Submitted:
13 January 2025
Posted:
14 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results and Discussion
Multivariate Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Karmakar, A.U.; Sultana, S.; Nishi, S.; Nath Biswas, N.; Hossain, L.; Sheikh, S. Antioxidant, analgesic, antimicrobial, and anthelmintic activity of the dried seeds of Bixa orellana (L). Int J Pharm 2018, 8, 150–163. [Google Scholar]
- Vidusha, A.; Gayatri Devi, R.; Selvaraj, J. Cytotoxic effects of Bixa Orellana bark extracts on human cell line (cell line HEPG2). Journal of Pharmaceutical Negative Results 2022, 1811–1816. [Google Scholar] [CrossRef]
- Perecin, M.B.; Bovi, O.A.; Maia, N.B. Pesquisa com plantas aromáticas, medicinais corantes: o papel do Instituto Agronómico”. O Agronómico, 2002, 54, 21. [Google Scholar]
- Cuong, T.V.; Chin Koo, B. Effects of annatto (Bixa orellana L.) seeds powder on physicochemical properties, antioxidant and antimicrobial activities of pork patties during refrigerated storage. Korean, J. Food Sci. An. 2016, 36, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Dike, I.P.; Ibojo, O.O.; Omonhinmin, D.F. Phytochemical and proximate analysis of foliage and seed of Bixa orellana Linn. Int. J. Pharm. Sci. Rev. Res. 2016, 36, 247–251. [Google Scholar]
- López, C.P.; Sumalapao, D.E.P.; Villarante, N.R. Hepatoprotective activity of aqueous and ethanolic Bixa orellana L. leaf extracts against carbon tetrachloride-induced hepatotoxicity. National Journal of Physiology, Pharmacology, 2017, 7, 972–976. [Google Scholar] [CrossRef]
- Zarza-García, A.L.; Sauri-Duch, E.; Raddatz-Mota, D.; Cuevas-Glory, L.F.; Pinzón-López, L.L.; Rivera-Cabrera, F.; Mendoza-Espinoza, J.A. Pharmacological, phytochemical and morphological study of three Mayan accessions of Bixa orellana L. leaves. Emirates Journal of Food and Agriculture, 2017, 29, 163–169. [Google Scholar] [CrossRef]
- De Oliveira, J.R.; Bonnet, A.; Braconnier, E.; Groult, H.; Prunier, G.; Beaugeard, L.; Grougnet, R.; Guedes, J.R.S.; Alves, C.A.F.; Picot, L. Bixin, an apocarotenoid isolated from Bixa orellana L.sensitizes human melanoma cells to dacarbazine-induced apoptosis through ROS-mediated cytotoxicity. Food and Chemical Toxicology, 2019, 125, 549–561. [Google Scholar] [CrossRef]
- Quintero, Q.J.; Naranjo, D.A.M.; Silva, G.M.; Ciro, G.G.L.; Rojas, C.J.J. Ultrasound-Assisted extraction of bioactive compounds from annatto seeds, evaluation of their antimicrobial and antioxidant activity, and identification of main compounds by LC/ESI-MS analysis. International Journal of Food Science, 2019, 2019, 3721828. [Google Scholar] [CrossRef]
- Kusmita, L.; Franyoto, Y.D.; Mutmainah, M.; Puspitaningrum, I.; Nurcahyanti, A.D. Bixa orellana L. carotenoids: antiproliferative activity on human lung cancer, breast cancer, and cervical cancer cells in vitro. Nat Prod Res. 2022, 36, 6421–6427. [Google Scholar] [CrossRef]
- Fleisher, T.C.; Ameade, E.P.K.; Mensah, M.L.K.; Sawe, I.K. Antimicrobial activity of the leaves and seeds of Bixa Orellana. Fitoterapia, 2003, 74, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Galindo, C.V.; Rankin, S.A. Bioautography and Chemical Characterization of Antimicrobial Compound(s) in Commercial Water-Soluble Annatto Extracts. J. Agric. Food Chem. 2005, 53, 2524–2529. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.J.; Ochoa, V.J.; Ocampo, S.A.; Muñoz, J.F. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complementary and Alternative Medicine, 2006, 6, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Raga, D.D.; Espiritu, R.A. A bioactive sesquiterpene from Bixa orellana. J Nat Med, 2011, 65, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Tamil, S.A.; Dinesh, M.G.; Satyan, R.S.; Chandrasekaran, B.; Rose, C. Leaf and seed extracts of Bixa Orellana, L. expert anti-microbial activity against bacterial pathogens. Journal of Applied Pharmaceutical Science, 2011, 1, 116–120. [Google Scholar]
- Gómez, C.G.; Quintana, C.J.; Alarcón, P.J.; Zapata, M.J. Ethanolic extrac from leaves of Bixa orellana L. : a potential natural food preservative. Interciencia, 2012, 37, 547–551. [Google Scholar]
- Kumar, Y.; Periyasam, L. GC-MS analysis and in-vitro cytotoxic studies of Bixa orellana seed extract against cancer cell line. International Journal of Pharmacy and Pharmaceutical Sciences, 2016, 8, 408–413. [Google Scholar]
- Viuda-Martos, M.; Ciro-Gómez, G.L.; Ruiz-Navajas, Y.; Zapata-Montoya, J.E.; Sendra, E.; Pérez-Alvarez, J.A.; Fernandez-López, J. In vitro antioxidant and antibacterial activities of extracts from annatto (Bixa Orellana L.) leaves and seeds. Journal of Food Safety, 2012, 32, 399–406. [Google Scholar] [CrossRef]
- Omonhinmin, A.C.; Dike, I.P.; Rotimi, S.O. Phytochemical, Cytotoxicity and Antioxidant Activities of Five Anti-malaria Plants. Research Journal of Medicinal Plant, 2015, 9, 81–89. [Google Scholar] [CrossRef]
- Prathima, D.; Sujitha, A.; Usha, R. Phytochemical screening and antimicrobial activity of Bixa orellana Linn. International Journal of Pharmacognosy and Phytochemical Research, 2016, 8, 1078–1082. [Google Scholar]
- Sepúlveda, R.C.; Gómez, G.L.; Zapata, M.J. Extracción de compuestos fenólicos y actividad antioxidante de hojas de Bixa orellana L. (achiote). Revista Cubana de Plantas Medicinales, 2016, 21, 133–144. [Google Scholar]
- Lima, L.V.; Zagmignan, A.; Lima, L.L.; Gomes, A.A.; Nascimento da Silva, L.C.; Cortez de Sá, J.; Andrade, M.C.; Ghilardi Lago, J.H.; Machado, G.L.; Cardoso, C.R.; Lima, N.L.; Martin de Sousa, E. Hydroalcoholic extract and ethyl acetate fraction of Bixa orellana leaves decrease the inflammatory response to Mycobacterium abscessus subsp. massiliense. Hindawi Evidence-Based. Complementary and Alternative Medicine 2018, 60911934. [Google Scholar] [CrossRef]
- Tarkany, B.R.; Barreto, A.P.M.; de Oliveira, S.I.M.; de Carvalho, J.E.; Nogueira, C.P.R.; Ann, F.M. Bixa orellana L. by-products’ fractions from an industrial process: antiproliferative activity on tumor cells and chemical profile. Natural Product Research Formerly Natural Product Letters, 2020, 85, 431–40. [Google Scholar] [CrossRef]
- Moraes, N.R.N.; Guedes, C.G.; Santos, A.A.C.; Oliveira, R.A.; Carnid, N.C.E.; Pontes, A.R.; Quintini, R.C.; Sousa, R.A.; Sousa, C.M.S.; Abreu, S.A.L.; Ferreira, S.I.V.; Rodrigues, S.C.B.; Meireles, G.R.N.; Melo, R.R.; Monteiro, N.V.; Martins, S.E.; Cardoso, C.R. Ethyl Acetate fraction of Bixa orellana and its component ellagic acid exert antibacterial and anti-inflammatory properties against Mycobacterium abscessus subsp. massiliens. Antibiotics, 2022, 11, 817. [Google Scholar] [CrossRef]
- Muddapur, U.M.; Turakani, B.; Jalal, N.A.; Ashgar, S.S.; Momenah, A.M.; Alshehri, O.M.; Mahnashi, M.H.; Shaikh, I.A.; Abddullatif, K.A.; Dafalla, S.E.; Malpani, J.; Manjunath, S.; Begum, T.; Khuwaja, G.; Iqubal, S. Phytochemical screening of Bixa orellana and preliminary antidiabetic, antibacterial, antifibrinolytic, anthelmintic, antioxidant, and cytotoxic activity against lung cancer (A549) cell lines. Journal of King Saud University-Science. 2023, 35, 102683. [Google Scholar] [CrossRef]
- Valencia, D.; Aguilar, G.D.I.; Ortega, G.J.; Godoy, H.G.; Leyva, P.M.A.; Moo, H.V.M.; Clarenc, A.R.; Quintero, V.J.; Mendoza, E.J.A.; Zarza, G.A.L. Phytochemical profile, antioxidant and antiproliferative activity from leaves and seeds of Bixa orellana L. from the Yucatán Peninsula, Mexico. Pharmacognosy Magazine, 2023, 19, 482–490. [Google Scholar] [CrossRef]
- Valarezo, E.; Torres, T.S.; Pineda, G.N.; Jaramillo, F.X.; Cartuche, L.; Morocho, V.; Meneses, M.A. Study of essential oil Isolated from achiote (Bixa orellana) leaves: chemical composition, enantiomeric distribution and antimicrobial, antioxidant and anticholinesterase activities. Antibiotics, 2023, 12, 710. [Google Scholar] [CrossRef]
- Kar, B.; Chandar, B.; Smruti, R.S.; Bhattacharya, H.; Bhattacharya, D. Antibacterial and genotoxic activity of Bixa orellana, a folk medicine and food supplement against multidrug resistant clinical isolates. Journal of Herbal Medicine, 2011, 32, 100502. [Google Scholar] [CrossRef]
- De Luna, E.; Guerrero, J.; Chew-Taracena, T. Sistemática biológica avances y direcciones en la teoría y los métodos de la reconstrucción filogenética. Hidrobiológica, 2005, 15, 351–370. [Google Scholar]
- Iñiguez-Luna, M.I.; Cadena-Iñiguez, J.; Soto-Hernández, R.M. Bioprospecting of Sechium spp. varieties for the selection of characters with pharmacological activity. Sci Rep. 2021, 11, 6185. [Google Scholar] [CrossRef]
- WinCladav.1.00.08. WinClada_FREE Download WinClada 1.00.08 Components & Libraries Development, 2020. https://www. winsite.com/Development/Components-Libraries/WinClada.
- Lanyon, S.M. Detección de inconsistencias internas en datos de distancias. Syst. Zool. 1985, 34, 397–403. [Google Scholar] [CrossRef]
- Frewer, L.J.; Vander, L.I.; Fisher, R.H.; Reinders, J.M.; Menozzi, D.; Zhang, X.; Vandenberg, I.; Zimmermann, L.K. Public perceptions of agri-food applications of genetic modification-a systematic review and meta-analysis. Trends Food Sci. Technol. 2013, 30, 142–152. [Google Scholar] [CrossRef]
- Franco, T.L.; Hidalgo, R. Análisis estadístico de datos de caracterización morfológica de recursos fitogenéticos, Boletín técnico, Instituto internacional de Recursos Fitogenéticos (IPGRI), 2003, Cali, Colombia, vol. 8, 89 págs. https://books.google.com.mx/books/about/An%C3%A1lisis_Estad%C3%ADstico_de_Datos_de_Carac.html?id=B55X-G3WiugC.
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. Journal of Statistical Software, 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. 2020. Package ‘factoextra’ [WWW Document]. URL 〈https://cran.r-project.org/web/packages/factoextra/factoextra.
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2023, Vienna. https://www.R-project.org/.
- Cadena-Iñiguez, J.; Avendaño, C.; Soto, M.; Ruiz, L.; Aguirre, J.; Arévalo, L. Infraspecific variation of Sechium edule (Jacq.) Sw. in the state of Veracruz, Mexico. Genetic Resources and Crop Evolution, 2008, 55, 835–847. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Soto, M.; Arévalo, M.; Avendaño, A.H.; Aguirre, J.; Ruiz, L. Caracterización bioquímica de variedades domesticadas de chayote Sechium edule (Jacq.) Sw. comparadas con parientes silvestres. Revista Chapingo. Serie horticultura, 2011, 17, 45–55. [Google Scholar]
- Pais, A.L.; Li, X.; Xiang, Q.Y. Discovering variation of secondary metabolite diversity and its relationship with disease resistance in Cornus florida L. Ecol Evol. 2018, 4, 5619–5636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lara, R.J.; Martínez, H.A. La Biodiversidad en Campeche: Estudio de Estado. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO), Gobierno del Estado de Campeche, Universidad Autónoma de Campeche, El Colegio de la Frontera Sur, 2010, México. 730 p.
- Márquez, L.A.; Chávez, P.M.; Hernández, G.J. Aspectos generales sobre los elagitaninos y su conversión a ácido elágico. Ciencia Nicolaita, 2019, 77, 36–58. [Google Scholar]
- Avendaño-Arrazate, C. Rescate y conservación del achiote (Bixa Orellana L.) en México. Agro Productividad, 2018, 5. https://revistaagroproductividad.org/index.php/agroproductividad/article/view/407.
- Miguel, K.; Pradines, A.; Favre, G. Farnesol and Geranylgeraniol induce actin cytoskeleton disorganization and apoptosis in a lung adenocarcinoma cells. Biochemical and Biophysical Research Communications, 1996, 225, 869–876. [Google Scholar] [CrossRef]
- Kumar, N.V.; Rani, E.M.; Rathinasamy, G.; Dhiraviam, K.N. Antioxidant and antimicrobial potential of natural colouring pigment derived from Bixa orellana L. seed aril. Proc. Natl. Acad. Sci.India, Sect. B Biol. Sci. 2017, 89, 137–143. [Google Scholar] [CrossRef]
- Samael, A.; Nachiketa, B.; Sayeed, S.; Masnoon, K.; Mohammad, H. (2016). In vitro antibacterial potential of Bixa orellana L. against some pathogenic bacteria and comparative investigation on some standard antibiotics. J Pharmacogn Phytochem, 2016, 5, 178–181. [Google Scholar]
- Dos Santos, D.; Silva Barboza, A.D.; Ribeiro, J.S.; Rodrigues, J.S.; Campos, Â.D.; Lund, R.G. Bixa orellana L. (Achiote, Annatto) as an antimicrobial agent: A scoping review of its efficiency and technological prospecting. J Ethnopharmacol, 2022, 287, 114961. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.M.; Coignard, F.; Johnson, I.; Chandler, S.; Palan, S.; Waller, A.; Wijkmans, J.; Hunter, M.G. Antibacterial activities and characterization of novel inhibitors of Lpx C. J Antimicrobial Agents and Chemotherapy, 2002, 46, 1793–1799. [Google Scholar] [CrossRef] [PubMed]

| Variable | Genotype | Research focus | References |
|---|---|---|---|
| Biological activity | África1; USA1; Colombia1; Philippines1; India1; Colombia 2; Colombia3; India2; Nigeria1; South Korea1; India3; Colombia4; Philippines2; Yucatán1; Brazil1; Bangladesh1; Brazil2; Colombia5; Brazil3; India4; Indonesia1; Brazil5; India5; Yucatán2; Ecuador1 | Antimicrobial, anticancer, antioxidant and hepatoprotective activity of leaves and/or seeds of Bixa orellana |
[1,4,6,7,8,9,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] |
| Biochemistry | África1; USA1; Colombia1; Philippines1; India1; Colombia2; Colombia3; India2; Nigeria1; South Korea1; Nigeria2; India3; Colombia4; Philippines2; Yucatán1; India4; Colombia5; Brazil3; India5; Yucatán2; Ecuador1 | Phytochemical characterization of extracts and essential oil of leaves and seeds of Bixa orellana |
[1,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] |
| Number | Variable | Variable status |
|---|---|---|
| 1 | Extraction organ | Leaves=1, Seeds=2 |
| 2 | Extraction method | Absent=0, Aqueous and ethanolic extracts=1, Solvent system=2, Methanol=3, Ethanol=4, Petroleum ether=5, Maceration=6, Steam Distillation=7 |
| 3 | Biochemistry class | Absent=0, Phenolic compounds=1, Terpenoids=2, Compounds with nitrogen=3 |
| 4 | Compound group | Phenols and phenolic acids=1, Flavonoids=2, Tannins=3, Monoterpenoids and sesquiterpenoids=4, Diterpenes=5, Triterpenoids=6, Tetraterpenoids=7, Alkaloids=8, Cyanogenic glycosides=9 |
| 5 | Phenols and phenolic acids | Absent=0, Phenylpropanoids=1, Coumarins=2, Anthraquinones=3, Procyanidins=4, Ellagic acid=5, Tannic acid=6, Gallic acid=7 |
| 6 | Flavonoids | Absent=0, Naringenin=1, Kaemferol=2, Anthocyanins=3, Isoflavonoids=4, Butein=5, Catechins=6, Chlorogenic acid=7, Hypolaetin=8 |
| 7 | Tannins | Absent=0, Granatin=1, Neostrictinin=2, Ellagitanin=3 |
| 8 | Monoterpenes | Absent=0, Poliprenol=1, Ocimene=2, Spathulenol=3, Isoledene=4, Bergamotene=5, Pinene=6, Aristolochene=7, Cadinene=8, Germacrene=9 |
| 9 | Sesquiterpenes | Absent=0, Farnesol=1, Elemene=2, Caryophyllene=3, Guaiol=4, Tomentosin=5, Ishwarane=6 |
| 10 | Diterpenes | Absent=0, Phytol=1, Geranylgeraniol=2, Geranyl terpinene=3, Geranyl linalool=4, Farnesyl=5 |
| 11 | Triterpenes | Absent=0, Saponins=1, Steroids=2, Stigmasterol=3, Sitosterol=4, Squalene=5 |
| 12 | Tetraterpenes | Absent=0, Carotenoids=1, 9'-cis-norbixin=2, Trans-norbixin=3, Bixin=4, Norbixin=5, Diapocarotenoids=6 |
| 13 | Alkaloids | Absent=0, Atrophin=1 |
| 14 | Cyanogenic glycosides | Absent=0, Saponins=1 |
| 15 | Biological activity | Absent=0, Chemo preventive=1, Anti-inflammatory=2, Hepatoprotective=3, Antioxidants=4, Cytotoxic=5 |
| 16 | Antimicrobial activity | Absent=0, Pseudomonas aeruginosa=1, Escherichia coli=2, Staphylococcus aureus=3, Salmonella sp=4, Candida albicans=6 |
| 17 | Anticancer activity | Absent=0, HepG2=1, U251=2, MCF-7=3, HeLa=4, NCI-H460=5, PC-3=6, HT-29=7, A549=8, MCF-7=9 |
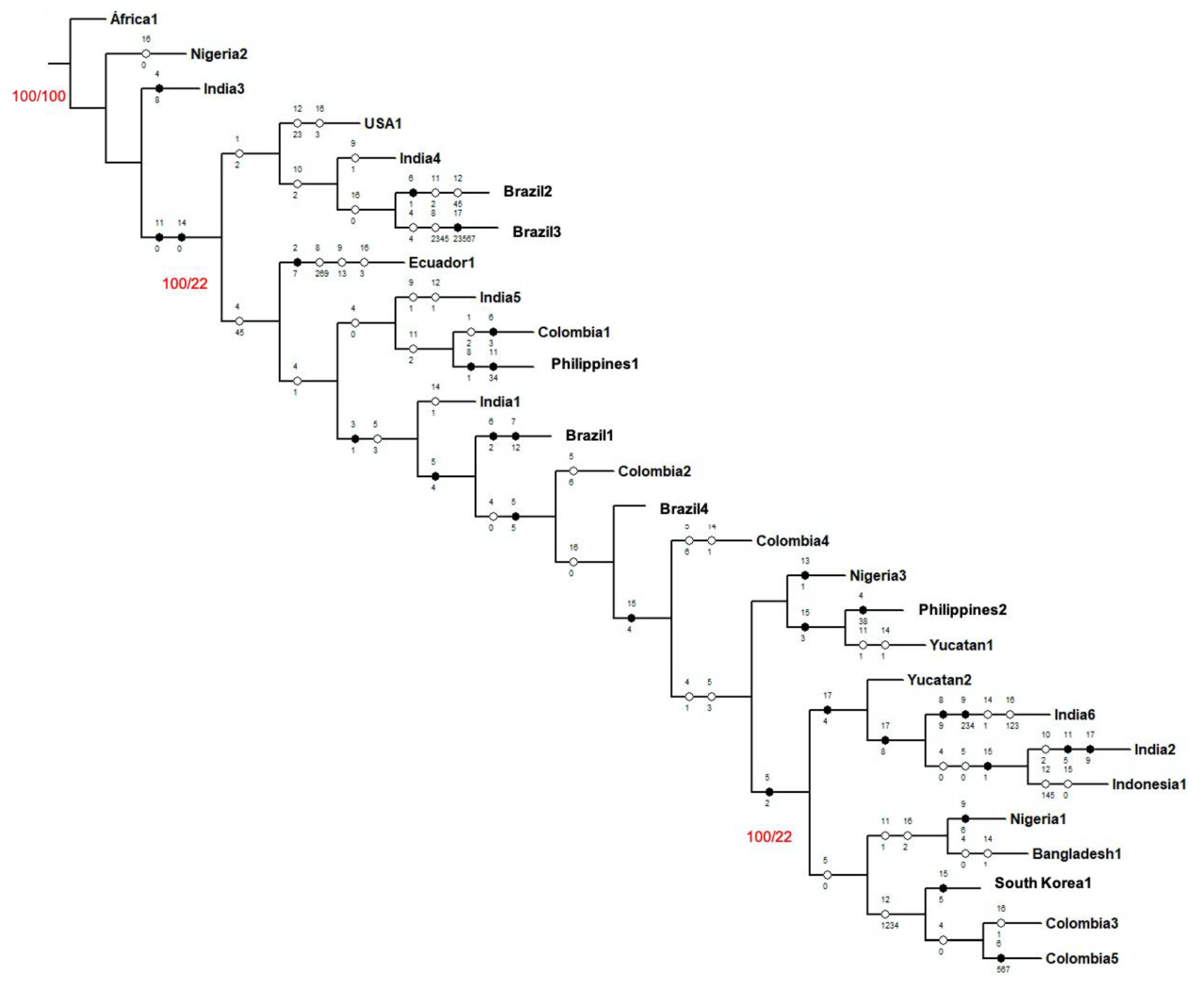
| Genotype | Character/character state | Biochemistry | Biological activity/antimicrobial activity/anticancer activity |
|---|---|---|---|
| Nigeria2 | |||
| India3 | |||
| USA1 | 10/2, 12/2, 12/3, 16/3, | Geranylgeraniol, cis-norbixin, trans-norbixin | Staphylococcus aureus |
| India4 | 9/1, 10/2, | Farnesol, geranylgeraniol | |
| Brazil2 | 11/2, 12/4, 12/5 | Steroids, bixin, norbixin | |
| Brazil3 | 4/4, 8/2, 8/3, 8/4, 8/5 | Mono and sesquiterpenoids, ocimene, spathulenol, isoledene, bergamotene | |
| Ecuador1 | 8/2, 8/6, 8/9, 9/1, 9/3, 16/3 | Ocimene, pinene, germacrene, farnesol, caryophyllene | Staphylococus aureus |
| India5 | 9/1, 11/2, 12/1 | Farnesol, saponins, carotenoids | |
| Colombia1 | 11/2 | Saponins | |
| Philippines1 | |||
| India1 | 14/1 | Saponins | |
| Brazil1 | |||
| Colombia2 | 5/6 | Tannic acid | |
| Brazil4 | |||
| Colombia4 | 5/6, 14/1 | Tannic acid, saponins | |
| Nigeria3 | |||
| Philippines2 | |||
| Yucatán1 | 11/1 | Saponins | |
| Yucatán2 | |||
| India6 | 14/1, 16/1, 16/2, 16/3 | Saponins | Pseudomonas aeruginosa, Escherichia coli, Staphylococus aureus |
| India2 | 10/2 | Geranylgeraniol | |
| Indonesia1 | 12/1, 12/4, 12/5 | Carotenoids, bixin, norbixin | |
| Nigeria1 | |||
| Bangladesh1 | 14/1 | Saponins | |
| South Korea1 | |||
| Colombia3 | 16/1 | Pseudomonas aeruginosa | |
| Colombia5 |
| Branch | Apomorphic character | Branch | Plesiomorphic character |
|---|---|---|---|
| 1 | 1 | Alkaloids | |
| 2 | Seeds, mono and sesquiterpenoids, ocimene, spathulenol, isoledene, bergamotene, farnesol, saponins, geranylgeraniol, cis-norbixin, trans-norbixin, bixin, norbixin, Staphylococcus aureus | 2 | Naringenin, U251, MCF-7, NCI-H460 PC-3 and HT-29 cell lines |
| 3 | Phenols and phenolic acids, mono and sesquiterpenoids, diterpenes, ocimene, pinene, germacrene, farnesol, caryophyllene, Staphylococcus aureus | 3 | Steam distillation |
| 4 | Seed, phenols and phenolic acids, farnesol, steroids, carotenoids | 4 | Anthocyanins, phenylpropanoids, stigmasterol, sitosterol |
| 5 | Phenols and phenolic acids, anthraquinones, tannic acid, saponins | 5 | Phenolic compounds, procyanidins, ellagic acid, kaempherol, granatin, neostrictinin, antioxidant |
| 6 | Saponins | 6 | Tannins, alkaloids, atrophin, hepatoprotective |
| 7 | Geranylgeraniol, carotenoids, bixin, norbixin, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus | 7 | Coumarins, germacrene, elemene, caryophyllene, squalene, chemo preventive, HeLa cell lines, A549, MCF-7 |
| 8 | Saponins, Staphylococcus aureus | 8 | Coumarins, caryophyllene |
| 9 | Carotenoides, cis-norbixina, trans-norbixina, bixina, Pseudomonas aeruginosa | 9 | Coumarins, butein, catechins, chlorogenic acid, cytotoxic |
| PC | Eigenvalues | Variance | Cumulative variance | % |
|---|---|---|---|---|
| 1 | 0.1046 | 0.0963 | 0.0963 | 9.63 |
| 2 | 0.0963 | 0.0887 | 0.1851 | 28.14 |
| 3 | 0.0717 | 0.0727 | 0.2578 | 53.92 |
| 4 | 0.0717 | 0.0660 | 0.3239 | 86.31 |
| Variable | Variable states | CP1 | CP2 | CP3 | CP4 |
| Extraction organ | Leaves Seeds |
0.40600 0.16010 |
0.02947 0.09092 |
0.00653 0.00378 |
0.00442 0.02480 |
| Extraction method | Aqueous and ethanolic extracts Solvent system Methanol Ethanol Petroleum ether Maceration Steam distillation |
0.08108 0.00741 0.00011 0.07360 0.01204 0.00519 0.00519 |
0.00111 0.00181 0.01826 0.03881 0.00217 0.29127 0.52403 |
0.04358 0.14477 0.00144 0.00227 0.00390 0.06014 0.09163 |
0.00607 0.01652 0.00843 0.04915 0.01648 0.00480 0.00072 |
| Biochemistry class | Phenolic compounds Terpenoids Compounds with nitrogen |
0.00470 0.18442 0.01603 |
0.01508 0.20710 0.00538 |
0.00001 0.05018 0.00219 |
0.00259 0.17695 0.16758 |
| Compound group | Phenols and phenolic acids Flavonoids Tannins Monoterpenoids and sesquiterpenoids Diterpenes Triterpenoids Tetraterpenoids Alkaloids Cyanogenic glycosides |
0.11318 0.29988 0.26420 0.00000 0.00000 0.18557 0.04881 0.26420 0.00000 |
0.00859 0.12431 0.12683 0.00000 0.00000 0.06410 0.00190 0.12683 0.00000 |
0.00795 0.16761 0.20160 0.00000 0.00000 0.08623 0.00214 0.20160 0.00000 |
0.01836 0.13349 0.15101 0.00000 0.00000 0.09697 0.01425 0.15101 0.00000 |
| Phenols and phenolic acids | Phenylpropanoids Coumarins Anthraquinones Procyanidins Ellagic acid Tannic acid Gallic acid |
0.15877 0.21741 0.26599 0.00656 0.00002 0.00108 0.00000 |
0.06654 0.02699 0.07780 0.00017 0.00002 0.00116 0.00000 |
0.12130 0.00046 0.04298 0.00004 0.00005 0.00310 0.00000 |
0.13140 0.35100 0.14762 0.06603 0.02223 0.04040 0.00000 |
| Flavonoids | Naringenin Kaemferol Anthocyanins Isoflavonoids Butein Catechins Chlorogenic acid Hypolaetin |
0.40034 0.00272 0.00059 0.00000 0.00013 0.00000 0.01195 0.00000 |
0.02715 0.00312 0.00027 0.00000 0.00063 0.00943 0.00235 0.00000 |
0.02587 0.00331 0.01002 0.00000 0.00117 0.00020 0.03611 0.00000 |
0.09716 0.10585 0.00397 0.00000 0.08565 0.03638 0.05006 0.00000 |
| Tannins | Granatin Neostrictinin Ellagitanin |
0.04169 0.00000 0.00000 |
0.03330 0.00000 0.00000 |
0.06398 0.00000 0.00000 |
0.01294 0.00000 0.00000 |
| Monoterpenes |
Poliprenol Ocimene Spathulenol Isoledene Bergamote Pinene Aristolochene Cadinene Germacrene |
0.04622 0.00098 0.00098 0.00000 0.00000 0.00098 0.00000 0.00000 0.00098 |
0.01371 0.77755 0.77755 0.00000 0.00000 0.77755 0.00000 0.00000 0.77755 |
0.20700 0.17288 0.17288 0.00000 0.00000 0.17288 0.00000 0.00000 0.17288 |
0.28666 0.00777 0.00777 0.00000 0.00000 0.00777 0.00000 0.00000 0.00777 |
| Sesquiterpenes |
Farnesol Elemene Caryophyllene Guaiol Tomentosin Ishwarane |
0.02186 0.00098 0.00098 0.00000 0.00000 0.00031 |
0.38639 0.77755 0.77755 0.00000 0.00000 0.02955 |
0.25792 0.17288 0.17288 0.00000 0.00000 0.35986 |
0.23356 0.00777 0.00777 0.00000 0.00000 0.01434 |
| Diterpenes |
Phytol Geranyl geraniol Geranyl terpinene Geranyl linalool Farnesyl |
0.00247 0.00183 0.01807 0.00011 0.00011 |
0.01756 0.00469 0.02596 0.01908 0.01908 |
0.09808 0.34644 0.47671 0.33076 0.33076 |
0.08027 0.04852 0.14103 0.00082 0.00082 |
| Triterpenes |
Saponins Steroids Stigmasterol Sitosterol Squalene |
0.10829 0.00186 0.10291 0.19469 0.12040 |
0.00292 0.01413 0.07116 0.00139 0.00217 |
0.00052 0.00282 0.16746 0.00820 0.00390 |
0.02278 0.00832 0.11540 0.14306 0.01648 |
| Tetraterpenes |
Carotenoids 9'-cis-norbixin Trans-norbixin Bixin Norbixin Diapocarotenoids |
0.40393 0.01080 0.01080 0.48865 0.48865 0.00000 |
0.02718 0.00040 0.00040 0.04809 0.04809 0.00000 |
0.03930 0.00393 0.00393 0.03452 0.03452 0.00000 |
0.09930 0.01509 0.01509 0.02605 0.02605 0.00000 |
| Alkaloids |
Atrophin | 0.21741 | 0.02699 | 0.00046 | 0.35100 |
| Cyanogenic glycosides | Saponins | 0.00000 |
0.00000 |
0.00000 |
0.00000 |
| Biological activity |
Chemo-preventive Anti-inflammatory Hepatoprotective Antioxidants Cytotoxic |
0.00568 0.00000 0.00544 0.02597 0.00000 |
0.00568 0.00000 0.00644 0.05981 0.00000 |
0.02083 0.00000 0.00785 0.07794 0.00000 |
0.06208 0.00000 0.02204 0.05050 0.00000 |
| Antimicrobial activity |
Pseudomonas aeruginosa Escherichia coli Staphylococcus aureus Salmonella sp Candida albicans |
0.16510 0.08361 0.05017 0.13572 0.17932 |
0.00000 0.01917 0.21822 0.04070 0.01001 |
0.14469 0.35559 0.00890 0.02404 0.03820 |
0.08732 0.02388 0.03508 0.00446 0.13449 |
| Anticancer activity | HepG2 U251 MCF-7 HeLa NCI-H460 PC-3 HT-29 A549 B16F10 |
0.00000 0.45618 0.47323 0.43024 0.04561 0.45618 0.45618 0.00048 0.00000 |
0.00000 0.02193 0.02313 0.00843 0.02193 0.02193 0.02193 0.00056 0.00000 |
0.00000 0.05048 0.03783 0.00906 0.05048 0.05048 0.05048 0.03869 0.00000 |
0.00000 0.22606 0.16672 0.00554 0.22606 0.22606 0.22606 0.15758 0.00000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





