Submitted:
21 January 2025
Posted:
22 January 2025
You are already at the latest version
Abstract
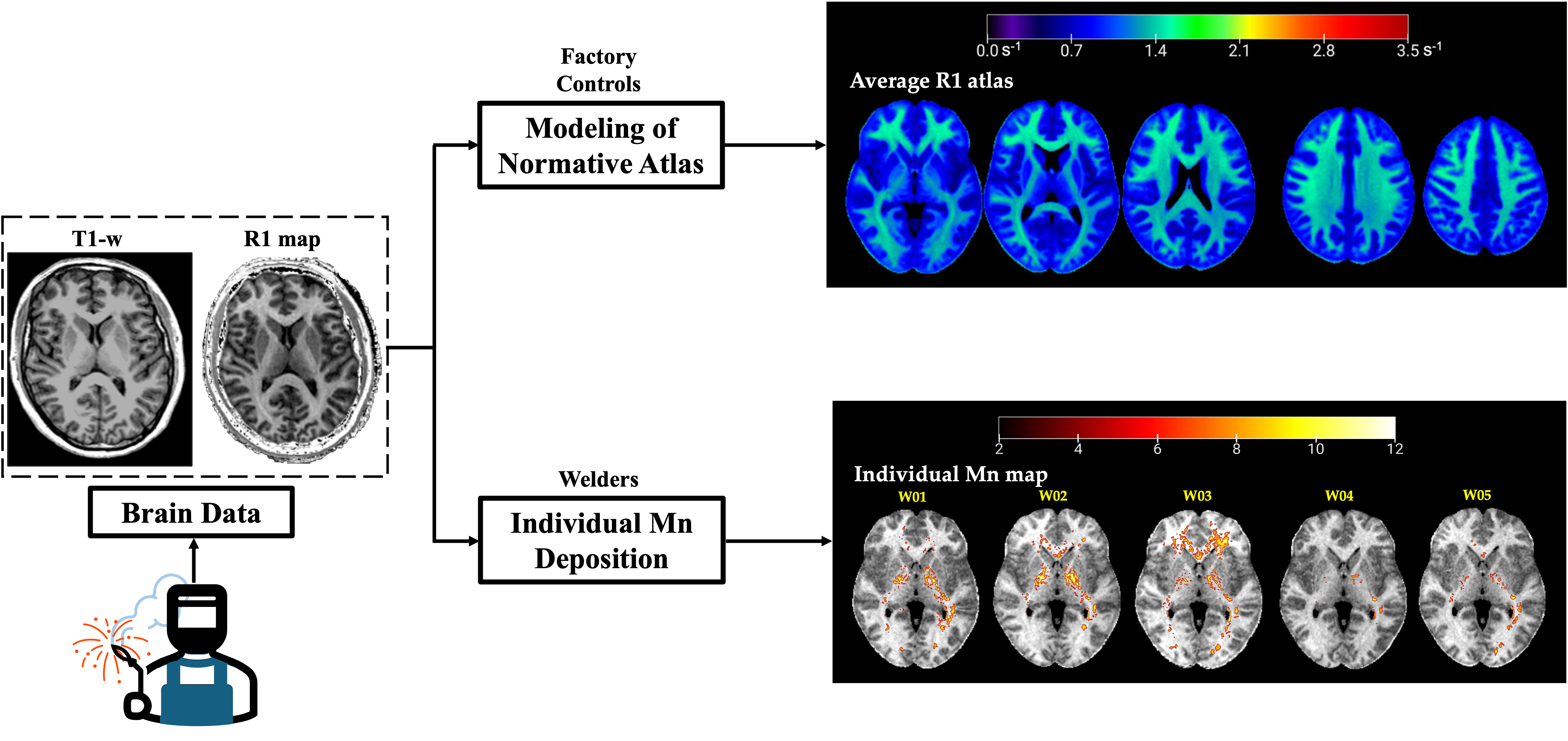
In this work we demonstrate a novel methodology for personalized diagnosis and spatial characterization of abnormal Magnetic Resonance Imaging R1 (R1 = 1/T1) relaxation rates arising from excessive manganese (Mn) accumulation in welders’ brains. Utilizing voxel-wise population-derived norms based on a frequency age-matched non-exposed group (n = 25), we demonstrate the ability to conduct subject-specific assessments and mapping of Mn exposure using MRI relaxometry. Our results show elevated R1 in multiple brain regions in individual welders, but also extreme between-subject variability in Mn accumulation, debasing the concept that high exposures correlate with uniformly high Mn deposition in the brain. Consequently, the presented personalized methodology serves as a counterpart to group-based comparison, which allows for understanding the level of individual exposure and the toxicokinetics of Mn accumulation. This work lays a foundation for improved occupational health assessments and preventive measures against neurotoxic metal exposure.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Whole-Brain R1 Mapping
2.3. Data Processing for Normative R1 Atlas
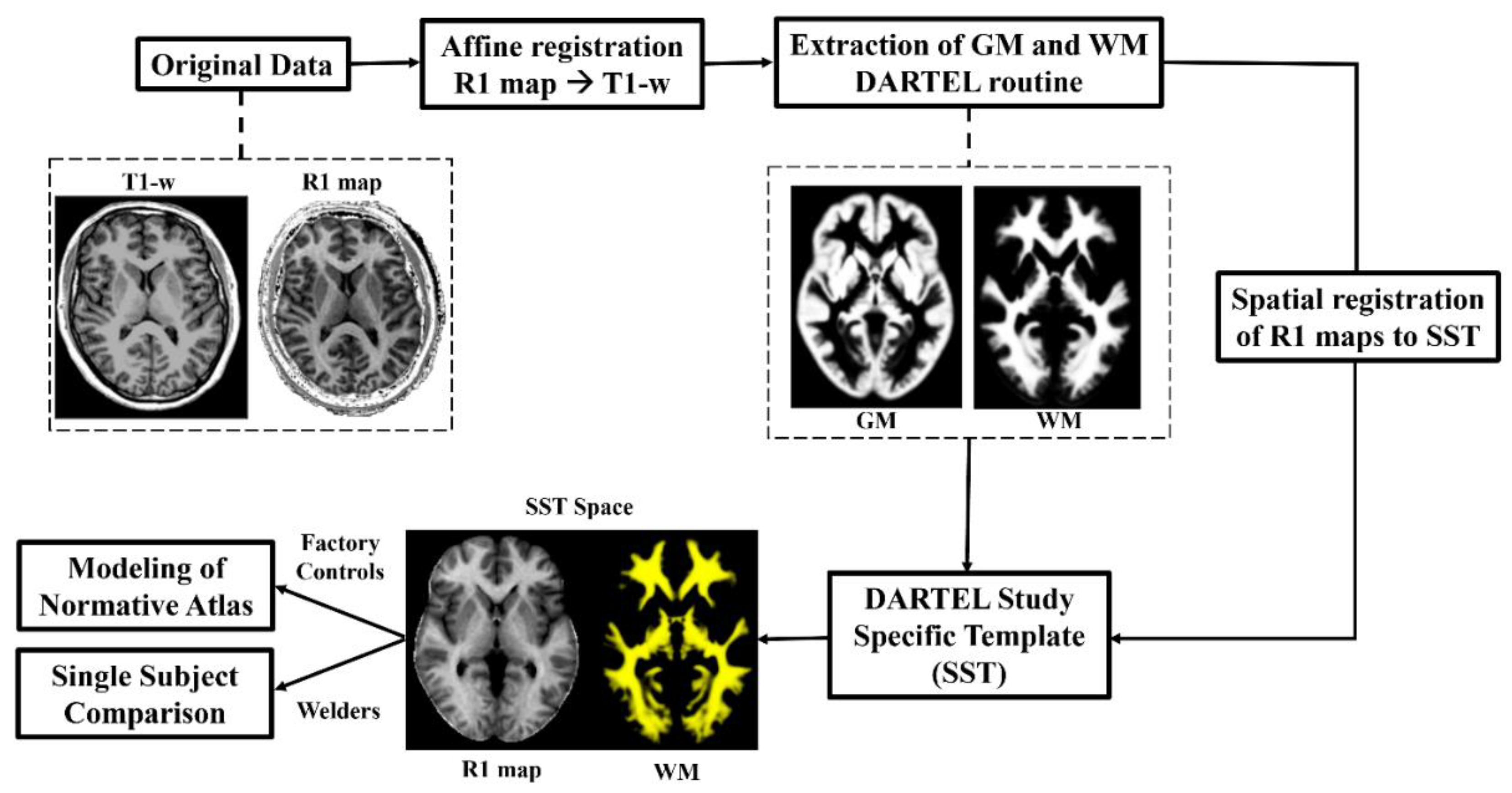
2.4. R1 Normative Atlas
5.5. Single-Subject Comparison
3. Results
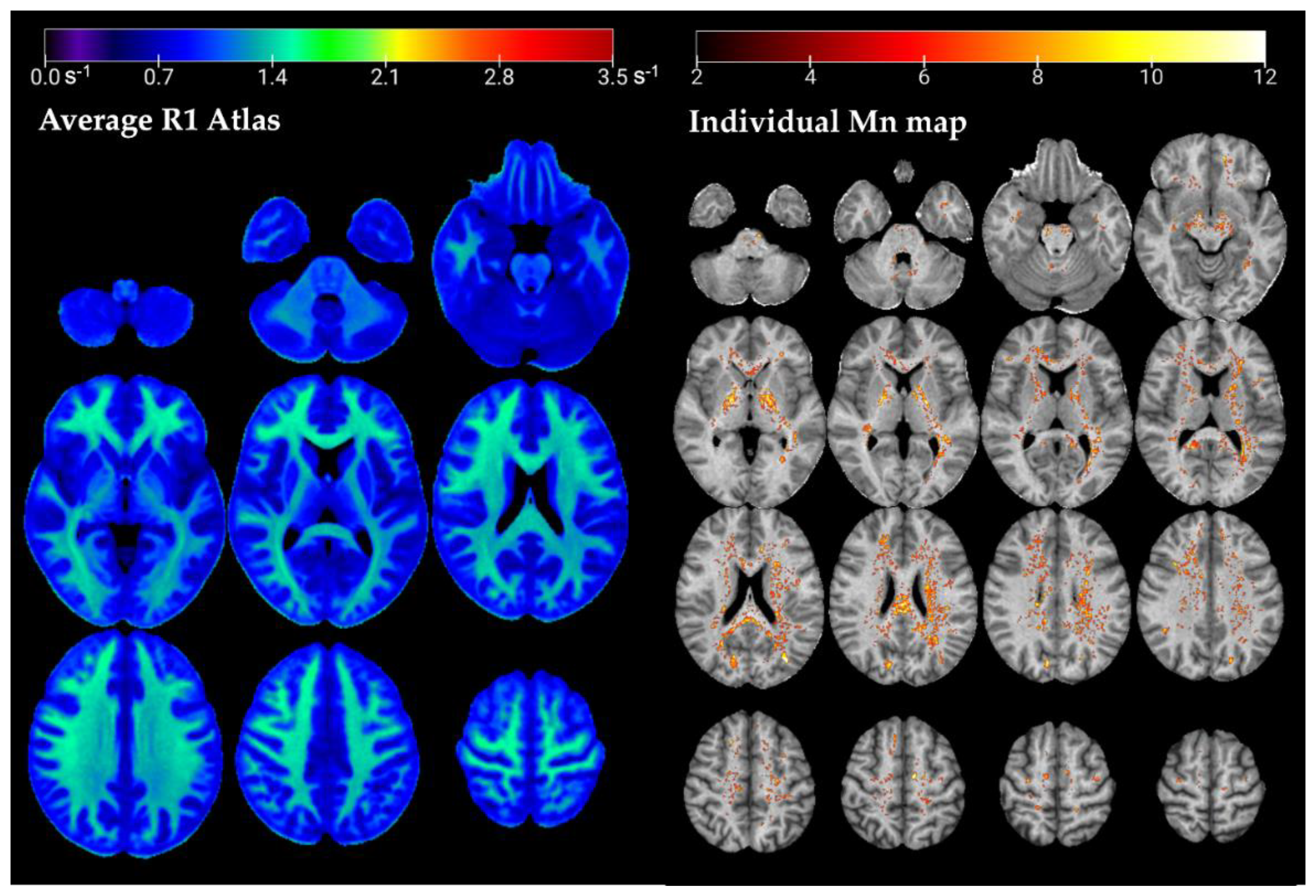
3.1. R1 Atlas
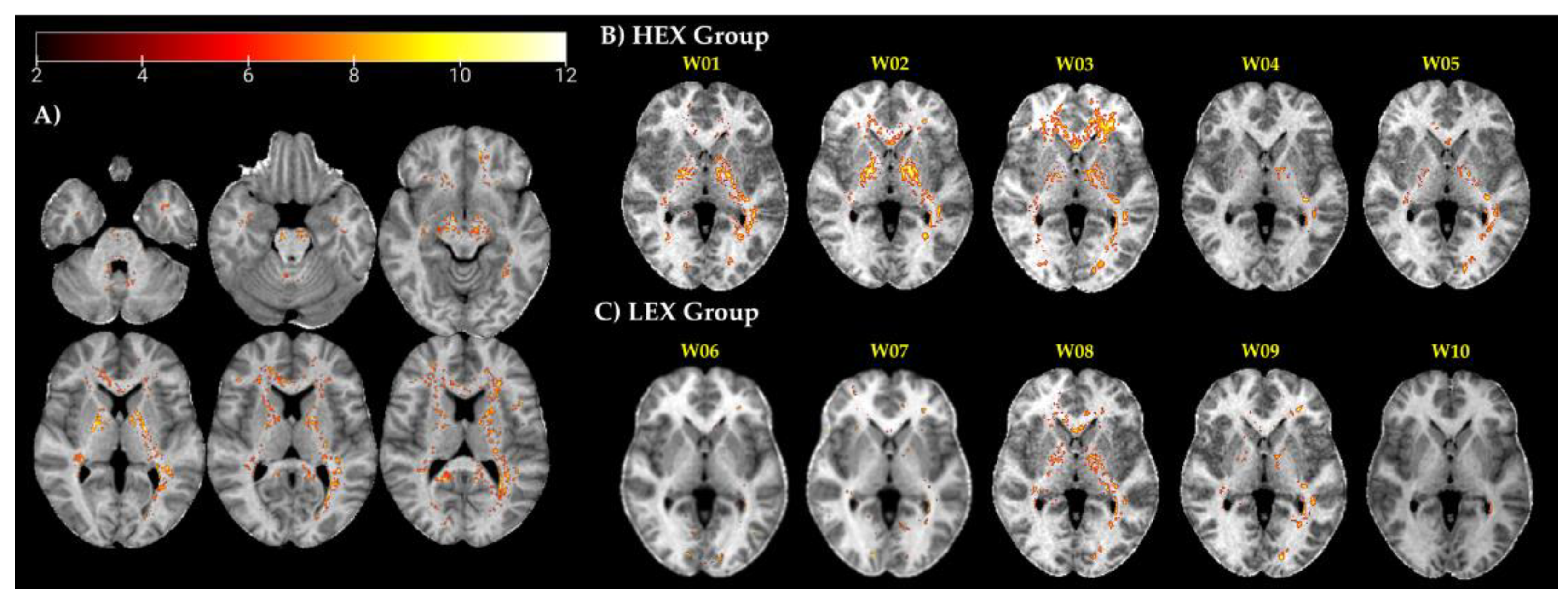
3.2. Case Reports
| HEX Group | |||||
| W01 | W02 | W03 | W04 | W05 | |
| Age [yrs] | 48 | 50 | 34 | 56 | 29 |
| Race | White | White | Hispanic | White | White |
| Welding time [yrs] | 11 | 24 | 3 | 36 | 6 |
| Welding type† | MIG-MS | MIG-MS | MIG-MS | MIG-MS | MIG-MS |
| Air Mn (mg/m3) | 0.16 | 0.12 | 0.12 | 0.48 | 0.48 |
| CEI3M [mg/m3·yr] | 0.12 | 0.05 | 0.05 | 0.05 | 0.06 |
| CEILife [mg/m3·yr] | 1.49 | 2.44 | 0.36 | 4.8 | 0.93 |
| LEX Group | |||||
| W06 | W07 | W08 | W09 | W10 | |
| Age [yrs] | 37 | 37 | 46 | 31 | 29 |
| Race | Hispanic | African Am. | African Am. | White | African Am. |
| Welding time [yrs] | 11 | 14 | 21 | 7 | 4 |
| Welding type† | MIG-MS | MIG-MS | MIG-HSS | MIG-HSS | MIG-MS |
| Air Mn (mg/m3) | 0.08 | 0.08 | 0.15 | 0.20 | 0.07 |
| CEI3M [mg/m3·yr] | 0.04 | 0.01 | 0.02 | 0.02 | 0.03 |
| CEILife [mg/m3·yr] | 0.56 | 0.68 | 1.37 | 0.51 | 0.31 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dean, D.C.; Sojkova, J.; Hurley, S.; Kecskemeti, S.; Okonkwo, O.; Bendlin, B.B.; Theisen, F.; Johnson, S.C.; Alexander, A.L.; Gallagher, C.L. Alterations of Myelin Content in Parkinson’s Disease: A Cross-Sectional Neuroimaging Study. PLoS ONE 2016, 11, e0163774. [Google Scholar] [CrossRef] [PubMed]
- Baudrexel, S.; Nürnberger, L.; Rüb, U.; Seifried, C.; Klein, J.C.; Deller, T.; Steinmetz, H.; Deichmann, R.; Hilker, R. Quantitative Mapping of T1 and T2* Discloses Nigral and Brainstem Pathology in Early Parkinson’s Disease. NeuroImage 2010, 51, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Bonnier, G.; Fischi-Gomez, E.; Roche, A.; Hilbert, T.; Kober, T.; Krueger, G.; Granziera, C. Personalized Pathology Maps to Quantify Diffuse and Focal Brain Damage. NeuroImage Clin. 2018, 21, 101607. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, N.; Mohammadi, S.; Lutti, A.; Callaghan, M.F. Advances in MRI-Based Computational Neuroanatomy: From Morphometry to in-Vivo Histology. Curr. Opin. Neurol. 2015, 28, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, K.S.; Yang, J.S.; Park, I.J.; Kim, E.; Jin, Y.; Kwon, K.R.; Chang, K.H.; Kim, J.W.; Park, S.H.; et al. Increase in Signal Intensities on T1-Weighted Magnetic Resonance Images in Asymptomatic Manganese-Exposed Workers. Neurotoxicology 1999, 20, 901–907. [Google Scholar]
- Criswell, S.R.; Perlmutter, J.S.; Huang, J.L.; Golchin, N.; Flores, H.P.; Hobson, A.; Aschner, M.; Erikson, K.M.; Checkoway, H.; Racette, B.A. Basal Ganglia Intensity Indices and Diffusion Weighted Imaging in Manganese-Exposed Welders. Occup. Environ. Med. 2012, 69, 437–443. [Google Scholar] [CrossRef]
- Dydak, U.; Criswell, S.R. Chapter 19. Imaging Modalities for Manganese Toxicity. In; 2014; pp. 477–512.
- Krieger, D.; Krieger, G.S.; Theilmann, L.; Krieger, D. Manganese and Chronic Hepatic Encephalopathy Departments of Neurology; 1995.
- Chang, Y.; Woo, S.T.; Kim, Y.; Lee, J.J.; Song, H.J.; Lee, H.J.; Kim, S.H.; Lee, H.; Kwon, Y.J.; Ahn, J.H.; et al. Pallidal Index Measured with Three-Dimensional T1-Weighted Gradient Echo Sequence Is a Good Predictor of Manganese Exposure in Welders. J. Magn. Reson. Imaging 2010, 31, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zheng, W.; Long, L.; Zhao, W.; Li, X.; Mo, X.; Lu, J.; Fu, X.; Li, W.; Liu, S.; et al. Brain Magnetic Resonance Imaging and Manganese Concentrations in Red Blood Cells of Smelting Workers: Search for Biomarkers of Manganese Exposure. NeuroToxicology 2007, 28, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Dietz, M.C.; Ihrig, A.; Wrazidlo, W.; Bader, M.; Jansen, O.; Triebig, G. Results of Magnetic Resonance Imaging in Long-Term Manganese Dioxide-Exposed Workers. In Proceedings of the Environmental Research, Academic Press Inc., 2001; Vol. 85, pp. 37–40. [Google Scholar]
- Shin, Y.C.; Kim, E.; Cheong, H.K.; Cho, S.; Sakong, J.; Kim, K.S.; Yang, J.S.; Jin, Y.W.; Kang, S.K.; Kim, Y. High Signal Intensity on Magnetic Resonance Imaging as a Predictor of Neurobehavioral Performance of Workers Exposed to Manganese. NeuroToxicology 2007, 28, 257–262. [Google Scholar] [CrossRef]
- Guilarte, T.R.; Gonzales, K.K. Manganese-Induced Parkinsonism Is Not Idiopathic Parkinson’s Disease: Environmental and Genetic Evidence. Toxicol. Sci. 2015, 146, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.A.; Ma, R.E.; Yeh, C.-L.; Ward, E.; Snyder, S.; Azizi, E.; Zauber, S.E.; Wells, E.M.; Dydak, U. Reversibility of Neuroimaging Markers Influenced by Lifetime Occupational Manganese Exposure. Toxicol. Sci. 2019, 172, 181–190. [Google Scholar] [CrossRef]
- Edmondson, D.A.; Yeh, C.L.; Hélie, S.; Dydak, U. Whole-Brain R1 Predicts Manganese Exposure and Biological Effects in Welders. Arch. Toxicol. 2020, 94, 3409–3420. [Google Scholar] [CrossRef] [PubMed]
- Monsivais, H.; Yeh, C.-L.; Edmondson, A.; Harold, R.; Snyder, S.; Wells, E.M.; Schmidt-Wilcke, T.; Foti, D.; Zauber, S.E.; Dydak, U. Whole-Brain Mapping of Increased Manganese Levels in Welders and Its Association with Exposure and Motor Function. NeuroImage 2024, 288, 120523. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Flynn, M.R.; Du, G.; Lewis, M.M.; Fry, R.; Herring, A.H.; Van Buren, E.; Van Buren, S.; Smeester, L.; Kong, L.; et al. T1 Relaxation Rate (R1) Indicates Nonlinear Mn Accumulation in Brain Tissue of Welders with Low-Level Exposure. Toxicol. Sci. 2015, 146, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Bowler, R.M.; Yeh, C.L.; Adams, S.W.; Ward, E.J.; Ma, R.E.; Dharmadhikari, S.; Snyder, S.A.; Zauber, S.E.; Wright, C.W.; Dydak, U. Association of MRI T1 Relaxation Time with Neuropsychological Test Performance in Manganese- Exposed Welders. NeuroToxicology 2018, 64, 19–29. [Google Scholar] [CrossRef]
- Choi, D.S.; Kim, E.A.; Cheong, H.K.; Khang, H.S.; Ryoo, J.W.; Cho, J.M.; Sakong, J.; Park, I. Evaluation of MR Signal Index for the Assessment of Occupational Manganese Exposure of Welders by Measurement of Local Proton T1 Relaxation Time. NeuroToxicology 2007, 28, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.E.; Ward, E.J.; Yeh, C.L.; Snyder, S.; Long, Z.; Gokalp Yavuz, F.; Zauber, S.E.; Dydak, U. Thalamic GABA Levels and Occupational Manganese Neurotoxicity: Association with Exposure Levels and Brain MRI. NeuroToxicology 2018, 64, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.J.; Edmondson, D.A.; Nour, M.M.; Snyder, S.; Rosenthal, F.S.; Dydak, U. Toenail Manganese: A Sensitive and Specific Biomarker of Exposure to Manganese in Career Welders. Ann. Work Expo. Health 2018, 62, 101–111. [Google Scholar] [CrossRef]
- Deoni, S.C.L.; Rutt, B.K.; Peters, T.M. Rapid Combined T1 and T2 Mapping Using Gradient Recalled Acquisition in the Steady State. Magn. Reson. Med. 2003, 49, 515–526. [Google Scholar] [CrossRef]
- Tabelow, K.; Balteau, E.; Ashburner, J.; Callaghan, M.F.; Draganski, B.; Helms, G.; Kherif, F.; Leutritz, T.; Lutti, A.; Phillips, C.; et al. hMRI – A Toolbox for Quantitative MRI in Neuroscience and Clinical Research. NeuroImage 2019, 194, 191–210. [Google Scholar] [CrossRef]
- Ashburner, J. A Fast Diffeomorphic Image Registration Algorithm. NeuroImage 2007, 38, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Pechaud, M.; Smith, S. BET2 - MR-Based Estimation of Brain, Skull and Scalp Surfaces.
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef]
- Piredda, G.F.; Hilbert, T.; Granziera, C.; Bonnier, G.; Meuli, R.; Molinari, F.; Thiran, J.P.; Kober, T. Quantitative Brain Relaxation Atlases for Personalized Detection and Characterization of Brain Pathology. Magn. Reson. Med. 2020, 83, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Badve, C.; Yu, A.; Rogers, M.; Ma, D.; Liu, Y.; Schluchter, M.; Sunshine, J.; Griswold, M.; Gulani, V. Simultaneous T1 and T2 Brain Relaxometry in Asymptomatic Volunteers Using Magnetic Resonance Fingerprinting. Tomography 2015, 1, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Draganski, B.; Ashburner, J.; Hutton, C.; Kherif, F.; Frackowiak, R.S.J.; Helms, G.; Weiskopf, N. Regional Specificity of MRI Contrast Parameter Changes in Normal Ageing Revealed by Voxel-Based Quantification (VBQ). Neuroimage 2011, 55, 1423–1434. [Google Scholar] [CrossRef]
- Slater, D.A.; Melie-Garcia, L.; Preisig, M.; Kherif, F.; Lutti, A.; Draganski, B. Evolution of White Matter Tract Microstructure across the Life Span. Hum. Brain Mapp. 2019, 40, 2252–2268. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Fabiano, G.; Procopio, A.C.; Aquila, I.; Pellicano, R.; Barone, S.; Morelli, M. Hepatic Encephalopathy by Manganese Deposition: A Case Report and a Review of Literature. Rev. Recent Clin. Trials 2022, 17, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Morishima, R. Pallidal Manganese Concentration in Hepatic Encephalopathy. Clin. Gastroenterol. Hepatol. 2021, 19, e114. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Bornhorst, J.B.; Aschner, M. Manganese Metabolism in Humans. Front. Biosci.-Landmark 2018, 23, 1655–1679. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Park, J.E.; Gugnani, K.; Betharia, S.; Pino-Figueroa, A.; Kim, J. Influence of Iron Metabolism on Manganese Transport and Toxicity. Metallomics 2017, 9, 1028–1046. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Böhlke, M.; Maher, T.; Kim, J. Alcohol Exposure Increases Manganese Accumulation in the Brain and Exacerbates Manganese-Induced Neurotoxicity in Mice. Arch. Toxicol. 2021, 95, 3665–3679. [Google Scholar] [CrossRef]
- Scarpazza, C.; Sartori, G.; De Simone, M.S.; Mechelli, A. When the Single Matters More than the Group: Very High False Positive Rates in Single Case Voxel Based Morphometry. NeuroImage 2013, 70, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, P.X.; Zheng, M.; Shi, W. A Diffeomorphic Unsupervised Method for Deformable Soft Tissue Image Registration. Comput. Biol. Med. 2020, 120, 103708. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Zhao, A.; Sabuncu, M.R.; Guttag, J.; Dalca, A.V. VoxelMorph: A Learning Framework for Deformable Medical Image Registration. IEEE Trans. Med. Imaging 2019. [Google Scholar] [CrossRef]
- Shao, S.; Pei, Z.; Chen, W.; Zhu, W.; Wu, X.; Zhang, B. A Multi-Scale Unsupervised Learning for Deformable Image Registration. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Rabin, O.; Hegedus, L.; Bourre, J. -M; Smith, Q.R. Rapid Brain Uptake of Manganese(II) Across the Blood-Brain Barrier. J. Neurochem. 1993, 61, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Prohaska, J.R. Functions of Trace Elements in Brain Metabolism; 1987; Vol. 67.
- Stockwell, T.; Zhao, J.; Greenfield, T.; Li, J.; Livingston, M.; Meng, Y. Estimating Under- and over-Reporting of Drinking in National Surveys of Alcohol Consumption: Identification of Consistent Biases across Four English-Speaking Countries. Addict. Abingdon Engl. 2016, 111, 1203. [Google Scholar] [CrossRef]
- Ellingsen, D.G.; Kusraeva, Z.; Bast-Pettersen, R.; Zibarev, E.; Chashchin, M.; Thomassen, Y.; Chashchin, V. The Interaction between Manganese Exposure and Alcohol on Neurobehavioral Outcomes in Welders. Neurotoxicol. Teratol. 2014, 41, 8–15. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Murray, D.L.; Thielen, K.R.; Williamson, E.E.; Eckel, L.J. Intracranial Gadolinium Deposition after Contrast-Enhanced MR Imaging. Radiology 2015, 275, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-Based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 2015, 276, 228–232. [Google Scholar] [CrossRef] [PubMed]
| Characteristics of participants | Welders (n = 36) | Controls (n = 25) |
| Age (y) [mean ± SD], range | 39.9 ± 10.7, (21, 57) | 38.9 ± 11.1, (20, 61) |
| Years of Education (y) [mean ± SD], range | 12.6 ± 1.3, (8, 12) | 12.9 ± 1.2, (12, 16) |
| Welding years (y) [mean ± SD], range | 12.5 ± 8.8, (1.7, 36) | 0 ± 0, - |
| Exposure (mg/m3·yr) [mean ± SD], range | ||
| Mean Airborne Mn Exposure [mg/m3] | 0.146 ± 0.109, (0.051, 0.477) | 0.005 ± 0.008, (0, 0.027) |
| Mn cumulative exposure index (Mn-CEI3M) | 0.038 ± 0.035, (0.006, 0.179) | 0.0005 ± 0.0003, (0, 0.006) |
| Mn cumulative exposure index (Mn-CEI7-12M) | 0.074 ± 0.080, (0.009, 0.404) | 0.001 ± 0.001, (0, 0.013) |
| Mn cumulative exposure index (Mn-CEILife) | 1.444 ± 1.292, (0.026, 4.807) | 0.059 ± 0.081, (0.003, 0.403) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





