Submitted:
22 January 2025
Posted:
22 January 2025
You are already at the latest version
Abstract
Keywords:
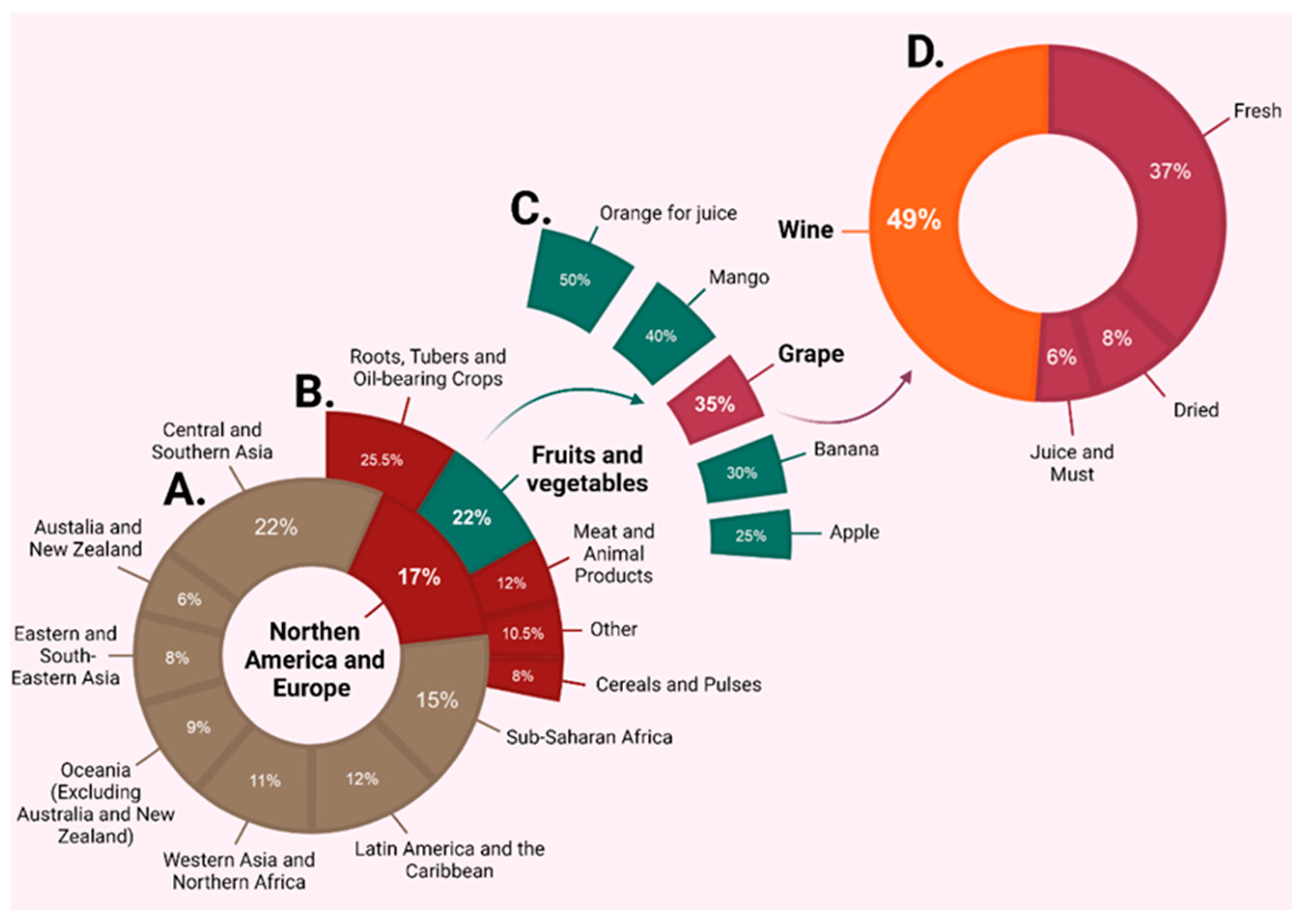
1. Introduction
2. Characterization of Aroma Compounds from Grapes: Profiles and their Impact on Flavor
2.1. C13 – Norisoprenoids
2.2. Terpenes
2.3. Sulfur-Derived Organic Compounds (Thiols)
2.4. Methoxypyrazines
3. Factors Influencing Aromatic Compounds in Viticulture and Winemaking Processes
3.1. Irrigation
3.2. Leaf Removal
3.3. Bunch Thinning
| Viticultural/Oenological practice | Grape variety | Registered aroma compound changes | Aroma descriptors* |
|---|---|---|---|
| Irrigation | Merlot [61] | C13-norisoprenois ↑ | Fruity [82], herbaceous, floral [83] |
| Tocai Friulano [65] | Monoterpenes ↑ | Rose, fruity, herbal, citric, floral [84] | |
| Merlot [65] | Monoterpenes ↕ | ||
| Bobal [67] | C13-norisoprenois ↑ | Fruity [82], herbaceous, floral [83] | |
| Leaf removal | Sauvignon Blanc [68] | Thiols ↕ | Fruity, flint, mineral [45,85] |
| Sauvignon Blanc [72] | Monoterpenes ↑ | Rose, fruity, herbal, citric, floral [84] | |
| Semillon [69] | Monoterpenes ↑ | Rose, fruity, herbal, citric, floral [84] | |
| C13-norisoprenois ↑ | Fruity [82], herbaceous, floral [83] | ||
| Cabernet Franc [73] | C13-norisoprenois ↓ | Fruity [82], herbaceous, floral [83] | |
| Petit Verdot [73] | C13-norisoprenois ↓ | Fruity [82], herbaceous, floral [83] | |
| Nero d’Avola [74] | Monoterpenes ↑ | Rose, fruity, herbal, citric, floral [84] | |
| Pinot Noir [75] | Monoterpenes ↑ | Rose, fruity, herbal, citric, floral [84] | |
| Bunch thinning | Syrah [76] | Varietal aromas ↑ | Black pepper, fruity, black olive [86] |
| Esters ↑ | Fruity [87,88] | ||
| Higher alcohols ↑ | Fruity, floral, honey [89] | ||
| Terpenes ↑ | Fruity, floral, muscatel [90] | ||
| Pinot Noir [77] | Monoterpenes ↑ | Rose, fruity, herbal, citric, floral [84] | |
| C13-norisoprenois ↑ | Fruity [82], herbaceous, floral [83] | ||
| Medium-chain fatty acids ↓ | Fruity [91] | ||
| Maraština [78] | Terpene ↑ | Fruity, floral, muscatel [90] | |
| Esters ↑ | Fruity [87,88] | ||
| Cabernet Sauvignon [79] | C13-norisoprenois ↑ | Fruity [82], herbaceous, floral [83] | |
| Terpene ↕ | Fruity, floral, muscatel [90] | ||
| Fertilization | Sauvignon Blanc [92] | Thiols ↑ | Fruity, flint, mineral [45,85] |
| Merlot [83] | Thiols ↑ | Fruity, flint, mineral [45,85] | |
| Sauvignon Blanc [93] | Thiols ↓ | Fruity, flint, mineral [45,85] | |
| Merlot [93] | Thiols ↓ | Fruity, flint, mineral [45,85] | |
| Cabernet Sauvignon [93] | Thiols ↓ | Fruity, flint, mineral [45,85] | |
| Pre-fermentation practices | |||
| Maceratin | Sauvignon Blanc [94] | Thiols ↑ | Fruity, flint, mineral [45,85] |
| Pyrazines ↕ | Green aromas [95] | ||
| Tannat [96] | Esters ↑ | Fruity [87,88] | |
| Higher alcohols ↑ | Fruity, floral, honey [89] | ||
| Monastrell [97] | Esters ↑ | Fruity [87,88] | |
| Acetates ↑ | Citrus, sweet/acid fruit, berry, floral [98] | ||
| Higher alcohols ↑ | Fruity, floral, honey [89] | ||
| Enzyme addition | Mencia [99] | Acetates ↑ | Citrus, sweet/acid fruit, berry, floral [98] |
| Albariño [100] | Terpenes ↑ | Fruity, floral, muscatel [90] | |
| C13-norisoprenois ↑ | Fruity [82], herbaceous, floral [83] | ||
| Pressing pressure | Sauvignon Blanc [101] | Thiols ↑ | Fruity, flint, mineral [45,85] |
| Fermentation practices(Temperature/yeast strain) | Merlot [102] | Terpenes ↑ | Fruity, floral, muscatel [90] |
| Moscatell [103] | Esters ↑ | Fruity [87,88] | |
| Aurore [104] | Esters ↑ | Fruity [87,88] | |
| 3-methyl-1-butanol ↓ | Earthy, solvent [105] | ||
| 2-phenylethanol ↓ | Floral, rose [105] | ||
| Amyl alcohols ↑ | Fusel [106], Herbaceous, whiskey, malt, burnt [107] | ||
| Aurore [104] | Esters ↑ | Fruity [87,88] | |
| 3-methyl-1-butanol ↓ | Earthy, solvent [105] | ||
| 2-phenylethanol ↓ | Floral, rose [105] | ||
| Amyl alcohols ↑ | Fusel [106], Herbaceous, whiskey, malt, burnt [107] | ||
| Syrah [108,109] | Terpenes ↑ | Fruity, floral, muscatel [90] | |
| Sauvognon Blanc [108,109] | Terpenes ↑ | Fruity, floral, muscatel [90] | |
| Tempranillo [110] | Higher alcohols ↑ | Fruity, floral, honey [89] | |
| Verdejo [111] | Higher alcohols ↑ | Fruity, floral, honey [89] | |
| Syrah [112] | Higher alcohols ↑ | Fruity, floral, honey [89] | |
| Soave, Chardonnay [113] | Higher alcohols ↓ | Fruity, floral, honey [89] | |
| Barbera [114] | Higher alcohols ↓ | Fruity, floral, honey [89] | |
| Verdicchio [114] | Esters ↑ | Fruity [87,88] |
3.4. Fertilization
3.5. Pre-Fermentation Practices
3.6. Fermentation Practices
3.6.1. Temperature
3.6.2. Yeast Strain
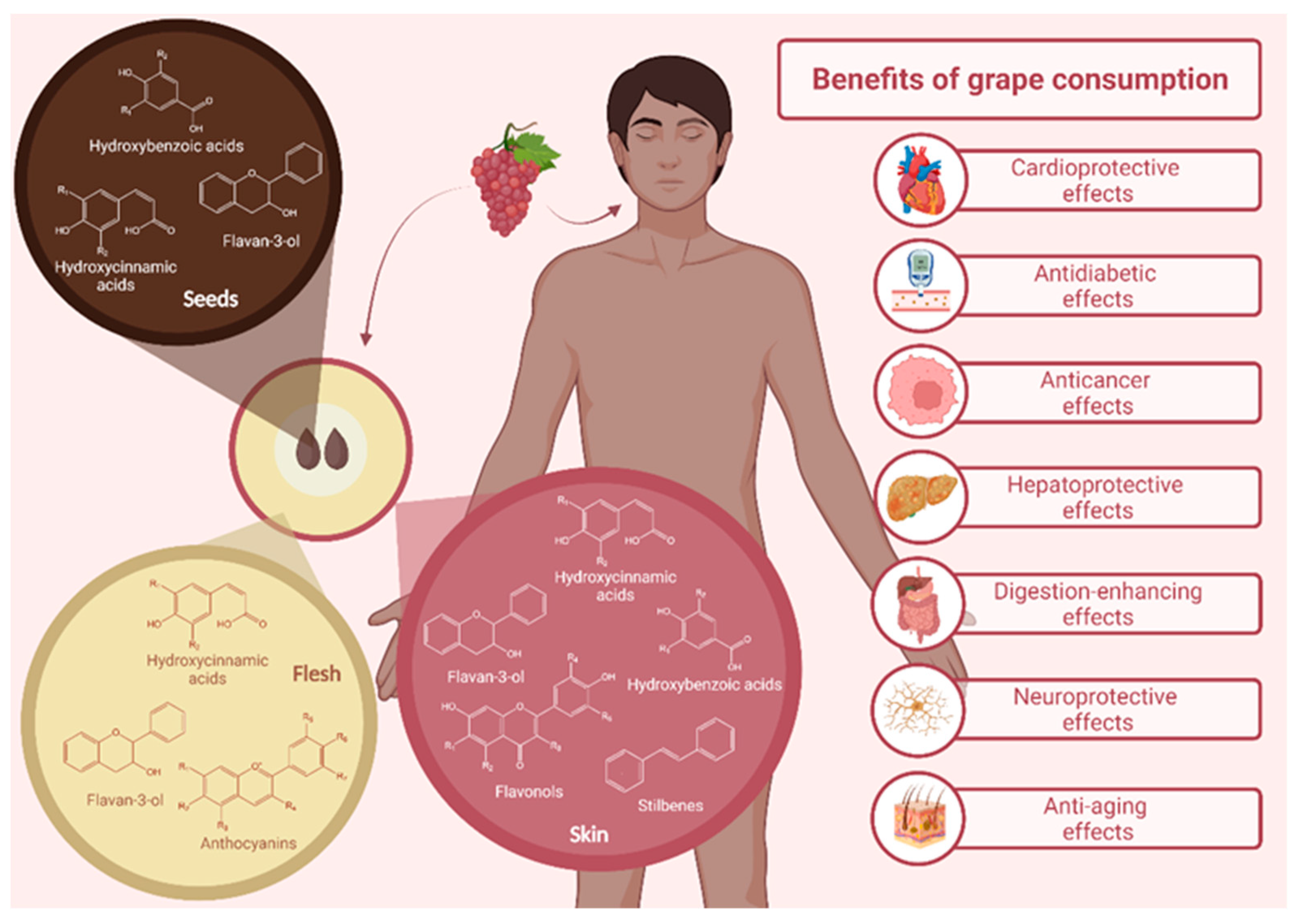
4. Volatile Compound Profile of the Grape Pomace
| Grape pomace (variety) | Region | Particular Examined Parameters | Main volatile compounds | Concentration (µg/mL) |
Aroma descriptor** | References |
|---|---|---|---|---|---|---|
| Chardonnay | Australia | Alcohols | 1-butanol | 0.84 – 0.96 | Alcohol, medicine [147] | [146] |
| 1-hexanol | 1.58 – 9.25 | Floral, cut grass, herbaceous [147,148], woody, resin [149] | ||||
| Esters | 3-methylbutyl acetate | 0.01 – 0.02 | Banana [150] | |||
| Ethyl benzoate | 0.01 – 0.02 | Floral, fruity, chamomile [151] | ||||
| Aldehydes | 2-undecanone | 0.01 | Pineapple, fruity notes [152] | |||
| Phenols | Phenylacetaldehyde | 0.08 – 0.87 | Fruit [152] | |||
| Benzyl alcohol | 0.17 – 0.92 | Chocolate, fig, tobacco [153] | ||||
| 2-Phenylethanol | 0.16 – 0.76 | Floral, rose [105] | ||||
| Terpenes | Nerol | 0.03 | Lime, roses [154] | |||
| Geraniol | 0.21 – 0.22 | Citrus, floral, geranium [150] | ||||
|
Nero d’Avola Frappato Nerello Mascalese Cabernet Sauvignon |
Italy | Esters | Ethyl hexanoate | 0.38 – 1.08 | Anise, caramel, fruit, wine [149] | [130] |
| Ethyl octanoate | 0 – 0.02 | Fruit, must, soap, sweet, waxy [149] | ||||
| Ethyl decanoate | 0.01 – 0.03 | Brandy, fruity, grape [107] | ||||
| Ethyl dodecanoate | 0.01 – 0.02 | Fruit, soap, sweet [149] | ||||
|
Orujos*** |
Spain Italy |
Alcohols | 2-butanol | 0.01 – 0.06 | Alcohol, medicine, off-flavour [147] | [139] |
| 1-propanol | 0.17 - 0.2 | Fusel, alcohol, ripe fruit [155] | ||||
| 3-methyl-1-butanol | 0.52 – 0.89 | Earthy, solvent [105] | ||||
| 2-methyl-1-butanol | 0.16 – 0.3 | Fermented, malt, wine [156] | ||||
| 1-hexanol | 0.03 | Floral, cut grass, herbaceous [147,148], woody, resin [149] | ||||
| 2-phenylethanol | 0.04 – 0.07 | Floral, rose [105] | ||||
| Acetates | Ethyl acetate | 0.3 – 0.45 | Fruity [153], nail polish remover [105] | |||
| Ethyl decanoate | 0.05 – 0.07 | Fruity, apple [151] | ||||
| Ethyl lactate | 0.06 – 0.23 | Solvent [105] | ||||
| Aldehydes and acetal | 1,1-diethoxyethane | 0.04 – 0.9 | Liquorices, nutty, pungent, wood [149] | |||
| Acetaldehyde | 0.23 – 0.25 | Unripe walnut, bruised fruit [157] | ||||
|
Variety not mentioned (red grapes) |
Romania | Aldehydes | Acetaldehyde | 0.03 – 0.04 | Unripe walnut, bruised fruit [157] | [143] |
| Acetates | Ethyl acetate | 0.1 – 0.16 | Fruity [153], nail polish remover [105] | |||
| Alcohols | Methanol | 0.65 - 0.73 | Alcohol [158] | |||
| 1-propanol | 0.03 | Alcohol [151] | ||||
| Isobutylic alcohol | 0.07 – 0.08 | Alcohol, nail polish, fusel [107] | ||||
| Isoamylic alcohol | 0.12 – 0.14 | Fusel [106], Herbaceous, whiskey, malt, burnt [107] | ||||
| Muscat de Frontignan | France | Alcohols | 1-hexanol | ns (+++) | Floral, cut grass, herbaceous [147,148], woody, resin [149] | [144] |
| Cis 2-hexen-1-ol | ns (++) | Grass aroma, appels [159] | ||||
| Cis 3-hexen-1-ol | ns (++) | Green aroma [160] | ||||
| 2-octen- 1-ol | ns (++) | Fresh mushroom [161] | ||||
| 2-phenyl-1-ethanol | ns (+) | Rose [89] | ||||
| Aldehydes | Hexanal | ns (+++) | Floral [152] | |||
| Benzaldehyde | ns (+) | Almond, fragrant [107] | ||||
| Phenylacetaldehyde | ns (+) | Fruit [152] | ||||
| Ketones | 3-hydroxy-2-butanone | ns (+) | Unctuous, milky [162] | |||
| Esters | 3-methylbutyl acetate | ns (+) | Fruity, bananas, pears, acetone [163] | |||
| Hexyl acetate | ns (+) | Redberry [105] | ||||
| Lactones | y-butyrolactone | ns (+) | Creamy, oily, fatty, caramel [164] | |||
| Terpenes | β-citronellol | ns (+++) | Citrus, clove, floral, fresh, green, rose, sour, sweet [107] | |||
| Geraniol | ns (+++) | Citrus, floral, geranium [150] | ||||
| Linalool | ns (+++) | Floral, lavender [107] | ||||
| Nerol | ns (+++) | Lime, roses [154] | ||||
| α-terpineol | ns (++) | Flowers, lilies, sweet [107] | ||||
| Kalambaki, Roditis, Xinomavro, Zambella, Agiorgitico | Greece | Alcohols | Methanol | 0.41 – 0.98 | Alcohol [158] | [137] |
| 1 - propanol | 0.06 – 0.28 | Alcohol [151] | ||||
| 2 – methyl - butanol | 0.13 – 0.83 | Fermented, malt, wine [156] | ||||
| 2 – methyl - propanol | 0.11 – 0.35 | Alcohol, nailpolish, fusel [107] | ||||
| Hexanol-1 | 0 – 0.17 | Floral, cut grass, herbaceous [147,148], woody, resin [149] | ||||
| 3-Methyl-butanol | 0.14 – 0.70 | Fusel [106], Herbaceous, whiskey, malt, burnt [107] | ||||
| Aldehydes | Acetaldehydes | 0.19 – 0.92 | Unripe walnut, bruised fruit [157] | |||
| Esters | Ethyl acetate | 0.15 – 0.66 | Fruity [153], nail polish remover [105] |
5. Exploring Sensorial Characteristics in Pomace Valorization: The Impact of Long-Maceration on White Wines
| Grape variety | Class of examined parameters | Volatile compounds | Conc (µg/L) | Aroma descriptor** |
|---|---|---|---|---|
|
Garganega [171] |
Monoterpenes | Linalool | 5.3 – 7.5 | Ginger, flowers ,grape-like , sweet, citrus [158] |
| α-Terpineol | 2.2 – 3.9 | Flowers, lilies, sweet [174] | ||
| Citronellol | 2.4 – 6.7 | Citrus, clove, floral, fresh, green, rose, sour, sweet [149] | ||
| Geraniol | 4.7 – 5.7 | Citrus, floral, geranium [150] | ||
| Esters | Isoamylacetate | 182.5 – 278.9 | Banana, pear [175] | |
| Ethyloctanoate | 305.7–413.0 | Fruit, must, soap, sweet, waxy [149] | ||
| Ethyldecanoate | 90.7–90.9 | Brandy, fruity, grape [174] | ||
| Norisoprenoids | 3-oxo--ionol | 81.2–90.9 | Burnt, spicy [175] | |
| Benzenoids | Vanillin | 12.8–51.3 | Sweet, vanilla [149] | |
|
Verdicchio [171] |
Monoterpenes | Linalool | 5.4 | Ginger, flowers,grape-like, sweet, citrus [158] |
| α-Terpineol | 8.6 | Flowers, lilies, sweet [174] | ||
| Citronellol | 3.1 | Citrus, clove, floral, fresh, green, rose, sour, sweet [149] | ||
| Geraniol | 2.2 | Citrus, floral, geranium [150] | ||
| Esters | Isoamylacetate | 572.8 | Banana, pear [175] | |
| Ethyloctanoate | 649.4 | Fruit, must, soap, sweet, waxy [149] | ||
| Ethyldecanoate | 144.9 | Brandy, fruity, grape [174] | ||
| Norisoprenoids | 3-oxo--ionol | 119.5 | Burnt, spicy [175] | |
| Benzenoids | Vanillin | 117.6 | Sweet, vanilla [149] | |
|
Malvazija istarska [172] |
Monoterpenes | Linalool | 111.65 - 113.91 | Ginger, flowers,grape-like, sweet, citrus [158] |
| Geraniol | 40.22 – 44.79 | Citrus, floral, geranium [150] | ||
| α-Terpineol | 46.52 – 76.68 | Flowers, lilies, sweet [174] | ||
| Norisoprenoides | β-damascenone | 11.08 – 19.27 | Floral, red berries [37] | |
| Alcohols | 1-Hexanol | 4329 – 4752 | Floral, cut grass, herbaceous [147,148] | |
| Fatty acids | Hexanoic acid | 3820 - 9834 | Fruit, Fresh, Sweet [176] | |
| Ethyl 3-methylbutanoate | 46.74 - 53.14 | Fusel [106], Herbaceous, whiskey, malt, burnt [107] | ||
| Ethyl octanoate | 732 - 871 | Fruit, must, soap, sweet, waxy [149] | ||
| 2-Phenethylacetate | 43.27 - 47.89 | Floral, rose [105] | ||
| Isoamyl acetate | 1030 - 1062 | Banana, pear [175] | ||
| Ethyl lactate | 152,14 - 160,33 | Floral, fruity, sweet [177] | ||
|
Malvazija istarska (in oak) [173] |
Monoterpenes | α-Terpineol | 114.7 | Flowers, lilies, sweet [174] |
| Linalool | 37.4 | Ginger, flowers,grape-like, sweet, citrus [158] | ||
| C13-norisoprenoids | Vitispirane | 37.1 | Camphor, spicy, wood [149] | |
| Higher alcohols | Isobutanol* | 48.3 | Alcohol, nail polish, fusel [174] | |
| Isoamyl alcohol* | 243.5 | Fusel [106], Herbaceous, whiskey, malt, burnt [107] | ||
| Esters | Ethyl acetate | 75.1 | Fruity [153], nail polish remover [105] | |
| Ethyl hexanoate | 145.7 | Anise, caramel, fruit, wine [149] | ||
| Ethyl octanoate | 128.1 | Fruit, must, soap, sweet, waxy [149] | ||
| Volatile phenols | 4-Ethylguaiacol | 445.7 | Phenolic, sweet [157] | |
| 4-Ethylphenol | 119.0 | Medicinal, stable [157] | ||
|
Chenin Blanc [178] |
Monoterpenes | Linalool | 1.94 | Ginger, flowers,grape-like, sweet, citrus [158] |
| Alcohols | Isopentanol | 353.3 | Fusel, alcoholic, fermented, pungent, bready, yeasty [179] | |
| Esters | Isoamyl acetate | 183.2 | Banana, pear [175] | |
| Ethyl hexanoate | 295.5 | Anise, caramel, fruit, wine [149] |
7. Exploring Future Applications of Aromatic Compounds Extracted from By-Products
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caldeira, C., et al. Quantification of food waste per product group along the food supply chain in the European Union: a mass flow analysis. Resour. Conserv. Recycl. 2019, 149, 479–488. [CrossRef] [PubMed]
- Godfray, H.C., et al.Food security: the challenge of feeding 9 billion people. Science 2010, 327, 812–818. [CrossRef]
- FAO, F.a.A.O.o.t.U.N. The future of food and agriculture: trends and challenges.; Food and Agriculture Organization of the United Nations, [2017] ©2017: Rome, 2017; pp. 163 pages.
- Comunian, T.A.; Silva, M.P.; Souza, C.J.F. The use of food by-products as a novel for functional foods: Their use as ingredients and for the encapsulation process. Trends in Food Science & Technology 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Sheahan, M.; Barrett, C.B. Review: Food loss and waste in Sub-Saharan Africa. Food Policy 2017, 70, 1–12. [Google Scholar] [CrossRef]
- Buzby, J.C.; Hyman, J. Total and per capita value of food loss in the United States. Food Policy 2012, 37, 561–570. [Google Scholar] [CrossRef]
- Beretta, C.; Stoessel, F.; Baier, U.; Hellweg, S. Quantifying food losses and the potential for reduction in Switzerland. Waste Manag 2013, 33, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Liu, G.; Parfitt, J.; Liu, X.; Van Herpen, E.; Stenmarck, A.; O'Connor, C.; Ostergren, K.; Cheng, S. Missing Food, Missing Data? A Critical Review of Global Food Losses and Food Waste Data. Environ Sci Technol 2017, 51, 6618–6633. [Google Scholar] [CrossRef] [PubMed]
- Kok, M.G.; Castelein, R.B.; Broeze, J.; Snels, J.C.M.A. The EFFICIENT protocol: A pragmatic and integrated methodology for food loss and waste quantification, analysis of causes and intervention design. 2021. [CrossRef]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M.W.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Research International 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Gomez, M.; Martinez, M.M. Fruit and vegetable by-products as novel ingredients to improve the nutritional quality of baked goods. Crit Rev Food Sci Nutr 2018, 58, 2119–2135. [Google Scholar] [CrossRef] [PubMed]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Frontiers in Sustainable Food Systems 2018, 2. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits By-Products - A Source of Valuable Active Principles. A Short Review. Front Bioeng Biotechnol 2020, 8, 319. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2019. Moving forward on food loss and waste reduction. 2019. [Google Scholar]
- OIV. Statistical Report on World Vitiviniculture. 2019, 3. [Google Scholar]
- Kammerer, D.; Gajdos Kljusuric, J.; Carle, R.; Schieber, A. Recovery of anthocyanins from grape pomace extracts (Vitis vinifera L. cv. Cabernet Mitos) using a polymeric adsorber resin. European Food Research and Technology 2005, 220, 431–437. [Google Scholar] [CrossRef]
- Garrido, M.D.; Auqui, M.; Martí, N.; Linares, M.B. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT - Food Science and Technology 2011, 44, 2238–2243. [Google Scholar] [CrossRef]
- Jin, Q.; O'Hair, J.; Stewart, A.C.; O'Keefe, S.F.; Neilson, A.P.; Kim, Y.T.; McGuire, M.; Lee, A.; Wilder, G.; Huang, H. Compositional Characterization of Different Industrial White and Red Grape Pomaces in Virginia and the Potential Valorization of the Major Components. Foods 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J Food Sci 2020, 85, 2977–2985. [Google Scholar] [CrossRef]
- Perdicaro, D.J.; Rodriguez Lanzi, C.; Fontana, A.R.; Antoniolli, A.; Piccoli, P.; Miatello, R.M.; Diez, E.R.; Vazquez Prieto, M.A. Grape pomace reduced reperfusion arrhythmias in rats with a high-fat-fructose diet. Food Funct 2017, 8, 3501–3509. [Google Scholar] [CrossRef]
- Averilla, J.N.; Oh, J.; Kim, H.J.; Kim, J.S.; Kim, J.S. Potential health benefits of phenolic compounds in grape processing by-products. Food Sci Biotechnol 2019, 28, 1607–1615. [Google Scholar] [CrossRef]
- Rodriguez Lanzi, C.; Perdicaro, D.J.; Antoniolli, A.; Fontana, A.R.; Miatello, R.M.; Bottini, R.; Vazquez Prieto, M.A. Grape pomace and grape pomace extract improve insulin signaling in high-fat-fructose fed rat-induced metabolic syndrome. Food Funct 2016, 7, 1544–1553. [Google Scholar] [CrossRef]
- Zhao, B.; Gong, H.; Li, H.; Zhang, Y.; Lan, T.; Chen, Z. Characterization of Chinese Grape Seed Oil by Physicochemical Properties, Fatty Acid Composition, Triacylglycrol Profiles, and Sterols and Squalene Composition. International Journal of Food Engineering 2019, 15. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9. [Google Scholar] [CrossRef]
- Jelena Cvejic Hogervorst, U.M., Vladimir Puškaš. 5 - Extraction of Bioactive Compounds from Grape Processing By-Products. Handbook of Grape Processing By-Products 2017. 105–135. [CrossRef]
- Ianni, A.; Martino, G. Dietary Grape Pomace Supplementation in Dairy Cows: Effect on Nutritional Quality of Milk and Its Derived Dairy Products. Foods 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Karovičová, J.; Kohajdová, Z.; Minarovičová, L.; Kuchtová, V. The Chemical Composition of Grape Fibre. Potravinarstvo Slovak Journal of Food Sciences 2015, 9, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.; Horita, K.; Sousa e Silva, J.P.; Almeida, I.F.; Amaral, M.H.; Lobao, P.A.; Costa, P.C.; Miranda, M.S.; Esteves da Silva, J.C.; Sousa Lobo, J.M. Photodegradation of avobenzone: stabilization effect of antioxidants. J Photochem Photobiol B 2014, 140, 36–40. [Google Scholar] [CrossRef]
- Coelho, M.C.; Pereira, R.N.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. The use of emergent technologies to extract added value compounds from grape by-products. Trends in Food Science & Technology 2020, 106, 182–197. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive Compounds from Vine Shoots, Grape Stalks, and Wine Lees: Their Potential Use in Agro-Food Chains. Foods 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.E.; Peinado, R.; Moreno, J.; Medina, M.; Moyano, L.; Zea, L. Selection of Volatile Aroma Compounds by Statistical and Enological Criteria for Analytical Differentiation of Musts and Wines of Two Grape Varieties. Journal of Food Science 2003, 68, 158–163. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Australian Journal of Grape and Wine Research 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Pedroza, M.A.; Zalacain, A.; Lara, J.F.; Salinas, M.R. Global grape aroma potential and its individual analysis by SBSE–GC–MS. Food Research International 2010, 43, 1003–1008. [Google Scholar] [CrossRef]
- J.D. Dunlevy, C.M.K., R.A. Keyzers, P.K. Boss. The Production of Flavour & Aroma Compounds in Grape Berries, 2 ed.; Roubelakis-Angelakis, K.A., Ed. Springer, Dordrecht: 2009.
- Parker, M.; Capone, D.L.; Francis, I.L.; Herderich, M.J. Aroma Precursors in Grapes and Wine: Flavor Release during Wine Production and Consumption. J Agric Food Chem 2018, 66, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.C.M.; Arraibi, A.A.; Ferreira, I.C.F.R. Bioactive and functional compounds in apple pomace from juice and cider manufacturing: Potential use in dermal formulations. Trends in Food Science & Technology 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Tomasino, E.; Bolman, S. The Potential Effect of beta-Ionone and beta-Damascenone on Sensory Perception of Pinot Noir Wine Aroma. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Fang Yuan, H.F., and Michael C. Qian. C13-Norisoprenoids in Grape and Wine Affected by Different Canopy Management. Advances in Wine Research, 147–160. [CrossRef]
- Mendes-Pinto, M.M. Carotenoid breakdown products the—norisoprenoids—in wine aroma. Archives of Biochemistry and Biophysics 2009, 483, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, X.-L.; Ullah, N.; Tao, Y.-S. Aroma Glycosides in Grapes and Wine. Journal of Food Science 2017, 82, 248–259. [Google Scholar] [CrossRef]
- Andrew L. Waterhouse, G.L.S., David W. Jeffery. Understanding Wine Chemistry; John Wiley & Sons: 2016.
- Ferreira, V.; Lopez, R. The Actual and Potential Aroma of Winemaking Grapes. Biomolecules 2019, 9. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the Aroma of Grapes and Wines: A Review. South African Journal of Enology & Viticulture 2017, 4. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Kievit, R.L.; Siebert, T.; Lattey, K.A.; Bramley, B.R.; Francis, I.L.; King, E.S.; Pretorius, I.S. The influence of yeast on the aroma of Sauvignon Blanc wine. Food Microbiol 2009, 26, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Roland, A.; Schneider, R.; Razungles, A.; Cavelier, F. Varietal thiols in wine: discovery, analysis and applications. Chem Rev 2011, 111, 7355–7376. [Google Scholar] [CrossRef]
- Pineau, B.; Trought, M.C.T.; Stronge, K.; Beresford, M.K.; Wohlers, M.W.; Jaeger, S.R. Influence of fruit ripeness and juice chaptalisation on the sensory properties and degree of typicality expressed by Sauvignon Blanc wines from Marlborough, New Zealand. Australian Journal of Grape and Wine Research 2011, 17, 358–367. [Google Scholar] [CrossRef]
- Jeffery, D.W. Spotlight on Varietal Thiols and Precursors in Grapes and Wines. Australian Journal of Chemistry 2016, 69. [Google Scholar] [CrossRef]
- Roland, A.; Schneider, R.; Razungles, A.; Le Guerneve, C.; Cavelier, F. Straightforward synthesis of deuterated precursors to demonstrate the biogenesis of aromatic thiols in wine. J Agric Food Chem 2010, 58, 10684–10689. [Google Scholar] [CrossRef] [PubMed]
- Peyrot Des Gachons, C.; Tominaga, T.; Dubourdieu, D. Sulfur aroma precursor present in S-glutathione conjugate form: identification of S-3-(hexan-1-ol)-glutathione in must from Vitis vinifera L. cv. Sauvignon blanc. J Agric Food Chem 2002, 50, 4076–4079. [Google Scholar] [CrossRef] [PubMed]
- Fedrizzi, B.; Pardon, K.H.; Sefton, M.A.; Elsey, G.M.; Jeffery, D.W. First identification of 4-S-glutathionyl-4-methylpentan-2-one, a potential precursor of 4-mercapto-4-methylpentan-2-one, in Sauvignon Blanc juice. J Agric Food Chem 2009, 57, 991–995. [Google Scholar] [CrossRef]
- Peña-Gallego, A.; Hernández-Orte, P.; Cacho, J.; Ferreira, V. S-Cysteinylated and S-glutathionylated thiol precursors in grapes. A review. Food Chemistry 2012, 131, 1–13. [Google Scholar] [CrossRef]
- Sarrazin, E.; Dubourdieu, D.; Darriet, P. Characterization of key-aroma compounds of botrytized wines, influence of grape botrytization. Food Chemistry 2007, 103, 536–545. [Google Scholar] [CrossRef]
- Cerreti, M.; Ferranti, P.; Benucci, I.; Liburdi, K.; De Simone, C.; Esti, M. Thiol precursors in Grechetto grape juice and aromatic expression in wine. European Food Research and Technology 2016, 243, 753–760. [Google Scholar] [CrossRef]
- C. Lund, M.K.T., F. Benkwitz, Mark W. Wohler, C. Triggs, R. Gardner, H. Heymann, Laura Nicolau New Zealand Sauvignon blanc Distinct Flavor Characteristics: Sensory, Chemical, and Consumer Aspects. American Journal of Enology and Viticulture 2009, 60, 1–12. [CrossRef]
- Lei, Y.; Xie, S.; Guan, X.; Song, C.; Zhang, Z.; Meng, J. Methoxypyrazines biosynthesis and metabolism in grape: A review. Food Chem 2018, 245, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Sala, C.; Mestres, M.; Martı́, M.P.; Busto, O.; Guasch, J. Headspace solid-phase microextraction analysis of 3-alkyl-2-methoxypyrazines in wines. Journal of Chromatography A 2002, 953, 1–6. [Google Scholar] [CrossRef]
- Sidhu, D.; Lund, J.; Kotseridis, Y.; Saucier, C. Methoxypyrazine analysis and influence of viticultural and enological procedures on their levels in grapes, musts, and wines. Crit Rev Food Sci Nutr 2015, 55, 485–502. [Google Scholar] [CrossRef]
- Hernandez-Orte, P.; Concejero, B.; Astrain, J.; Lacau, B.; Cacho, J.; Ferreira, V. Influence of viticulture practices on grape aroma precursors and their relation with wine aroma. J Sci Food Agric 2015, 95, 688–701. [Google Scholar] [CrossRef]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: a review. J Sci Food Agric 2019, 99, 975–985. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Mattii, G.B. Effect of Agronomic Techniques on Aroma Composition of White Grapevines: A Review. Agronomy 2021, 11. [Google Scholar] [CrossRef]
- Song, J.; Shellie, K.C.; Wang, H.; Qian, M.C. Influence of deficit irrigation and kaolin particle film on grape composition and volatile compounds in Merlot grape (Vitis vinifera L.). Food Chem 2012, 134, 841–850. [Google Scholar] [CrossRef]
- Bindon, K.A.; Dry, P.R.; Loveys, B.R. Influence of Plant Water Status on the Production of C13-Norisoprenoid Precursors in Vitis vinifera L. Cv. Cabernet Sauvignon Grape Berries. Journal of Agricultural and Food Chemistry 2007, 55, 4493–4500. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.C.; Fang, Y.; Shellie, K. Volatile Composition of Merlot Wine from Different Vine Water Status. Journal of Agricultural and Food Chemistry 2009, 57, 7459–7463. [Google Scholar] [CrossRef]
- Koundouras, S.; Marinos, V.; Gkoulioti, A.; Kotseridis, Y.; van Leeuwen, C. Influence of Vineyard Location and Vine Water Status on Fruit Maturation of Nonirrigated Cv. Agiorgitiko (Vitis vinifera L.). Effects on Wine Phenolic and Aroma Components. Journal of Agricultural and Food Chemistry 2006, 54, 5077–5086. [Google Scholar] [CrossRef] [PubMed]
- Savoi, S.; Herrera, J.C.; Carlin, S.; Lotti, C.; Bucchetti, B.; Peterlunger, E.; Castellarin, S.D.; Mattivi, F. From grape berries to wines: drought impacts on key secondary metabolites. OENO One 2020, 54, 569–582. [Google Scholar] [CrossRef]
- Delgado, J.A.; Osorio Alises, M.; Alonso-Villegas, R.; Sánchez-Palomo, E.; González-Viñas, M.A. Effects of the Irrigation of Chelva Grapevines on the Aroma Composition of Wine. Beverages 2022, 8. [Google Scholar] [CrossRef]
- Lizama, V.; Pérez-Álvarez, E.P.; Intrigliolo, D.S.; Chirivella, C.; Álvarez, I.; García-Esparza, M.J. Effects of the irrigation regimes on grapevine cv. Bobal in a Mediterranean climate: II. Wine, skins, seeds, and grape aromatic composition. Agricultural Water Management 2021, 256. [Google Scholar] [CrossRef]
- Sivilotti, P.; Falchi, R.; Herrera, J.C.; Škvarč, B.; Butinar, L.; Sternad Lemut, M.; Bubola, M.; Sabbatini, P.; Lisjak, K.; Vanzo, A. Combined Effects of Early Season Leaf Removal and Climatic Conditions on Aroma Precursors in Sauvignon Blanc Grapes. Journal of Agricultural and Food Chemistry 2017, 65, 8426–8434. [Google Scholar] [CrossRef]
- Alessandrini, M.; Battista, F.; Panighel, A.; Flamini, R.; Tomasi, D. Effect of pre-bloom leaf removal on grape aroma composition and wine sensory profile of Semillon cultivar. J Sci Food Agric 2018, 98, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Verdenal, T.; Zufferey, V.; Dienes-Nagy, A.; Bieri, S.; Bourdin, G.; Reynard, J.-S.; Spring, J.-L. Exploring grapevine canopy management: effects of removing main leaves or lateral shoots before flowering. IVES Technical Reviews, vine and wine, 2087. [Google Scholar] [CrossRef]
- Bubola, M.; Lukic, I.; Radeka, S.; Sivilotti, P.; Grozic, K.; Vanzo, A.; Bavcar, D.; Lisjak, K. Enhancement of Istrian Malvasia wine aroma and hydroxycinnamate composition by hand and mechanical leaf removal. J Sci Food Agric 2019, 99, 904–914. [Google Scholar] [CrossRef]
- Yue, X.; Ma, X.; Tang, Y.; Wang, Y.; Wu, B.; Jiao, X.; Zhang, Z.; Ju, Y. Effect of cluster zone leaf removal on monoterpene profiles of Sauvignon Blanc grapes and wines. Food Research International 2020, 131. [Google Scholar] [CrossRef] [PubMed]
- Hickey, C.C.; Kwasniewski, M.T.; Wolf, T.K. Leaf Removal Effects on Cabernet franc and Petit Verdot: II. Grape Carotenoids, Phenolics, and Wine Sensory Analysis. American Journal of Enology and Viticulture 2018, 69, 231–246. [Google Scholar] [CrossRef]
- Verzera, A.; Tripodi, G.; Dima, G.; Condurso, C.; Scacco, A.; Cincotta, F.; Giglio, D.M.; Santangelo, T.; Sparacio, A. Leaf removal and wine composition of Vitis vinifera L. cv. Nero d'Avola: the volatile aroma constituents. J Sci Food Agric 2016, 96, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Skinkis, P.A.; Qian, M.C. Pinot noir wine volatile and anthocyanin composition under different levels of vine fruit zone leaf removal. Food Chemistry 2017, 214, 736–744. [Google Scholar] [CrossRef]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Sparacio, A.; Giglio, D.M.L.; Sparla, S.; Verzera, A. Effects of cluster thinning on wine quality of Syrah cultivar (Vitis vinifera L.). European Food Research and Technology 2016, 242, 1719–1726. [Google Scholar] [CrossRef]
- Rutan, T.E.; Herbst-Johnstone, M.; Kilmartin, P.A. Effect of Cluster Thinning Vitis vinifera cv. Pinot Noir on Wine Volatile and Phenolic Composition. J Agric Food Chem 2018, 66, 10053–10066. [Google Scholar] [CrossRef] [PubMed]
- Mucalo, A.; Lukšić, K.; Budić-Leto, I.; Zdunić, G. Cluster Thinning Improves Aroma Complexity of White Maraština (Vitis vinifera L.) Wines Compared to Defoliation under Mediterranean Climate. Applied Sciences 2022, 12. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.N.; He, L.; He, F.; Chen, W.; Duan, C.Q.; Wang, J. Changes in global aroma profiles of Cabernet Sauvignon in response to cluster thinning. Food Res Int 2019, 122, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kok, D. Influences of pre- and post-veraison cluster thinning treatments on grape composition variables and monoterpene levels of Vitis vinifera L. cv. Sauvignon Blanc. Journal of Food Agriculture and Environment 2011, 9, 22–26. [Google Scholar]
- Diago, M.P.; Vilanova, M.; Blanco, J.A.; Tardaguila, J. Effects of mechanical thinning on fruit and wine composition and sensory attributes of Grenache and Tempranillo varieties (Vitis vinifera L.). Australian Journal of Grape and Wine Research 2010, 16, 314–326. [Google Scholar] [CrossRef]
- Yuan, F.; Feng, H.; Qian, M.C. C13-Norisoprenoids in Grape and Wine Affected by Different Canopy Management. 2015, 1203, 147–160. [CrossRef]
- Mendez-Costabel, M.P.; Wilkinson, K.L.; Bastian, S.E.P.; Jordans, C.; McCarthy, M.; Ford, C.M.; Dokoozlian, N.K. Effect of increased irrigation and additional nitrogen fertilisation on the concentration of green aroma compounds inVitis viniferaL. Merlot fruit and wine. Australian Journal of Grape and Wine Research 2014, 20, 80–90. [Google Scholar] [CrossRef]
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and their role in wine flavour: recent advances. Australian Journal of Grape and Wine Research 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Espinase Nandorfy, D.; Siebert, T.; Bilogrevic, E.; Likos, D.; Watson, F.; Barter, S.; Pisaniello, L.; Kulcsar, A.; Shellie, R.A.; Keast, R.; et al. The Role of Potent Thiols in “Empyreumatic” Flint/Struck-Match/Mineral Odours in Chardonnay Wine. Australian Journal of Grape and Wine Research 2023, 2023, 1–17. [Google Scholar] [CrossRef]
- Geffroy, O.; Morere, M.; Lopez, R.; Pasquier, G.; Condoret, J.S. Investigating the Aroma of Syrah Wines from the Northern Rhone Valley Using Supercritical CO(2)-Dearomatized Wine as a Matrix for Reconstitution Studies. J Agric Food Chem 2020, 68, 11512–11523. [Google Scholar] [CrossRef] [PubMed]
- Lytra, G.; Tempere, S.; Le Floch, A.; de Revel, G.; Barbe, J.-C. Study of Sensory Interactions among Red Wine Fruity Esters in a Model Solution. Journal of Agricultural and Food Chemistry 2013, 61, 8504–8513. [Google Scholar] [CrossRef] [PubMed]
- Gammacurta, M.; Marchand, S.; Albertin, W.; Moine, V.; de Revel, G. Impact of Yeast Strain on Ester Levels and Fruity Aroma Persistence during Aging of Bordeaux Red Wines. Journal of Agricultural and Food Chemistry 2014, 62, 5378–5389. [Google Scholar] [CrossRef] [PubMed]
- Cordente, A.G.; Espinase Nandorfy, D.; Solomon, M.; Schulkin, A.; Kolouchova, R.; Francis, I.L.; Schmidt, S.A. Aromatic Higher Alcohols in Wine: Implication on Aroma and Palate Attributes during Chardonnay Aging. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.A.; Kang, H.M.; Lee, Y.T.; Islam, M.Z. Grape terpenoids: flavor importance, genetic regulation, and future potential. Crit Rev Food Sci Nutr 2021, 61, 1429–1447. [Google Scholar] [CrossRef]
- Csutoras, C.; Bakos-Barczi, N.; Burkus, B. Medium chain fatty acids and fatty acid esters as potential markers of alcoholic fermentation of white wines. Acta Alimentaria 2022, 51, 33–42. [Google Scholar] [CrossRef]
- Lacroux, F.; Trégoat, O.; Van Leeuwen, C.; Pons, A.; Tominaga, T.; Lavigne-Cruège, V.; Dubourdieu, D. Effect of foliar nitrogen and sulphur application on aromatic expression of <em>Vitis vinifera</em> L. cv. Sauvignon blanc. OENO One 2008, 42. [Google Scholar] [CrossRef]
- Darriet, P.B., P; Poupot, C; Bugaret, Y; Clerjeau, M; Sauris, P; Medina, B; Dubourdieu, D Effects of copper fungicide spraying on volatile thiols of the varietal aroma of Sauvignon blanc, Cabernet Sauvignon and Merlot wines. Vitis 2001, 40, 93–99.
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Influence of harvesting technique and maceration process on aroma and phenolic attributes of Sauvignon blanc wine. Food Chem 2015, 183, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Wamhoff, H.; Gribble, G.W. Wine and Heterocycles. 2012, 106, 185–225. [CrossRef]
- Gonzalez-Neves, G.; Favre, G.; Gil, G.; Ferrer, M.; Charamelo, D. Effect of cold pre-fermentative maceration on the color and composition of young red wines cv. Tannat. J Food Sci Technol 2015, 52, 3449–3457. [Google Scholar] [CrossRef]
- Álvarez, I.; Aleixandre, J.L.; García, M.J.; Lizama, V. Impact of prefermentative maceration on the phenolic and volatile compounds in Monastrell red wines. Analytica Chimica Acta 2006, 563, 109–115. [Google Scholar] [CrossRef]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile. Foods 2020, 10. [Google Scholar] [CrossRef]
- Mihnea, M.; González-SanJosé, M.L.; Ortega-Heras, M.; Pérez-Magariño, S. A comparative study of the volatile content of Mencía wines obtained using different pre-fermentative maceration techniques. LWT - Food Science and Technology 2015, 64, 32–41. [Google Scholar] [CrossRef]
- Rogerson, F.S.S.; Vale, E.; Grande, H.J.; Silva, M.C.M. Alternative Processing of Port-Wine Using Pectolytic Enzymes Procesado Alternativo Del Vino De Oporto Usando Enzimas PectolÍticos Procesado Alternativo Do ViÑo De Oporto Usando Enzimas PectolÍticos. Ciencia y Tecnologia Alimentaria 2000, 2, 222–227. [Google Scholar] [CrossRef]
- Maggu, M.; Winz, R.; Kilmartin, P.A.; Trought, M.C.T.; Nicolau, L. Effect of Skin Contact and Pressure on the Composition of Sauvignon Blanc Must. Journal of Agricultural and Food Chemistry 2007, 55, 10281–10288. [Google Scholar] [CrossRef] [PubMed]
- Massera, A.; Assof, M.; Sari, S.; Ciklic, I.; Mercado, L.; Jofré, V.; Combina, M. Effect of low temperature fermentation on the yeast-derived volatile aroma composition and sensory profile in Merlot wines. LWT 2021, 142, 111069. [Google Scholar] [CrossRef]
- Beltran, G.; Novo, M.; Guillamon, J.M.; Mas, A.; Rozes, N. Effect of fermentation temperature and culture media on the yeast lipid composition and wine volatile compounds. Int J Food Microbiol 2008, 121, 169–177. [Google Scholar] [CrossRef]
- Samoticha, J.; Wojdyło, A.; Chmielewska, J.; Nofer, J. Effect of Different Yeast Strains and Temperature of Fermentation on Basic Enological Parameters, Polyphenols and Volatile Compounds of Aurore White Wine. Foods 2019, 8, 599. [Google Scholar] [CrossRef]
- Mayr, C.M.; Geue, J.P.; Holt, H.E.; Pearson, W.P.; Jeffery, D.W.; Francis, I.L. Characterization of the key aroma compounds in Shiraz wine by quantitation, aroma reconstitution, and omission studies. J Agric Food Chem 2014, 62, 4528–4536. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Campo, E.; Escudero, A.; Grana, M.; Masa, A.; Cacho, J. Volatile composition and sensory properties of Vitis vinifera red cultivars from north west Spain: correlation between sensory and instrumental analysis. Anal Chim Acta 2012, 720, 104–111. [Google Scholar] [CrossRef]
- Buican, B.-C.; Colibaba, L.C.; Luchian, C.E.; Kallithraka, S.; Cotea, V.V. “Orange” Wine—The Resurgence of an Ancient Winemaking Technique: A Review. Agriculture 2023, 13, 1750. [Google Scholar] [CrossRef]
- Beckner Whitener, M.E.; Carlin, S.; Jacobson, D.; Weighill, D.; Divol, B.; Conterno, L.; Du Toit, M.; Vrhovsek, U. Early fermentation volatile metabolite profile of non-Saccharomyces yeasts in red and white grape must: A targeted approach. LWT - Food Science and Technology 2015, 64, 412–422. [Google Scholar] [CrossRef]
- Beckner Whitener, M.E.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME–GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12. [Google Scholar] [CrossRef]
- Belda, I.; Navascues, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl Microbiol Biotechnol 2015, 99, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in varietal thiol (3-SH and 4-MSP) release in wine sequential fermentations. Int J Food Microbiol 2017, 257, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res Int 2015, 76, 325–333. [Google Scholar] [CrossRef]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J Microbiol Biotechnol 2015, 31, 277–293. [Google Scholar] [CrossRef]
- Nardi, T.; Panero, L.; Petrozziello, M.; Guaita, M.; Tsolakis, C.; Cassino, C.; Vagnoli, P.; Bosso, A. Managing wine quality using Torulaspora delbrueckii and Oenococcus oeni starters in mixed fermentations of a red Barbera wine. European Food Research and Technology 2018, 245, 293–307. [Google Scholar] [CrossRef]
- Herbst-Johnstone, M.; Araujo, L.D.; Allen, T.A.; Logan, G.; Nicolau, L.; Kilmartin, P.A. Effects of Mechanical Harvesting on 'Sauvignon Blanc' Aroma. Acta Horticulturae, 2013; 18, 179–186. [Google Scholar] [CrossRef]
- Allen, T.; Herbst-Johnstone, M.; Girault, M.; Butler, P.; Logan, G.; Jouanneau, S.; Nicolau, L.; Kilmartin, P.A. Influence of grape-harvesting steps on varietal thiol aromas in Sauvignon blanc wines. J Agric Food Chem 2011, 59, 10641–10650. [Google Scholar] [CrossRef] [PubMed]
- Cejudo-Bastante, M.J.; Castro-Vazquez, L.; Hermosin-Gutierrez, I.; Perez-Coello, M.S. Combined effects of prefermentative skin maceration and oxygen addition of must on color-related phenolics, volatile composition, and sensory characteristics of Airen white wine. J Agric Food Chem 2011, 59, 12171–12182. [Google Scholar] [CrossRef] [PubMed]
- Diana De Santis, M.T.F. Effect of prefermentative cold maceration on the aroma and phenolic profiles of a Merlot red wine Italian. Italian Journal of Food Science 2010, 22, 2–8. [Google Scholar]
- Rocha, S.M.; Coutinho, P.; Delgadillo, I.; Cardoso, A.D.; Coimbra, M.A. Effect of enzymatic aroma release on the volatile compounds of white wines presenting different aroma potentials. Journal of the Science of Food and Agriculture 2005, 85, 199–205. [Google Scholar] [CrossRef]
- Armada, L.; Fernández, E.; Falqué, E. Influence of several enzymatic treatments on aromatic composition of white wines. LWT - Food Science and Technology 2010, 43, 1517–1525. [Google Scholar] [CrossRef]
- Patel, P.; Herbst-Johnstone, M.; Lee, S.A.; Gardner, R.C.; Weaver, R.; Nicolau, L.; Kilmartin, P.A. Influence of Juice Pressing Conditions on Polyphenols, Antioxidants, and Varietal Aroma of Sauvignon blanc Microferments. Journal of Agricultural and Food Chemistry 2010, 58, 7280–7288. [Google Scholar] [CrossRef]
- Minas, M.; Dimitrios, T. Contribution of Yeast in Wine Aroma and Flavour. In Yeast, Antonio, M., Iris, L., Eds. IntechOpen: Rijeka, 2017; 10.5772/intechopen.70656p. Ch. 5.
- Morales, M.L.; Fierro-Risco, J.; Callejón, R.M.; Paneque, P. Monitoring volatile compounds production throughout fermentation by Saccharomyces and non-Saccharomyces strains using headspace sorptive extraction. J Food Sci Technol 2017, 54, 538–557. [Google Scholar] [CrossRef]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl Microbiol Biotechnol 2007, 77, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Lerno, L.; Reichwage, M.; Ponangi, R.; Hearne, L.; Block, D.E.; Oberholster, A. Effects of Cap and Overall Fermentation Temperature on Phenolic Extraction in Cabernet Sauvignon Fermentations. American Journal of Enology and Viticulture 2015, 66, 444–453. [Google Scholar] [CrossRef]
- Ruiz, J.; de Celis, M.; de Toro, M.; Mendes-Ferreira, A.; Rauhut, D.; Santos, A.; Belda, I. Phenotypic and transcriptional analysis of Saccharomyces cerevisiae during wine fermentation in response to nitrogen nutrition and co-inoculation with Torulaspora delbrueckii. Food Res Int 2020, 137, 109663. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii for secondary fermentation in sparkling wine production. Food Microbiology 2018, 74, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.-C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. International Journal of Food Microbiology 2015, 207, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Del Fresno, J.M.; Morata, A.; Loira, I.; Bañuelos, M.A.; Escott, C.; Benito, S.; González Chamorro, C.; Suárez-Lepe, J.A. Use of non-Saccharomyces in single-culture, mixed and sequential fermentation to improve red wine quality. European Food Research and Technology 2017, 243, 2175–2185. [Google Scholar] [CrossRef]
- Ruberto, G.; Renda, A.; Amico, V.; Tringali, C. Volatile components of grape pomaces from different cultivars of Sicilian Vitis vinifera L. Bioresour Technol 2008, 99, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Oliveros, A.; Bautista-Hernandez, I.; Castro-Lopez, C.; Aguilar-Zarate, P.; Meza-Carranco, Z.; Rojas, R.; Michel, M.R.; Martinez-Avila, G.C.G. Grape Pomace-Advances in Its Bioactivity, Health Benefits, and Food Applications. Foods 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, A.; Gancel, A.L.; Jourdes, M.; Teissedre, P.L. Valorization of Grape Pomace: A Review of Phenolic Composition, Bioactivity, and Therapeutic Potential. Antioxidants (Basel) 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Abreu, T.; Sousa, P.; Gonçalves, J.; Hontman, N.; Teixeira, J.; Câmara, J.S.; Perestrelo, R. Grape Pomace as a Renewable Natural Biosource of Value-Added Compounds with Potential Food Industrial Applications. Beverages 2024, 10. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. Structural, functional and physicochemical properties of pectin from grape pomace as affected by different extraction techniques. Int J Biol Macromol 2023, 224, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Lourenço, S.; Silva, C.; Lopes, A.; Andrade, C.; Perestrelo, R. Exploring the potential of wine industry by-products as source of additives to improve the quality of aquafeed. Microchemical Journal 2020, 155. [Google Scholar] [CrossRef]
- Ledauphin, J.; Guichard, H.; Saint-Clair, J.F.; Picoche, B.; Barillier, D. Chemical and sensorial aroma characterization of freshly distilled Calvados. 2. Identification of volatile compounds and key odorants. J Agric Food Chem 2003, 51, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Gerogiannaki-Christopoulou, M.; Kyriakidis, N.V.; Athanasopoulos, P.E. The evaluation of grape pomace distillates from selected red grape varieties. International Journal of Food Science & Technology 2006, 41, 854–860. [Google Scholar] [CrossRef]
- Soles, R.M.; Ough, C.S.; Kunkee, R.E. Ester Concentration Differences in Wine Fermented by Various Species and Strains of Yeasts. American Journal of Enology and Viticulture 1982, 33, 94–98. [Google Scholar] [CrossRef]
- Cortés, S.; Rodríguez, R.; Salgado, J.M.; Domínguez, J.M. Comparative study between Italian and Spanish grape marc spirits in terms of major volatile compounds. Food Control 2011, 22, 673–680. [Google Scholar] [CrossRef]
- EUR-Lex. REGULATION (EU) 2019/787 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL. Official Journal of the European Union 2019, 62, 1–55.
- Aurand, J.-M.; Hodson, G.; Wilkes, E.; Azevedo, S.; Battaglene, T. Methanol in wine. BIO Web of Conferences 2017, 9, 02028. [Google Scholar] [CrossRef]
- Plata, C.; Millán, C.; Mauricio, J.C.; Ortega, J.M. Formation of ethyl acetate and isoamyl acetate by various species of wine yeasts. Food Microbiology 2003, 20, 217–224. [Google Scholar] [CrossRef]
- Coldea, T.E.; Mudura, E.; Ranta, N.; Hadarean, D. The Impact of Grape Marc Distillation Process on the Major Volatile Compounds. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Animal Science and Biotechnologies 2013, 70, 223–229. [Google Scholar]
- Etievant, P.X.; Bayonove, C.L. Aroma components of pomaces and wine from the variety muscat de frontignan. Journal of the Science of Food and Agriculture 1983, 34, 393–403. [Google Scholar] [CrossRef]
- TGSC. The good scents company information system. Available online: http://www.thegoodscentscompany.com (accessed on 1 January 2025).
- Liang, Z.; Pai, A.; Liu, D.; Luo, J.; Wu, J.; Fang, Z.; Zhang, P. Optimizing extraction method of aroma compounds from grape pomace. J Food Sci 2020, 85, 4225–4240. [Google Scholar] [CrossRef] [PubMed]
- Lopez de Lerma, N.; Bellincontro, A.; Mencarelli, F.; Moreno, J.; Peinado, R.A. Use of electronic nose, validated by GC–MS, to establish the optimum off-vine dehydration time of wine grapes. Food Chemistry 2012, 130, 447–452. [Google Scholar] [CrossRef]
- Vazquez-Pateiro, I.; Arias-Gonzalez, U.; Miras-Avalos, J.M.; Falque, E. Evolution of the Aroma of Treixadura Wines during Bottle Aging. Foods 2020, 9. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Camara, J.S. Madeira Wine Volatile Profile. A Platform to Establish Madeira Wine Aroma Descriptors. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Vilela, A. Modulating Wine Pleasantness Throughout Wine-Yeast Co-Inoculation or Sequential Inoculation. Fermentation 2020, 6, 22. [Google Scholar] [CrossRef]
- Kim, B.-H.; Park, S.K. Volatile aroma and sensory analysis of black raspberry wines fermented by different yeast strains. Journal of the Institute of Brewing 2015, 121, 87–94. [Google Scholar] [CrossRef]
- Xia, Y.-n.; Suo, R.; Wang, H.; Cerbin, S.; Wang, J. Analysis on Flavor Compounds of Jujube Brandy from Different Fermentation Heights by HS-SPME-GC/MS, E-nose and E-tongue. American Journal of Food Technology 2017, 12, 332–344. [Google Scholar] [CrossRef]
- Martin, V.; Giorello, F.; Farina, L.; Minteguiaga, M.; Salzman, V.; Boido, E.; Aguilar, P.S.; Gaggero, C.; Dellacassa, E.; Mas, A.; et al. De Novo Synthesis of Benzenoid Compounds by the Yeast Hanseniaspora vineae Increases the Flavor Diversity of Wines. J Agric Food Chem 2016, 64, 4574–4583. [Google Scholar] [CrossRef]
- Ribéreau-Gayon P., G.Y., Maujean A., Dubourdieu D. Varietal Aroma. In Handbook of Enology, 2006. 205–230. [CrossRef]
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Research International 2015, 69, 244–255. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.M.; Song, S.H.; Kim, Y.S. Characterization of volatile components in makgeolli, a traditional Korean rice wine, with or without pasteurization, during storage. Molecules 2013, 18, 5317–5325. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of Yeasts on the Sensory Component of Wines. Foods 2022, 11. [Google Scholar] [CrossRef]
- Tarasov, A.; Giuliani, N.; Dobrydnev, A.; Schuessler, C.; Volovenko, Y.; Rauhut, D.; Jung, R. 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) Sensory Thresholds in Riesling Wine. Foods 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-l.; Kang, L.; Hu, J.-j.; Li, X.-f.; Shen, X. Aroma Volatile Compound Analysis of SPME Headspace and Extract Samples from Crabapple (Malus sp.) Fruit Using GC-MS. Agricultural Sciences in China 2008, 7, 1451–1457. [Google Scholar] [CrossRef]
- Nie, C.N.; Gao, Y.; Du, X.; Bian, J.L.; Li, H.; Zhang, X.; Wang, C.M.; Li, S.Y. Characterization of the effect of cis-3-hexen-1-ol on green tea aroma. Sci Rep 2020, 10, 15506. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, D.; Sun, B.; Ren, F.; Zhang, Y.; Chen, H. Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem 2018, 258, 260–268. [Google Scholar] [CrossRef]
- Bayarri, S.; Costell, E. Sensory Evaluation of Fruit and Vegetable Flavors. 2010, 10.1002/9780470622834.ch3, 45-57. [CrossRef]
- Scutarașu, E.C.; Luchian, C.E.; Vlase, L.; Nagy, K.; Colibaba, L.C.; Trinca, L.C.; Cotea, V.V. Influence Evaluation of Enzyme Treatments on Aroma Profile of White Wines. Agronomy 2022, 12, 2897. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Zhang, B.; Shen, C.; Xu, Y.; Tang, K. Identification, quantitation and sensorial contribution of lactones in brandies between China and France. Food Chem 2021, 357, 129761. [Google Scholar] [CrossRef] [PubMed]
- Howard, C. Skin contact whites: Perhaps amber is the new “orange”. Wine Vitic. 2017, 32. [Google Scholar]
- Palomo, E.S.; Díaz-Maroto, M.C.; Viñas, M.A.G.; Soriano-Pérez, A.; Pérez-Coello, M.S. Aroma profile of wines from Albillo and Muscat grape varieties at different stages of ripening. Food Control 2007, 18, 398–403. [Google Scholar] [CrossRef]
- Tominaga, T.; Murat, M.-L.; Dubourdieu, D. Development of a method for analyzing the volatile thiols involved in the characteristic aroma of wines made from vitis vinifera l. Cv. Sauvignon blanc. Journal of Agricultural and Food Chemistry 1998, 46, 1044–1048. [Google Scholar] [CrossRef]
- Waterhouse, A.; Sacks, G.; Jeffery, D. Understanding Wine Chemistry; 2016. [CrossRef]
- Şener, H. Effect of Temperature and Duration of Maceration on Colour and Sensory Properties of Red Wine: A Review. South African Journal of Enology & Viticulture 2018, 32. [Google Scholar] [CrossRef]
- Lukic, I.; Lotti, C.; Vrhovsek, U. Evolution of free and bound volatile aroma compounds and phenols during fermentation of Muscat blanc grape juice with and without skins. Food Chem 2017, 232, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Fedrizzi, B.; Versini, G.; Finato, F.; Casarotti, E.M.; Nicolis, E.; Ferrarini, R. Evaluation of the Impact of an Archaic Protocol on White Wine Free Aroma Compounds. 2012, 1104, 117–131. [CrossRef]
- Radeka, S.; Bestulić, E.; Rossi, S.; Orbanić, F.; Bubola, M.; Plavša, T.; Lukić, I.; Jeromel, A. Effect of Different Vinification Techniques on the Concentration of Volatile Aroma Compounds and Sensory Profile of Malvazija Istarska Wines. Fermentation 2023, 9, 676. [Google Scholar] [CrossRef]
- Lukic, I.; Jedrejcic, N.; Ganic, K.K.; Staver, M.; Persuric, D. Phenolic and Aroma Composition of White Wines Produced by Prolonged Maceration and Maturation in Wooden Barrels. Food Technol Biotechnol 2015, 53, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chemistry 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Slaghenaufi, D.; Ugliano, M. Norisoprenoids, Sesquiterpenes and Terpenoids Content of Valpolicella Wines During Aging: Investigating Aroma Potential in Relationship to Evolution of Tobacco and Balsamic Aroma in Aged Wine. Frontiers in Chemistry 2018, 6. [Google Scholar] [CrossRef]
- Ferreira, V. Volatile aroma compounds and wine sensory attributes. 2010, 3-28. [CrossRef]
- Prezioso, I.; Fioschi, G.; Rustioni, L.; Mascellani, M.; Natrella, G.; Venerito, P.; Gambacorta, G.; Paradiso, V.M. Influence of prolonged maceration on phenolic compounds, volatile profile and sensory properties of wines from Minutolo and Verdeca, two Apulian white grape varieties. Lwt 2024, 192, 115698. [Google Scholar] [CrossRef]
- Wang, J.; Huo, S.; Zhang, Y.; Liu, Y.; Fan, W. Impact of various maceration techniques on the phenolic and volatile composition of Chenin Blanc wines. International Journal of Food Science & Technology 2016, 51, 2360–2366. [Google Scholar] [CrossRef]
- Du Plessis, H.; Du Toit, M.; Nieuwoudt, H.; Van der Rijst, M.; Kidd, M.; Jolly, N. Effect of Saccharomyces, Non-Saccharomyces Yeasts and Malolactic Fermentation Strategies on Fermentation Kinetics and Flavor of Shiraz Wines. Fermentation 2017, 3, 64. [Google Scholar] [CrossRef]
- Fernandez, C.M.; Ramos, M.J.; Perez, A.; Rodriguez, J.F. Production of biodiesel from winery waste: extraction, refining and transesterification of grape seed oil. Bioresour Technol 2010, 101, 7030–7035. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Environ Pollut 2021, 278, 116796. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Rodríguez-Bencomo, J.J.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.Á. Recovery of Aromatic Aglycones from Grape Pomace Winemaking By-Products by Using Liquid-Liquid and Pressurized-Liquid Extraction. Food Analytical Methods 2013, 7, 47–57. [Google Scholar] [CrossRef]
- Jelley, R.E.; Herbst-Johnstone, M.; Klaere, S.; Pilkington, L.I.; Grose, C.; Martin, D.; Barker, D.; Fedrizzi, B. Optimization of Ecofriendly Extraction of Bioactive Monomeric Phenolics and Useful Flavor Precursors from Grape Waste. ACS Sustainable Chemistry & Engineering 2016, 4, 5060–5067. [Google Scholar] [CrossRef]
- Rebecchi, S.; Pinelli, D.; Bertin, L.; Zama, F.; Fava, F.; Frascari, D. Volatile fatty acids recovery from the effluent of an acidogenic digestion process fed with grape pomace by adsorption on ion exchange resins. Chemical Engineering Journal 2016, 306, 629–639. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Carbonell-Barrachina Á, A. Flavor and Aroma Analysis as a Tool for Quality Control of Foods. Foods 2021, 10. [Google Scholar] [CrossRef]
- Vilela, A.; Bacelar, E.; Pinto, T.; Anjos, R.; Correia, E.; Gonçalves, B.; Cosme, F. Beverage and Food Fragrance Biotechnology, Novel Applications, Sensory and Sensor Techniques: An Overview. Foods 2019, 8. [Google Scholar] [CrossRef]
- Shaaban, H.; Mahmoud, K.; Amin, A.; Banna, H. Application of Biotechnology to the Production of Natural Flavor and Fragrance Chemicals. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2016, 7, 2670–2717. [Google Scholar]
- Harlander, S. Biotechnology for the Production of Flavoring Materials. In Source Book of Flavors, Reineccius, G., Ed. Springer US: Boston, MA, 1994; pp. 155-175. 10.1007/978-1-4615-7889-5_6.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).