.; Nakajima, H.; Field, L.J. Spontaneous and evoked intracellular calcium transients in donor-derived myocytes following intracardiac myoblast transplantation. J Clin Invest 2004, 114, 775–783. [Google Scholar] [CrossRef]
- Eisenberg, L.M.; Eisenberg, C.A. Embryonic myocardium shows increased longevity as a functional tissue when cultured in the presence of a noncardiac tissue layer. Tissue Eng 2006, 12, 853–865. [Google Scholar] [CrossRef]
- Eisenberg, L.M.; Kaur, K.; Castillo, J.M.; Edwards, J.G.; Eisenberg, C.A. Dexamethasone Treatment Preserves the Structure of Adult Cardiac Explants and Supports Their Long-Term Contractility In Vitro. Int J Transl Med 2023, 3, 360–373. [Google Scholar] [CrossRef]
- Kaur, K.; Yang, J.; Edwards, J.; Eisenberg, C.; Eisenberg, L. G9a histone methyltransferase inhibitor BIX 01294 promotes expansion of adult cardiac progenitor cells without changing their phenotype or differentiation potential. Cell Prolif 2016, 49, 373–385. [Google Scholar] [CrossRef]
- Yang, J.; Kaur, K.; Edwards, J.G.; Eisenberg, C.A.; Eisenberg, L.M. Inhibition of histone methyltransferase, histone deacetylase, and β-catenin synergistically enhance the cardiac potential of bone marrow cells. Stem Cells Int 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Rémond, M.C.; Iaffaldano, G.; O’Quinn, M.P.; Mezentseva, N.V.; Garcia, V.; Harris, B.S.; Gourdie, R.G.; Eisenberg, C.A.; Eisenberg, L.M. GATA6 reporter gene reveals myocardial phenotypic heterogeneity that is related to variations in gap junction coupling. Am J Physiol Heart Circ Physiol 2011, 301, H1952–H1964. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kaur, K.; Ong, L.L.; Eisenberg, C.A.; Eisenberg, L.M. Inhibition of G9a histone methyltransferase converts bone marrow mesenchymal stem cells to cardiac competent progenitors. Stem Cells Int 2015, 2015, 270428. [Google Scholar] [CrossRef] [PubMed]
- Messina, E.; De Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battaglia, M.; Latronico, M.V.; Coletta, M. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 2004, 95, 911–921. [Google Scholar] [CrossRef]
- Sugi, Y.; Markwald, R.R. Formation and early morphogenesis of endocardial endothelial precursor cells and the role of endoderm. Dev Biol 1996, 175, 66–83. [Google Scholar] [CrossRef]
- Eisenberg, C.A.; Bader, D. QCE-6: a clonal cell line with cardiac myogenic and endothelial cell potentials. Dev Biol 1995, 167, 469–481. [Google Scholar] [CrossRef]
- Eisenberg, C.A.; Gourdie, R.G.; Eisenberg, L.M. Wnt-11 is expressed in early avian mesoderm and required for the differentiation of the quail mesoderm cell line QCE-6. Development 1997, 124, 525–536. [Google Scholar] [CrossRef]
- Kaur, K.; Yang, J.; Eisenberg, C.A.; Eisenberg, L.M. 5-azacytidine promotes the transdifferentiation of cardiac cells to skeletal myocytes. Cell Reprogram 2014, 16, 324–330. [Google Scholar] [CrossRef]
- Clause, K.C.; Tinney, J.P.; Liu, L.J.; Gharaibeh, B.; Huard, J.; Kirk, J.A.; Shroff, S.G.; Fujimoto, K.L.; Wagner, W.R.; Ralphe, J.C. A Three-Dimensional Gel Bioreactor for Assessment of Cardiomyocyte Induction in Skeletal Muscle–Derived Stem Cells. Tissue Eng Part C Methods 2010, 16, 375–385. [Google Scholar] [CrossRef]
- Winitsky, S.O.; Gopal, T.V.; Hassanzadeh, S.; Takahashi, H.; Gryder, D.; Rogawski, M.A.; Takeda, K.; Yu, Z.X.; Xu, Y.H.; Epstein, N.D. Adult murine skeletal muscle contains cells that can differentiate into beating cardiomyocytes in vitro. PLoS biol 2005, 3, e87. [Google Scholar] [CrossRef]
- Judson, R.N.; Zhang, R.H.; Rossi, F.M. Tissue-resident mesenchymal stem/progenitor cells in skeletal muscle: collaborators or saboteurs? FEBS J 2013, 280, 4100–4108. [Google Scholar] [CrossRef] [PubMed]
- Pannérec, A.; Marazzi, G.; Sassoon, D. Stem cells in the hood: the skeletal muscle niche. Trends Mol Med 2012, 18, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Sesillo, F.B.; Wong, M.; Cortez, A.; Alperin, M. Isolation of muscle stem cells from rat skeletal muscles. Stem Cell Res 2020, 43, 101684. [Google Scholar] [CrossRef] [PubMed]
- Shahini, A.; Vydiam, K.; Choudhury, D.; Rajabian, N.; Nguyen, T.; Lei, P.; Andreadis, S.T. Efficient and high yield isolation of myoblasts from skeletal muscle. Stem Cell Res 2018, 30, 122–129. [Google Scholar] [CrossRef]
- Matsuura, K.; Nagai, T.; Nishigaki, N.; Oyama, T.; Nishi, J.; Wada, H.; Sano, M.; Toko, H.; Akazawa, H.; Sato, T. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem 2004, 279, 11384–11391. [Google Scholar] [CrossRef]
- Paquin, J.; Danalache, B.A.; Jankowski, M.; McCann, S.M.; Gutkowska, J. Oxytocin induces differentiation of P19 embryonic stem cells to cardiomyocytes. Proc Natl Acad Sci U S A 2002, 99, 9550–9555. [Google Scholar] [CrossRef]
- Gaughan, J.P.; Hefner, C.A.; Houser, S.R. Electrophysiological properties of neonatal rat ventricular myocytes with α1-adrenergic-induced hypertrophy. Am J Physiol Heart Circ Physiol 1998, 275, H577–H590. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Ehrlich, B.E. Signaling in muscle contraction. Cold Spring Harb Perspect Biol 2015, 7, a006023. [Google Scholar] [CrossRef]
- Constantin, B.; Cognard, C.; Raymond, G. Myoblast fusion is not a prerequisite for the appearance of calcium current, calcium release, and contraction in rat skeletal muscle cells developing in culture. Exp Cell Res 1995, 217, 497–505. [Google Scholar] [CrossRef]
- Eisenberg, C.A.; Eisenberg, L.M. WNT11 promotes cardiac tissue formation of early mesoderm. Dev Dyn 1999, 216, 45–58. [Google Scholar] [CrossRef]
- Gharaibeh, B.; Lu, A.; Tebbets, J.; Zheng, B.; Feduska, J.; Crisan, M.; Péault, B.; Cummins, J.; Huard, J. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc 2008, 3, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Neef, K.; Treskes, P.; Xu, G.; Drey, F.; Srinivasan, S.P.; Saric, T.; Nembo, E.; Semmler, J.; Nguemo, F.; Stamm, C. Dynamic Support Culture of Murine Skeletal Muscle-Derived Stem Cells Improves Their Cardiogenic Potential In Vitro. Stem Cells Int 2015, 2015, 247091. [Google Scholar] [CrossRef] [PubMed]
- Invernici, G.; Cristini, S.; Madeddu, P.; Brock, S.; Spillmann, F.; Bernasconi, P.; Cappelletti, C.; Calatozzolo, C.; Fascio, U.; Bisleri, G. Human adult skeletal muscle stem cells differentiate into cardiomyocyte phenotype in vitro. Exp Cell Res 2008, 314, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Hatami, L.; Valojerdi, M.R.; Mowla, S.J. Effects of oxytocin on cardiomyocyte differentiation from mouse embryonic stem cells. Int J Cardiol 2007, 117, 80–89. [Google Scholar] [CrossRef]
- White, S.M.; Constantin, P.E.; Claycomb, W.C. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol 2004, 286, H823–H829. [Google Scholar] [CrossRef]
- Natarajan, A.R.; Rong, Q.; Katchman, A.N.; Ebert, S.N. Intrinsic cardiac catecholamines help maintain beating activity in neonatal rat cardiomyocyte cultures. Pediatr Res 2004, 56, 411–417. [Google Scholar] [CrossRef]
- Li, J.; Stouffs, M.; Serrander, L.; Banfi, B.; Bettiol, E.; Charnay, Y.; Steger, K.; Krause, K.-H.; Jaconi, M.E. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell 2006, 17, 3978–3988. [Google Scholar] [CrossRef]
- Guo, S.; Okyere, A.D.; McEachern, E.; Strong, J.L.; Carter, R.L.; Patwa, V.C.; Thomas, T.P.; Landy, M.; Song, J.; Lucchese, A.M. Epidermal growth factor receptor-dependent maintenance of cardiac contractility. Cardiovasc Res 2022, 118, 1276–1288. [Google Scholar] [CrossRef]
- Tenin, G.; Clowes, C.; Wolton, K.; Krejci, E.; Wright, J.A.; Lovell, S.C.; Sedmera, D.; Hentges, K.E. Erbb2 is required for cardiac atrial electrical activity during development. PLoS One 2014, 9, e107041. [Google Scholar] [CrossRef]
- Sanchez-Soria, P.; Camenisch, T.D. ErbB signaling in cardiac development and disease. Seminars in cell & developmental biology 2010, 21, 929–935. [Google Scholar] [CrossRef]
- Ebner, R.; Derynck, R. Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regul 1991, 2, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Forough, R.; Scarcello, C.; Perkins, M. Cardiac biomarkers: a focus on cardiac regeneration. J Tehran Heart Cent 2011, 6, 179–186. [Google Scholar] [PubMed]
- Clément, S.; Stouffs, M.; Bettiol, E.; Kampf, S.; Krause, K.-H.; Chaponnier, C.; Jaconi, M. Expression and function of α-smooth muscle actin during embryonic-stem-cell-derived cardiomyocyte differentiation. J Cell Sci 2007, 120, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Ozerdem, U.; Grako, K.A.; Dahlin-Huppe, K.; Monosov, E.; Stallcup, W.B. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn 2001, 222, 218–227. [Google Scholar] [CrossRef]
- Valente, M.; Nascimento, D.S.; Cumano, A.; Pinto-Do-Ó, P. Sca-1+ cardiac progenitor cells and heart-making: a critical synopsis. Stem Cells Dev 2014, 23, 2263–2273. [Google Scholar] [CrossRef]
- Israeli-Rosenberg, S.; Manso, A.M.; Okada, H.; Ross, R.S. Integrins and integrin-associated proteins in the cardiac myocyte. Circ Res 2014, 114, 572–586. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




