Submitted:
16 January 2025
Posted:
16 January 2025
You are already at the latest version
Abstract
Background: Alzheimer’s disease (AD) is the fifth leading cause of death for Americans older than 65. Though fluctuations have been noticed over the past two decades, the mortality of Alzheimer’s patients increased considerably during the COVID-19 pandemic. This study aims to explore the temporal trends in AD-associated mortality (ADAM) and disparities in these trends, and we aim to discern changes to these trends during the COVID-19 pandemic. Methods: The CDC WONDER Multiple Cause-of-Death Public Use Records from 1999–2022 was used to extract population data on deaths related to AD and stratify them based on age, biological sex, race, ethnicity, place of death, census region, and state. ICD-10 codes G30.0, G30.1, G30.8, and G30.9 were used to identify AD-related mortality. Statistical analysis was performed using the Joinpoint Regression Program version 5.0.2. Results: We confirmed an increase in mortality rate in all races, sexes, places of death, age groups above 65, and states/census regions. Interestingly, the AAMR of AD was consistently higher in females compared to males. Non-Hispanic whites had the highest AD mortality by race and ethnicity. At the intersection of race and biological sex, white females had the highest AAMR with AD. Lastly, we noted an increase in AD mortality at hospice facilities as compared to other places of death. Our findings demonstrate not only that the number of deaths due to AD were exacerbated by the recent pandemic, but also that certain subgroups of AD patients were disproportionately affected. Conclusion: Our findings demonstrate that the number of deaths due to AD were exacerbated by the recent pandemic, and that white females were disproportionately affected. The disparities relating to ADAM uncovered in this study may assist healthcare administrators and policymakers in their decisions. Additionally, the findings might help initiate larger studies focusing on these disparities to explore novel risk/prognostic factors for AD.
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Overall Findings
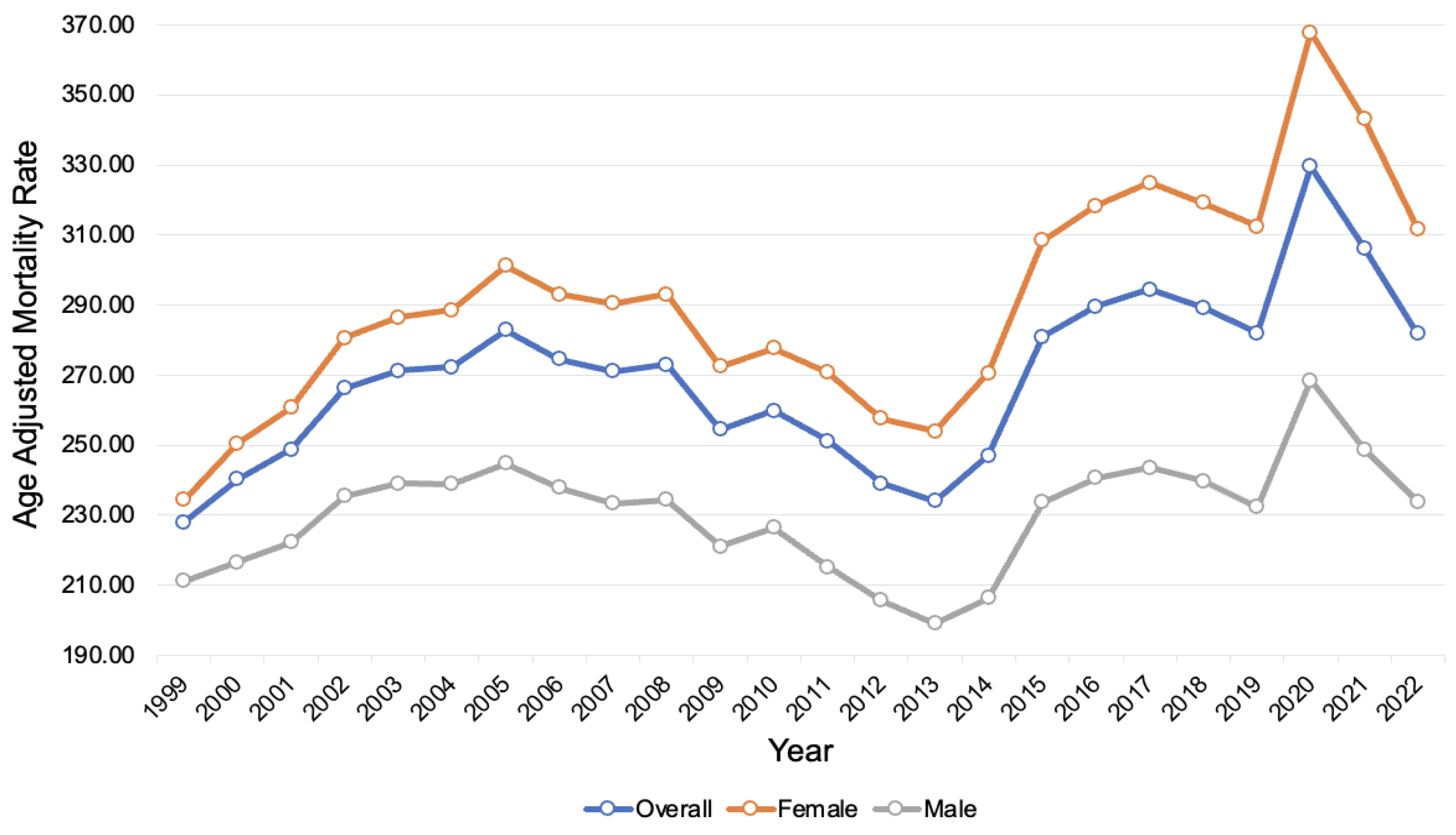
3.2. Disparities in AD-Related Mortality by Sex
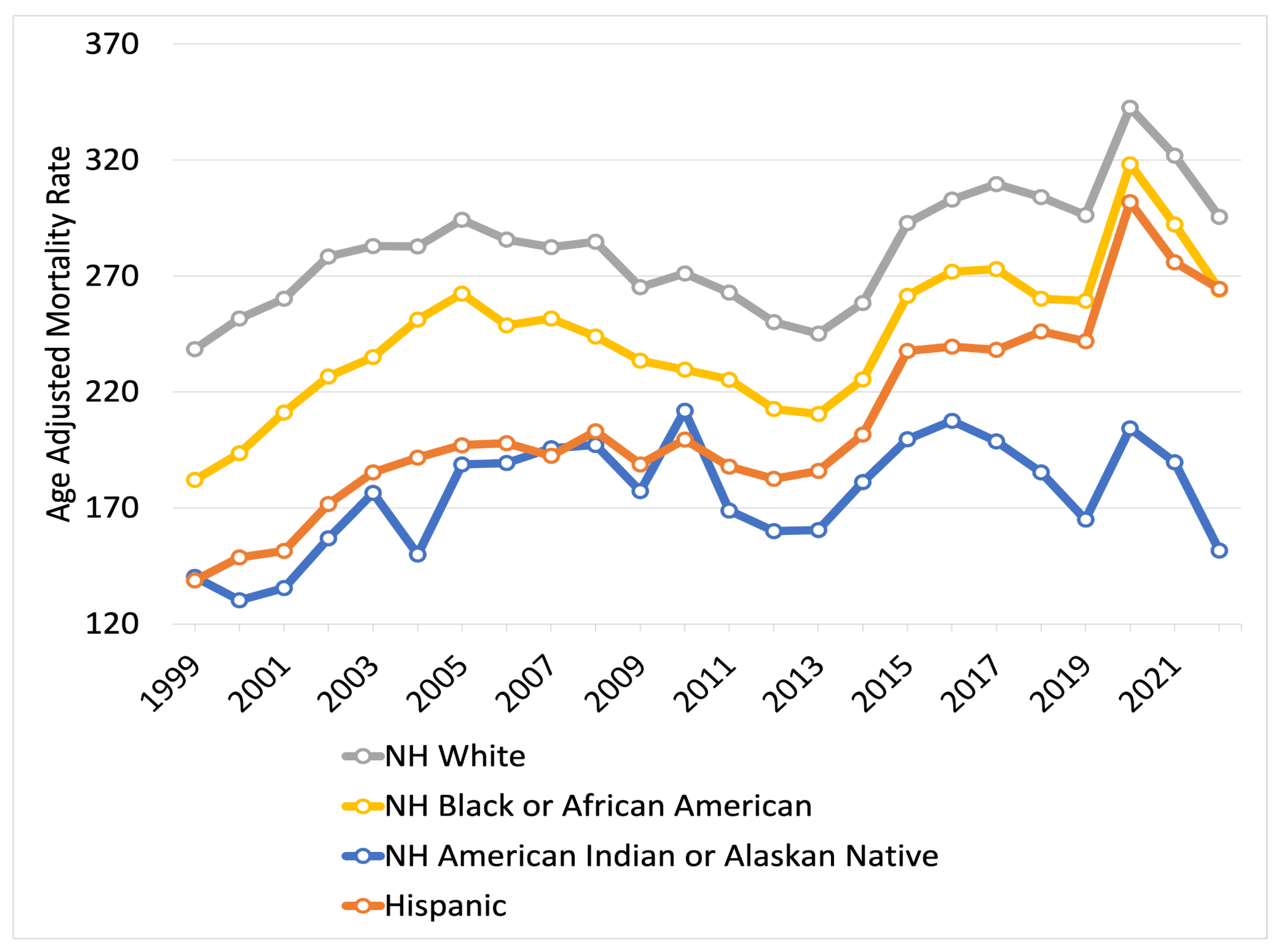
3.3. Disparities in AD-Related Mortality by Race and Ethnicity
3.4. Disparities in AD-Related Mortality by Race and Sex
3.5. Disparities of AD-Related Mortality by Place of Death
3.6. Disparities in AD-Related Mortality by Age Group
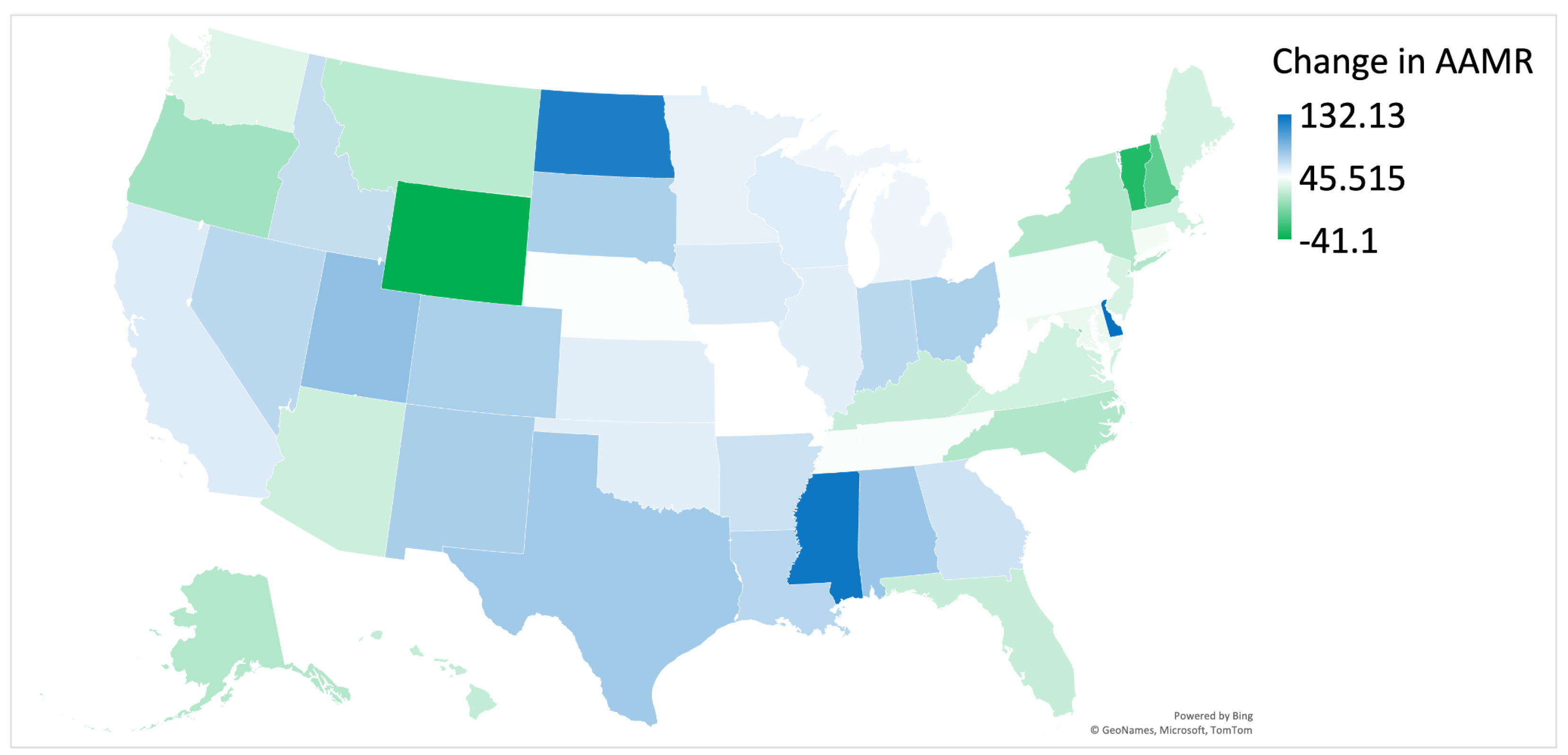
3.7. Regional Disparities in AD-Related Mortality by State
3.8. Census-Region Disparities in AD-Related Mortality
3.9. Disparities in AD-Related Mortality by Census-Region and Sex
4. Discussion
4.1. Overall Findings
4.2. Disparities in AD-Related Mortality by Sex
4.3. Disparities in AD-Related Mortality by Race and Ethnicity
4.4. Place of Death
4.5. Regional Differences
4.6. Limitations
5. Conclusions
Supplementary Materials
Authors’ Contributions
Funding
Institutional Review Board Statement
Consent for Publication
Availability of Data and Materials
Conflicts of Interest
Abbreviations
References
- Dzamba D, Harantova L, Butenko O, Anderova M. Glial Cells - The Key Elements of Alzheimer’s Disease. Curr Alzheimer Res. 2016;13(8):894-911. PMID: 26825092. [CrossRef]
- Balmiki Ray, Debomoy K Lahiri, Neuroinflammation in Alzheimer’s disease: different molecular targets and potential therapeutic agents including curcumin, Current Opinion in Pharmacology, Volume 9, Issue 4, 2009, Pages 434-444, ISSN 1471-4892. [CrossRef]
- Bhole RP, Chikhale RV, Rathi KM. Current biomarkers and treatment strategies in Alzheimer disease: An overview and future perspectives. IBRO Neurosci Rep. 2023 Nov 30;16:8-42. PMID: 38169888; PMCID: PMC10758887. [CrossRef]
- 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023 Apr;19(4):1598-1695. Epub 2023 Mar 14. PMID: 36918389. [CrossRef]
- Mayo Foundation for Medical Education and Research. (2023, June 7). Alzheimer’s stages: How the disease progresses. Mayo Clinic.
- 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021 Mar;17(3):327-406. Epub 2021 Mar 23. PMID: 33756057. [CrossRef]
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System, Mortality 1999-2020 on CDC WONDER Online Database, released in 2021. Data are from the Multiple Cause of Death Files, 1999-2020, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed at http://wonder.cdc.gov/mcd-icd10.html on January 30, 2024.
- Anderson RN, Rosenberg HM. Age standardization of death rates; implementation of the year 2000 standard.
- Joinpoint Regression Program, Version 5.0.2 - May 2023; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute.
- Kim HJ, Fay MP, Feuer EJ, Midthune DN. “Permutation tests for joinpoint regression with applications to cancer rates” Statistics in Medicine 2001;20:655.
- Zhao X, Li C, Ding G, Heng Y, Li A, Wang W, Hou H, Wen J, Zhang Y. The burden of Alzheimer’s disease mortality in the United States, 1999-2018. Journal of Alzheimer’s Disease. 2021 Jan 1;82(2):803-13.
- Mobaderi T, Kazemnejad A, Salehi M. Exploring the impacts of risk factors on mortality patterns of global Alzheimer’s disease and related dementias from 1990 to 2021. Scientific Reports. 2024 Jul 6;14(1):15583. [CrossRef]
- Gilstrap L, Zhou W, Alsan M, Nanda A, Skinner JS. Trends in mortality rates among Medicare enrollees with Alzheimer disease and related dementias before and during the early phase of the COVID-19 pandemic. JAMA neurology. 2022 April 1;79(4):342-8. [CrossRef]
- Mogharab V, Ostovar M, Ruszkowski J, Hussain SZ, Shrestha R, Yaqoob U, Aryanpoor P, Nikkhoo AM, Heidari P, Jahromi AR, Rayatdoost E. Global burden of the COVID-19 associated patient-related delay in emergency healthcare: a panel of systematic review and meta-analyses. Globalization and health. 2022 Jun 8;18(1):58. [CrossRef]
- Mattiuzzi C, Lippi G. Mortality for Alzheimer’s disease during the COVID-19 pandemic. European Geriatric Medicine. 2024 Nov 1:1-3. [CrossRef]
- Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT. Age, Alzheimer disease, and brain structure. Neurology. 2009 Dec 1;73(22):1899-905.
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Archives of general psychiatry. 1998 Sep 1;55(9):809-15.
- Fisher DW, Bennett DA, Dong H. Sexual dimorphism in predisposition to Alzheimer’s disease. Neurobiology of aging. 2018 Oct 1;70:308-24. [CrossRef]
- Zhu D, Montagne A, Zhao Z. Alzheimer’s pathogenic mechanisms and underlying sex difference. Cellular and Molecular Life Sciences. 2021 Jun;78:4907-20.
- Casaletto KB, Nichols E, Aslanyan V, Simone SM, Rabin JS, La Joie R, Brickman AM, Dams-O’Connor K, Palta P, Kumar RG, George KM. Sex-specific effects of microglial activation on Alzheimer’s disease proteinopathy in older adults. Brain. 2022 Oct 3;145(10):3536-45. [CrossRef]
- Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, D’agostino RB. Lifetime risk of dementia and Alzheimer’s disease: the impact of mortality on risk estimates in the Framingham Study. Neurology. 1997 Dec;49(6):1498-504.
- Avila JF, Vonk JMJ, Verney SP, Witkiewitz K, Arce Rentería M, Schupf N, Mayeux R, Manly JJ. Sex/gender differences in cognitive trajectories vary as a function of race/ethnicity. Alzheimers Dement. 2019 Dec;15(12):1516-1523. Epub 2019 Oct 9. PMID: 31606366; PMCID: PMC6925640. [CrossRef]
- Mielke MM, Aggarwal NT, Vila-Castelar C, Agarwal P, Arenaza-Urquijo EM, Brett B, Brugulat-Serrat A, DuBose LE, Eikelboom WS, Flatt J, Foldi NS. Consideration of sex and gender in Alzheimer’s disease and related disorders from a global perspective. Alzheimer’s & dementia. 2022 Dec;18(12):2707-24. [CrossRef]
- Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N, Stern Y. Survival in Alzheimer disease: a multiethnic, population-based study of incident cases. Neurology. 2008 Nov 4;71(19):1489-95.
- Mehta KM, Yaffe K, Perez-Stable EA, Stewart A, Barnes D, Kurland BF, Miller BL. Race/ethnic differences in AD survival in US Alzheimer’s Disease Centers. Neurology. 2008 Apr 1;70(14):1163-70. [CrossRef]
- Morris JC, Schindler SE, McCue LM, Moulder KL, Benzinger TL, Cruchaga C, Fagan AM, Grant E, Gordon BA, Holtzman DM, Xiong C. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA neurology. 2019 Mar 1;76(3):264-73. [CrossRef]
- Belloy ME, Andrews SJ, Le Guen Y, Cuccaro M, Farrer LA, Napolioni V, Greicius MD. APOE genotype and Alzheimer disease risk across age, sex, and population ancestry. JAMA neurology. 2023 Dec 1;80(12):1284-94.
- Akushevich I, Kolpakov S, Yashkin AP, Kravchenko J. Vulnerability to hypertension is a major determinant of racial disparities in Alzheimer’s disease risk. American journal of hypertension. 2022 Aug 1;35(8):745-51. [CrossRef]
- Bell ML, Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environmental health perspectives. 2012 Dec;120(12):1699-704. [CrossRef]
- Younan D, Wang X, Gruenewald T, Gatz M, Serre ML, Vizuete W, Braskie MN, Woods NF, Kahe K, Garcia L, Lurmann F. Racial/ethnic disparities in Alzheimer’s disease risk: role of exposure to ambient fine particles. The Journals of Gerontology: Series A. 2022 May 1;77(5):977-85. [CrossRef]
- Kosar CM, Mor V, Werner RM, Rahman M. Risk of discharge to lower-quality nursing homes among hospitalized older adults with Alzheimer disease and related dementias. JAMA Network Open. 2023 Feb 1;6(2):e2255134-. [CrossRef]
- Cross SH, Kaufman BG, Taylor Jr DH, Kamal AH, Warraich HJ. Trends and factors associated with place of death for individuals with dementia in the United States. Journal of the American Geriatrics Society. 2020 Feb;68(2):250-5.
- Schorr CA, Angelo M, John G, LeCompte K, Dellinger RP. The association of increasing hospice use with decreasing hospital mortality: an analysis of the national inpatient sample. Journal of Healthcare Management. 2020 Mar 1;65(2):107-20. [CrossRef]
- Hallas L, Hatibie A, Majumdar S, Pyarali M, Hale T. Variation in US states’ responses to COVID-19. University of Oxford. 2021 May.
- Ailshire JA, Walsemann KM, Fisk CE. Regional variation in US dementia trends from 2000-2012. SSM-Population Health. 2022 Sep 1;19:101164. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




