3.2.2. The Molecular Structure of Covalent Compounds
Due to the highest charge flux at the vertex of the ellipsoid of a single electron orbital dynamic entity, the electron spin field density per unit time is highest at that location. If the ellipsoidal single electron orbital dynamic entity of another atom approaches, then the two ellipsoidal single electron orbital dynamic entities must have the maximum
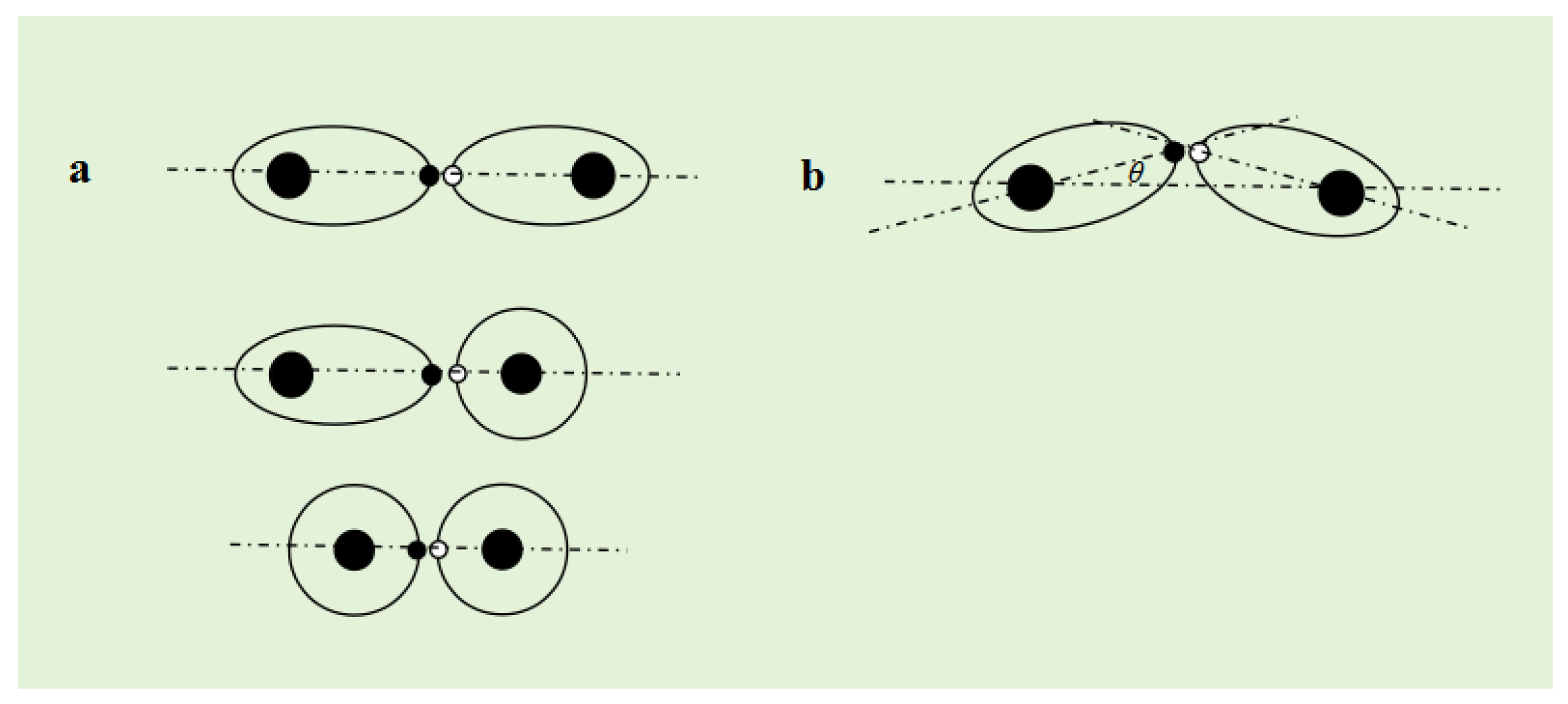
spin magnetic force at the vertex in a "head to head" manner, which determines the directionality of the covalent bond. When single electron orbits are attracted to each other by spin magnetic force, if the symmetry axes of the two single electron orbits are connected to the atomic nucleus on the same straight line, the covalent bond formed in this way is called an
α bond (
Figure 7a). If the symmetry axes of the two single electron orbits are not connected to the atomic nucleus on the same straight line, the covalent bond formed in this way is called a
β bond (
Figure 7b).
Assuming that A atom forms an α bond with B atom, and the "charge spin quantity" of A atom's single electron orbit is , and the "charge spin quantity" of B atom's single electron orbit is , then the spin magnetic force of their spin pairing is:
Due to the coincidence of the electron orbital symmetry axis of the α bond with the line connecting the two atomic nuclei of AB, the spin magnetic force of spin pairing is the binding force between AB atoms.
Assuming that A atom and B atom form a β bond, and the angle between the symmetry axis of the single electron orbit and the line connecting the atomic nucleus is θ, the binding force between AB atoms is . Therefore, the beta bond is slightly weaker than the α bond.
Based on the sum of the energies of each atomic system and the difference in energy between the molecular system, we can determine the bond energy of covalent bonds
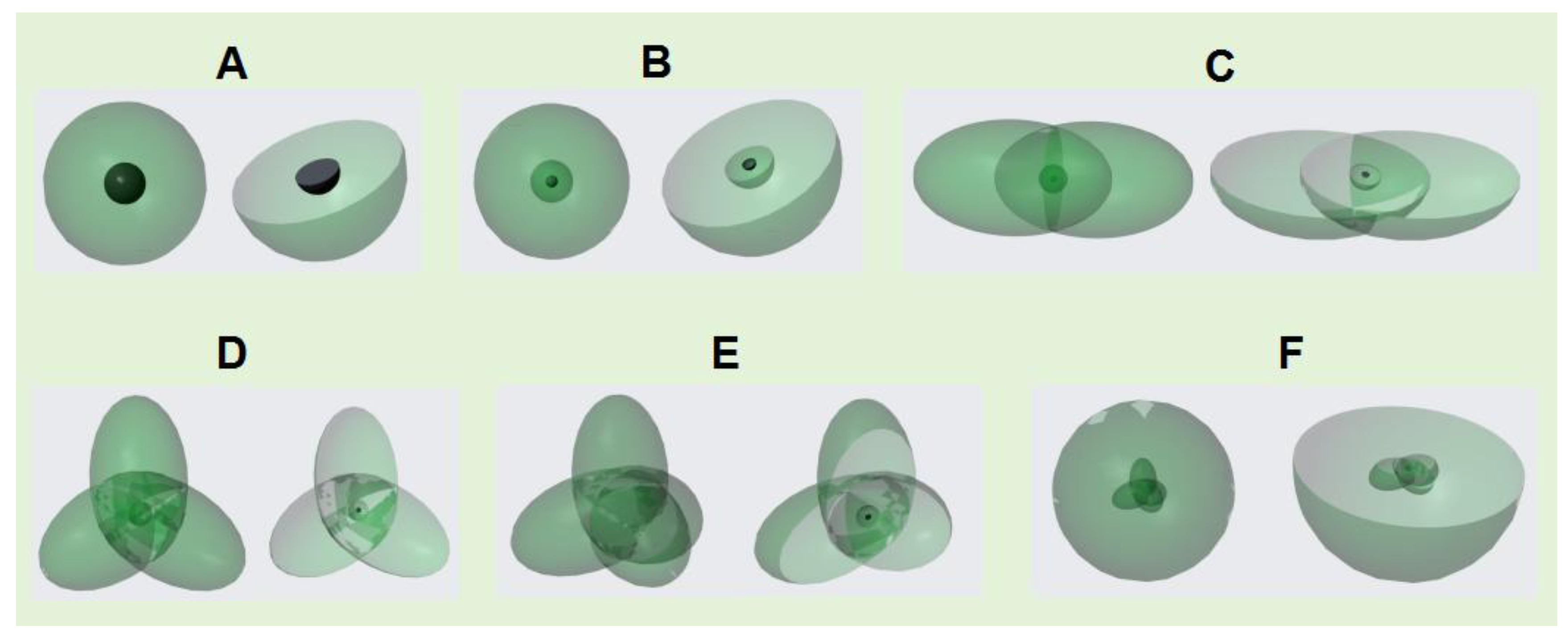
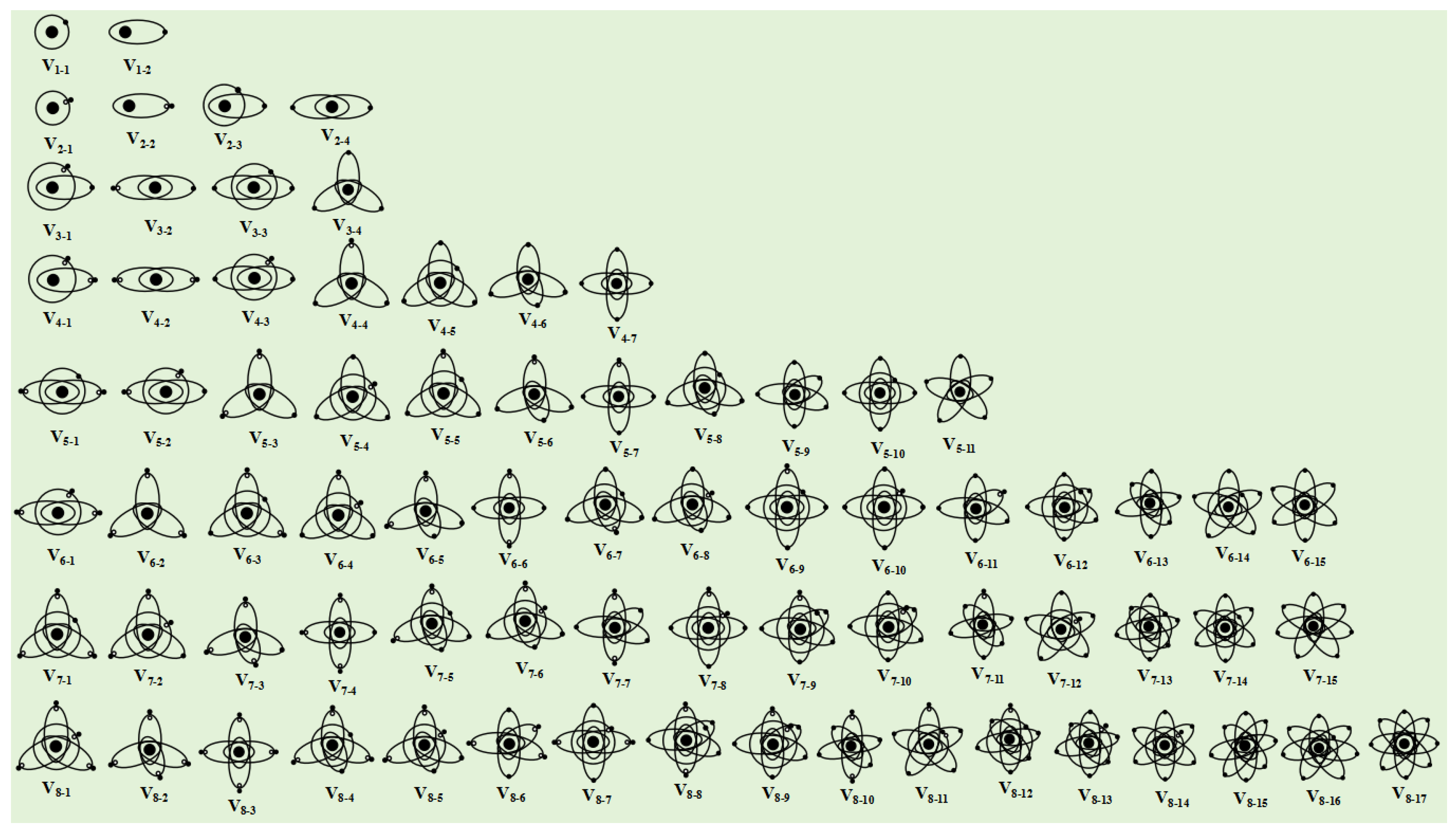
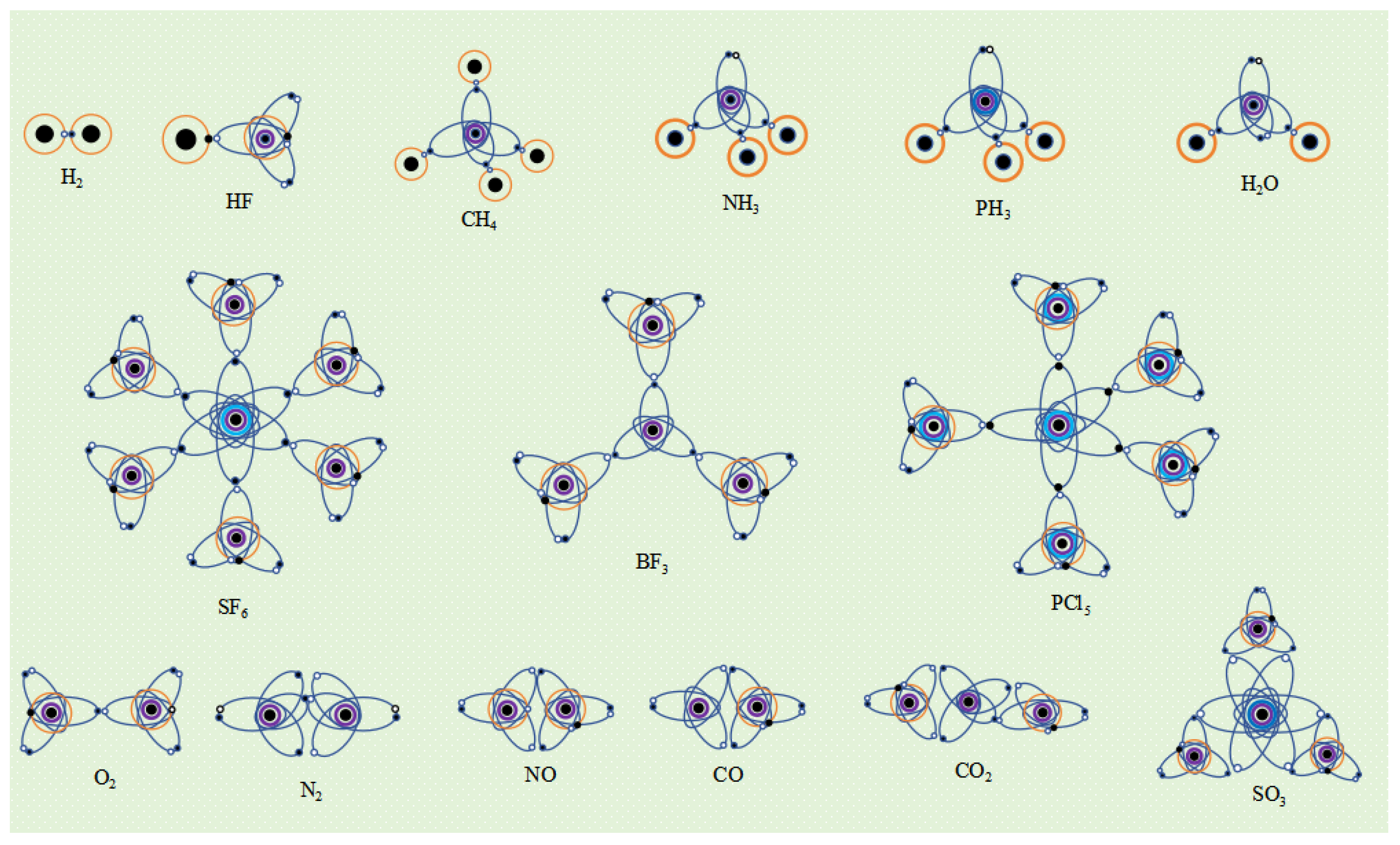
If an atom has only one single electron orbital in its valence electron configuration, then the covalent bond it forms with other atom that has only one single electron orbital must be an α bond. For example, a hydrogen atom has only one single electron orbital, and any covalent bond formed between it and any atom is an α bond. In molecules such as SF6, BF3, and PCl3, the valence layer electron configuration of F and Cl atoms is V7-2, with only one single electron ellipsoidal orbital. Therefore, the S-F, B-F, and P-Cl covalent bonds are all α bonds.
If an atom has multiple single electron orbits in its valence layer, then the covalent bond it forms with other atoms that only have one single electron orbital must also be an α bond. For example, the valence layer electron configuration of carbon atoms in methane molecules (CH4) is V4-6, with four ellipsoidal single electron orbits that spin pair with the single electron orbits of four hydrogen atoms to form four covalent bonds, all of which are α bonds.
Since an atom with multiple single electron orbits can form covalent bonds with multiple atoms, such an atom is the central atom of covalent molecule. The spatial configuration of the valence electron orbits of the central atom usually determines the spatial structure of the molecule. For example, the electronic orbital spatial configuration of the carbon atom valence layer in methane molecules is V
4-6, which is a tetrahedral spatial structure composed of four ellipsoidal single electron orbital dynamic entities. Therefore, the spatial configuration of methane molecules is tetrahedral. The electronic configuration of the valence layer of sulfur atoms in SF
6 molecules is V
6-12, which is a regular octahedral structure composed of six single electron orbits. Therefore, the spatial structure of SF
6 molecules is a regular octahedron. The valence layer electronic configuration of boron atoms in BF
3 molecule is V
3-4, which is a equilateral triangle structure composed of three ellipsoidal single electron orbital dynamic entities. Therefore, the spatial structure of BF
3 molecule is a planar equilateral triangle. The valence layer electron configuration of phosphorus atom in PCl
5 molecule is V
5-9, which is a triangular bipyramid structure composed of five single electron orbits. Therefore, the spatial structure of PCl
5 molecule is a triangular bipyramid (
Figure 8).
The valence layer electron configuration of nitrogen atoms in ammonia molecules (NH
3) is V
5-6, which is a tetrahedral structure composed of three single electron orbitals and one double electron orbital. Due to the fact that the double electron orbitals do not participate in the formation of covalent bonds, the spatial structure of ammonia molecules is not a regular tetrahedron but a triangular pyramid Due to the higher charge of the double electron orbital dynamic entity compared to the single electron orbital, it forms a stronger electrostatic repulsion squeezing effect on the other three single electron orbitals. Therefore, the H-N-H bond angle is not 109.47 °, but 107 °. Similarly, the valence layer electronic configuration V
5-6 of phosphorus atoms in phosphine molecules (PH
3) is a tetrahedral spatial structure composed of three ellipsoidal single electron orbitals and one ellipsoidal single electron orbital dynamic entity. Due to the absence of double electron orbitals in the formation of covalent bonds, the spatial structure of PH
3 molecule is not a regular tetrahedron but a triangular pyramid, and the H-P-H bond angle is not 109.47 °, but 93.5 °. The valence layer electron configuration of oxygen atoms in water molecules (H
2O) is V
6-5, which is a tetrahedral structure composed of two single electron orbitals and two double electron orbitals. Due to the fact that two double electron orbitals do not participate in the formation of covalent bonds, only two single electron orbitals form covalent bonds, the spatial structure of water molecules is a triangular structure. Due to the electrostatic squeezing effect of the two electron orbital dynamic entity, the H-O-H bond angle of water molecules is not 109.47 °, but 104.5 ° (
Figure 8).
If an atom has multiple single electron orbits in its valence electron configuration, it may form 2 or 3 covalent bonds when it approaches another atom that also has multiple single electron orbits. For example, the electronic configuration of the valence layer of an excited oxygen atom is V
6-3, with 2 double electron orbits and 2 single electron orbits. Two oxygen atoms form two
α bonds by spin pairing with an ellipsoidal single electron orbit and a spherical single electron orbit, respectively, and combine to form an oxygen molecule (O
2) (
Figure 8). Due to the exposure of spherical single electron orbits in the outer layer of the molecule, oxygen molecules are susceptible to the influence of external magnetic fields, thus exhibiting paramagnetism. In addition, due to the large distance between the spherical single electron orbits of two oxygen atoms, the formed
α bond is weak and easy to break, thus oxygen molecules have strong chemical activity. When two
α bonds are formed between atoms due to the simultaneous existence of ellipsoidal and spherical single electron orbits, we call the
α bond formed between ellipsoidal single electron orbits "type I
α bond" and the
α bond formed between spherical single electron orbits "type II
α bond".
If there are multiple ellipsoidal single electron orbits in the valence layer electronic configuration of an atom, due to the presence of certain angles in their spatial distribution, the 2 or 3 covalent bonds formed between such atoms must be
β bonds. For example, the valence layer electron configuration of an excited nitrogen N atom is V
5-6, with one ellipsoidal double electron orbits and three ellipsoidal single electron orbits. Two nitrogen atoms spin pair with three ellipsoidal single electron orbits to form three
β bonds, forming a nitrogen molecule (N
2). Nitric oxide molecule (NO) forms two
β bonds between the two ellipsoidal single electron orbits of N atom and the two ellipsoidal single electron orbits of O atom. Due to the presence of an unpaired single electron orbital in the N atom, NO molecules exhibit paramagnetism. The valence layer electronic configuration of carbon atoms in carbon monoxide (CO) molecules is V
4-4, and the valence layer electronic configuration of oxygen atoms is V
6-4. The two ellipsoidal single electron orbits of C atoms and the two ellipsoidal single electron orbits of O atoms form two
β bonds. There are no unpaired single electron orbits in CO molecules, therefore it has diamagnetism. In carbon dioxide molecules (CO
2), the valence layer electron configuration of carbon atoms is V
4-6, with 4 single electron orbits. One carbon atom can form 4
β bonds with 2 oxygen atoms, and the O-C-O bond angle is 180°. The spatial structure is linear. The valence layer electronic configuration of sulfur atoms in sulfur trioxide molecules (SO
3) is V
6-14, with six single electron orbits uniformly distributed in the same plane at an angle of 60°. Therefore, one sulfur atom can form six
β bonds with three oxygen atoms, with an O-S-O bond angle of 120°. The spatial structure of SO
3 molecules is a planar triangle (
Figure 8).
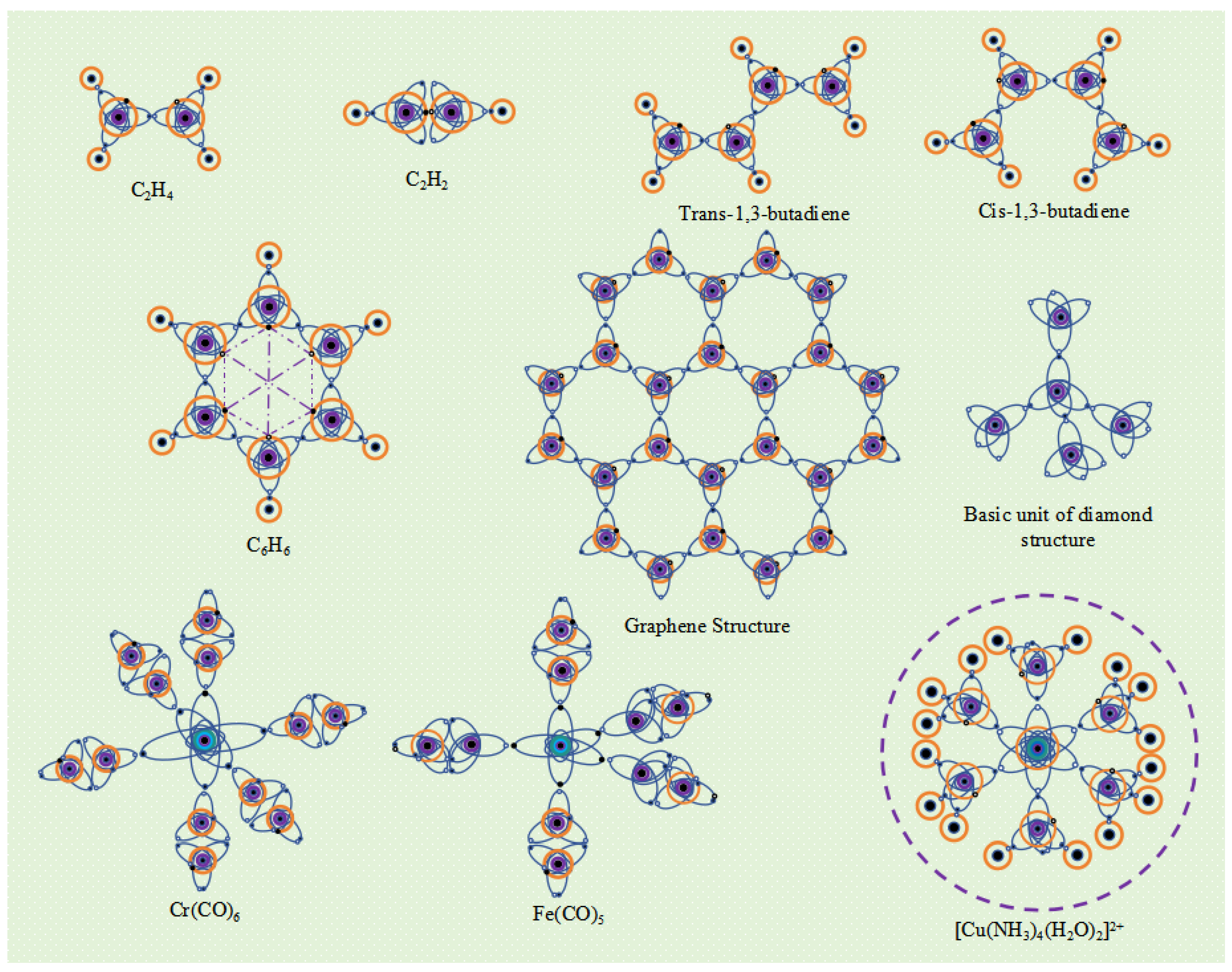
The two carbon atoms of ethylene molecule (C
2H
4) have a V
4-5 valence layer electronic configuration. The two ellipsoidal single electron orbits in the valence layer of each carbon atom spin pair with the single electron orbits of hydrogen atoms to form two "C-H" type I
α bonds. Two carbon atoms form a "C-C" type I
α bond with ellipsoidal single electron orbit spin pairing, and a "C-C" type II
α bond with spherical single electron orbit spin pairing. Type II
α bonds are weaker than type I
α bonds and are more prone to breakage, making ethylene molecules more reactive. The two carbon atoms of acetylene molecule are also in the V
4-5 valence layer electronic configuration, forming two "C-C"
β bonds and one "C-C" α bond between the two carbon atoms. Due to the presence of more
β bonds in the covalent bonds of acetylene molecules, they are more reactive than ethylene molecules. The carbon atoms in butadiene (including trans and cis 1,3-butadiene) are also in the V
4-5 valence layer electronic configuration. In addition to forming an I type
α bond, adjacent carbon atoms also form a "C-C" II type
α bond. The four carbon atoms in the butadiene molecule are tightly bound together by three type I
α bonds and three type II
α bonds, further reducing the energy of the molecular system. Therefore, the butadiene molecule has high stability. The valence layer electronic configuration of the six carbon atoms in benzene molecule (C
6H
6) is V
4-5, with each carbon atom having a "C-C" type I
α bond and a "C-C" type II
α bond with adjacent carbon atoms. In addition, the spherical single electron orbits between carbon atoms in opposite positions on the benzene ring have opposite electron spin directions, resulting in spin magnetism and the formation of "C-C" type II
α bonds. In this way, a strong bond is formed between the six carbon atoms of the benzene ring molecule, and the potential energy of the molecular system is greatly reduced, making the benzene ring molecule very stable (
Figure 9).
The electronic configuration of the valence layer of an atom reflects its energy state. Different energy states determine the different valence electron configurations of atoms, and when covalent compound molecules are formed, they form different spatial structures. Therefore, the spatial structure of a molecule is essentially determined by the energy state of the atoms at the time of its formation. For example, in excited states with lower energy levels, the valence electron configuration of carbon atoms is V
4-5, and adjacent carbon atoms can form type I
α bonds and type II
α bonds. Six carbon atoms can form a stable hexagonal structure like a benzene ring. By using this as a unit, a planar network structure composed of regular hexagonal structures can be formed, which is the basic structure of graphene molecules. Graphene with a planar network structure is stacked layer by layer, and the spherical single electron orbits of carbon atoms between layers exhibit spin magnetic interactions, forming sheet-like graphite. If the edges of such a planar network structure are raised and connected end-to-end, fullerene molecular structures can be formed, which can be spherical, ellipsoidal, cylindrical, or tubular in shape. At higher energy levels of excitation, the valence electron configuration of carbon atoms changes to V
4-6, with each carbon atom serving as the central atom and forming four Type I
α bonds with four carbon atoms. The four carbon atoms form a three-dimensional network structure at the vertices of a regular tetrahedron, which is known as the diamond molecular structure (
Figure 9). Due to the strong covalent bonds formed between each carbon atom and four carbon atoms, releasing a large amount of energy, the spatial potential energy of the entire molecular system is extremely low, making diamond molecules very stable. Elemental allotropes like graphene and diamond are giant covalent molecules with different structures formed under excited states of different energy states.
Transition metal elements are less active than alkali and alkaline-earth metal elements, and electrons outside the atomic nucleus are less likely to become free electrons. Therefore, if a transition metal element has single electron orbits in its valence electron configuration, these single electron orbits can form covalent bonds with the single electron orbits of other atoms. For example, the outermost layer of Cr atom has 6 electrons, and the excited valence layer electron configuration is V
6-12, which is a regular octahedral structure. When the CO molecule is in an excited state, the valence layer electronic configuration of carbon atoms changes to V
4-5, and one single electron orbital of the Cr atom forms a covalent bond with one single electron orbital of the carbon atom. Each of the six single electron orbits of the Cr atom can bond with a CO molecule, forming the Cr(CO)
6 molecule (
Figure 9).
The metal element Fe has 8 electrons in its outermost shell. In an excited state, 3 electrons become free and the resulting ion is called Fe
3+, leaving 5 electrons in the outermost shell, which can form a V
5-9 electron configuration. When Fe atoms are mixed with CO molecules, 3 CO molecules receive 3 electrons from the Fe atom to become 3 CO
- ions. These CO
- ions then combine with Fe
3+ ions under electrostatic attraction, releasing energy that further excites the O and C atoms within the CO
- ions, changing their valence electron configurations to V
7-6 and V
4-6, respectively, and forming three
β-bonds between the O and C atoms. Each C atom also has an elliptical single-electron orbital that forms 3 α-bonds with the three coplanar elliptical single-electron orbits in the V
5-9 electron configuration of the Fe atom. For CO molecules that have not acquired electrons, the bonding between the C and O atoms remains unchanged, but in the excited state, the valence electron configuration of the C atom changes to V
4-5, gaining an additional elliptical single-electron orbital. Two such excited CO molecules form two α-bonds with the two single-electron orbits in the V
5-9 electron configuration of the Fe atom that are perpendicular to the planar triangle. In this way, five CO molecules combine with one Fe atom to form a Fe(CO)
5 molecule (
Figure 9). The Fe(CO)
5 molecule contains both ionic bonds between the Fe
3+ ion and the three CO
- ions, and five covalent bonds between the Fe atom and C atom, making it a special compound that possesses both ionic and covalent bonds.
The electron arrangements of Group VIII elements, Group IB elements, lanthanide and actinide elements have special characteristics, and the arrangement of their inner and outermost electrons usually needs to be adjusted according to the energy state of the atomic system. When the number of outermost electrons changes, such as losing n electrons, the configuration of the valence layer electrons needs to be adjusted compared to the atoms with the previous n atomic numbers. For example, when the Cu atom of element 29 loses two electrons to become Cu
2+, its number of electrons outside the nucleus is the same as that of element 27 Co, and the valence layer electrons are both 7. In the excited state, Cu
2+ can form the V
7-13 electronic configuration, while the N atom in NH
3 and the O atom in H
2O form the V
5-8 and V
6-7 electronic configurations, respectively. Four excited NH
3 molecules (with N atom having an ellipsoidal single electron orbital) and two excited water molecules (with O atom having an ellipsoidal single electron orbital) form six covalent bonds with excited Cu
2+ (with six ellipsoidal single electron orbits), forming stable [Cu (NH3) 4 (H2O) 2]
2+ complex ions (
Figure 9).
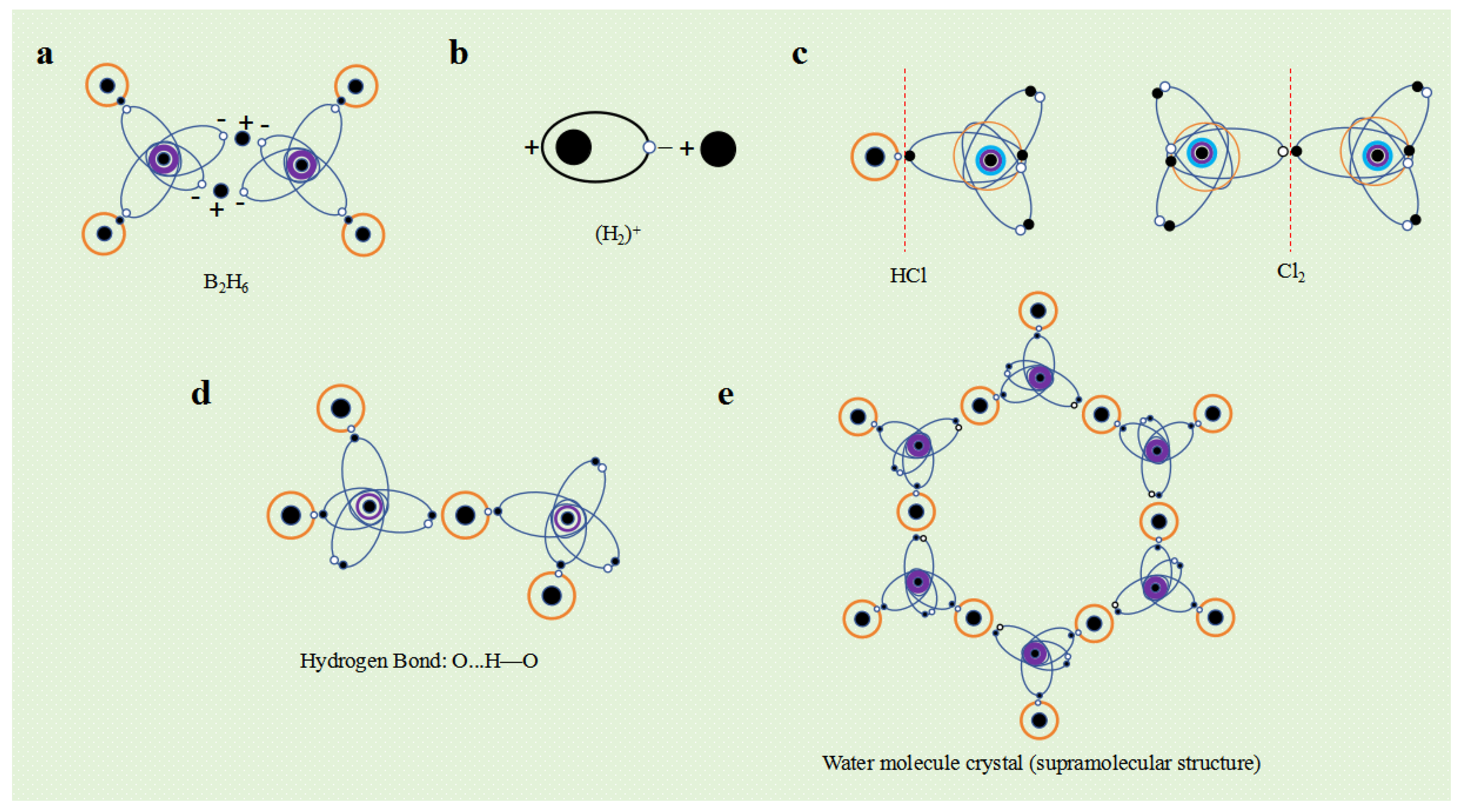
Some non-metallic elements also undergo changes in their valence layer electronic configuration after obtaining electrons. For example, when excited, the B atom forms a V
3-4 electronic configuration and can form a BH
3 covalent molecule with three hydrogen atoms. But under certain conditions, after B atom captures an electron from one of the hydrogen atoms, its valence layer electron configuration changes to V
4-6, forming two ellipsoidal single electron orbits. BH
3 becomes (BH
2)
- ion, and one hydrogen atom becomes a hydrogen ion (H
+). The (BH
2)
- ion as a whole is negatively charged, and its ellipsoidal single electron orbit exhibits a negative charge property. Therefore, two (BH
2)
- ions can tightly bind with the electrostatic attraction between two hydrogen ions H
+ to form diborane molecules (B
2H
6) (
Figure 10a). This type of compound is called a hydrogen bridge compound, which combines two negative ions with single electron orbits through electrostatic attraction using hydrogen ions (protons).
3.2.3. Polarity of Molecules and Intermolecular Forces
The atoms that make up a molecule form different spatial structures due to their different valence electron configurations. If the electronic configuration space structure of the entire molecule is uniformly symmetrical, then the charge distribution of the entire molecular system is uniform, and the molecule exhibits non-polar characteristics, which we call non-polar molecules. If the electronic configuration space structure of a molecule is asymmetric, non-uniform, or symmetric but non-uniform, then the charge distribution of the entire molecular system is non-uniform, and the molecule exhibits polar characteristics, which we call polar molecules. In polar molecules, they exhibit negative charge properties in directions with more charge distribution and positive charge properties in directions with less charge distribution.
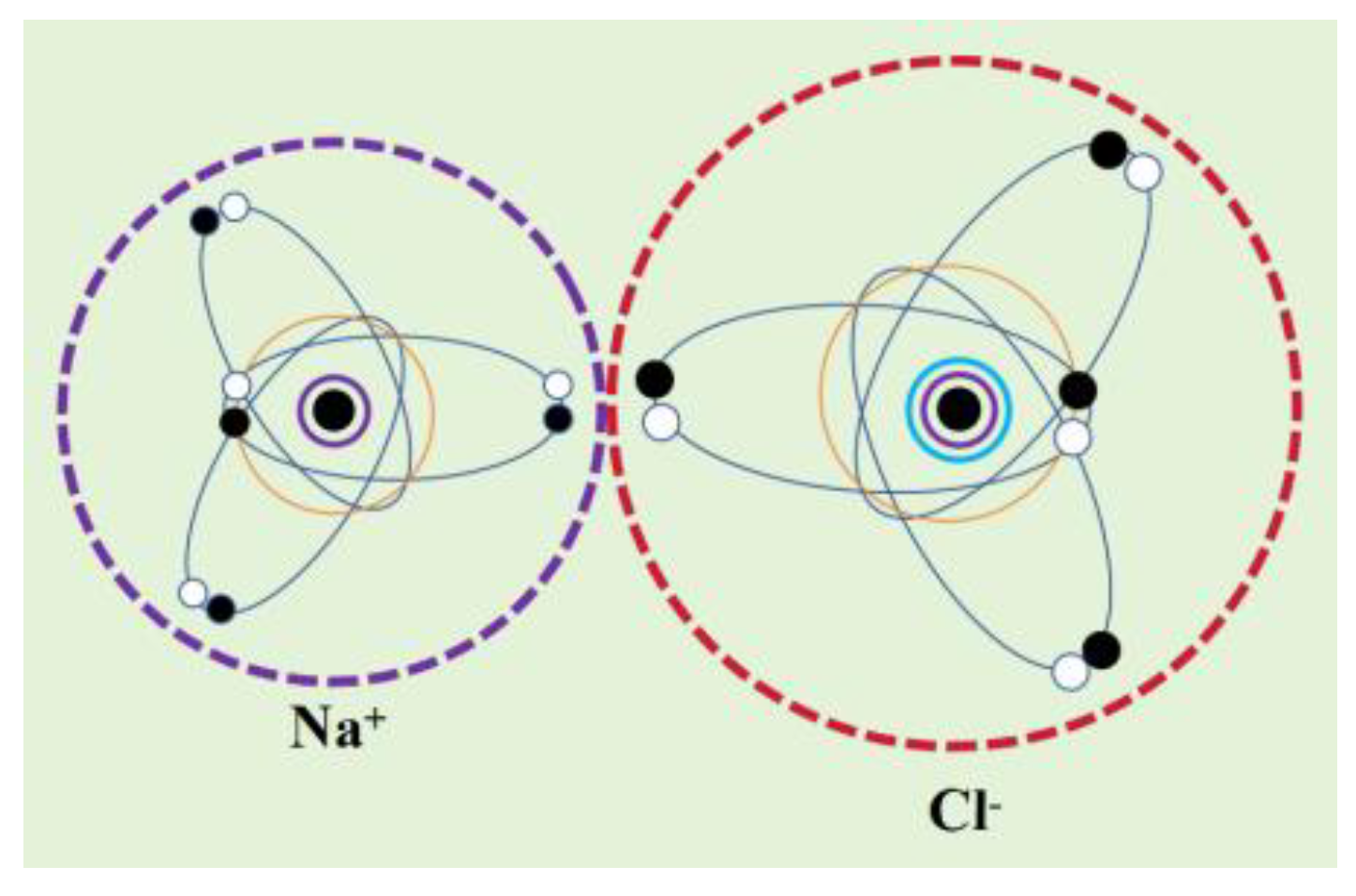
Taking hydrogen chloride (HCl) molecules as an example, chlorine atoms have 7 valence electrons, while hydrogen atoms only have 1 valence electron. The distribution of "electron orbital dynamic entities" in the molecular system is significantly uneven, and the charge distribution is significantly more in the direction of chlorine atoms than in the direction of hydrogen atoms. Therefore, HCl molecules are polar molecules. The chlorine molecule (Cl
2) has two identical chlorine atoms, and due to the completely uniform and symmetrical spatial distribution of the valence layer "electron orbital dynamic entity" of the two chlorine atoms, the charge distribution is uniform and exhibits non polarity (
Figure 10c).
The simplest atom is a hydrogen atom, where an electron orbits a proton in a circular or elliptical motion. Observing over a period of time, a hydrogen atom is a dynamic entity with a spherical or ellipsoidal electron orbit surrounding a proton. However, in the case of instantaneous observation, hydrogen atoms can be regarded as an electric dipole composed of protons and electrons at any moment. In the direction close to the electron, hydrogen atoms have a negative charge property, while in the direction close to the proton, hydrogen atoms have a positive charge property. Therefore, hydrogen atoms exhibit polarity under instantaneous observation. The hydrogen molecular ion (H
2)
+ is actually the product of the instantaneous electrostatic attraction between hydrogen atoms and hydrogen ions (H
+) in a polar state (
Figure 10b).
Polarized molecules (HX) containing hydrogen atoms are usually positively charged in the direction of the hydrogen atom, and correspondingly negatively charged in the direction of other atoms. The electrostatic attraction between polar molecules (HX ··· HX) occurs between the hydrogen atom and other atoms, which is called hydrogen bonding. Since hydrogen atoms have only one electron outside the nucleus, the molecular charge distribution formed by their combination with other atoms is the most uneven, resulting in a higher polarity. Hydrogen bonds have stronger electrostatic attraction than other polar molecules.
Figure 10d illustrates the hydrogen bonds between water molecules.
Since the overall effect of dynamic entities is not only related to the periodic motion speed but also to the observation distance, the overall effect of electronic orbital dynamic entities becomes more pronounced when the distance between molecules is far, and less pronounced when the distance between molecules is close. When the distance between molecules is very close, the instantaneous relationship between electrons and atomic nuclei exhibits an electric dipole mode, and the instantaneous interaction between molecules is manifested as the interaction between dipole moments. Due to the inherent polarity of polar molecules, the dynamic electronic orbits of non-polar molecules are attracted to the positive charge side of polar molecules and tilt, resulting in induced dipole moments. The so-called dispersion force, induction force, and orientation force are all short-range forces, manifested as attractive forces between molecules at close distances, with a range of only a few picometers. These intermolecular forces are collectively referred to as van der Waals forces, which are essentially electrostatic forces generated by molecular or atomic polarity.
Just like metal elements and ionic compounds can form crystal structures, there is electrostatic attraction between molecules, which can also form molecular crystal structures. Due to the spatial structure of molecules and the specific valence electron configurations of the atoms that make up the molecules, the electrostatic attraction between molecules exhibits significant directionality and saturation. The polarity or instantaneous polarity between molecules generates electrostatic attraction, causing molecules to condense and form different molecular crystals or supramolecular structures. For example, water molecules interact with each other through hydrogen bonds to form water molecule crystals (
Figure 10e).
When the energy state of the molecular system is low, the volume of the "dynamic entity of electron orbits" outside the ground state atomic nucleus is small, manifested as the small volume of atoms and molecules, which can tightly bind and condense into a solid "molecular cluster". When the energy state is high, the volume of atoms and molecules is relatively large, and the binding between atoms and molecules is not very tight, resulting in a condensed "molecular cluster" that appears as liquid. When the energy state is higher, the volume of atoms and molecules becomes larger, the bonding between atoms and molecules becomes less tight, the cohesion between molecules becomes weak, and the molecular aggregates appear in a gaseous state. The change of a substance composed of molecules from solid to liquid and gas is a manifestation of the energy state change of the molecular system, which is usually referred to as physical change. The change in state of matter usually refers to the change in the composition of substances composed of the same molecular aggregates. If molecules of different substances are mixed together, chemical changes may occur simultaneously with the change in the state of matter.
3.2.4. The Physical Mechanism of Chemical Reactions
The essence of a chemical reaction is the formation of new ionic or covalent bonds between different atoms that make up a substance molecule, the formation of new substance molecules from different atoms, or the formation of different molecular structures from molecules composed of the same atoms. At normal temperature and pressure, the chemical reaction in which different types of substance molecules mix to form new substance molecules is called spontaneous chemical reaction. A chemical reaction that cannot occur spontaneously at room temperature and pressure and requires conditions such as heating, pressure, or light exposure is called a non spontaneous chemical reaction.
Mixing different types of liquid molecules, or mixing between liquids and solids, or between liquids and gases, is prone to spontaneous chemical reactions. Usually, acid-base reactions, ion exchange reactions, and so-called coordination reactions are spontaneous chemical reactions. In acid-base reactions, such as mixing hydrochloric acid and sodium hydroxide solutions, hydrogen ions (H+) , chloride ions, sodium ions, and hydroxide ions (OH-) mix together, and positive and negative ions recombine under the interaction of electrostatic attraction. H+and OH- attract each other under electrostatic attraction. The excess electron outside the oxygen nucleus in the OH- is captured by H+, and the valence layer electron of the oxygen atom is lost, forming an elliptical shape and becoming a single electron orbit. The H+ obtains an electron and becomes a hydrogen atom with a single electron orbit. The single electron orbits outside the hydrogen nucleus can form covalent bonds with the single electron orbits of oxygen atoms in OH- , generating H2O molecules.
Sodium chloride solution is a homogeneous mixture of sodium ions and chloride ions (Cl-), while silver nitrate solution is a homogeneous mixture of nitrate ions and silver ions (Ag+). When sodium chloride solution and silver nitrate solution are mixed, these four ions will also be evenly distributed, which means that recombination will occur between these ions. Cl- combines with Ag+ under electrostatic attraction to form AgCl, which precipitates due to its insolubility in water.
Sodium carbonate dissolves in water and dissociates into carbonate ions (CO32-) and sodium ions. If hydrochloric acid is added to a sodium carbonate solution, a large amount of hydrogen ions (H+) are dissociated from hydrochloric acid in water. H+ combines with CO32- under electrostatic attraction, and H+ obtains an electron from CO32- to form a hydrogen atom. Two hydrogen atom single electron orbits form two covalent bonds with two ellipsoidal single electron orbits of oxygen atoms, generating H2CO3. At room temperature and pressure, the H2CO3 structure is unstable and spontaneously forms CO2 and H2O.
Copper sulfate solution contains Cu2+ and SO42-. When excessive ammonia water is added, due to the strong polarity of NH3 molecules, H2O molecules can dissociate into H+ and OH- in aqueous solution. Cu2+and OH- combine through electrostatic attraction to form Cu (OH)2 precipitate. The released energy will excite some atoms, transforming double electron orbits into single electron orbits and changing the valence layer electron configuration. For example, the valence layer electron configuration of Cu2+ is excited to V7-13 electron configuration, the valence layer electron configuration of N atoms in NH3 is excited to V5-8, and the valence layer electron configuration of O atoms in water molecules is excited to V6-7. Therefore, Cu2+ can form 6 covalent bonds with 4 NH3 and 2 H2O, generating stable [Cu (NH3)4(H2O)2]2+ ions that combine with SO42- to form [Cu(NH3)4(H2O)2]SO4.
These spontaneous chemical reactions have one thing in common, which is that after the reactants are mixed, there is electrostatic interaction between ions, and different ions recombine to form a new molecular system. The potential energy decreases, releasing energy and forming more stable ionic compounds, or exciting the valence layer electronic configuration change of atoms in ions or polar molecules (from double electron orbits to single electron orbits), and then forming stable covalent compounds.
If different substance molecules cannot be combined by electrostatic attraction after mixing, and the atoms that make up the molecules cannot form new bonds by spin magnetic force, then spontaneous chemical reactions will not occur. Usually, additional energy needs to be provided, such as heating, pressure, or light, in order for chemical reactions to occur. These additional energies increase the potential energy of molecules and atomic systems, breaking existing chemical bonds or transforming double electron orbits into single electron orbits, changing the electronic configuration of the atomic valence layer, thus allowing new bonds to form between atoms and form new compounds.
For example, in the valence layer electronic configuration of a nitrogen atom in nitrogen gas, three single electron orbits form three covalent bonds with three single electron orbits of another nitrogen atom. The two hydrogen atoms in a hydrogen molecule also form covalent bonds with two single electron orbits (
Figure 8). Due to the lack of ionic interactions and single electron orbits between covalent molecules nitrogen and hydrogen, spontaneous chemical reactions do not occur at room temperature and pressure. Under high temperature and pressure, nitrogen and hydrogen molecules enter a high-energy state where the covalent bonds within the molecules are broken. Subsequently, the single-electron orbits of nitrogen atoms can form covalent bonds with the single-electron orbits of hydrogen atoms, leading to the production of ammonia.