1. Introduction
Peritoneal dialysis (PD) is a high-quality and cost-effective renal replacement therapy (RRT), with an estimated global prevalence of 38.1 per million population [
1]. In Mexico, PD accounts for 59% of all RRT cases, representing 35,255 patients and assuming an approximately cost of
$6,000 USD per patient annually, according to reported by Mexican Institute of Social Security [
2]. PD functions by the ability of the peritoneal membrane to serve as a semipermeable membrane enabling selectively filtration of metabolic waste products to a dialysis fluid introduced into peritoneal cavity via a catheter. Dialysis fluid is drained after a dwell time, according to specific patients’ clinical requirements [
3,
4].
Despite its advantages, PD induces peritoneal inflammation [
5], due to exposure of the peritoneal mesothelium to PD components (e.g. catheter, acidic pH, high glucose, glucose degradation products, and advanced glycation end products) and the uremic state [
3], that contributes to the release of transforming growth factor beta (TGF-β) from the mesothelial cells, resulting in proliferation and its transdifferentiating to myofibroblast, followed by the secretion of pro-inflammatory cytokines involved in leukocyte migration, and vascular endothelial growth factor A (VEGF-A), which promotes angiogenesis [
6]. The combined effect of these pathways merge in peritoneal fibrosis, which progressively reduces PD efficiency and leads to peritoneal membrane failure. Addressing these mechanisms is essential for prolonging treatment viability and improving the quality of life in PD patients [
7].
Protein-energy wasting (PEW) is characterized by the decrease in body stores of energy reserves [
8]. PEW development is a complex multifactorial process involving inflammation, decreased food intake, dialysate nutrient losses, metabolic acidosis, hormonal disorders, and diminished antioxidant levels, among other factors [
9]. With an estimated prevalence of 75%, PEW is an important factor which increases mortality in PD patients’ principle due to the loss of the adipose tissue reserves [
10]. Uremia-caused anorexia is a higher contributor to PEW, and it’s exacerbated by alteration in production of orexigenic and anorexigenic hormones [
11]. In this context, nutritional supplementation is a critical strategy to mitigate the impact of PEW due to preventing weight loss and alterations in food intake, increasing quality of like in patients undergoing PD [
9].
Arthrospira maxima is a cyanobacterium used as food by humans due to its high nutritional value [
12]. Previous studies have demonstrated its potential as a suitable strategy for nutritional recovery and combating malnutrition [
13]. C-phycocyanin (CPC) is the major contributor to anti-inflammatory properties of
A. maxima [
14]. CPC exhibited anti-angiogenic effects in silico [
15], and immunomodulatory and antifibrotic effects in vivo models [
16,
17,
18]. However, its potential role in preventing peritoneal inflammation and protein-energy wasting in peritoneal dialysis remains unexplored.
This study aims to evaluate the protective effect of A. maxima in peritoneal inflammation and protein-energy wasting in a short-term uremic peritoneal dialysis (UPD) model.
2. Materials and Methods
2.1. Animals
24 male Wistar rats weighing 300-350 g were acclimatized for two weeks in an animal room with regulated temperature at 21 ± 2 °C, 40-60% of relativity humidity, a 12 h light-dark cycle (lights on at 08:00), and
at libidum access to standard feed (RatChow 5001, LabDiet®, Richmond, USA.) and tap water in individual cages. Care and procedures were performed in accordance with the regulation set in NOM-062-ZOO-1999 [
19], and were approved by the Institutional Animal Ethical Committee for the care and use of laboratory animals with number ZOO-003-2024.
2.2. Uremic Peritoneal Dialysis Model
Animals were anesthetized with sodium pentobarbital (35 mg/kg, ip) and bilateral nephrectomy to induce uremic state or sham surgery was performed. Blood samples were collected from caudal vein, and ventral laparotomy was conducted to expose both kidneys [
20]. The blood vessels and ureter were occluded, and the kidneys were extirped. A peritoneal catheter was implanted into the peritoneal cavity and fixed to the abdominal wall using 3-0 silk suture. The catheter was tunneled subcutaneously until interscapular back. 30 mL/kg of 1.5% glucose pre-warmed (37° C) dialysis solution (Dianeal, Baxter, Deerfield, USA) was infused through the catheter and drained after 10 min to prove the permeability of catheter [
21], then it was secured and closed by a disinfected silicone tip. Finally, animals were administered with 10 mg/kg/d of enrofloxacin and tramadol to prevent infections and avoid pain, during all the experiment [
22].
2.3. Experimental Design
Following surgery, animals were divided into four groups (n=6): (1) Sham (Standard diet); (2) Enriched diet; (3) UPD; and (4) UPD + Enriched diet, afterwards standard diet (Laboratory Rodent Diet 5001, LabDiet) or enriched diet were proportionate. The enriched diet was prepared by mixing grounded standard diet feed with 20% of
A. maxima (Spiral Spring, Pozo Almonte, Chile). The composition of the diets is shown in
Table 1.
At 24 h after surgery, PD was performed instilling 30 mL/kg of pre-warmed 1.5% glucose dialysis solution through the catheter and drained after 2 hours. Before and after every PD, blood samples from caudal vein were collected. Peritoneal dialysis was performed daily, as well as feed intake and body weight measure. Energy intake was calculated according to energy supply of diets (
Table 1) and the data are presented as normalized energy intake in kJ per 100 g of body weight.
On the fifth day after PD, animals were euthanized by sodium pentobarbital (150 mg/kg, ip) and briefly, 10 mL of sterile isotonic saline solution (ISS) was injected into the peritoneal cavity and held for 2 minutes with a soft massage. The washed was recovered in 1 mL of new ISS and immediately centrifugated at 600 ×
g for 10 min and supernatant was removed and cellular pellet was immediately processed. Afterwards, intracardiac blood was collected and five liver imprints of each animal were obtained using glass slides coated with 6% (p/v) gelatin [
23]. One portion of the abdominal wall close to the end of catheter was dissected and frizzed at -80° C to molecular evaluation. Mesenteric, retroperitoneal and gonadal adipose tissue were dissected and weighted. Results of adipose tissue are presented as g of adipose tissue per 100 g of body weight.
2.4. Biochemical Analysis
Serum was obtained by centrifugation at 3500 x g for 5 min. Assays were conducted using a 96 well microplate adjusting the final volume to 100 µL, following the proportion of reactive:sample according to kits from Spinreact® (Girona, Spain), Randox (Crumlin, Irland) or Wiener Lab (Rosario, Argentin). The absorbances were measured using a spectrophotometer MultiSkan Go (ThermoFisher Scientific, Waltham, EE. UU.).
Blood urea nitrogen (BUN, 41042), uric acid (41002), creatinine (CR524), lactate (LC2389), sodium (1001385) and potassium (PT1600) were measured before and after dialysis sessions. Calcium (1152002), inorganic phosphorus (1382321), albumin (1001020), total protein (PT1630A), triglycerides (TR1697) and cholesterol (CH200) were measured at the end of the experiment.
2.5. Evaluation of Cellular Alterations in Liver Peritoneum
Cellular composition in peritoneum was analyzed by resuspending the cellular pellet in 1 mL of ISS and vortexed for 30 sec. Afterwards, suspension was mingled with Türk reagent (1:1) and total cells were quantified in a hematocytometer, and differential cellular count was made using Wright stain. The results are expressed as cell type x10
6 per mL [
24]. Liver imprints were stained with Wright’s and mesothelial cells and alterations were counted in 20 fields by a blind observer to the experiment. Results are presented as mesothelial cells x10
2 per mm
2 and percentage of activated mesothelial cells in UPD groups [
23].
2.6. Determination of Remodeling Markers in Parietal Peritoneum by Western Blot
Western blot was performed as previously reported with slight modification [
25]. Briefly the abdominal walls were homogenized in 1.5 mL of 10 mM phosphate buffer pH 7.4 and protein quantification was made using Bradford technique. Primary antibodies (Santa Cruz Biotechnology, Dallas, TX) iNOS (sc-7271), collagen α-III (sc-271249) and eNOS (sc-376751) were diluted 1:1000. Diluted 1:1500 β-actin (sc-47778) was used as constitutive protein expression and loading control. The optical density (O.D.) from protein bands were analyzed by ImageJ2, and the results are shown as the O.D. of protein/O.D. of β-actin ratio.
2.7. Statistical Analysis
GraphPad Prism (v8.0.1, GraphPad Software, MA, USA) was used for statistics. Activated mesothelial cells are expressed as median ± interquartile range (IQR) and were analyzed by U-test. The rest of the results are expressed as mean ± standard error of mean (SEM). Two-way analysis of variance (ANOVA) was performed using procedure (Sham or UDP) and treatment (Standard diet or Enriched diet) as factors, while Two-way repeated measures (RM) ANOVA were performed using groups and time as factors. The ANOVA were followed by Tukey post hoc test. In all test P<0.05 was regarded as statistically significative difference.
3. Results
3.1. Uremic State, Metabolic Acidosis and Hydroelectrolytic Balance Evaluation
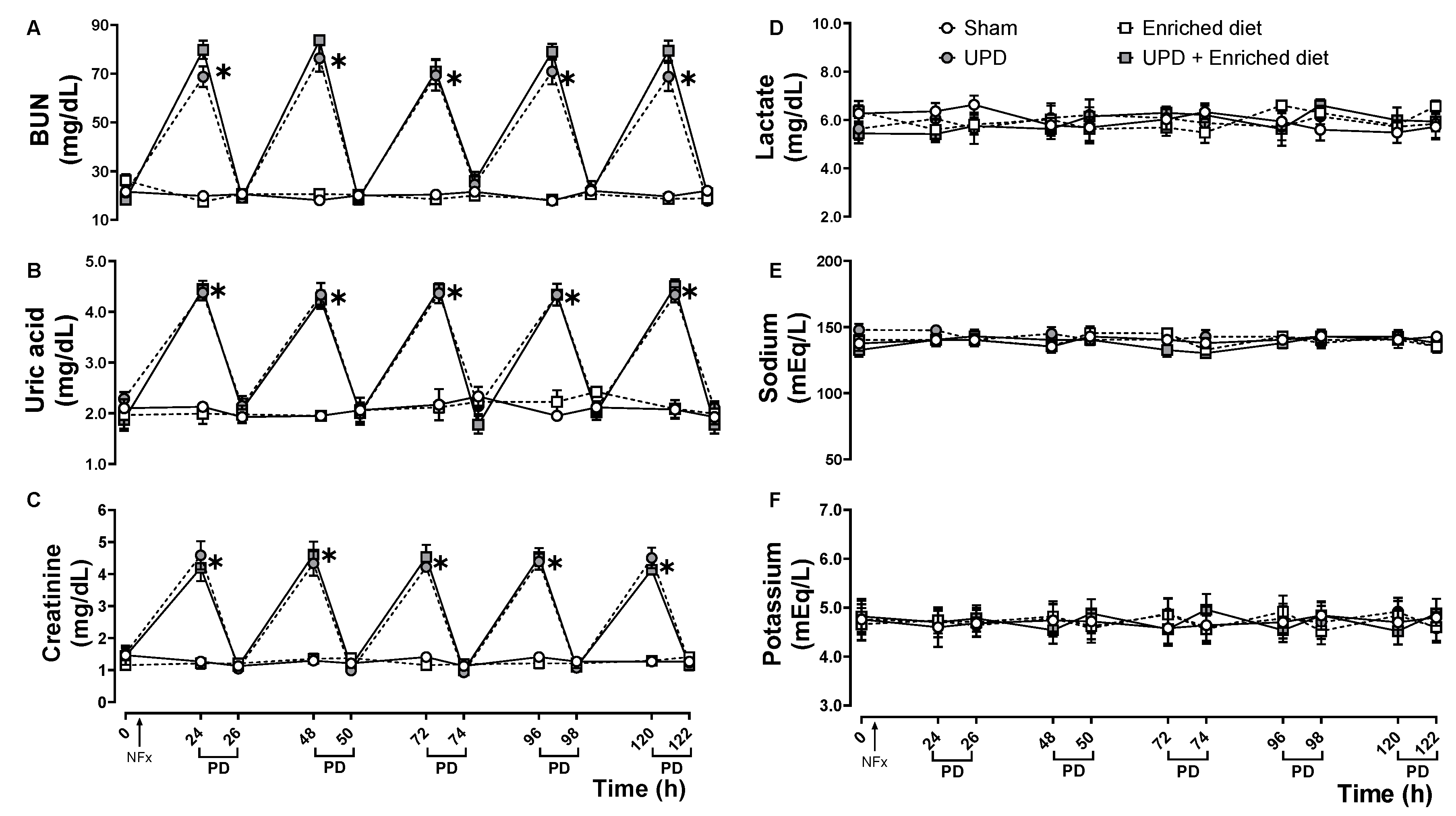
Panels A, B, and C of
Figure 1 represent biochemical data demonstrating the uremic state in the experimental animals, as well as the changes in uremic markers following peritoneal dialysis. These markers, including BUN, uric acid, and creatinine, exhibit a cyclic pattern of increases and decreases, but without any influence from dietary changes. Panel D shows lactate levels as a biomarker for metabolic acidosis. Panels E and F display the dynamics of sodium and potassium, respectively, which serve as indicators of alterations in hydroelectrolytic balance. The peritoneal dialysis procedure did not affect lactate, sodium, or potassium levels in serum of the experimental animals during the experiment.
3.2. Peritoneal Leukocytes Composition
The UPD group demonstrated a ~1.92-fold increase in basophils; ~1.89-fold in eosinophils; ~10.77-fold in neutrophils; ~2.05-fold in lymphocytes, and ~2.25-fold in macrophages, with no changes in monocytes compared to Sham group. However, UPD + enriched diet prevented the alterations in basophils, eosinophils, neutrophils and lymphocytes, while no changes in the macrophage’s population compared to UPD group, as shown in
Table 2.
3.3. Peritoneal Remodeling Markers
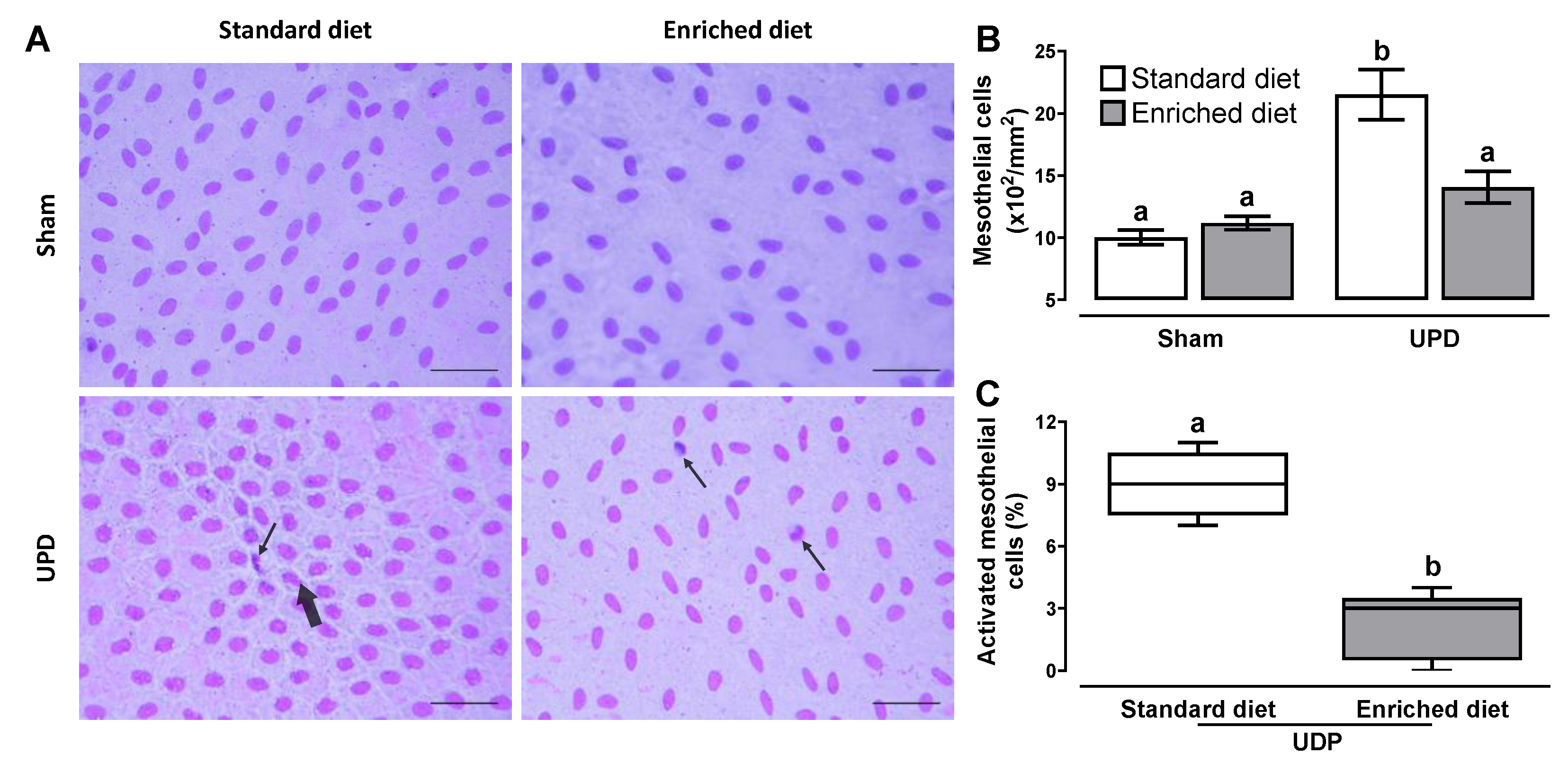
Figure 2 illustrates the effect of
A. maxima on peritoneal remodeling markers. Panel B shows that the UPD rats exhibited a ~2.15-fold increase in cellularity, while UPD + enriched diet showed a ~1.14-fold increase compared to the Sham group. Additionally, the UPD groups displayed the presence of activated mesothelial cells, characterized by bone-shape nuclei (thick arrow) and indicating the first phase of transdifferentiate myofibroblast as shown in panel C. This activation was reduced to ~24.4 in the UPD + enriched diet group establishing as 100% the UPD group. Other cells (e.g. leukocytes) were also observed (thin arrow).
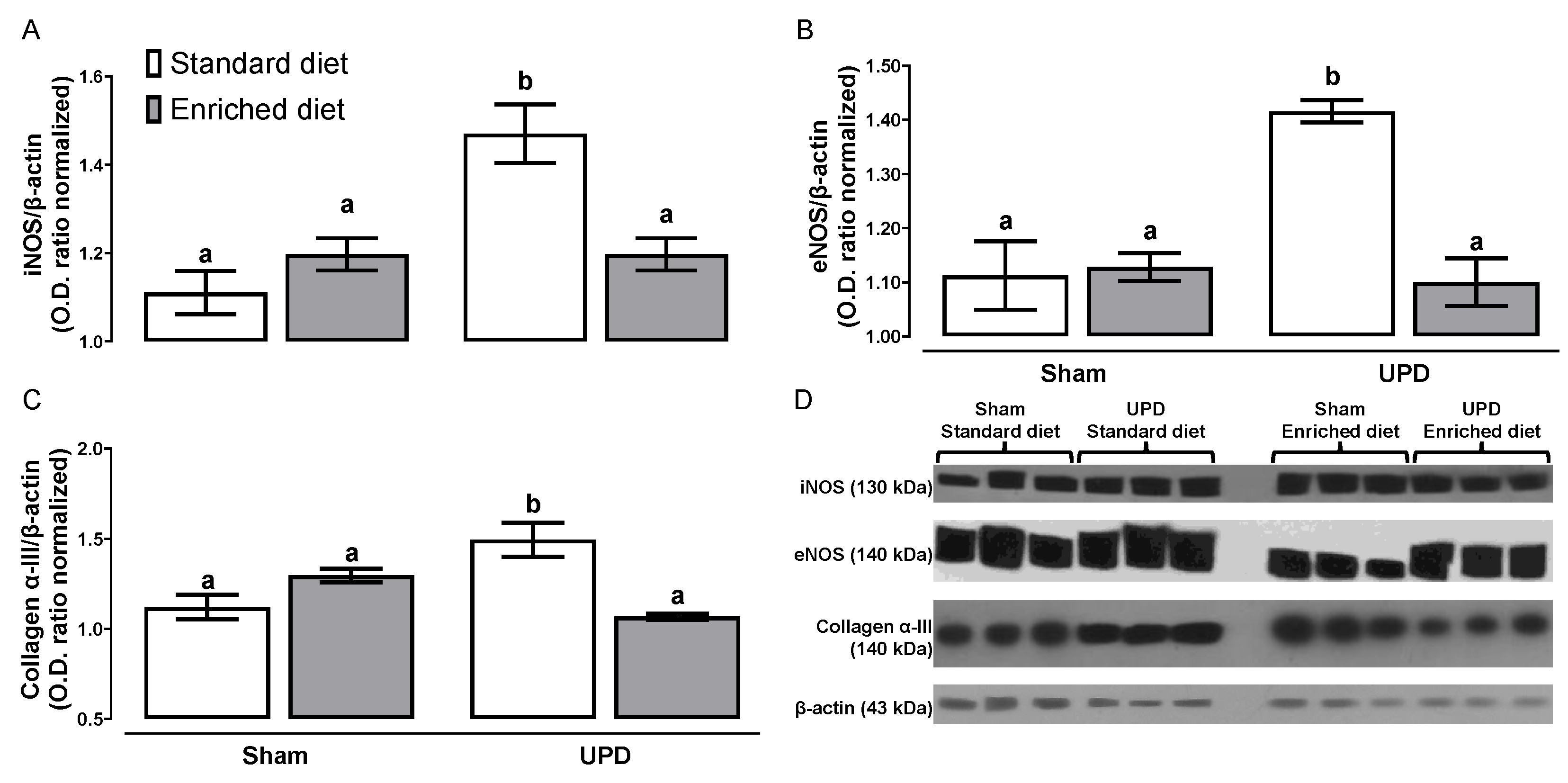
The effect of
A. maxima on the expression of proteins associated with peritoneal remodeling is illustrated in
Figure 3. The UPD groups exhibited higher levels of iNOS (~1.32-fold), eNOS(~1.27-fold), and collagen α-III expression(~1.33-fold), respect Sham group. In contrast, the UPD + enriched diet group did not show an increase in the expression of these proteins compared to Sham.
3.4. Protein-Energy Wasting Evaluation
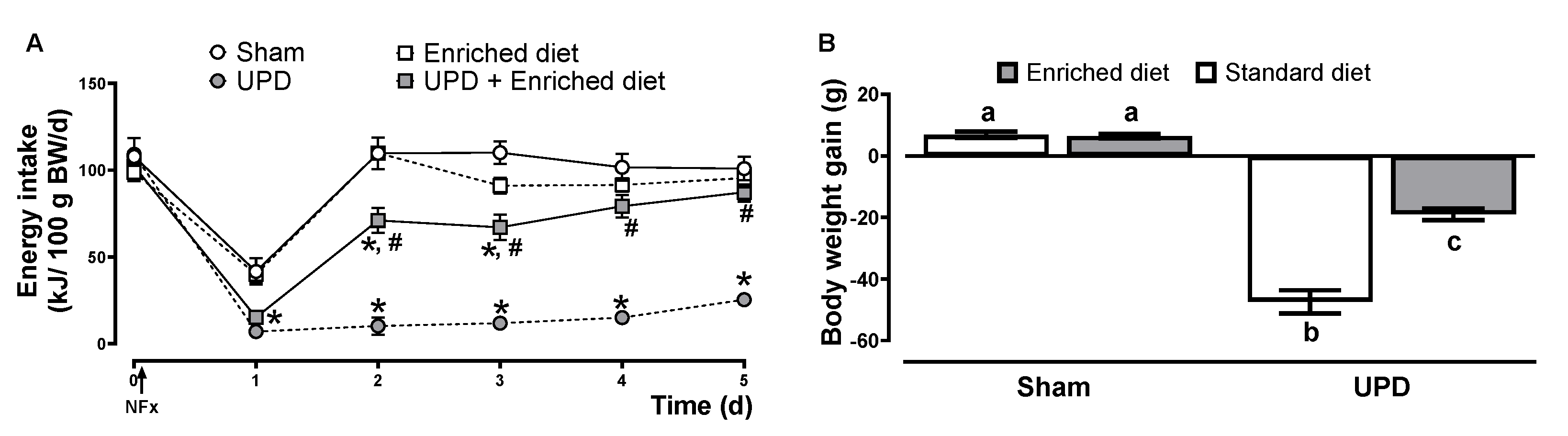
The effects of the enriched diet on energy intake and body weight in UPD rats are presented in
Figure 2. All groups experienced a reduction in energy intake the day following surgery with a decrease of ~60-62% for Sham groups, while the UPD groups exhibited a larger reduction of ~82-86%. In the following days, sham and enriched diet groups fully restored their energy intake, while the UPD group (receiving the standard diet) did not. However, providing the enriched diet to the UPD group resulted in a gradual recovery, reaching near baseline levels by day 3. In terms of body weight, the UPD group showed a ~17% weight loss, while UPD + enriched diet experienced a moderate loss of ~6%, both respect to Sham group.
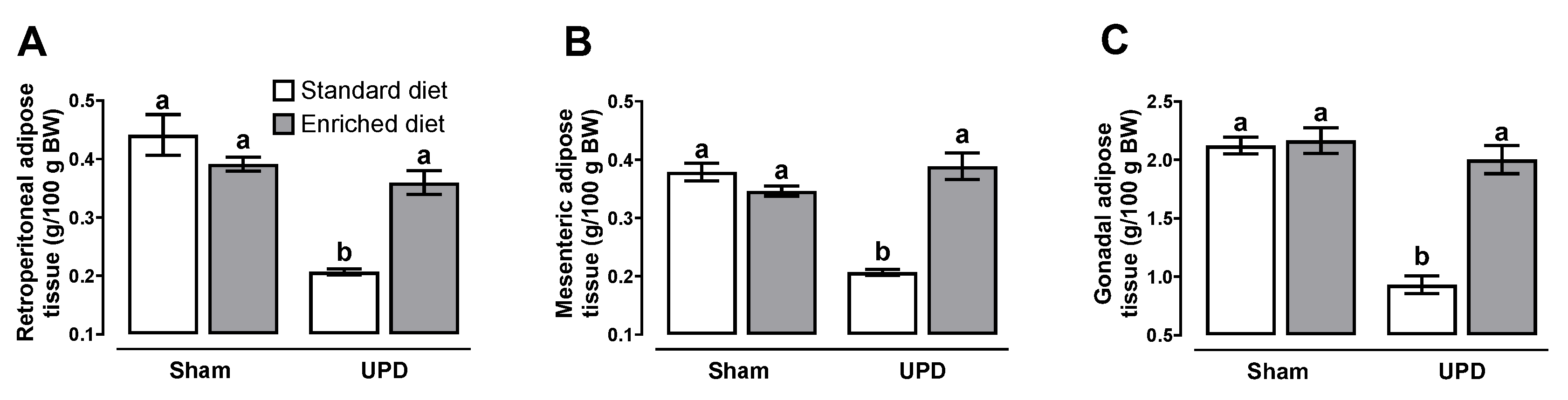
As it is deployed in
Figure 5, the UPD group exhibited reductions in retroperitoneal (~45%), mesenteric (~66%), and gonadal (~46%) adipose tissues, and in contrast, no alterations in adipose tissue deposits were observed in the UPD + Enriched diet group, both compared to Sham group.
The biochemical serum markers of protein-energy wasting are shown in
Table 3, the UPD group showed decrease in albumin (~25%), total proteins (~20%), triglycerides (~22%), and cholesterol (~35%) concentrations compared to Sham group. In the other hand, the UPD + enriched diet group exhibited a lesser reduction in albumin (~9%), and total proteins (~16%) concentration, while maintained levels of triglycerides and cholesterol, respect to Sham group.
4. Discussion
Previous research shew that
A. maxima possess nephroprotective effects against acute kidney injury and chronic kidney disease [
26,
27,
28,
29], primarily through its antioxidant, anti-inflammatory effects [
14]. Our study demonstrates that
Arthrospira maxima could modulate key pathophysiological processes in a uremic peritoneal dialysis (PD)-induced peritoneal remodeling and protein-energy wasting model, highlighting its potential as a therapeutic intervention in renal failure management.
Renal failure was induced through bilateral nephrectomy, leading to an accumulation of serum nitrogen compounds. Following PD sessions, nitrogenous compound levels normalized, confirming model’s efficacy [
22]. Furthermore, neither natremia nor kalemia were disturbed through the study, despite the inherent risk of hypokalemia associated with potassium-free dialysis solutions [
30,
31]. These findings indicate that the implemented UPD model not only reduces uremic state effectively but also prevented hydroelectrolytic imbalances, highlighting its potential as a platform to the study of short-term uremic peritoneal complications.
To evaluate the beneficial effects of
A. maxima as functional food in UPD, it was provided a 20%
A. maxima enriched diet to assess its effect in peritoneal remodeling and protein-energy wasting. The peritoneal inflammation is the principal contributor to peritoneal dialysis complications development [
5]. Our results show that 20%
A. maxima enriched diet prevents UPD-induced fibrosis and PEW. These effects may be attributed to its principal bioactive compound, CPC [
15,
28]. After ingestion, it is released from
A. maxima [
17], and undergoes digestion both gastric and intestinal phases, leads to the breakdown of CPC into bioactive peptides and phycocyanobilin (PCB), which is proposed to be the principal mediator of CPC's biological activity [
32,
33]. In agreement with this hypothesis, PCB prevents peritoneal fibrosis by downregulating type III α-collagen, an integral component of the fibrotic collagen matrix, potentially via inhibition of the TGF-β/Smad3 signaling pathway [
34]. Moreover, the expression of eNOS, which is correlated with the degree of angiogenesis [
35], was reduced due to
A. maxima, may interfering with the VEGF-A/ VEGFR-2 axis [
15]. In parallel, iNOS, which is typically associated with acute inflammation [
36], was also downregulated indicating that
A. maxima modulates UPD-induced peritoneal inflammation and it’s supported by the reduction in basophils, eosinophils, neutrophils, and lymphocytes levels in peritoneum. These reductions are endorsed mediated through the inhibition of proinflammatory cytokines, like IL-1, TNF-α, IL-6, IL-8, IL-5, limiting the migration of leukocyte to the peritoneum and selectively suppressing the acute inflammatory response. [
12,
16]. Furthermore, our data supports that CPC might influence macrophage reprograming, favoring a transition towards a pro-resolving M2 phenotype [
18].This shift in macrophage polarization is consistent with a reduction in inflammatory activity and may contribute to a more favorable microenvironment for resolving inflammation and reducing fibrosis [
37].
Previous studies have highlighted the efficacy of
A. maxima in nutritional recovery as nutritional supplementation due to its high protein source [
38]. Elevated cortisol level and uremic toxins are high correlated with inflammation process and exacerbate protein and lipid catabolism, contributing anorexia [
39]. By reducing cortisol levels,
A. maxima may play a crucial role in improving appetite recovery, as evidenced by increased energy intakes in UPD with provided 20%
A. maxima enriched diet animals [
40]. This effect may be also mediated through the decrease in leptin and glucagon-like peptide-1, and increase in agouti-related protein and ghrelin, which are critical regulators of appetite and energy balance [
41,
42]. In this context, the current results show that
A. maxima prevents the reduction of serum protein and lipids. Additionally,
A. maxima mitigates the reduction of adipose tissue deposits, which leads to the release of accumulated uremic toxins, contributing to systemic toxicity and exacerbating PEW by increasing catabolism and inducing anorexia [
8,
11]. In summary,
Figure 6 shows the potential mechanisms of
A. maxima in inflammatory and metabolic complications in the uremic peritoneal dialysis. These findings highlight the beneficial effects of
A. maxima, by protecting the reserves of adipose tissue.
5. Conclusions
Our findings provide evidence that dietary supplementation with 20% A. maxima effectively modulates peritoneal dialysis-induced peritoneal inflammation and protein-energy wasting. By targeting inflammatory pathways. A. maxima demonstrates its potential not only to reduce complications associated with peritoneal dialysis but also to prevent anorexia, minimize weight loss, and preserve adipose tissue. These results highlight A. maxima as a promising therapeutic strategy for improving the nutritional status of patients undergoing peritoneal dialysis. Future research should further explore its molecular mechanisms and long-term clinical applications to optimize its use in combating systemic complications of renal failure.
Author Contributions
Conceptualization, Oscar Florencio-Santiago and Edgar Cano-Europa; Formal analysis, Oscar Florencio-Santiago; Funding acquisition, Edgar Cano-Europa, Placido Rojas-Franco and Margarita Franco-Colín; Investigation, Oscar Florencio-Santiago; Methodology, Oscar Florencio-Santiago, Zayra Mundo-Franco, Alejandro Londoño-Moreno and Rosa Angélica González-Estrella; Resources, Edgar Cano-Europa, Placido Rojas-Franco and Margarita Franco-Colín; Software, Oscar Florencio-Santiago; Writing – original draft, Oscar Florencio-Santiago; Writing – review & editing, Zayra Mundo-Franco, Alejandro Londoño-Moreno, Rosa Angélica González-Estrella, Edgar Cano-Europa and Margarita Franco-Colín.
Funding
This study was supported by Secretaría De Investigación Y Posgrado, Instituto Politécnico Nacional (SIP-IPN), grant numbers: 20240554, 20241464 & 20240520.
Institutional Review Board Statement
The animal study protocol was approved by Ethics Committee of ESCUELA NACIONAL DE CIENCIAS BIOLÓGICAS (protocol code ZOO-003-2024, approval date 25/05/2024).
Data Availability Statement
The datasets generated during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Instituto Politécnico Nacional, SIP-IPN, and Consejo Nacional de Humanidades, Ciencias y Tecnologías for their financial support. The E.C.-E., P.R.-F., and M.F.-C. are members of SNI, while E.C.-E., and M.F.-C. are fellows of COFA and EDI.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PD |
Peritoneal dialysis |
| RRT |
Renal replacement therapy |
| TGF-β |
growth factor β |
| VEGF-A |
vascular endothelial growth factor A |
| PEW |
Protein energy wasting |
| CPC |
C-phycocyanin |
| UPD |
Uremic peritoneal dialysis |
| ISS |
Isotonic saline solution |
| O.D. |
Optical density |
| IQR |
Interquartile range |
| SEM |
Standard error |
| ANOVA |
Analysis of variance |
| RM |
Repeated measures |
| BUN |
Blood urea nitrogen |
| BW |
Body weight |
| WAT |
White adipose tissue |
| RAT |
Retroperitoneal adipose tissue |
| GAT |
Gonadal adipose tissue |
| MAT |
Mesenteric adipose tissue |
| ECM |
Extracellular matrix |
| SIP-IPN |
Secretaría De Investigación Y Posgrado, Instituto Politécnico Nacional |
References
- Bello, A.K.; Okpechi, I.G.; Osman, M.A.; Cho, Y.; Cullis, B.; Htay, H.; Jha, V.; Makusidi, M.A.; McCulloch, M.; Shah, N.; et al. Epidemiology of Peritoneal Dialysis Outcomes. Nat Rev Nephrol 2022, 18, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Jimenez, E.; Madero, M. Global Dialysis Perspective: Mexico. Kidney360 2020, 1, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Dounousi, E.; Salmas, M.; Eleftheriadis, T.; Liakopoulos, V. Unfavorable Effects of Peritoneal Dialysis Solutions on the Peritoneal Membrane: The Role of Oxidative Stress. Biomolecules 2020, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, F.A.; de Jesus, J.S.; Cordeiro, L.; Piraciaba, M.C.T.; de Araujo, L.K.R.P.; Steller Wagner Martins, C.; Dalboni, M.A.; Pereira, B.J.; Silva, B.C.; Moysés, R.M.A.; et al. Hypokalemia and Hyperkalemia in Patients on Peritoneal Dialysis: Incidence and Associated Factors. Int Urol Nephrol 2020, 52, 393–398. [Google Scholar] [CrossRef]
- Zareie, M.; De Vriese, A.S.; Hekking, L.H.P.; ter Wee, P.M.; Schalkwijk, C.G.; Driesprong, B.A.J.; Schadee-Eestermans, I.L.; Beelen, R.H.J.; Lameire, N.; van den Born, J. Immunopathological Changes in a Uraemic Rat Model for Peritoneal Dialysis. Nephrology Dialysis Transplantation 2005, 20, 1350–1361. [Google Scholar] [CrossRef]
- Uiterwijk, H.; Franssen, C.F.M.; Kuipers, J.; Westerhuis, R.; Nauta, F.L. Glucose Exposure in Peritoneal Dialysis Is a Significant Factor Predicting Peritonitis. Am J Nephrol 2020, 51, 237–243. [Google Scholar] [CrossRef]
- Aroeira, L.S.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; del Peso, G.; Jiménez-Heffernan, J.A.; Selgas, R.; López-Cabrera, M. Epithelial to Mesenchymal Transition and Peritoneal Membrane Failure in Peritoneal Dialysis Patients. Journal of the American Society of Nephrology 2007, 18, 2004–2013. [Google Scholar] [CrossRef]
- Dukkipati, R.; Kopple, J.D. Causes and Prevention of Protein-Energy Wasting in Chronic Kidney Failure. Semin Nephrol 2009, 29, 39–49. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.M.; et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement From the International Society of Renal Nutrition and Metabolism (ISRNM). Journal of Renal Nutrition 2013, 23, 77–90. [Google Scholar] [CrossRef]
- Yamada, S.; Nakano, T.; Tsuneyoshi, S.; Arase, H.; Shimamoto, S.; Taniguchi, M.; Tokumoto, M.; Hirakata, H.; Ooboshi, H.; Tsuruya, K.; et al. Association between Modified Simple Protein-Energy Wasting (PEW) Score and All-Cause Mortality in Patients Receiving Maintenance Hemodialysis. Ren Replace Ther 2020, 6, 39. [Google Scholar] [CrossRef]
- Bonanni, A.; Mannucci, I.; Verzola, D.; Sofia, A.; Saffioti, S.; Gianetta, E.; Garibotto, G. Protein-Energy Wasting and Mortality in Chronic Kidney Disease. Int J Environ Res Public Health 2011, 8, 1631–1654. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. The Bioactivities of Phycocyanobilin from Spirulina. J Immunol Res 2022, 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.M.; Behling, B. del S.; Rodrigues, R. da S.; Costa, J.A.V.; Soares, L.A. de S. Spirulina as a Protein Source in the Nutritional Recovery of Wistar Rats. Brazilian Archives of Biology and Technology 2013, 56, 447–456. [Google Scholar] [CrossRef]
- Acharya, B. ; Swami Narsingh; Bhasker Joshi; Rajesh Kumar Mishra Spirulina: A Miraculous Alga with Pharmaco-Nutraceutical Potential as Future Food. International Journal of Food, Nutrition and Dietetics 2023, 11, 127–136. [Google Scholar]
- Jayanti, D.A.P.I.S.; Abimanyu, I.G.A.; Azzamudin, H. Spirulina Platensis’s Phycocyanobilin as an Antiangiogenesis by Inhibiting VEGFR2-VEGFA Pathway in Breast Cancer: In Silico Study. JSMARTech 2021, 2, 87–91. [Google Scholar] [CrossRef]
- Liu, R.; Qin, S.; Li, W. Phycocyanin: Anti-Inflammatory Effect and Mechanism. Biomedicine & Pharmacotherapy 2022, 153, 113362. [Google Scholar] [CrossRef]
- Blas-Valdivia, V.; Moran-Dorantes, D.; Rojas Franco, P.; Franco-Colin, M.; Mirhosseini, N.; Davarnejad, R.; Halajisani, A.; Tavakoli, O.; Cano-Europa, E. C-Phycocyanin Prevents Acute Myocardial Infarction-Induced Oxidative Stress, Inflammation and Cardiac Damage. Pharm Biol 2022, 60, 755–763. [Google Scholar] [CrossRef]
- Mundo-Franco, Z.; Luna-Herrera, J.; Castañeda-Sánchez, J.I.; Serrano-Contreras, J.I.; Rojas-Franco, P.; Blas-Valdivia, V.; Franco-Colín, M.; Cano-Europa, E. C-Phycocyanin Prevents Oxidative Stress, Inflammation, and Lung Remodeling in an Ovalbumin-Induced Rat Asthma Model. Int J Mol Sci 2024, 25, 7031. [Google Scholar] [CrossRef]
- DOF NOM-062-ZOO-1999: Especificaciones Técnicas Para La Producción, Cuidado y Uso de Los Animales de Laboratorio. 1999.
- Cavallini, N.; Wieslander, A.; Braide, M. Substituting Citrate for Lactate in Peritoneal Dialysis Fluid Improves Ultrafiltration in Rats. Peritoneal Dialysis International 2009, 29, 36–43. [Google Scholar] [CrossRef]
- Méndez-García, S.R.; Cano-Europa, E.; Ocotitla-Hernández, J.; Franco-Colín, M.; Florencio-Santiago, O.I.; Torres-SanMiguel, C.R. Experimental Lab Tests on Rabbits for the Optimization and Redesign of Low-Cost Equipment for Automated Peritoneal Dialysis. Bioengineering 2024, 11, 114. [Google Scholar] [CrossRef]
- Fujii, Y.; Yamauchi, K.; Kokuba, Y.; Kikuchi, T. New Peritoneal Dialysis Model in Rats with Bilateral Nephrectomy. Ren Fail 2009, 31, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Hekking, L.H.P.; Zareie, M.; Driesprong, B.A.J.; Faict, D.; Welten, A.G.A.; de Greeuw, I.; Schadee-Eestermans, I.L.; Havenith, C.E.G.; van den Born, J.; Ter Wee, P.M.; et al. Better Preservation of Peritoneal Morphologic Features and Defense in Rats after Long-Term Exposure to a Bicarbonate/Lactate-Buffered Solution. Journal of the American Society of Nephrology 2001, 12, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Połubinska, A.; Pawlaczyk, K.; Kużlan-Pawlaczyk, M.; Wieczorowska-Tobis, K.; Chen, C.; Moberly, J.B.; Martis, L.; Breçborowicz, A.; Oreopoulos, D.G. Dialysis Solution Containing Hyaluronan: Effect on Peritoneal Permeability and Inflammation in Rats. Kidney Int 2000, 57, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Santiago, O.I.; Blas-Valdivia, V.; Serrano-Contreras, J.I.; Rojas-Franco, P.; Escalona-Cardoso, G.N.; Paniagua-Castro, N.; Franco-Colin, M.; Cano-Europa, E. C-Phycoerythrin Prevents Chronic Kidney Disease-Induced Systemic Arterial Hypertension, Avoiding Oxidative Stress and Vascular Dysfunction in Remanent Functional Kidney. Mar Drugs 2024, 22, 337. [Google Scholar] [CrossRef]
- Memije-Lazaro, I.N.; Blas-Valdivia, V.; Franco-Colín, M.; Cano-Europa, E. Arthrospira Maxima (Spirulina) and C-Phycocyanin Prevent the Progression of Chronic Kidney Disease and Its Cardiovascular Complications. J Funct Foods 2018, 43, 37–43. [Google Scholar] [CrossRef]
- Rojas-Franco, P.; Franco-Colín, M.; Camargo, M.E.M.; Carmona, M.M.E.; Ortíz-Butrón, M. del R.E.; Blas-Valdivia, V.; Cano-Europa, E. Phycobiliproteins and Phycocyanin of Arthrospira Maxima ( Spirulina ) Reduce Apoptosis Promoters and Glomerular Dysfunction in Mercury-Related Acute Kidney Injury. Toxicology Research and Application 2018, 2. [Google Scholar] [CrossRef]
- Rojas-Franco, P.; Franco-Colín, M.; Blas-Valdivia, V.; Melendez-Camargo, M.E.; Cano-Europa, E. Arthrospira Maxima (Spirulina) Prevents Endoplasmic Reticulum Stress in the Kidney through Its C-Phycocyanin. Journal of Zhejiang University-SCIENCE B 2021, 22, 603–608. [Google Scholar] [CrossRef]
- Rojas-Franco, P.; Garcia-Pliego, E.; Vite-Aquino, A.G.; Franco-Colin, M.; Serrano-Contreras, J.I.; Paniagua-Castro, N.; Gallardo-Casas, C.A.; Blas-Valdivia, V.; Cano-Europa, E. The Nutraceutical Antihypertensive Action of C-Phycocyanin in Chronic Kidney Disease Is Related to the Prevention of Endothelial Dysfunction. Nutrients 2022, 14, 1464. [Google Scholar] [CrossRef]
- Borrelli, S.; De Nicola, L.; Minutolo, R.; Perna, A.; Provenzano, M.; Argentino, G.; Cabiddu, G.; Russo, R.; La Milia, V.; De Stefano, T.; et al. Sodium Toxicity in Peritoneal Dialysis: Mechanisms and “Solutions. ” J Nephrol 2020, 33, 59–68. [Google Scholar] [CrossRef]
- Mirmiran, P.; Nazeri, P.; Bahadoran, Z.; Khalili-Moghadam, S.; Azizi, F. Dietary Sodium to Potassium Ratio and the Incidence of Chronic Kidney Disease in Adults : A Longitudinal Follow-Up Study. Prev Nutr Food Sci 2018, 23, 87–93. [Google Scholar] [CrossRef]
- Gligorijević, N.; Minić, S.; Radibratović, M.; Papadimitriou, V.; Nedić, O.; Sotiroudis, T.G.; Nikolić, M.R. Nutraceutical Phycocyanobilin Binding to Catalase Protects the Pigment from Oxidation without Affecting Catalytic Activity. Spectrochim Acta A Mol Biomol Spectrosc 2021, 251, 119483. [Google Scholar] [CrossRef] [PubMed]
- Pentón-Rol, G.; Marín-Prida, J.; Falcón-Cama, V. C-Phycocyanin and Phycocyanobilin as Remyelination Therapies for Enhancing Recovery in Multiple Sclerosis and Ischemic Stroke: A Preclinical Perspective. Behavioral Sciences 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Peng, W.; Zhang, Z.; Pei, X.; Sun, Z.; Ou, Y. A Phycocyanin Derived Eicosapeptide Attenuates Lung Fibrosis Development. Eur J Pharmacol 2021, 908, 174356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, N.; Ni, Z. Strategies for Preventing Peritoneal Fibrosis in Peritoneal Dialysis Patients: New Insights Based on Peritoneal Inflammation and Angiogenesis. Front Med 2017, 11, 349–358. [Google Scholar] [CrossRef]
- Pérez, S.; Rius-Pérez, S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Balzer, M.S. Molecular Pathways in Peritoneal Fibrosis. Cell Signal 2020, 75, 109778. [Google Scholar] [CrossRef]
- Sinha, S.; Patro, N.; Patro, I.K. Maternal Protein Malnutrition: Current and Future Perspectives of Spirulina Supplementation in Neuroprotection. Front Neurosci 2018, 12. [Google Scholar] [CrossRef]
- Haque, Z.; Akbar, N.; Yasmin, F.; Haleem, M.A.; Haleem, D.J. Inhibition of Immobilization Stress-Induced Anorexia, Behavioral Deficits, and Plasma Corticosterone Secretion by Injected Leptin in Rats. Stress 2013, 16, 353–362. [Google Scholar] [CrossRef]
- Mishra, P.; Kumar, S.; Malik, J.K. Molecular Mechanistic Insight Spirulina as Anti-Stress Agent. Middle East Research Journal of Pharmaceutical Sciences 2023, 3, 25–30. [Google Scholar] [CrossRef]
- Abadjieva, D.; Nedeva, R.; Marchev, Y.; Jordanova, G.; Chervenkov, M.; Dineva, J.; Shimkus, A.; Shimkiene, A.; Teerds, K.; Kistanova, E. Arthrospira (Spirulina) Platensis Supplementation Affects Folliculogenesis, Progesterone and Ghrelin Levels in Fattening Pre-Pubertal Gilts. J Appl Phycol 2018, 30, 445–452. [Google Scholar] [CrossRef]
- Korczynska, J.; Czumaj, A.; Chmielewski, M.; Swierczynski, J.; Sledzinski, T. The Causes and Potential Injurious Effects of Elevated Serum Leptin Levels in Chronic Kidney Disease Patients. Int J Mol Sci 2021, 22, 4685. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Evaluation biochemical markers on anephrotic rats fed with 20% A maxima enriched diet during PD. (A) BUN, (B) uric acid, and (C) creatinine were measured as markers of uremic state; (D) lactate as metabolic acidosis; (E) sodium, and (F) potassium as hydroelectrolytic balance. The values represent mean ± SEM. Two-way RM ANOVA and Tukey post hoc test. (*) P<0.05 vs Sham at same time.
Figure 1.
Evaluation biochemical markers on anephrotic rats fed with 20% A maxima enriched diet during PD. (A) BUN, (B) uric acid, and (C) creatinine were measured as markers of uremic state; (D) lactate as metabolic acidosis; (E) sodium, and (F) potassium as hydroelectrolytic balance. The values represent mean ± SEM. Two-way RM ANOVA and Tukey post hoc test. (*) P<0.05 vs Sham at same time.
Figure 2.
(A) Representative photomicrographs of liver imprints of anephrotic rats fed with A maxima enriched diet after PD. Activated bone-shaped nuclei (thick arrow) and other cells (thin arrow). Wright’s stain at 400× magnification. The lower right bar represents 250 µm. (B) Morphometric cellularity and (C) activated mesothelial cells. In B the values represent mean ± SEM and were evaluated by Two-way RM ANOVA and Tukey post hoc test. In C the values represent median ± IQR and were evaluated by Mann-Whitney U-test. Different letters indicate significant differences (a≠b) P<0.05.
Figure 2.
(A) Representative photomicrographs of liver imprints of anephrotic rats fed with A maxima enriched diet after PD. Activated bone-shaped nuclei (thick arrow) and other cells (thin arrow). Wright’s stain at 400× magnification. The lower right bar represents 250 µm. (B) Morphometric cellularity and (C) activated mesothelial cells. In B the values represent mean ± SEM and were evaluated by Two-way RM ANOVA and Tukey post hoc test. In C the values represent median ± IQR and were evaluated by Mann-Whitney U-test. Different letters indicate significant differences (a≠b) P<0.05.
Figure 3.
Effect of A. maxima enriched diet on (A) iNOS, (B) eNOS, and (C) Collagen α- III as peritoneal remodeling markers in parietal peritoneum of anephrotic rats after PD. The values represent mean ± SEM. Two-way ANOVA and Tukey post hoc test. Different letters indicate significant differences (a ≠ b ≠ c) P<0.05.
Figure 3.
Effect of A. maxima enriched diet on (A) iNOS, (B) eNOS, and (C) Collagen α- III as peritoneal remodeling markers in parietal peritoneum of anephrotic rats after PD. The values represent mean ± SEM. Two-way ANOVA and Tukey post hoc test. Different letters indicate significant differences (a ≠ b ≠ c) P<0.05.
Figure 4.
Effect of A. maxima enriched diet on (A) energy intake, and (B) body weight of anephrotic rats after PD. The values represent mean ± SEM. A was evaluated by RM Two-Way ANOVA and Tukey post hoc test. (*) P< 0.05 vs Sham at same time; (#) P< 0.05 vs UPD at same time. B was evaluated by Two-Way ANOVA and Tukey post hoc test. Different letters indicate significant differences (a ≠ b ≠ c) P<0.05.
Figure 4.
Effect of A. maxima enriched diet on (A) energy intake, and (B) body weight of anephrotic rats after PD. The values represent mean ± SEM. A was evaluated by RM Two-Way ANOVA and Tukey post hoc test. (*) P< 0.05 vs Sham at same time; (#) P< 0.05 vs UPD at same time. B was evaluated by Two-Way ANOVA and Tukey post hoc test. Different letters indicate significant differences (a ≠ b ≠ c) P<0.05.
Figure 5.
Effect of A. maxima enriched diet on (A) retroperitoneal, (B) mesenteric, and (C) gonadal adipose tissue deposits of anephrotic rats after PD. The values represent mean ± SEM. Two-Way ANOVA and Tukey post hoc test. Different letters indicate significant differences (a ≠ b) P<0.05. .
Figure 5.
Effect of A. maxima enriched diet on (A) retroperitoneal, (B) mesenteric, and (C) gonadal adipose tissue deposits of anephrotic rats after PD. The values represent mean ± SEM. Two-Way ANOVA and Tukey post hoc test. Different letters indicate significant differences (a ≠ b) P<0.05. .
Figure 6.
(A In the peritoneum, the presence of a catheter, high glucose concentrations, low pH, uremia, and the catheter itself trigger the release of TGF-β1 and other proinflammatory cytokines. The autocrine action of TGF-β1 induces the expression of iNOS, VEGF-A, and subsequent angiogenesis, which is evidenced by the upregulation of eNOS. Additionally, TGF-β1 mediates the activation of mesothelial cells to myofibroblasts through mesothelial-to-mesenchymal transition (MMT), thereby initiating fibrosis within the extracellular matrix (ECM). Concurrently, proinflammatory cytokines promote leukocyte migration, further enhancing peritoneal inflammation. A. maxima, through its bioactive compounds released upon digestion, attenuates the angiogenic and fibrotic processes in the ECM, while also modules leukocyte migration to an anti-inflammatory process. (B) Renal failure and inflammation contribute to the development of protein-energy wasting (PEW) by increasing the catabolism of proteins and lipids, leading to hypoproteinemia and hypolipidemia resulting in the depletion of white adipose tissue (WAT). An elevation in leptin and other anorexigenic hormones suppress appetite and facilitate weight loss. A. maxima showed to prevent the onset of PEW by mitigating the disturbances in lipidemia and proteinemia, while also promoting appetite and preventing weight loss. Created with Biorender.com.
Figure 6.
(A In the peritoneum, the presence of a catheter, high glucose concentrations, low pH, uremia, and the catheter itself trigger the release of TGF-β1 and other proinflammatory cytokines. The autocrine action of TGF-β1 induces the expression of iNOS, VEGF-A, and subsequent angiogenesis, which is evidenced by the upregulation of eNOS. Additionally, TGF-β1 mediates the activation of mesothelial cells to myofibroblasts through mesothelial-to-mesenchymal transition (MMT), thereby initiating fibrosis within the extracellular matrix (ECM). Concurrently, proinflammatory cytokines promote leukocyte migration, further enhancing peritoneal inflammation. A. maxima, through its bioactive compounds released upon digestion, attenuates the angiogenic and fibrotic processes in the ECM, while also modules leukocyte migration to an anti-inflammatory process. (B) Renal failure and inflammation contribute to the development of protein-energy wasting (PEW) by increasing the catabolism of proteins and lipids, leading to hypoproteinemia and hypolipidemia resulting in the depletion of white adipose tissue (WAT). An elevation in leptin and other anorexigenic hormones suppress appetite and facilitate weight loss. A. maxima showed to prevent the onset of PEW by mitigating the disturbances in lipidemia and proteinemia, while also promoting appetite and preventing weight loss. Created with Biorender.com.
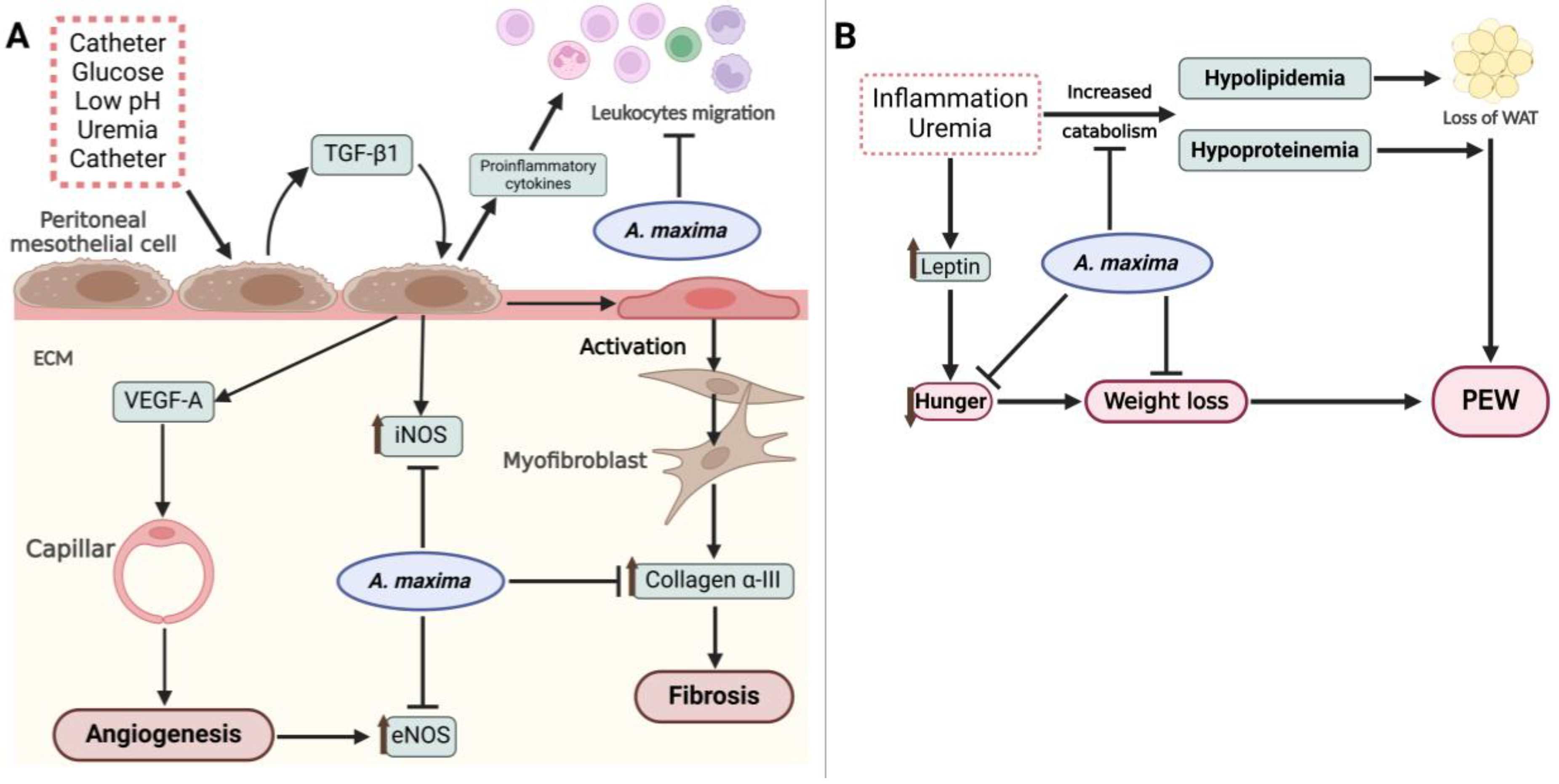
Table 1.
Composition of Chow and 20% A. maxima enriched diet.
Table 1.
Composition of Chow and 20% A. maxima enriched diet.
| Component (%) |
Standard diet |
Enriched diet |
| Proteins |
24.1 |
32.28 |
| Carbohydrates |
57.94 |
49.6 |
| Lipids |
5 |
5.2 |
| Energy supply (kJ/g) |
14.31 |
14.30 |
Table 2.
Effect of of A. maxima enriched diet on peritoneal leukocytes populations of anephrotic rats after PD.
Table 2.
Effect of of A. maxima enriched diet on peritoneal leukocytes populations of anephrotic rats after PD.
| Cells (106/mL) |
Sham |
Enriched diet |
UPD |
UPD +
Enriched diet |
| Basophils |
0.48 ± 0.08 |
0.44 ± 0.10 |
0.92 ± 0.11* |
0.68 ± 0.09# |
| Eosinophils |
8.5 ± 1.25 |
8.44 ± 1.3 |
16.06 ± 0.41* |
9.64 ± 0.84# |
| Neutrophils |
1.04 ± 0.17 |
0.8 ± 0.34 |
11.2 ± 1.39* |
4.02 ± 1# |
| Lymphocytes |
4.02 ± 0.43 |
4.46 ± 0.93 |
8.6 ± 1.13* |
4.42 ± 0.37# |
| Monocytes |
3.64 ± 0.48 |
3.96 ± 0.93 |
4.84 ± 0.29 |
5.1 ± 0.26 |
| Macrophages |
9.74 ± 1.07 |
9.66 ± 1.29 |
22 ± 1.09* |
25.9 ± 2.55* |
Table 3.
Effect of A. maxima enriched diet on metabolic parameters of anephrotic rats after PD.
Table 3.
Effect of A. maxima enriched diet on metabolic parameters of anephrotic rats after PD.
| Parameter |
Sham |
Enriched diet |
UPD |
UPD +
Enriched diet |
| Albumin (g/dL) |
3.20 ± 0.09 |
3.40 ± 0.1 |
2.58 ± 0.21* |
3.1 ± 0.12# |
| Total proteins (g/dL) |
6.42 ± 0.18 |
6.46 ± 0.24 |
5.24 ± 0.15* |
6.22 ± 0.1# |
| Triglycerides (mg/dL) |
73.91 ± 1.68 |
74.70 ± 2.64 |
58.63 ± 2.18* |
69.42 ± 2.35*,# |
| Cholesterol (mg/dL) |
68.05 ± 2.97 |
68.73 ± 2.67 |
44.82 ± 3.48* |
61.67 ± 2.79*,# |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).