1. Introduction
Peripheral nerve injury (PNI) is a common occurrence resulting in functional, physiological, and psychological consequences that are the target of frequent clinical approaches in both human medicine and veterinary medicine. It is estimated that cases of PNI occur in around 3% of trauma patients [
1], and that around 13 to 23 per 1,00,000 people suffer from this condition [
2]. The cause behind this type of injury is multiple and can range from external trauma, penetrating injuries, neoplasms, metabolic changes and iatrogenic interventions such as surgery or perineural administrations [
3]. The deeper nerves protected by surrounding structures tend to be affected by more aggressive injuries in cases of bone fractures, perforating damages, or the development of compressive masses; the more superficial nerves, such as the radial or common peroneal nerve in part of their course, can easily be externally compressed and suffer a crushing injury. In particularly severe cases, when the nerve injury is just an additional element in a multiple trauma scenario, PNI is often undervalued or addressed late, missing the small window of opportunity that guarantees the success of a therapeutic approach, be it surgical or medical [
4]. This reality gains even more importance in veterinary medicine, where diagnostic tools available in a timely manner and at affordable prices are even more scarce.
Therapeutic options for treating PNI cases range from conservative treatment to drug approaches and surgical interventions. The choice of the method to use will depend on several factors such as the type of injury, the injured nerve, the lesion location within the nerve, the state of the surrounding tissues, the time elapsed since the injury and, last but not least, the options available [
5]. In complete transection injuries, a more aggressive type of injury with no capacity for spontaneous recovery, where there is a total loss of nerve continuity with affection not only of the axons but also of all the nerve envelopes of myelin and connective tissue [
6], surgical techniques such as end-to-end sutures and neurografts continue to be the gold standard approaches [
7]. In crush injuries, since nerve continuity is maintained with different levels of loss of connective tissue coverings, spontaneous recovery is possible, although slow [
6,
8]. In these cases, conservative treatments and neurorehabilitation takes on greater importance [
9]. Whatever the type of injury, no method can currently be considered as ideal, and the results tend to be imperfect, with long-term motor, sensory and autonomic impairment, and loss of quality of life for the patient.
Regenerative medicine is an alternative medical field that aims to establish innovative therapeutic approaches to medical problems whose surgical, medicinal or conservative resolution is currently unsatisfactory [
10]. The promotion of peripheral nerve regeneration has been the focus of multiple studies over the last decade [
11]. The therapeutic fields explored within PNI field are multiple, ranging from the development of new biomaterials that function as scaffolds to replace grafts, neural tube guides as physical bridges to guide and protect the site of regeneration or cell-based therapies that promote regeneration of the peripheral nerve while modulating the local and systemic immune response. More specifically, combined therapies, which use two or more pro-regenerative methods simultaneously, have gained special attention. The need to test therapies developed in animal models before applying them in real clinical scenarios has led to the use of traditional animal models such as rats, mice and rabbits, on which the overwhelming majority of work published in the last decade is based [
12]. In these models, the lesional paradigms explored are essentially the neurotmesis and axonotmesis, corresponding to complete transection and crush injuries, with the animals being evaluated regarding their functional recovery in vivo and the structural reorganization of the intervened nerves and effector muscles post-mortem [
8]. Advances in the area have been notable, but there is an excess of work developed in low-complexity animal models with little capacity for direct translation into clinical reality. Even though more complex animal models such as dogs or pigs are considered, they face ethical and emotional constraints that limit their wider use [
12]. More recently, sheep have gained attention as an advantageous model for studying regeneration after PNI [
13,
14] due to its availability, low price, ease of maintenance and handling, affable behavior, and fewer ethical restrictions. Furthermore, the position, distribution, functions and dimensions of peripheral nerves, as well as the functional consequences of their injury, are identical to those observed in humans [
13].
Our research group has been developing new treatments for PNI over the last two decades, mainly exploring combined therapies based on the use of biomaterials and mesenchymal stem cells. Most of the advances achieved were made in vitro and in the rat model [
15,
16], but recently the ovine model began to be explored, focusing on the common peroneal nerve instead of the sciatic nerve traditionally used in rodents. This polyfasciculated nerve is one of the branches of the sciatic nerve, with the advantage of having a superficial position in part of its route along the lateral side of the hindlimb, which facilitates surgical access for inducing injuries and for therapeutic approaches. The functional consequences of its injury are not as serious and deleterious to the animal's quality of life as a sciatic nerve injury, allowing the animal to maintain the ability to bear weight and explore the environment, while its mixed nature leads to motor and sensory changes that can be easily observed and quantified over time [
13,
17]. In a previous work, a protocol for the ovine model was established and validated, including the establishment of a method for surgical access and induction of nerve lesions of neurotmesis and axonotmesis in the common peroneal nerve, for the application of therapeutic approaches, for methods of functional evaluation and for ideal times for monitoring animals after injury. Subsequently, the intervened nerves were also collected and stereologically evaluated, preliminary evaluating the recovery behavior of the injured nerves after being subjected to the established treatments. This assay allowed to obtain control values for both functional tests and stereological studies in animals subject to neurotmesis, which can now be used for comparison in future tests. Despite efforts, in animals subject to axonotmesis injuries, a complete descriptive study was not carried out since stereologically, after inducing crushing with a clamp exerting a pressure of 80N, the observation of some degenerated/regenerated fascicles in conjunction with healthy ones indicated that the induced crush injury was not effective from the structural point of view, even if it has been translated into compatible symptoms in vivo [
13].
These results left open the standardization of an axonotmesis injury protocol in the ovine model, in which it will be necessary to establish a new value of exerted pressure, greater than 80N, to guarantee the complete crushing of the nerve fibers of the common peroneal nerve. From a 3Rs perspective, and to avoid using new animals for this purpose, it was decided to carry out a preliminary biomechanical characterization study of the sheep's common peroneal nerve before moving on to new in vivo studies. The aim of this work is to study and compare the biomechanical behavior of the sheep common peroneal nerve after crushing by different pressures, to establish which parameters will be used in future in in vivo assays to induce effective axonotmesis injuries.
4. Discussion
The scientific literature has devoted much less attention to the study of peripheral nerve crush injuries than to complete transection ones. The crushing of a peripheral nerve is caused by an external compressive force or stretching, whether naturally occurring or iatrogenic, and happens mainly in large-caliber nerves that are close to bone structures or that have a superficial course [
8]. It has long been known that peripheral nerves undergo mechanical changes when subjected to crushing, which will have important consequences on the integrity and functionality of the injured nerve [
18]. In Seddon's classification criteria, this type of injury is called axonotmesis, and is characterized by a lesion that affects the axons and the myelin sheath, with the maintenance of the integrity in the connective tissue envelopes and support structures. In the Sunderland classification, axonotmesis is subdivided into three categories depending on whether the
endoneurium and
perineurium may also be involved, although the
epineurium always remains intact [
19]. The
vasa-nervorum can rupture with consequent ischemia, or tearing of the intraneural connective tissue can lead to hemorrhage and necrosis [
20]. In any case, the occurrence of axonal injury leads to the development of the normal Wallerian degeneration sequence at the edges of the crushed area with dragging of the ruptured nerve structures away from the point of greatest pressure and towards the limits of crushing [
21], but the preservation of the nerve connective tissue allows regeneration to be guided with potential for full spontaneous recovery, even if it takes longer than a simpler neuropraxia injury. The functional consequences manifest distally to the site of injury with sensory, motor and autonomic changes, pain and muscle wasting, and symptoms compatible with retrograde degeneration may also be observed. Spontaneous nerve growth and regeneration generally occurs at a rate of 1 mm/day (approximately 30 mm per month), and regeneration and full recovery may take months or years to occur [
22]. The degree of nerve damage in an axonotmesis injury is influenced by the applied pressure and the crushing duration [
23]. Furthermore, the regenerative capacity in this type of injury depends on the extent of the lesion, the distance from the injury site to the cell body and to the effector organ and the occurrence of additional complications in the surrounding tissues and organs. Generally, nerves with a simple branching pattern that are purely sensory or motor are more likely to regenerate successfully than mixed ones [
24].
Due to its ability to regenerate spontaneously and the maintenance of nervous continuity, the therapeutic options developed for cases of axonotmesis are more limited than those available for neurotmesis injuries, often opting for conservative approaches such as physiotherapy or, in more complicated and severe cases, surgical approaches for nerve decompression [
22,
24]. Experimentally, options such as wrapping with biomaterials or cell-based therapies to protect and act directly on the crush site were also explored [
25], but therapeutic options for these clinical situations are still scarce. Even considering that spontaneous regeneration occurs after a few months, the consequences and physical and psychological limitations of those affected during this period are relevant and it is important to explore new therapeutic approaches.
As expected, the overwhelming majority of experimental studies on this topic are carried out in low-complexity animal models such as rats and mice [
12], and the use of other models is rare [
14,
26]. In the past, Varejão
et al. developed a method of inducing a sciatic nerve crushing injury in the rat model using a non-serrated clamp applying a force of 54N for 30 seconds, resulting in an applied pressure of 9MPa and in a 3 mm long lesion [
27]. This model has been used frequently over the years, proving to be effective in this animal model. The standardization of axonotmesis models is not as well described in other species, a flaw that the authors consider essential to be addressed. From a translational perspective, and to allow the application of therapies already explored in the rat [
15,
16] in a more complex model before its application in clinical trials in humans or in veterinary species of clinical interest, our group developed neurotmesis and axonotmesis protocols to be applied in the common peroneal nerve of the ovine model [
13]. For the axonotmesis model, adapting the protocol applied to the rat model, a non-serrated clamp was tested exerting a force of 80N over 1 minute, inducing a 5 mm lesion. Despite the manifestation of functional deficits after the injury, after 12 weeks of follow-up this crushing did not translate into a histopathological scenario compatible with axonotmesis, with healthy fibers being identified in the middle of fibers undergoing regeneration. The crushing probably did not translate into an effective axonotmesis since, considering the force applied and the variation in the diameter of the nerve before and after the injury, the pressure exerted to the common peroneal nerve of the sheep was only 4 MPa. Additionally, the common peroneal nerve in this species is mixed, has a sizable diameter, large amounts of connective tissue and greater amounts of infiltrated adipose tissue than the nerves of the rat. With around 70% of the fibers in this nerve having less than 11 µm [
13], integral crushing of all fibers is significantly more challenging. For the same pressure to be exerted on the sheep's common peroneal nerve, it would be necessary to apply a force of 180N for the same period (1 minute) and inducing a lesion of equal extent (5 mm). Thus, the aim of this work was precisely to study ex vivo the biomechanical consequences observed in common peroneal nerves of the sheep when subjected to different crushing forces. In this way, it was possible to determine the force necessary to be applied in vivo without the need to use more animals before carrying out effective pre-clinical tests.
Previously a study was performed using human digital nerves subjected to different crushing forces. In this work, a direct influence of the different crushing forces applied on the physical behavior of the nerves and on the biomechanical parameters considered was not identified [
28]. An identical and adapted protocol was used here in the common peroneal nerve of the sheep.
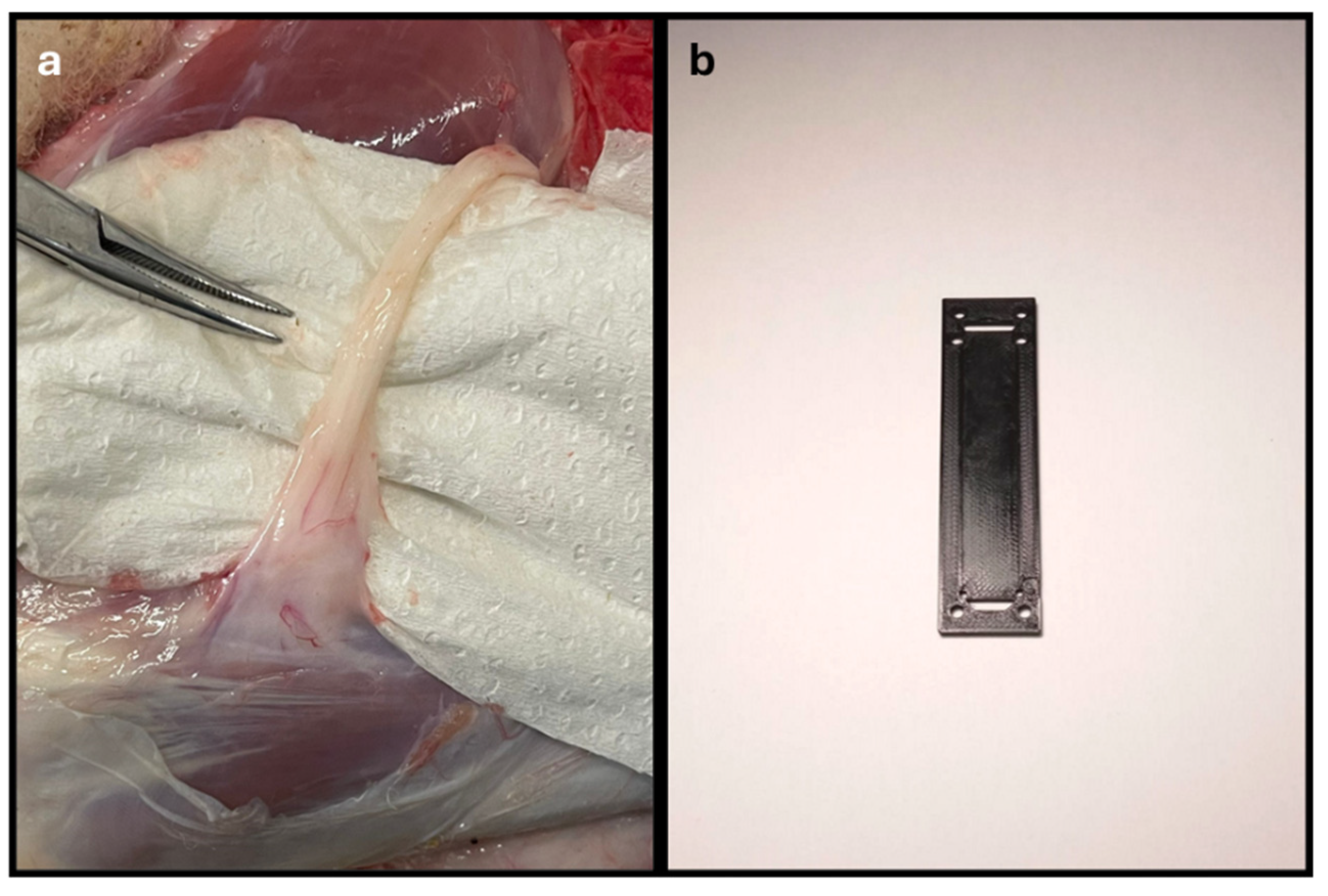
The device developed for harvesting the common peroneal nerves, shown in
Figure 1, proved to be effective in facilitating the harvesting of properly oriented nerves, without getting curled and all with the same length of 7 cm. This fact facilitated the subsequent application of crushing forces exactly to the middle of the nerve, ensuring an equal distribution of forces at the midpoint and placing all nerves in equal physical conditions for evaluation.
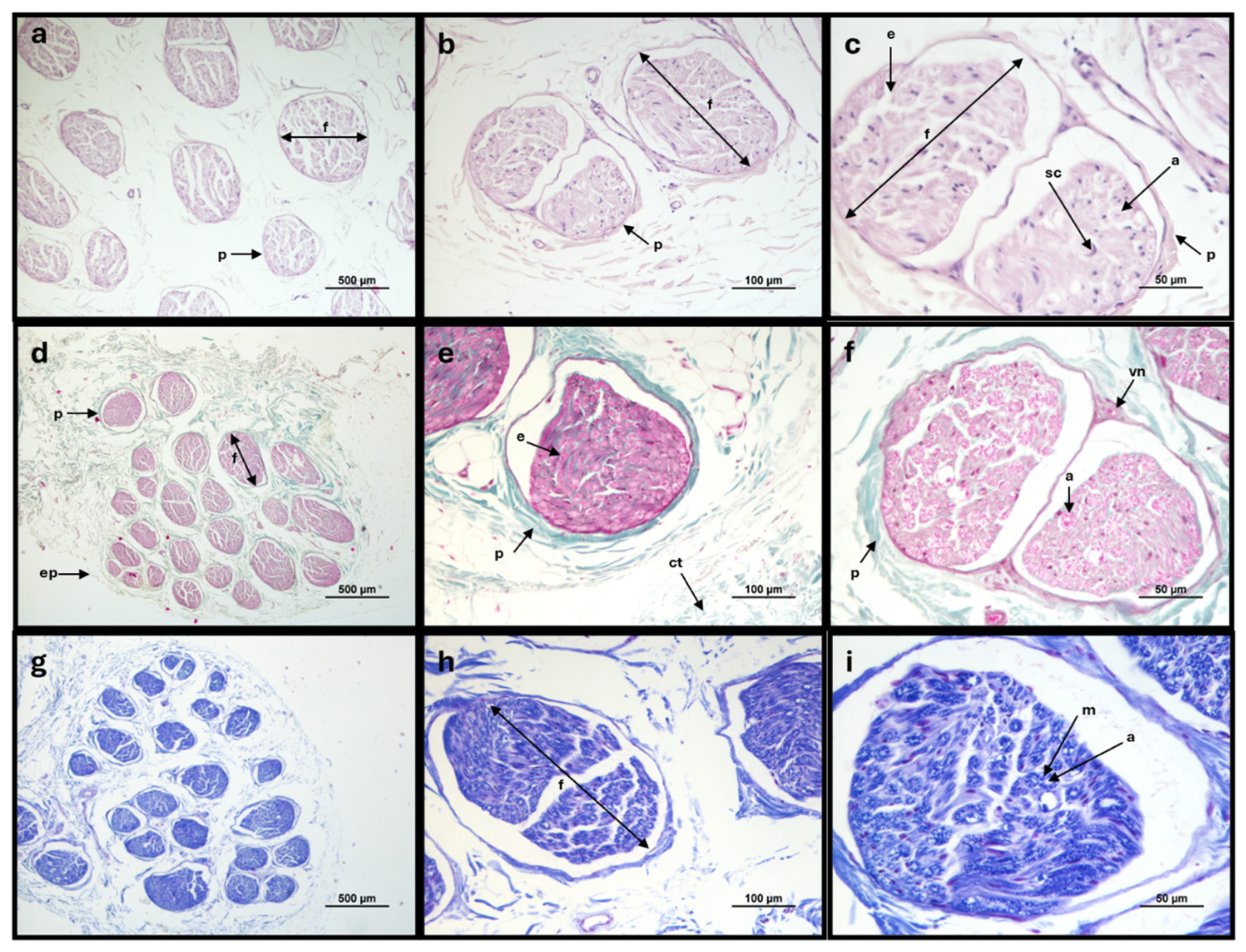
The histological and immunohistochemical evaluation of the collected nerves allowed to observe the microscopic morphology expected for this peripheral nerve, with a polyfasciculated and myelinated nature. The amount of connective tissue is significant, and appears to be proportionally higher when compared to other species such as dogs [
29]. A greater amount of connective tissue represents additional protection of the axons within the nerve trunk, which may help explain the difficulty in inducing an effective crush injury when using insufficient pressure. Additionally, macroscopically, a significant amount of adipose tissue infiltrated between the nerve envelopes is noticeable, which facilitates nerve sliding and makes it difficult to stabilize when using the non-serrated clamp. Finally, the diameter of the fibers identified in the ovine nerve appears to be smaller than the corresponding average diameter in the human nerve [
30]. A greater proportion of small diameter fibers, with a greater amount of connective tissue and adipose tissue infiltration may justify the difficulty in inducing effective crushing of all fibers within the nerve trunk.
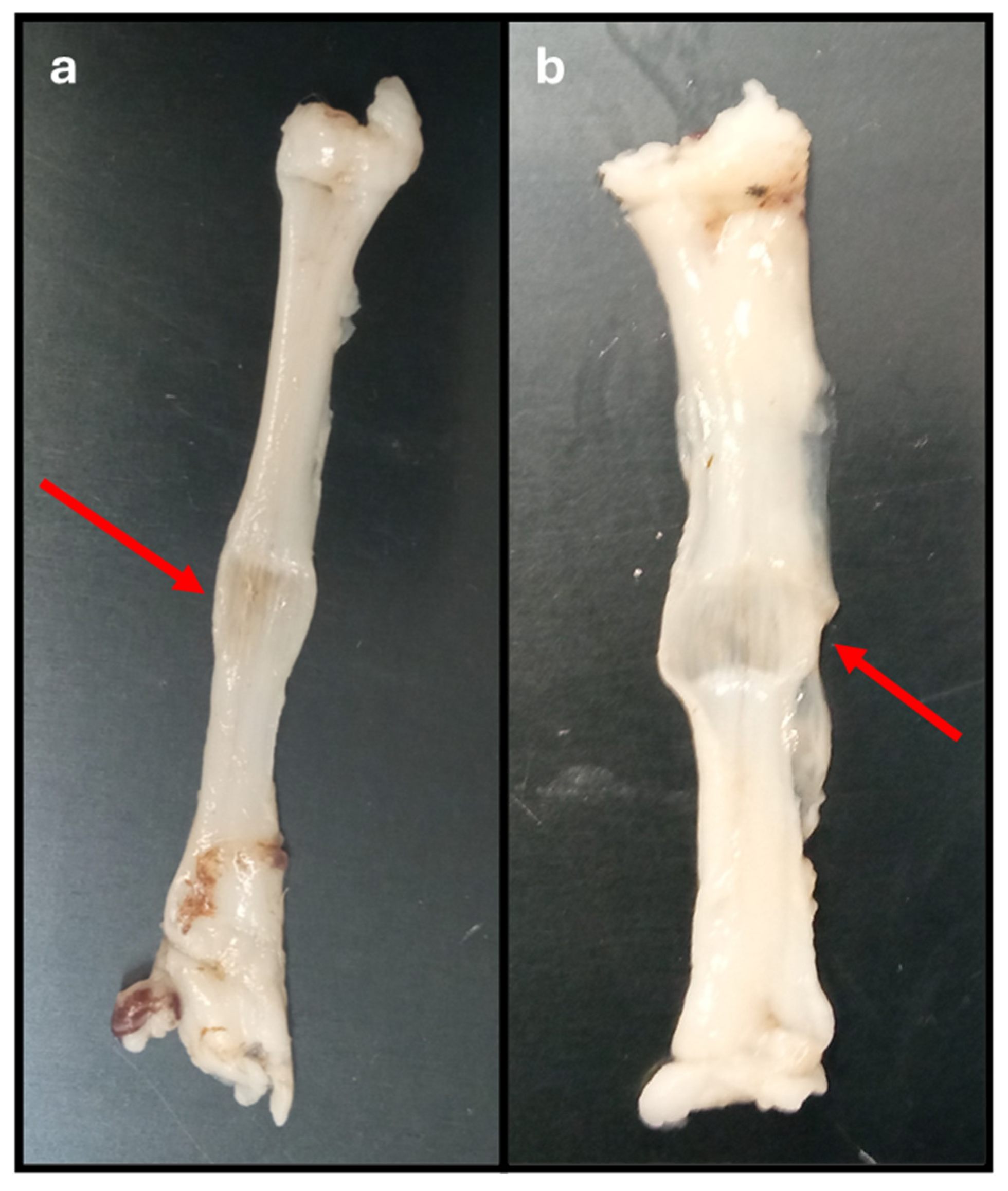
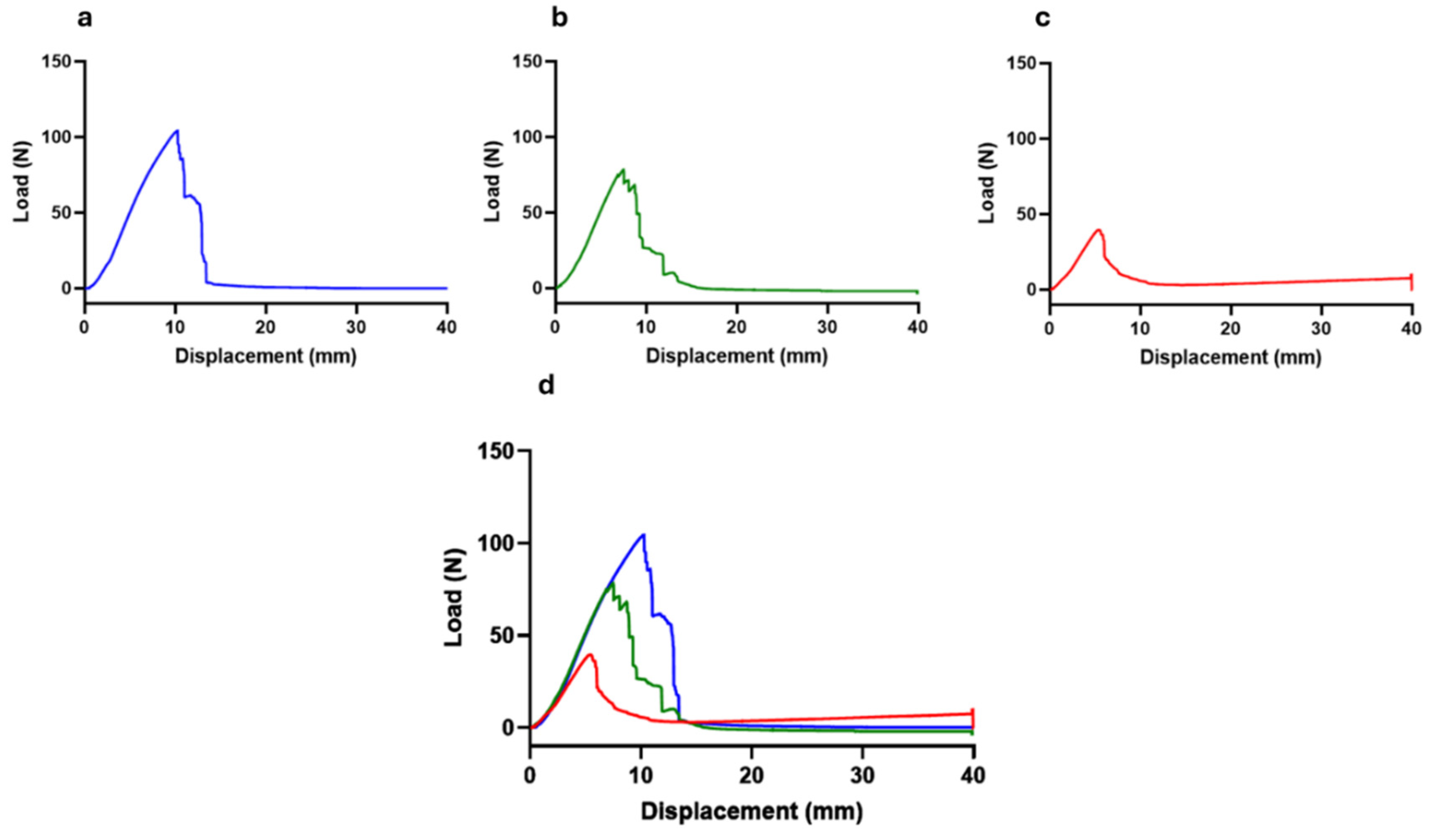
The crushing and pulled to failure tests proved to be effective in allowing assessments of the intended biomechanical parameters. The crushing test made it possible to effectively mimic the axonotmesis injury intended to be applied in vivo, with the application of two different forces, 80 and 180N, for one minute and with an extension of 5 mm. Macroscopically, after crushing the nerve, it was possible to observe the translucent and enlarged crushed area, as observed in vivo [
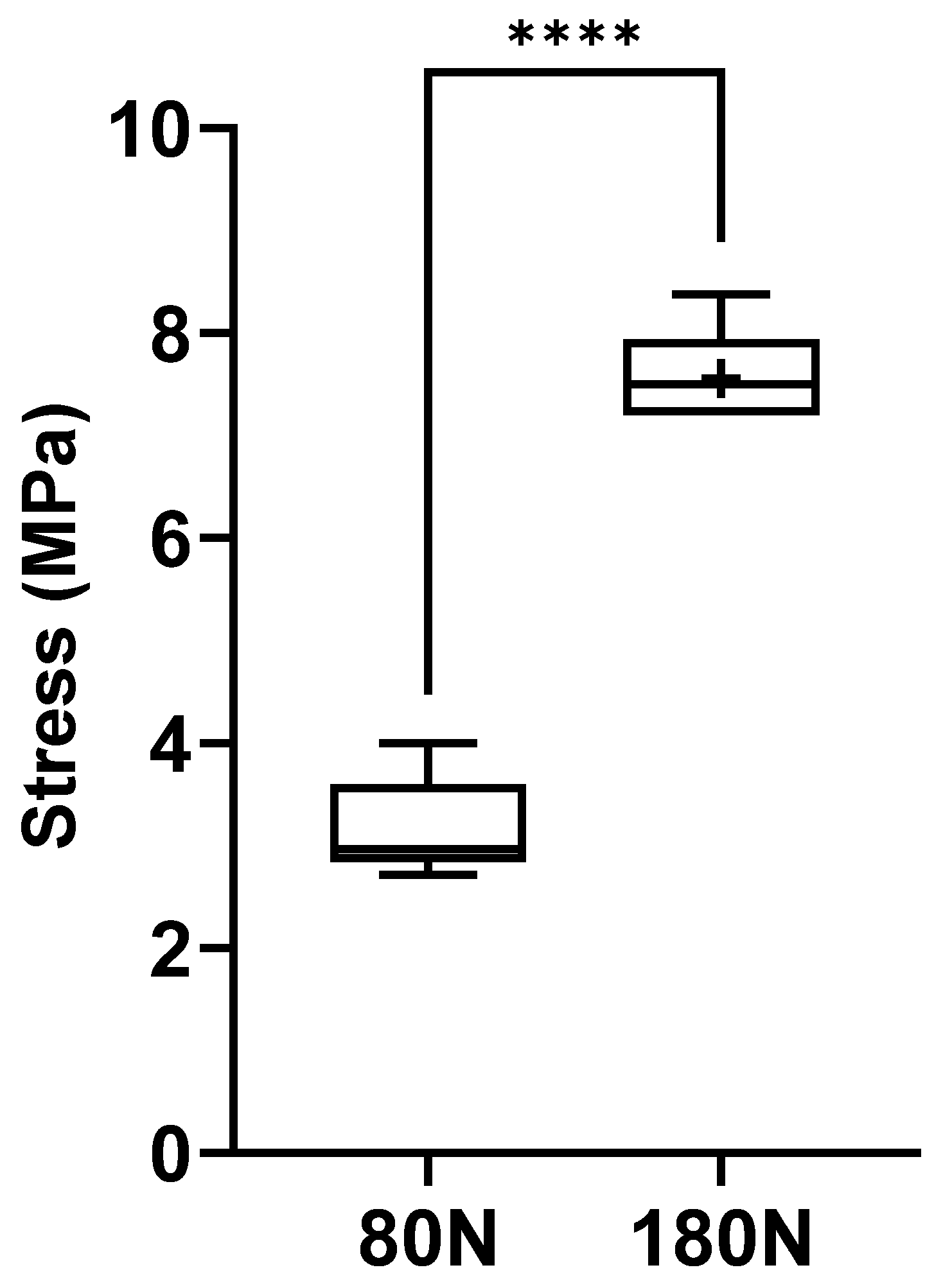
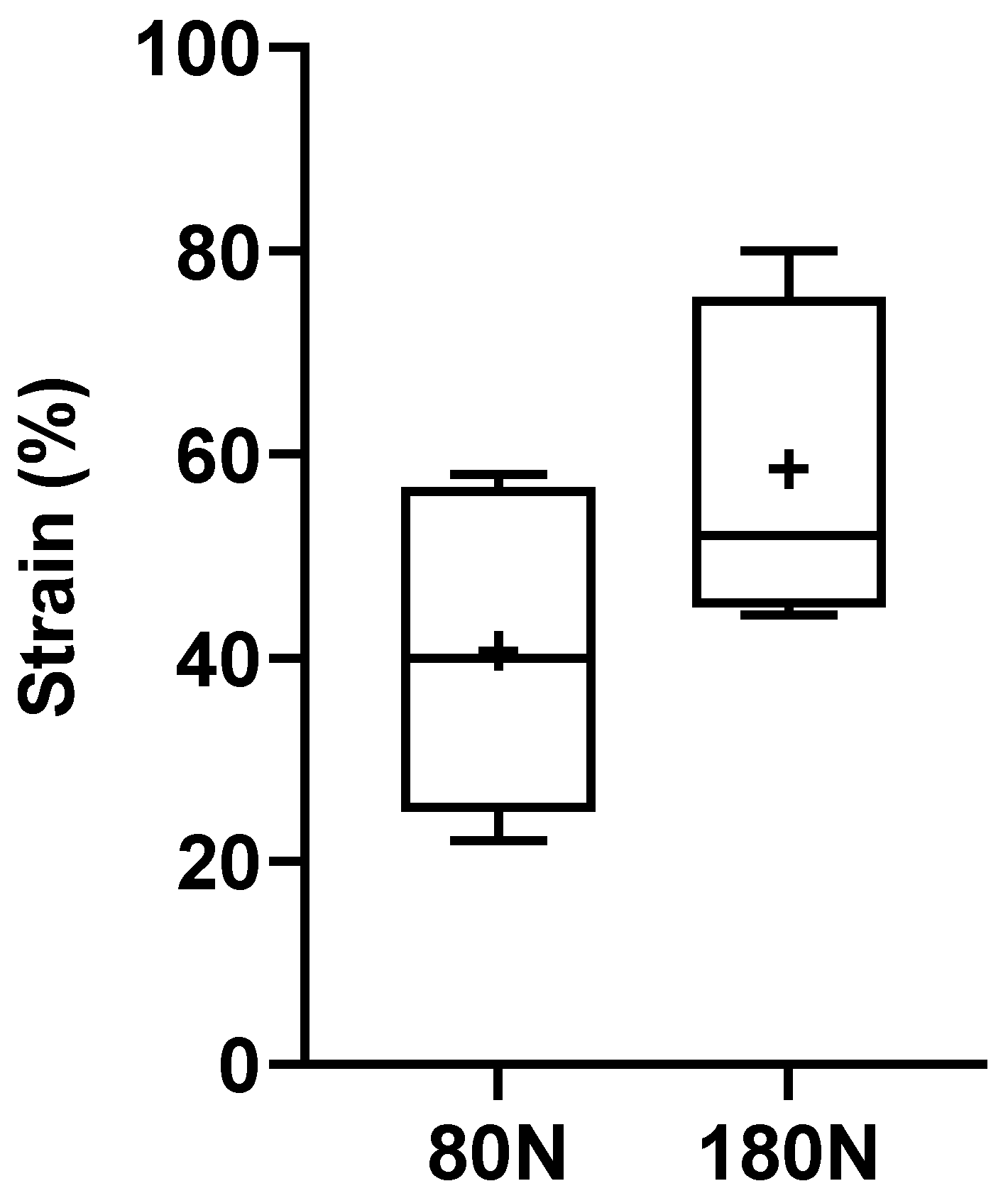
13]. In both applied forces there was an increase in the diameter at the crushing site, which biomechanically translated into a variation in strain that represents a change in shape or size of a material under the action of a certain force. The deformation in the common peroneal nerves was higher in the nerves subjected to a force of 180N, although without statistical differences compared to the group where the force of 80N was applied. Statistically relevant were the differences observed in the stress to which the nerves were subjected during crushing, that is, the internal force that the material experiences when subjected to an external force. Stress was substantially higher in nerves subjected to a force of 180N, with statistically significant differences compared to nerves subjected to 80N.
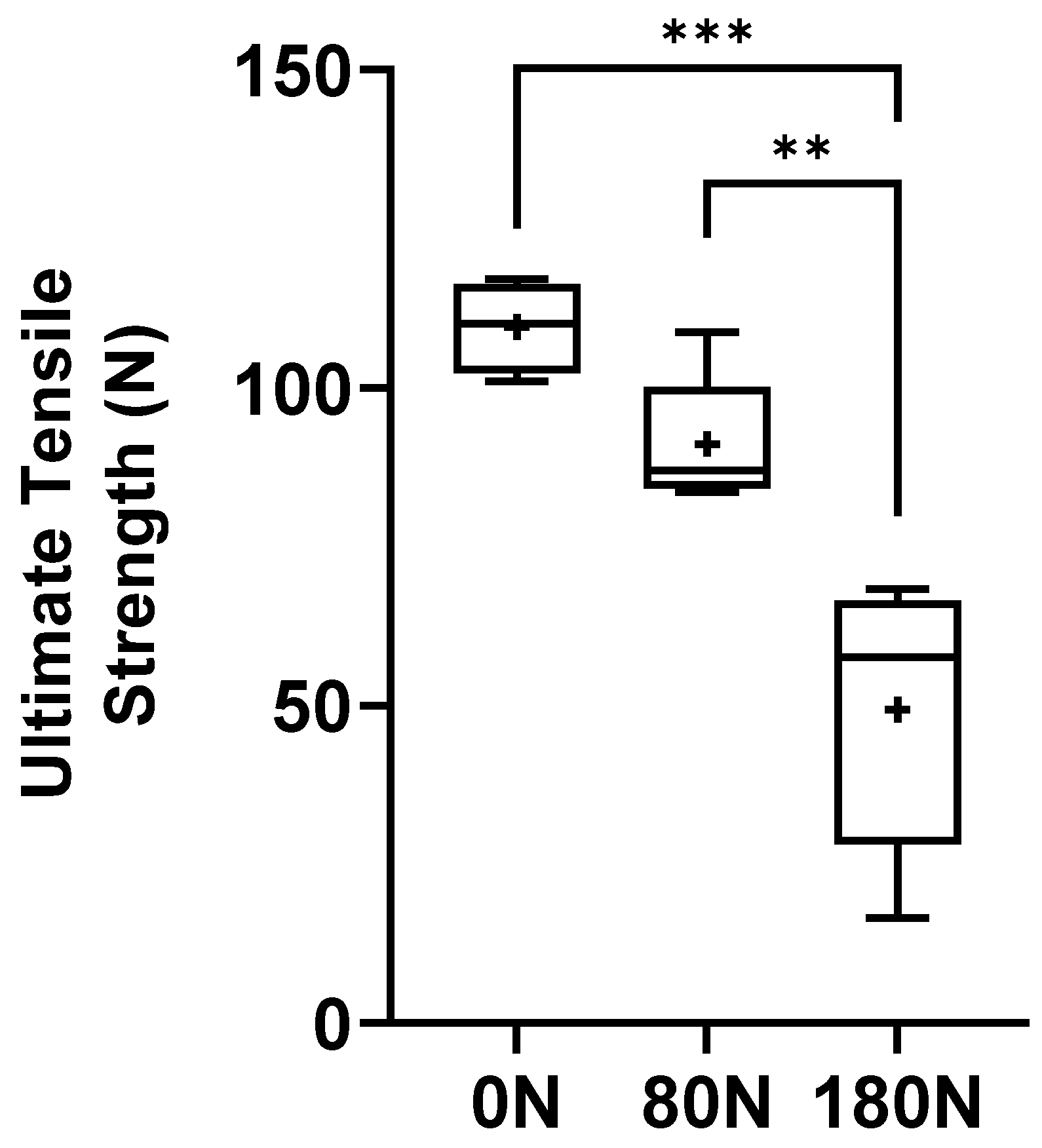
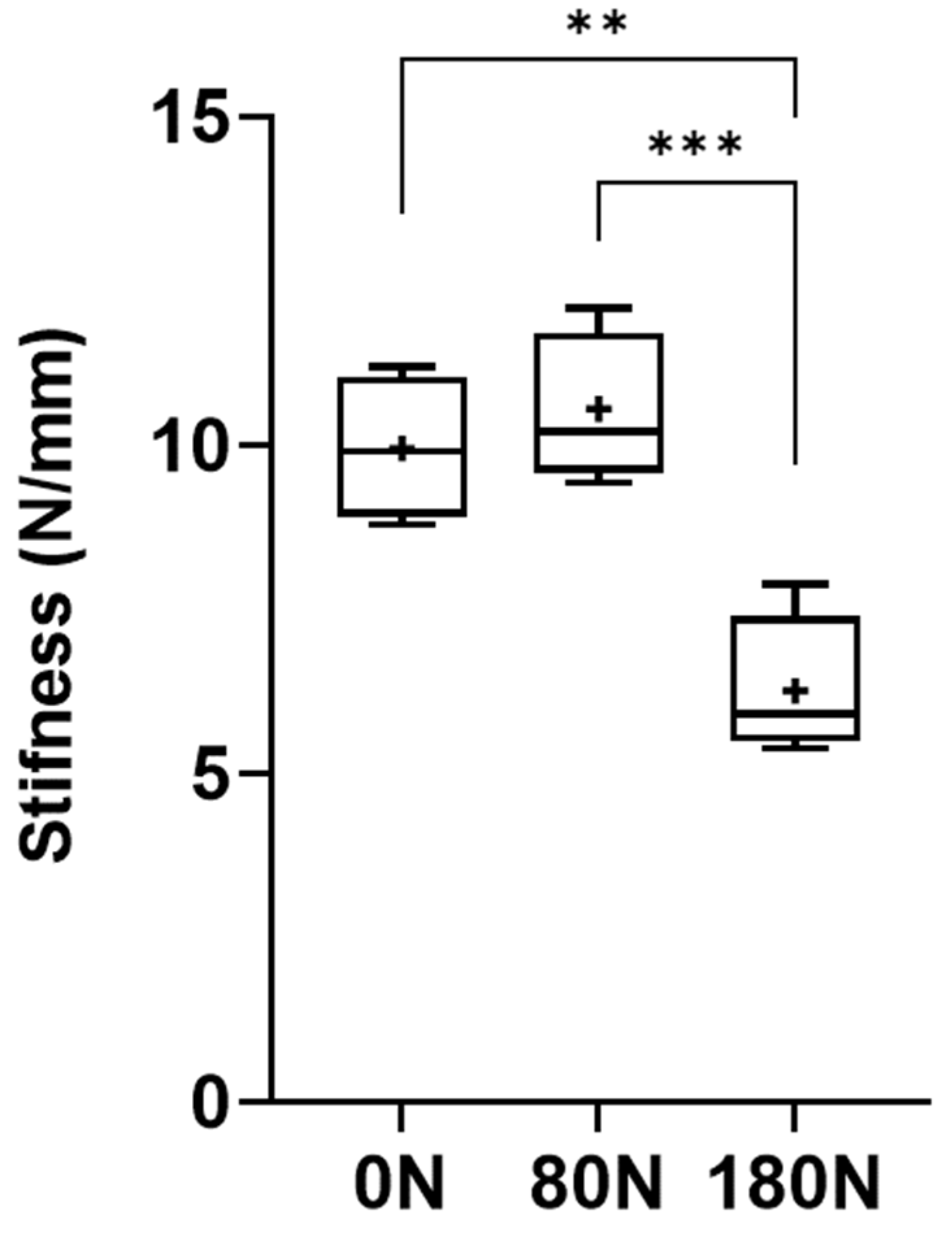
Pulled to failure aims to apply an increasing traction force over a material until rupture or irreversible deformation occurs. In this case, it allowed to determine at what level of traction force the nerve rupture occurred, considering that a crushed nerve with destruction of its internal support structures will have less resistance to rupture than an intact one. Nerves not subjected to crushing or subjected to a crushing force of 80N ruptured later than those subjected to 180N, with statistical differences being observed between the groups. Directly related to this parameter is stiffness, which determines the ability of a material to resist deformation, relating the ultimate tensile load force to the displacement observed at the moment of rupture. In this case, it was also in the nerves subjected to a force of 180N that lower stiffness and resistance were observed, once again with statistical differences regarding the different conditions tested.
Unlike the studies developed by Wong
et al. [
28] on human digital nerves, in our study the biomechanical tests and evaluations carried out demonstrate that compression has a significant influence on the parameters evaluated, with an increase in strain, stress and a decrease in ultimate tensile strength and stiffness in the nerves subjected to a higher force, with statistical differences between groups. The absence of statistical differences in the different parameters between the control groups and the group subjected to 80N, in addition to the histomorphological characteristics described above, justifies the ineffectiveness of axonotmesis when the animals were subjected to this crushing force in vivo. The ultimate tensile load value in the intact common peroneal nerve of the sheep is higher than the value observed for the same nerve in humans, where the stiffness was also inferior [
31], reinforcing the greater mechanical resistance of this nerve in the sheep species. The value of 180N, calculated so that a pressure of 9MPa can be applied (the one that efficiently produces an axonotmesis lesion in the rat) effectively induces a change in all biomechanical parameters studied, and the statistical differences observed between the nerves subjected to this force and those crushed with 80N, appears to indicate that this force will be effective in creating a complete axonotmesis when applied to sheep nerves in vivo. As far as the authors know, there are no studies that have evaluated crushing forces of this magnitude in the sheep model. In the rat model, forces of 150N allowed, after identification of post-crushing neurological deficits, full functional recovery over time [
32]. Even considering the dimensional and biomechanical differences between species, and the different forces applied, the same is expected to happen in sheep.
It is important, however, to list the limitations of the present study, which are related to the relatively small number of nerves used, the differences in the diameter of the nerves used in the different groups (even if randomly distributed) and the conditions in which the biomechanical tests were performed, dissimilar from the organic conditions in which spontaneous axonotmesis injuries occur. Despite the conservation and maintenance of tissue hydration, the cadaveric nerve does not reflect all the neurophysiological changes observed in the living nerve nor is it influenced by the biomechanical environment resulting from interactions with neighboring tissues and physiological conditions. The crushing force established here should in the future be tested in the ovine model so that it can be confirmed that in addition to the functional deficits already achieved by lower forces, histologically and stereologically there will be an effective axonotmesis with crushing of all nerve fibers and disruption of myelin wraps and connective tissue coatings. Considering the previous observations and results, and the statistical differences observed, the authors believe in the effectiveness of the conditions established here.
Figure 1.
Process for collecting the common peroneal nerve in sheep: a) common peroneal nerve duly individualized from neighboring tissues; b) device used to collect the common peroneal nerve and to avoid curling, guaranteeing equal length for all samples (7 cm).
Figure 1.
Process for collecting the common peroneal nerve in sheep: a) common peroneal nerve duly individualized from neighboring tissues; b) device used to collect the common peroneal nerve and to avoid curling, guaranteeing equal length for all samples (7 cm).
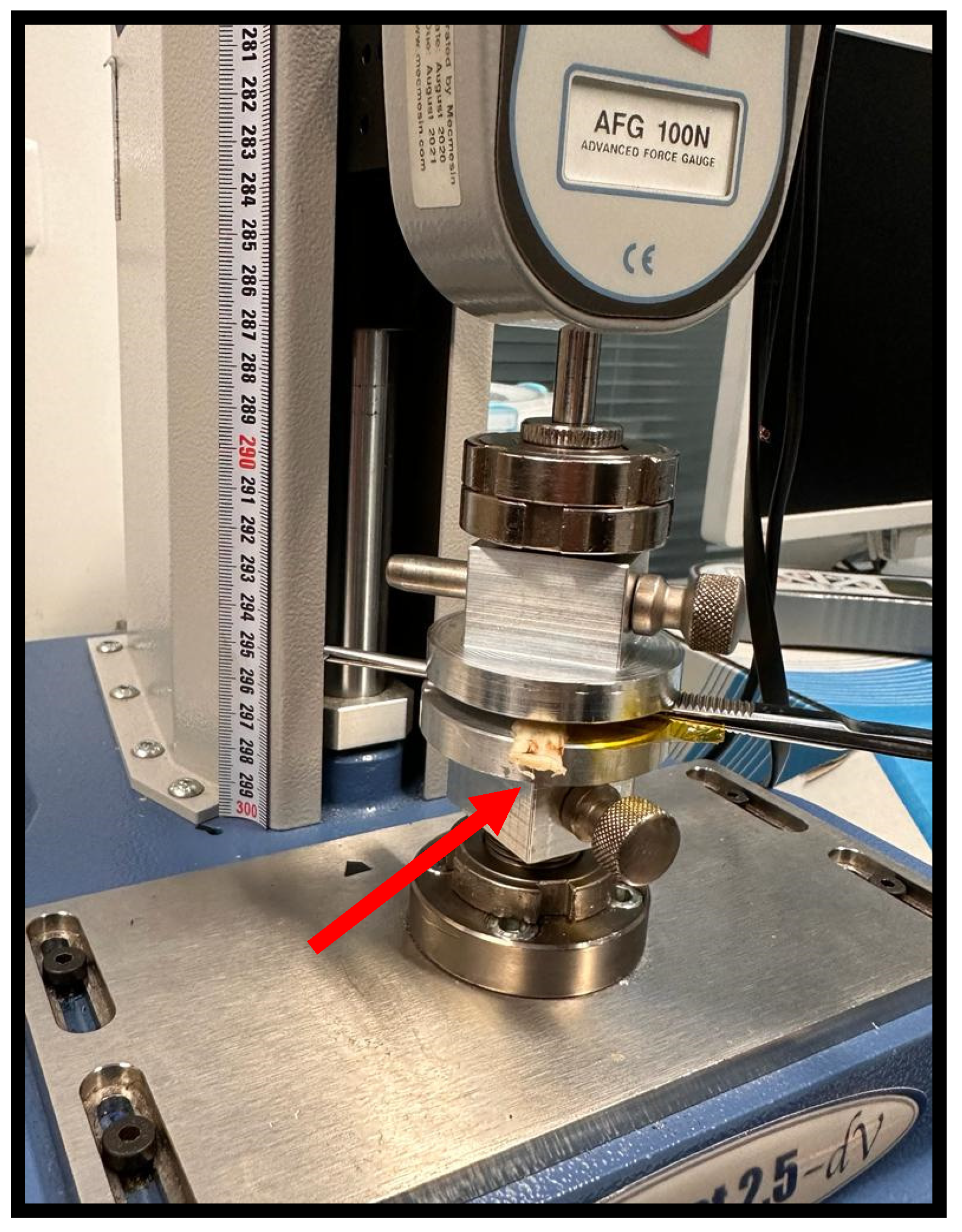
Figure 2.
Process of crushing the sheep's common peroneal nerve. The nerve was placed between the claws of the surgical forceps, so that the application of a constant perpendicular force by the Force Tester platforms could induce crushing at the midpoint of the collected nerve segment, to an extent of 5 mm. Red arrow – end of the common peroneal nerve under crushing.
Figure 2.
Process of crushing the sheep's common peroneal nerve. The nerve was placed between the claws of the surgical forceps, so that the application of a constant perpendicular force by the Force Tester platforms could induce crushing at the midpoint of the collected nerve segment, to an extent of 5 mm. Red arrow – end of the common peroneal nerve under crushing.
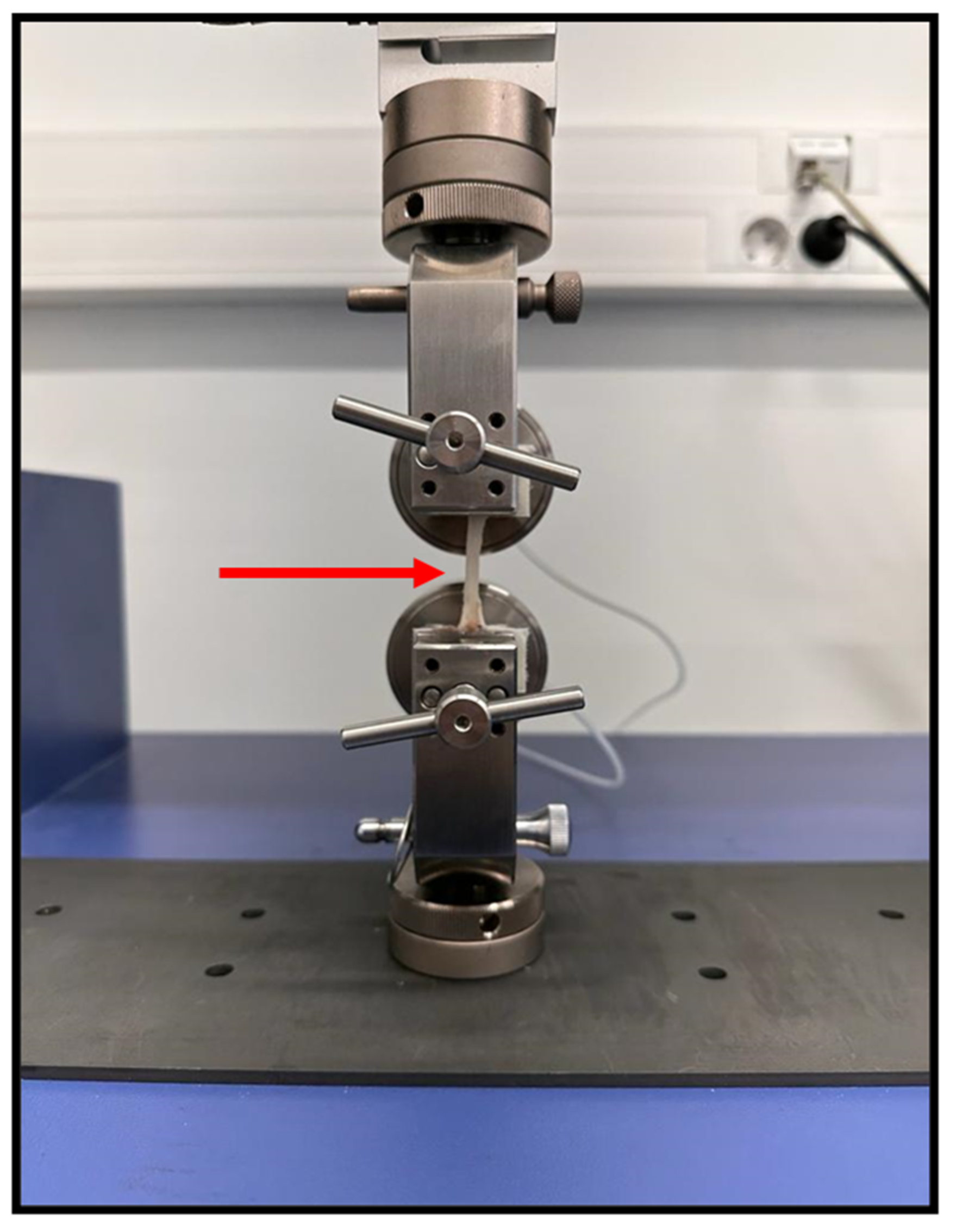
Figure 3.
Biomechanical testing of the common peroneal nerve using the pulled to failure process. Both ends of the nerves were held between a pair of pneumatic grippers using a Velcro covering to prevent slippage. Red arrow - common peroneal nerve suspended between the pneumatic grippers, with a gouge length of 3.5 cm.
Figure 3.
Biomechanical testing of the common peroneal nerve using the pulled to failure process. Both ends of the nerves were held between a pair of pneumatic grippers using a Velcro covering to prevent slippage. Red arrow - common peroneal nerve suspended between the pneumatic grippers, with a gouge length of 3.5 cm.
Figure 4.
Histologic cross-section of the sheep common peroneal nerve: a-c - Haematoxylin and Eosin stain; d-f - Masson's Trichrome stain; g-i – Lugol Fast Blue stain. Staining with Hematoxylin and Eosin allowed the identification of the ultrastructural characteristics of the peripheral nerve, with visualization of the nerve fascicles, connective tissue sheats, nerve fibers and Schwann cells around the myelinated fibers. Staining with Masson's Trichrome allowed to observe the epineurium, perineurium and endoneurium in addition to abundant interfascicular connective tissue. Staining with Lugol Fast Blue allowed the observation of the myelin sheaths surrounding the nerve fibers. f, nerve fascicule; ep, epineurium; p, perineurium; e, endoneurium; ct, interfascicular connective tissue; a, axons; sc, Schwann cells; m, myelin sheath.
Figure 4.
Histologic cross-section of the sheep common peroneal nerve: a-c - Haematoxylin and Eosin stain; d-f - Masson's Trichrome stain; g-i – Lugol Fast Blue stain. Staining with Hematoxylin and Eosin allowed the identification of the ultrastructural characteristics of the peripheral nerve, with visualization of the nerve fascicles, connective tissue sheats, nerve fibers and Schwann cells around the myelinated fibers. Staining with Masson's Trichrome allowed to observe the epineurium, perineurium and endoneurium in addition to abundant interfascicular connective tissue. Staining with Lugol Fast Blue allowed the observation of the myelin sheaths surrounding the nerve fibers. f, nerve fascicule; ep, epineurium; p, perineurium; e, endoneurium; ct, interfascicular connective tissue; a, axons; sc, Schwann cells; m, myelin sheath.
Figure 5.
Common peroneal nerves after being subjected to crushing with different forces for one minute. a) 80N; b) 180N. Red arrow - crushing site, with flattening of the site subject to the crushing force and variation in the diameter of the nerve trunk.
Figure 5.
Common peroneal nerves after being subjected to crushing with different forces for one minute. a) 80N; b) 180N. Red arrow - crushing site, with flattening of the site subject to the crushing force and variation in the diameter of the nerve trunk.
Figure 6.
Load-displacement curves. a) Nerves not subject to crushing (0N - control); b) Nerves subject to a force of 80N; c) Nerves subjected to a force of 180N; d) Superposition of the three load-displacement curves.
Figure 6.
Load-displacement curves. a) Nerves not subject to crushing (0N - control); b) Nerves subject to a force of 80N; c) Nerves subjected to a force of 180N; d) Superposition of the three load-displacement curves.
Figure 7.
Stress of the common peroneal nerves during the crushing test. The plots represent the 25 percentile (Q1) and 75 percentile (Q3) values. The lower whiskers correspond to Q1 + 1.5 IQR and the upper whiskers Q3 + 1.5 IQR. +, mean value. * corresponds to 0.01 ≤ p < 0.05, ** to 0.001 ≤ p < 0.01, *** to 0.0001 ≤ p < 0.001, and **** to p < 0.0001.
Figure 7.
Stress of the common peroneal nerves during the crushing test. The plots represent the 25 percentile (Q1) and 75 percentile (Q3) values. The lower whiskers correspond to Q1 + 1.5 IQR and the upper whiskers Q3 + 1.5 IQR. +, mean value. * corresponds to 0.01 ≤ p < 0.05, ** to 0.001 ≤ p < 0.01, *** to 0.0001 ≤ p < 0.001, and **** to p < 0.0001.
Figure 8.
Strain of the common peroneal nerves during the crushing test. +, mean value. * corresponds to 0.01 ≤ p < 0.05, ** to 0.001 ≤ p < 0.01, *** to 0.0001 ≤ p < 0.001, and **** to p < 0.0001.
Figure 8.
Strain of the common peroneal nerves during the crushing test. +, mean value. * corresponds to 0.01 ≤ p < 0.05, ** to 0.001 ≤ p < 0.01, *** to 0.0001 ≤ p < 0.001, and **** to p < 0.0001.
Figure 9.
Ultimate tensile strength of the common peroneal nerves to rupture. +, mean value. * corresponds to 0.01 ≤ p < 0.05, ** to 0.001 ≤ p < 0.01, *** to 0.0001 ≤ p < 0.001, and **** to p < 0.0001.
Figure 9.
Ultimate tensile strength of the common peroneal nerves to rupture. +, mean value. * corresponds to 0.01 ≤ p < 0.05, ** to 0.001 ≤ p < 0.01, *** to 0.0001 ≤ p < 0.001, and **** to p < 0.0001.
Figure 10.
Stifness of the common peroneal nerves under tensile testing. +, mean value. * corresponds to 0.01 ≤ p < 0.05, ** to 0.001 ≤ p < 0.01, *** to 0.0001 ≤ p < 0.001, and **** to p < 0.0001.
Figure 10.
Stifness of the common peroneal nerves under tensile testing. +, mean value. * corresponds to 0.01 ≤ p < 0.05, ** to 0.001 ≤ p < 0.01, *** to 0.0001 ≤ p < 0.001, and **** to p < 0.0001.
Table 1.
Values of the biomechanical parameters of each nerve subjected to different crushing forces. Values expressed as Mean + SEM.
Table 1.
Values of the biomechanical parameters of each nerve subjected to different crushing forces. Values expressed as Mean + SEM.
Crushing Force
(N) |
Stress (MPa) |
Strain (%) |
Ultimate Tensile Strength (N) |
Displacement at Rupture (mm) |
Stifness (N/mm) |
| 0N |
// |
// |
109.59 + 6.33 |
11.12 + 1.30 |
9.95 + 0.98 |
| 80N |
3.17 + 0.17 |
40.67 + 14.39 |
91.16 + 9.19 |
8.78 + 1.60 |
10.56 + 1.00 |
| 180N |
7.55 + 0.43 |
58.57 + 14.30 |
49.38 + 19.13 |
7.27 + 1.39 |
6.26 + 0.96 |