1. Introduction
Colorectal cancer (CRC) is a major global health issue, since CRC represents the third most common cancer and the second leading cause of cancer mortality worldwide [
1,
2]. Colorectal tumor progression and metastasis are significantly promoted by neoangiogenesis, which consists in the growth of new blood vessels [
3]. Several anti-angiogenic drugs have been developed and a large number of randomised clinical trials showed clinical benefit from the employment of this class of antitumoral agents in patients with metastatic colorectal cancer [
4,
5,
6,
7]. This led to the approval of various antiangiogenics for CRC treatment [
8]. As a result, anti-angiogenic drugs have become a cornerstone in therapeutic algorithms of metastatic CRC [
9]. Despite advances in anti-angiogenic therapies, several challenges still need to be addressed—including drug resistance development and limited efficacy in subgroups of patients [
10]. Compensatory mechanisms often drive resistance to vascular endothelial growth factor (VEGF) inhibition [
11]. Therefore, novel combinations and antiangiogenic drugs are needed [
12]. Additionally, more effective predictive biomarkers for antiangiogenic therapies are considered necessary and should be developed to improve patient selection and maximize clinical benefit [
13].
In such a context, several cellular subtypes involved in endothelial homeostasis, such as circulating endothelial cells (CEC) and circulating endothelial progenitor cells (CEPC) have been investigated as potential biomarker [
14,
15]. CEC are mature endothelial cells that enter into the bloodstream after detaching from vessel walls because of vascular damage or physiological turnover [
16,
17,
18] Blood levels of CEC can be hypothetically modulated by vascular remodelling, thus supporting their role as potential circulating reporters of cancer neoangiogenesis and putative biomarkers for antiangiogenic treatment [
19]. Conversely, CEPC are mobilized from the bone marrow and contribute to vascular repair by differentiating into endothelial cells—thus promoting angiogenesis [
20,
21]. Several reports highlighted correlations between high blood levels of CEPC and more advanced disease in patients with solid tumors have been described [
21,
22]. In addition, CEPCs are able to cross the blood-brain barrier, thus contributing to tumor vascularization and progression [
23]. Of note, higher levels of CEPC have been correlated with negative clinical outcomes including poor treatment response and worse cancer-related overall survival both in solid tumors and hematological malignancies [
24,
25,
26,
27]. CEPCs can also represent targets for anticancer treatments. In this regard, FTY720—an immunomodulatory drug—reduced CEPC levels and suppressed liver tumor metastasis in a rat model, thus suggesting the potential of this drug in preventing tumor recurrence [
28]. Some other agents like phloroglucinol have been shown to block tumor angiogenesis by specifically inhibiting CEPC bioactivities [
29].
Unfortunately, the identification and quantification of blood CEC and CEPC is challenging. In particular, there is a phenotypic overlap among CEC, CEPC and other cell types hampering the standardization of methods for their detection [
30,
31]. Flow cytometry is commonly employed to identify CEC and CEPC populations by using specific surface markers.
Therefore, here we undertook the task of studying different circulating endothelial subtypes in patients with metastatic colorectal cancer (mCRC) by applying an optimized flow cytometry method based on the use of a large panel of endothelial and progenitor markers. We further used computational flow cytometry methods to automatically identify new putative circulating endothelial subsets related with mCRC outcomes.
2. Materials and Methods
2.1. Patients
This prospective observational study enrolled adult patients with a histologically or cytologically confirmed diagnosis of stage IV colorectal cancer, candidates for antitumoral systemic treatment. Patients were recruited from the Clinical Oncology Unit of the SS Annunziata Hospital in Chieti (Italy) from January 2017 to August 2022. All procedures involving human participants were carried out in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or with comparable ethical standards. This study was approved by the local ethics committee on 25 February 2016. All patients gave a written informed consent.
2.2. Peripheral Blood Collection
For each patient, peripheral blood (5 mL) was collected at the baseline by using ethylenediamine tetraacetic acid (EDTA) tubes (BD Biosciences, San Jose, CA, USA, cat. 368861). Peripheral blood samples were processed within 8 h from venipuncture.
2.3. Flow Cytometry Assay for the Identification and Count of Circulating Endothelial Cells, Circulating Progenitor Endothelial Cells and Their Subsets.
The identification and count of each detected population was carried out according to a previously published flow cytometry protocol, already optimised and standardized by a network of six laboratories [
16,
17,
18]. The panel of markers used for multiparametric flow cytometry analysis is detailed in
Supplementary Table S1.
2.3.1. Blood Processing and Cell Staining
Peripheral blood samples were processed by a common flow cytometry lyse and wash method [
16,
17,
18]. Briefly, for each sample, 5 mL of peripheral blood, harvested in EDTA tubes, as above specified (paragraph 2.2), underwent an erythrocyte-lysis step, being treated with 45 mL of Pharm Lyse solution (BD Biosciences), for 15 min at room temperature, under gentle agitation. Samples were then centrifuged at 400g for 10 min at room temperature and washed by adding 2 mL of PBS. The pellet was resuspended with 100 µL of 1X binding buffer (BD Biosciences) and the surface staining was carried out by adding the mixture of reagents summarised in
Supplementary Table S1. Samples were then incubated for 30 min at 4°C and washed with 2 mL of 1X binding buffer. Before the acquisition, samples were re-suspended in 1.5 mL of 1X binding buffer (BD Biosciences) and filtered using 70 μm filters. Finally, 10×10
6 events
per sample were acquired by flow cytometry (BD FACSCanto II, BD Biosciences).
2.3.2. Flow Cytometry Computational Analysis
Unsupervised computational analysis of flow cytometry data was carried out by applying t-distributed Stochastic Neighbor Embedding (t-SNE) and FlowSom algorithms. To this purpose, plugins of the FlowJo software (BD Bioscience) v 10.10.0 were employed. Flow cytometry data—derived from patient samples within the same study group—were merged into a single file. T-SNE was run on concatenated data with a perplexity parameter of 30 and 1000 iterations. The FlowJo plugin FlowSOM (v.4.1.0) was applied on concatenated data by setting a metacluster number of 6 and a SOM grid size of 10x10. Compensated parameters were used for both t-SNE and FlowSom calculations.
2.3.3. Identification and Enumeration of Cell Subsets by Manual Gating
Data were analysed using FACSDiva v 6.1.3 (BD Biosciences), and FlowJo v 10.10.0 (BD Bioscience) lying a dual platform counting method using the lymphocyte subset as the reference population and applying the following formula [
16,
17,
18].
Abs Population of Interest/ml= (Population of Interest Abs Count*(Lymphocyte Count)/ml)/(# Lymphocyte count ) where Abs: absolute; Abs Population of Interest/ml: Concentration.
The possibility to parallel the results all along the whole study was ensured by the daily Cytometer Setup and Tracking (CS&T) Beads, used to generate both initially the instrument setting target values and further to ensure the proper performance of the instrument.
2.5. Statistical Analysis
Statistical analysis was conducted using SPSS v25.0 (IBM SPSS, Chicago, IL, USA) and Graphpad Prism 9 (GraphPad Software Inc.; San Diego, CA, USA). Normality of the data was assessed using the Shapiro-Wilk test. Comparisons were made by applying the unpaired t-test for normally distributed data, whereas the Mann-Whitney and Kruskal-Wallis non-parametric tests were used for non-normally distributed data. Multiple comparisons were assessed using Dunn’s test. Correlations between blood levels of rare cell subpopulation and clinical-pathological variables was conducted by using Spearman’s rank correlation coefficients. A proportion of clinical variables including Estearn Cooperative Oncology Group (ECOG) performance status (PS), number and site of metastasis, diabetes, cardiovascular disease, arterial hypertension, body mass index (BMI), tumor grading, serum blood CEA concentration, tumor location and mutational status of K-RAS gene were collected retrospectively and included in the correlation analysis. Radiological response was evaluated according to RECIST criteria v1.1. The overall response rate (ORR), defined as the percentage of patients achieving complete response (CR) or partial response (PR), was calculated to discriminate between responders and non responders. ORRs were compared between patient groups by using Fisher's exact test. Receiving operative curves (ROC) of response vs. non-response were calculated to evaluate the predictive ability of selected cell subsets. The Youden Index was employed to calculate the optimal cut-off points using ROC curve data. Univariate and multivariate Cox proportional hazards models were applied to calculate Hazard Ratio (HRs) with 95% of confidence intervals (CIs). Internal validation was conducted with the SPSS biased-corrected and accelerated bootstrap method with 1000 bootstrap samples and a 95% confidence interval. The Kaplan-Meier (KM) curve estimator was applied to estimate median overall survival (mOS) and the log-rank test was employed to examine differences in mOS across patient groups. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Machine Learning Algorithms Reveal Specific Subsets of Cells with a CD34+/CD45-/dim Phenotype in mCRC Responders vs Non-Responders to Antitumoral Systemic Therapies
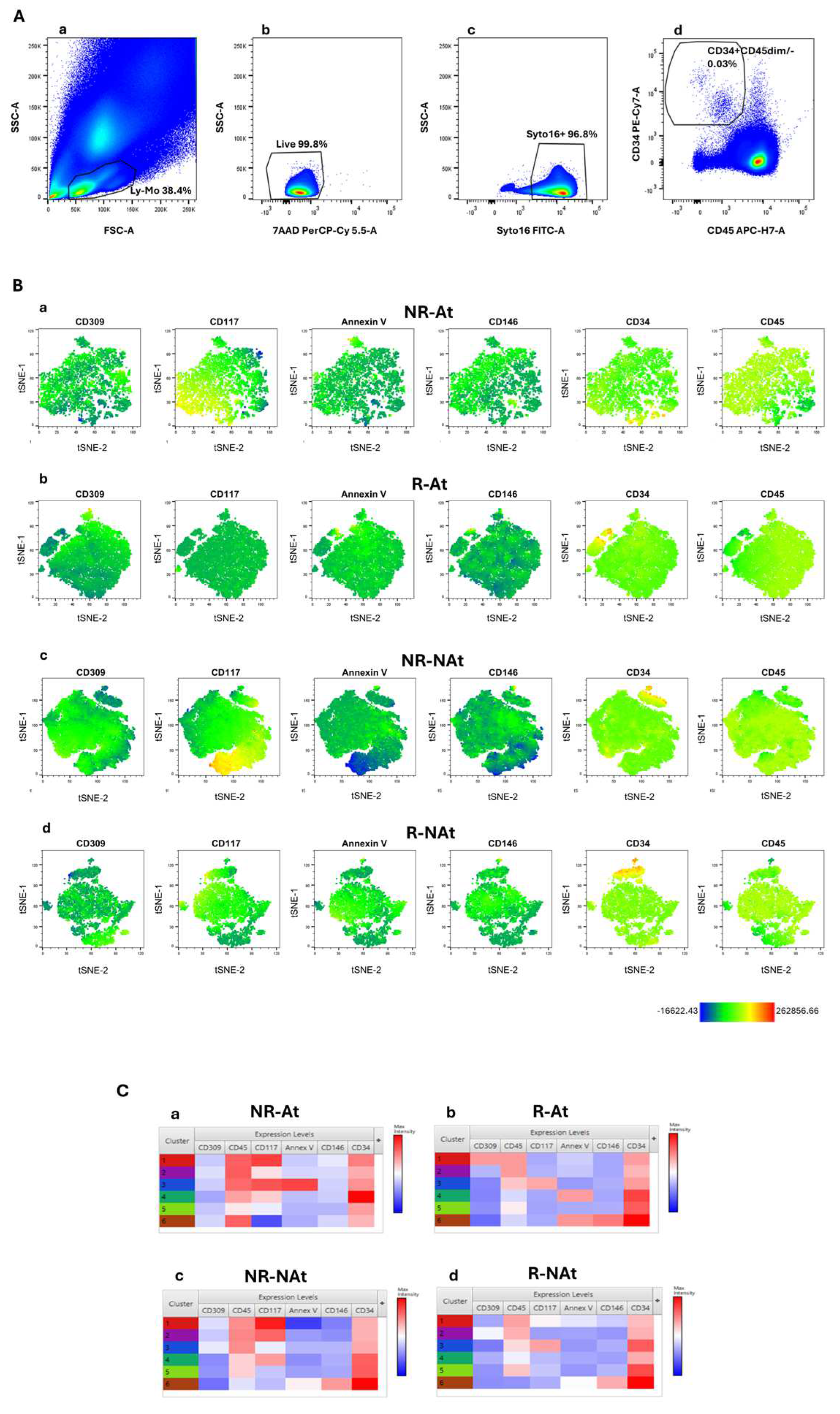
We carried out an exploratory computational analysis to explore flow cytometry data generated using a multiparametric panel, as described in the method section. This panel was applied to identify blood circulating endothelial cells, progenitor cells, and related subsets, as reported. In line with the power of analysis of this machine learning approach, this exploratory analysis was carried out in four groups of 3 mCRC patients—responders to antiangiogenic-based therapy (R-At), non-responders to antiangiogenic therapy (NR-At), responders to non-antiangiogenic therapy (R-NAt) and unresponsive patients to non-antiangiogenic therapy (NR-NAt). All patients were candidates to a first line antitumoral systemic treatment. Flow cytometry data from single patients were merged within each group before computational analysis. This approach aimed at automatically identifying clinically relevant cell clusters for further analyses, while avoiding noise and overfitting that could be generated in large heterogeneous cohorts. Endothelial and pro-angiogenic cell subsets lack the expression or express at dim levels the hematopoietic cell marker CD45 [
32,
33], while express CD34 [
34]. We applied t-SNE to analyze flow cytometry data from the whole CD34+CD45-/dim blood cell population, while reducing data dimensionality to visualize cell clusters. The gating strategy used to identify CD34+CD45-/dim cells was depicted in
Figure 1A. T-SNE was run with the following parameters: CD45, CD34, CD146, CD309, Annexin V, CD117. These markers were selected given that they have been associated with mature (CD146) or progenitor (CD309 and CD117) endothelial cell phenotypes [
18,
35,
36]. Results from t-SNE analysis were represented in
Figure 1. Globally, t-SNE plots showed a separation between the subset of cells with the CEC phenotype (CD34 bright expression) and cell clusters with lower CD34 surface expression. Of note, the analysis of t-SNE plots by single markers revealed that CD117-expressing cells were more represented in non-responders of both treatment groups, as compared to responders (
Figure 1B). We further analyzed flow cytometry data using FlowSOM to carry out hierarchical clustering and improve the identification of distinct cell subsets. FlowSOM was run with the same parameters selected for t-SNE analysis.
Figure 1C shows heatmaps depicting the phenotypic features of cell clusters derived by the application of the FlowSOM algorithm to flow cytometry data in each patient group. Notably, confirming t-SNE results, cell clusters with the CD34+/CD45dim phenotype and expressing CD117 were predominant in non-responders, as compared to responders. The subset characterized by the CD34+/CD45dim/CD117+ phenotype presented heterogeneity for phosphatidylserine surface expression (revealed by annexin V) in non-responders, whereas these cell subsets did not appear to co-express CD146 and CD309 (VEGFR-2) both in responders and non-responders. Additionally, a cluster of VEGFR-2-expressing CD34+/CD45dim cells was detectable in the group of responders to antiangiogenic agents (
Figure 1C). The subset of cells with high CD34 expression and negative to CD45–referred to as CEC phenotype–was equally represented across all patient groups. Overall this exploratory analysis with machine learning algorithms suggested that blood CD34+/CD45dim/CD117+ cells might be associated with tumor resistance to both antiangiogenic and non-antiangiogenic therapies. Of note, heterogeneity in phosphatidylserine expression was observed among cell clusters with a CD34+/CD45dim/CD117+ phenotype.
3.2. Blood Levels of Peripheral Blood Cells with a CD34+/CD45dim/CD117+/AnnV- Phenotype Are Correlated with Overall Response Rate to Antitumoral Systemic Therapies in Patients with mCRC
To validate the results obtained by exploratory flow cytometry computational analyses, we investigated the relationship between blood concentrations of cells with CD34+/CD45dim/CD117+ phenotype and response to antitumoral systemic therapy in the whole cohort of enrolled mCRC patients (n=40). Overall baseline demographic and clinical features of patients included in the study are summarized in
Supplementary Table S2.
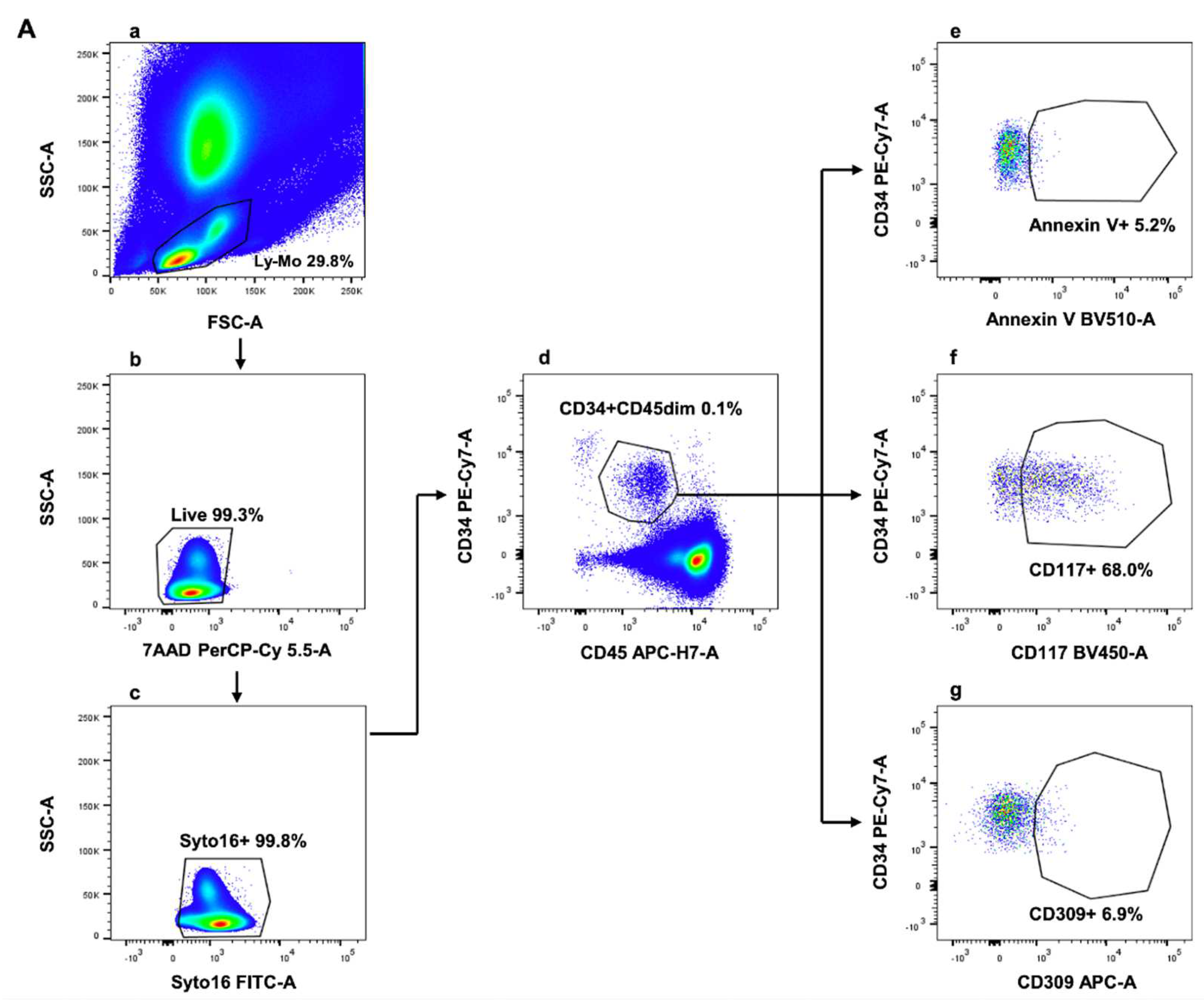
Blood CD34+/CD45dim/CD117+ cells were identified and enumerated by conventional polychromatic flow cytometry, as reported. In line with findings from hierarchical clustering analysis, Annexin V positive and negative events were also evaluated by manual gating. Results underlined that two distinct cell subsets—CD34+/CD45dim/CD117+/AnnV- and CD34+/CD45dim/CD117+/AnnV+—composed the CD34+/CD45dim/CD117+ circulating cell population. The used flow cytometry gating strategy was depicted in
Figure 2.
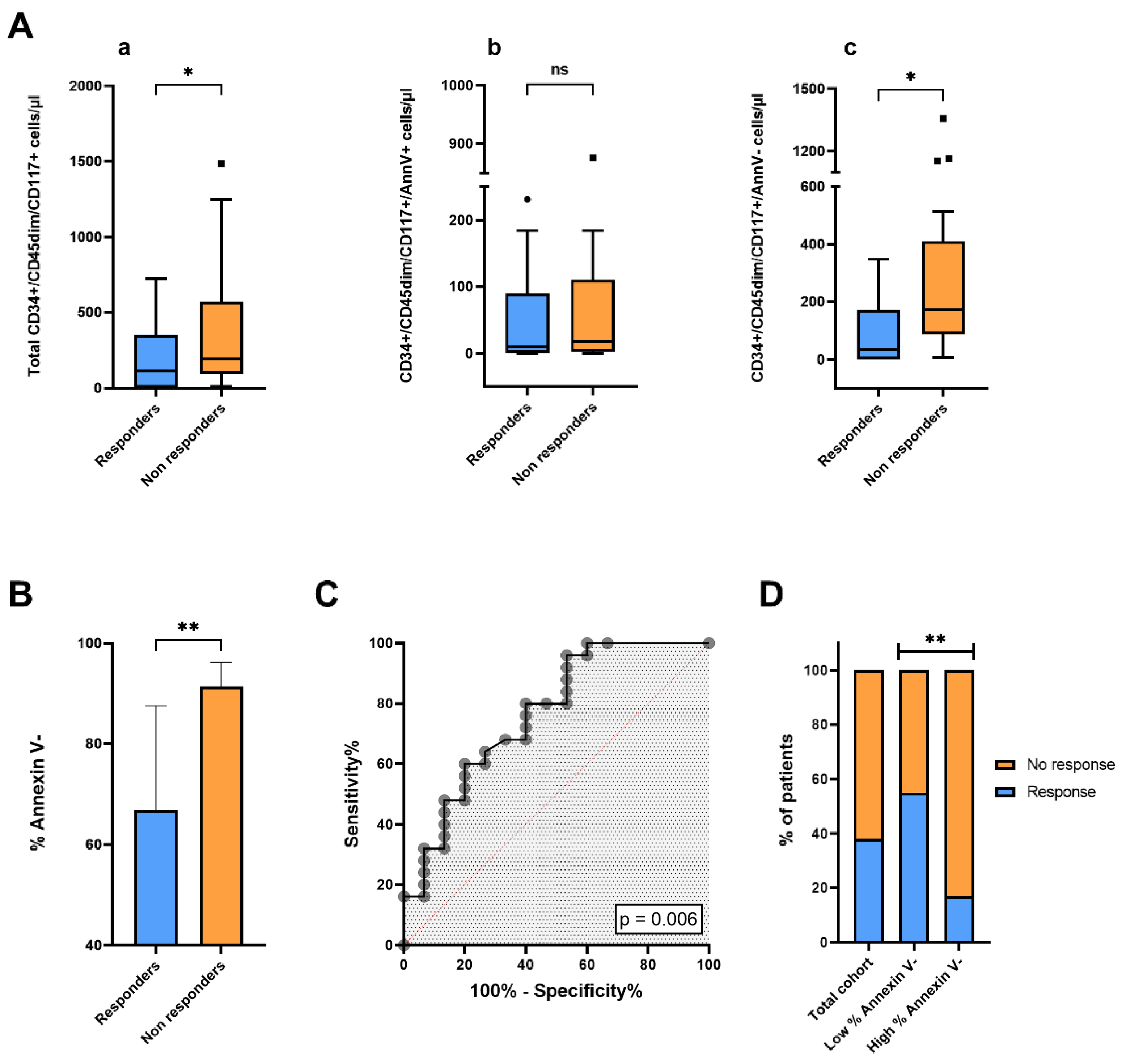
Patients were separated in two subgroups according to overall response rate (ORR). Partial or complete response after antitumoral systemic therapy was achieved in 15 of 40 patients (ORR=37.5%), while progressive or stable disease was observed in 25 of 40 patients. We compared blood concentrations of total CD34+/CD45dim/CD117+, CD34+/CD45dim/CD117+/AnnV+ and CD34+/CD45dim/CD117+/AnnV- cells at baseline between responders (n=15) and non-responders (n=25) (
Figure 3Aa-c). Blood levels of total CD34+/CD45dim/CD117+ cells were significantly lower in responders, as compared to non-responders (respectively, p = 0.03). Interestingly, the difference in blood concentrations of CD34+/CD45dim/CD117+ cells between responders and non-responders was mainly driven by the different concentrations of CD34+/CD45dim/CD117+/AnnV- cells between the two groups of patients (p = 0.01). Indeed, no significant difference in blood concentrations of CD34+/CD45dim/CD117+/AnnV+ cells was observed between responders and non-responders (p = 0.58). Accordingly, we observed that the median percentage of CD34+/CD45dim/CD117+ cells negative for Annexin V at baseline was significantly lower in patients with tumor response, as compared to those with stable or progressive disease (p = 0.005) (
Figure 3B). As shown in
Figure 3C, the receiving operator characteristic (ROC) curve analysis confirmed a correlation between treatment response and blood percentage of Annexin V- cells within the CD34+/CD45dim/CD117+ subset (AUC = 0.764 [CI 95% 0.607–0.921]; p = 0.006). By applying the Youden index to ROC curve data, we calculated the optimal cutoff to dichotomize the population in patients with high and low percentages of Annexin V- cells (cut off=90%); we further compared overall response rates between the two groups (
Figure 3D). Of note, patients in the group with higher percentages of Annexin V- cells presented a 4-fold lower ORR, as compared with patients with lower % of Annexin V- events within blood circulating CD34+/CD45dim/CD117+ cells (ORR% 16.7 vs 54.5; p = 0.005).
Furthermore, machine learning analysis suggested a potential predictive role of CD34+/CD45dim/CD309(VEGFR-2)+ cells in patients treated with antiangiogenic-based therapies. Therefore, we analysed by blood concentrations of this subset of blood-derived VEGFR2+-expressing cells at baseline in the subgroup of mCRC patients who received antiangiogenic agents (n=15). We did not observe, however, any difference in blood concentration of VEGFR-2+ cells between responders and non-responders to antiangiogenic drugs (p = 0.27) (
Supplementary Figure S1).
3.3. Blood-Circulating Concentration of CD34+/CD45dim/CD117+/Annexin V- Cells Correlates with the Number of Metastatic Sites
We evaluated the correlation between clinical–pathological factors and blood levels of circulating CD34+/CD45dim/CD117+, as well as CD34+/CD45dim/CD117+/AnnV- cells. The correlation analysis included the following clinical-pathological variables: sex, ECOG PS, age, number of metastatic sites, lung metastasis, liver metastasis, BMI, tumor grading, primary tumor location, serum blood CEA concentration, K-RAS mutational status, as well as number of previous lines of systemic therapies in the overall patient cohort (
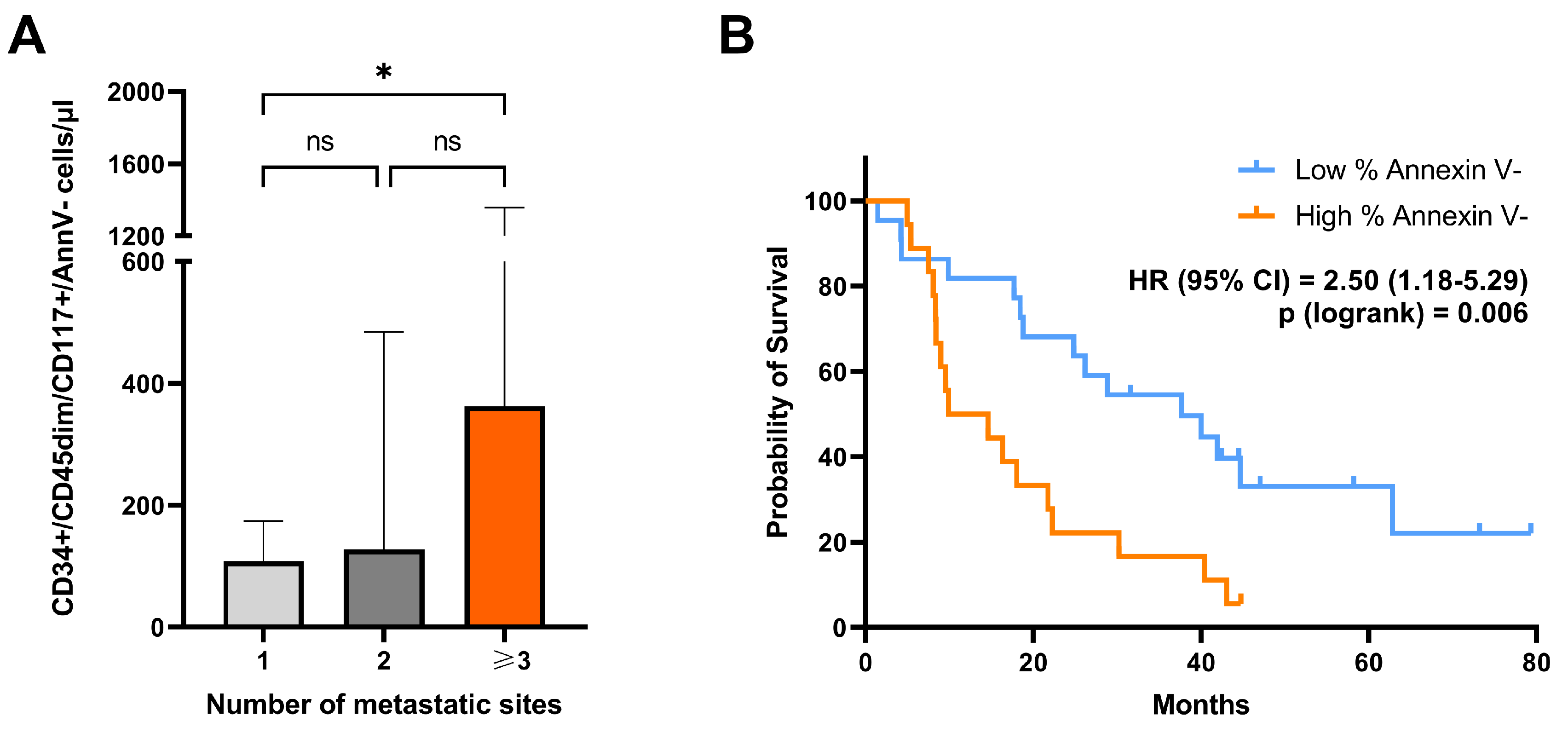
Supplementary Table S3). Notably, blood concentration of CD34+/CD45dim/CD117+/AnnV- cells was correlated with the number of metastatic sites (p = 0.03). Median blood concentration of cells with CD34+/CD45dim/CD117+/AnnV- phenotype was almost 2-fold higher in patients with multiple organ involvement (>3 site of metastasis), as compared with those with single-site metastasis (p = 0.03) (
Figure 4A). Additionally, blood concentrations of total CD34+/CD45dim/CD117+ cells were significantly and positively correlated with lung metastatic spread (p = 0.04) (
Supplementary Table S3). There was a weaker trend for a positive correlation also between CD34+/CD45dim/CD117+/AnnV- and lung metastasis, but it did not reach statistical significance (p 0.06). No other significant correlations were observed (
Supplementary Table S3).
3.4. High Percentage of Blood Annexin V- Cells with a CD34+/CD45dim/CD117+ Phenotype Independently Predict Worse Survival in Patients with mCRC
Considering the association observed between CD117+ cell subsets and tumor response, we investigated whether baseline blood concentrations of CD117-expressing CD34+/CD45dim cells and the percentage of Annexin V-negative cells within this cell subset were associated with survival in patients with mCRC (n = 40). Univariate and multivariate Cox proportional hazards regression analyses were employed to investigate the correlation between patient survival and cell subsets. On univariate analysis, a significant correlation between overall survival and the percentage of Annexin V- blood cells with a CD34+/CD45dim/CD117+ phenotype was observed (p = 0.04) (
Table 2). No correlation between blood concentration of the whole CD34+/CD45dim/CD117+ cell population and survival was found (p = 0.23). Univariate Cox proportional hazards regression analysis was used to evaluate the association between OS and clinical–pathological factors including ECOG PS, age, number of metastatic sites, BMI, tumor grading, primary tumor location, serum blood CEA concentration, K-RAS mutational status, line and type of systemic therapy (
Table 2). In this regard, ECOG PS and CEA levels correlated with survival (p = 0.001; p = 0.03, respectively). Cox regression univariate analyses were confirmed via bootstrap validation. All variables significantly correlated with OS (p < 0.05) in the univariate analysis and those considered clinically meaningful including line and type of systemic therapy received after study enrollment were selected as candidate variables for the multivariate analysis. A Cox proportional hazards regression multivariate analysis using a stepwise backward procedure was employed to derive a final model of the variables that had a significant independent relationship with survival. In this model, a variable was stepwise removed if the corresponding p value was > 0.10. Intriguingly, in the final multivariate model, percentage of Annexin V expression within blood CD34+/CD45dim/CD117+ cells resulted independently associated with survival in our cohort of mCRC (
Table 2).
Difference in overall survival between groups with high and low percentage of annexin V- cells (cut off=90%) is depicted in the Kaplan-Meier plot reported in
Figure 4. Kaplan-Meier (KM) survival curves showed that patients with higher percentage of annexin V- cells in the circulating CD34+/CD45dim/CD117+ cell compartment presented a remarkably reduced survival, as compared to patients with lower percentage of annexin V- events (p = 0.006) (
Figure 4B). No difference was observed between patients with different blood levels of total CD117-expressing CD34+/CD45dim cells (cut off= 135 cells/µl; (p = 0.24) (
Supplementary Figure S2).
4. Discussion
Tumour growth is sustained by the formation of new blood vessels—a process called neoangiogenesis. Targeting neoangiogenesis has represented a challenge for cancer therapy in the last decades [
37]. Several anti-angiogenic drugs have been recently developed, such as monoclonal antibodies or tyrosine kinase inhibitors [
38]. They play crucial roles in the treatment of colorectal cancer by inhibiting the formation of new blood vessels necessary for tumor growth and metastasis. These drugs target the vascular endothelial growth factor (VEGF) pathway, which is of pivotal importance for neo angiogenesis in CRC and other solid tumors [
39]. Despite progress in anti-angiogenic therapy for advanced CRC, there are still unmet challenges to address, including drug resistance and limited efficacy of this treatment strategy in patient subgroups. Therefore, the assessment of more effective predictive biomarkers for antiangiogenic and, more widely, antitumoral systemic therapy is a clinical need of growing interest [
13]. Several cellular subtypes involved in endothelial homeostasis, such as circulating endothelial cells (CEC), circulating endothelial progenitor cells (CEPC), and pro-angiogenic hematopoietic stem cells (HSC) have a potential as biomarkers in this context. Therefore, we undertook the task to deeply analyze and correlate blood levels of circulating endothelial cells and their putative progenitor cells with clinical outcomes in mCRC patients.
In this study, we used a computational flow cytometry analysis for identifying novel cell subsets of clinical relevance. This approach allows for automatic detection of cell populations and extraction of meaningful biological information from high-dimensional data sets [
40,
41]. Interestingly, we applied such a method to a large flow cytometry panel that included markers of putative CEC and CEPC. It is known that endothelial and pro-angiogenic cell subsets lack the expression or express the hematopoietic cell marker CD45 at dim levels [
32,
33]. Conversely, endothelial cells, endothelial progenitors or pro-angiogenic circulating cells express CD34 [
34]. Interestingly, by applying automatic data analysis to the circulating CD34+CD45dim/neg cell population, we observed—in an unbiased fashion—distinct distributions in cell subsets between mCRC responders and non-responders to antitumoral systemic therapies. Thus,
in silico analysis provided specific flow cytometry signatures related to tumor response that would be hardly obtained with classical analysis of bidimensional data. In detail, flow cytometry computational analysis of circulating CD34+CD45dim/- cells showed a differential expression of CD117+ cell clusters between responders and non-responders. In line with these findings, conventional flow cytometry data analysis of blood concentrations of circulating CD34+/CD45dim subsets in a cohort of 40 patients with mCRC confirmed that non-responders displayed higher circulating levels of CD117-expressing cells, as compared with responders. This phenotype may correspond to cells with endothelial progenitor features [
42,
43,
44,
45].
Of note, we observed that a subset of the CD34+/CD45dim/CD117+ parental population not exposing phosphatidylserine (AnnV-) had a high capability to predict treatment efficacy. More in detail, non-responders displayed higher concentrations of circulating CD34+/CD45dim/CD117+/AnnV- than responder patients. Interestingly, phosphatidylserine (PS) exposure on endothelial cells can be induced by different stimuli, such as oxidative stress and inflammatory cytokines [
46]. PS is externalized on the vascular endothelium in different tumor models and this externalization is driven by tumor-associated oxidative stress and activating cytokines [
47]. On the other hand, PS is externalized from the inner leaflet to the outer leaflet of the plasma membrane, acting as an "eat me" signal to direct phagocytes to engulf PS expressing cells [
48,
49]. It is also known that stem cell factor (SCF), the ligand of CD117 protects tumor cells from apoptosis
via an autocrine loop [
50]. Thus, Annexin V+ cells may represent cellular elements undergoing apoptosis or detaching from vessel walls. Conversely, cells with a CD34+/CD45dim/CD117+/AnnV- phenotype may compose an active proliferating subpopulation of circulating progenitors with a potential role in tumor progression [
32]. These data were corroborated by the observation that high frequencies of AnnV- cells within the circulating CD34+/CD45dim/CD117+ population were independently associated with worse survival in our cohort of mCRC patients. Patients displaying a population of CD34+/CD45dim/CD117+ circulating cells, almost totally composed of AnnexinV negative events (>90%), harbored a more aggressive disease. This may be due to the potential role of AnnexinV-/CD117+ cells that could be recruited from the bloodstream to the tumor, where they may become active players of the tumorigenesis [
51]. This hypothesis is sustained by a large body of literature showing that the expression of c-Kit (CD117) within solid tumors was associated with cancer stemness, treatment resistance, tumor progression and metastasis [
52,
53,
54].
Additionally, we observed that concentrations of the same CD34+/CD45dim/CD117+/AnnV- cell subpopulation in peripheral blood was correlated with the number of metastatic sites. Specifically, median blood cell concentration of CD34+/CD45dim/CD117+/AnnV- cells was almost 2-fold higher in patients with multiple organ involvement (≥ 3 sites of metastasis), as compared to those with single-site metastasis. Expression of CD117 on cells of the tumor microenvironnement (TME) may influence the metastatic tumor spread through various mechanisms. In a mouse model of breast cancer associated with arthritis, the interaction between mast cell CD117+ and stem cell factor (SCF) released by tumor cells enhances metastasis by remodeling both the TME and the metastatic niche [
55]. Furthermore, CD117+ adipose tissue-derived mesenchymal stem cells promote breast cancer growth and angiogenesis, further supporting the role of CD117 in metastasis [
56]. Conversely, it can be conceivable that expansion of blood CD34+/CD45dim/CD117+/AnnV- cell compartment can be secondary to increased tumor burden, which may perturbate blood levels of this cell subset [
51].
5. Conclusions
Altogether, our data suggest a role for blood CD34+/CD45dim/CD117+/AnnV- cells in mCRC treatment resistance and progression. Therefore, blood circulating CD34+/CD45dim/CD117+/AnnV- cells may represent a candidate biomarker to predict clinical outcomes in patients with mCRC. This intriguing observation calls for further analysis in larger cohorts in order to gain a deeper understanding of the pathological significance of this cell subpopulation and its potential as biomarker in colorectal cancer.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: List of flow cytometry specificities and reagent for cellular analysis of circulating endothelial cells, hematopoietic stem cells and their subtypes; Table S2: Demographic characteristics of enrolled mCRC patients (n=40); Figure S1: Blood levels of CD34+/CD45dim/CD309+ cells in responders and non-responders to antiangiogenic treatments; Table S3: Spearman rank correlation coefficients between blood CD34+CD45dim cell subsets and selected clinical-pathological features in patients with mCRC (n=40); Figure S2: Overall survival according to blood levels of CD34+/CD45dim/CD117+ cells.
Author Contributions
Conceptualization, D.B., P.L. and N.T.; data curation, D.B.; investigation, D.B, P.D.M., P.S., D.D.B., F.D.A., G.C., A.G., M.D.T., R.F.; methodology, P.S., D.B. and P.L.; resources, P.L., M.D.I. and N.T.; software, D.B.; supervision, M.D.I, A.C., N.T., and P.L.; writing—original draft, D.B. and P.L.; writing—review and editing, M.D.I and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the European Union—NextGenerationEU, under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2—M4C2 Investment 1.4—Call for tender No. 3138 of 16 December 2021, Italian Ministry of University Award Number: CN_00000041, Project Title: “National Center for Gene Therapy and Drugs based on RNA Technology.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Chieti-Pescara and University “G. d’Annunzio”, Chieti-Pescara (V. 1.0, 25 February 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roshandel, G.; Ghasemi-Kebria, F.; Malekzadeh, R. Colorectal Cancer: Epidemiology, Risk Factors, and Prevention. Cancers 2024, 16, 1530. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Dakowicz, D.; Zajkowska, M.; Mroczko, B. Relationship between VEGF Family Members, Their Receptors and Cell Death in the Neoplastic Transformation of Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 3375. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Hurwitz, H.I. Bevacizumab-Based Therapies in the First-Line Treatment of Metastatic Colorectal Cancer. Oncol. 2012, 17, 513–524. [Google Scholar] [CrossRef]
- Hurwitz, H.I.; Tebbutt, N.C.; Kabbinavar, F.; Giantonio, B.J.; Guan, Z.-Z.; Mitchell, L.; Waterkamp, D.; Tabernero, J. Efficacy and Safety of Bevacizumab in Metastatic Colorectal Cancer: Pooled Analysis From Seven Randomized Controlled Trials. Oncol. 2013, 18, 1004–1012. [Google Scholar] [CrossRef]
- Denda, T.; Sakai, D.; Hamaguchi, T.; Sugimoto, N.; Ura, T.; Yamazaki, K.; Fujii, H.; Kajiwara, T.; Nakajima, T.E.; Takahashi, S.; et al. Phase II trial of aflibercept with FOLFIRI as a second-line treatment for Japanese patients with metastatic colorectal cancer. Cancer Sci. 2019, 110, 1032–1043. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Martinelli, E.; Cascinu, S.; Sobrero, A.; Banzi, M.; Seitz, J.-F.; Barone, C.; Ychou, M.; Peeters, M.; Brenner, B.; et al. Regorafenib for Patients with Metastatic Colorectal Cancer Who Progressed After Standard Therapy: Results of the Large, Single-Arm, Open-Label Phase IIIb CONSIGN Study. Oncol. 2018, 24, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.; Siebler, J.; Grützmann, R.; Stürzl, M.; Naschberger, E. Blood Vessel-Targeted Therapy in Colorectal Cancer: Current Strategies and Future Perspectives. Cancers 2024, 16, 890. [Google Scholar] [CrossRef] [PubMed]
- Mody, K.; Baldeo, C.; Bekaii-Saab, T. Antiangiogenic Therapy in Colorectal Cancer. Cancer J. 2018, 24, 165–170. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Xiao, L.; Ullah, M.W.; Yu, M.; Ouyang, C.; Yang, G. Current Challenges of Cancer Anti-angiogenic Therapy and the Promise of Nanotherapeutics. Theranostics 2018, 8, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Haibe, Y.; Kreidieh, M.; El Hajj, H.; Khalifeh, I.; Mukherji, D.; Temraz, S.; Shamseddine, A. Resistance Mechanisms to Anti-angiogenic Therapies in Cancer. Front. Oncol. 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Killock, D. New anti-angiogenic option for mCRC. Nat. Rev. Clin. Oncol. 2023, 20, 1–1. [Google Scholar] [CrossRef] [PubMed]
- Sveen, A.; Kopetz, S.; Lothe, R.A. Biomarker-guided therapy for colorectal cancer: strength in complexity. Nat. Rev. Clin. Oncol. 2019, 17, 11–32. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, M.R.; Bruzzese, G.; Crimi, E.; Grimaldi, V.; Liguori, A.; Brongo, S.; Barbieri, M.; Picascia, A.; Schiano, C.; Sommese, L.; et al. Severe Type 2 Diabetes Induces Reversible Modifications of Endothelial Progenitor Cells Which are Ameliorate by Glycemic Control. Int. J. Stem Cells 2016, 9, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-B.; Gong, Y.-F.; Yu, C.-J.; Sun, Y.-Y.; Li, X.-Y.; Zhao, D.; Zhang, Z.-R. Endothelial progenitor cells in cardiovascular diseases: from biomarker to therapeutic agent. Regen. Med. Res. 2013, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, R.; Lanuti, P.; Miscia, S. OMIP-011: Characterization of circulating endothelial cells (CECs) in peripheral blood. Cytom. Part A 2012, 81A, 549–551. [Google Scholar] [CrossRef]
- Lanuti, P.; Simeone, P.; Rotta, G.; Almici, C.; Avvisati, G.; Azzaro, R.; Bologna, G.; Budillon, A.; Di Cerbo, M.; Di Gennaro, E.; et al. A standardized flow cytometry network study for the assessment of circulating endothelial cell physiological ranges. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Lanuti, P.; Rotta, G.; Almici, C.; Avvisati, G.; Budillon, A.; Doretto, P.; Malara, N.; Marini, M.; Neva, A.; Simeone, P.; et al. Endothelial progenitor cells, defined by the simultaneous surface expression of VEGFR2 and CD133, are not detectable in healthy peripheral and cord blood. Cytom. Part A 2016, 89, 259–270. [Google Scholar] [CrossRef]
- Malka, D.; Boige, V.; Jacques, N.; Vimond, N.; Adenis, A.; Boucher, E.; Pierga, J.Y.; Conroy, T.; Chauffert, B.; François, E.; et al. Clinical value of circulating endothelial cell levels in metastatic colorectal cancer patients treated with first-line chemotherapy and bevacizumab. Ann. Oncol. 2011, 23, 919–927. [Google Scholar] [CrossRef]
- Garmy-Susini, B.; A Varner, J. Circulating endothelial progenitor cells. Br. J. Cancer 2005, 93, 855–858. [Google Scholar] [CrossRef]
- Yang, B.; Gu, W.; Peng, B.; Xu, Y.; Liu, M.; Che, J.; Geng, J.; Zheng, J. High Level of Circulating Endothelial Progenitor Cells Positively Correlates with Serum Vascular Endothelial Growth Factor in Patients with Renal Cell Carcinoma. J. Urol. 2012, 188, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zheng, L.; Wang, Q.; Li, W.; Cai, Z.; Xiong, S.; Bao, J. Quantity and clinical relevance of circulating endothelial progenitor cells in human ovarian cancer. J. Exp. Clin. Cancer Res. 2010, 29, 27–27. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Wang, Q.; Wang, L.; Wang, H.; Shen, Y.; Li, X.; Fu, Y.; Shen, Y.; Yu, Y. Circulating endothelial progenitor cells are involved in VEGFR-2-related endothelial differentiation in glioma. Oncol. Rep. 2014, 32, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Marçola, M.; Rodrigues, C.E. Endothelial Progenitor Cells in Tumor Angiogenesis: Another Brick in the Wall. Stem Cells Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Zahran, A.M.; Abdel-Rahim, M.H.; Refaat, A.; Sayed, M.; Othman, M.M.; Khalak, L.M.R.; Hetta, H.F. Circulating hematopoietic stem cells, endothelial progenitor cells and cancer stem cells in hepatocellular carcinoma patients: contribution to diagnosis and prognosis. Acta Oncol. 2019, 59, 33–39. [Google Scholar] [CrossRef]
- Wierzbowska, A.; Robak, T.; Krawczyńska, A.; Pluta, A.; Wrzesień-Kuś, A.; Cebula, B.; Robak, E.; Smolewski, P. Kinetics and apoptotic profile of circulating endothelial cells as prognostic factors for induction treatment failure in newly diagnosed acute myeloid leukemia patients. Ann. Hematol. 2007, 87, 97–106. [Google Scholar] [CrossRef]
- Wang, L.; Du, F.; Zhang, H.; Zhang, W.; Wang, H. Changes in circulating endothelial progenitor cells predict responses of multiple myeloma patients to treatment with bortezomib and dexamethasone. Braz. J. Med Biol. Res. 2015, 48, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Shao, Y.; Ng, K.T.P.; Liu, X.B.; Ling, C.C.; Ma, Y.Y.; Geng, W.; Fan, S.T.; Lo, C.M.; Man, K. FTY720 Suppresses Liver Tumor Metastasis by Reducing the Population of Circulating Endothelial Progenitor Cells. PLOS ONE 2012, 7, e32380. [Google Scholar] [CrossRef]
- Kwon, Y.-H.; Jung, S.-Y.; Kim, J.-W.; Lee, S.-H.; Lee, J.-H.; Lee, B.-Y.; Kwon, S.-M. Phloroglucinol Inhibits the Bioactivities of Endothelial Progenitor Cells and Suppresses Tumor Angiogenesis in LLC-Tumor-Bearing Mice. PLOS ONE 2012, 7, e33618. [Google Scholar] [CrossRef] [PubMed]
- Campioni, D.; Zauli, G.; Gambetti, S.; Campo, G.; Cuneo, A.; Ferrari, R.; Secchiero, P. In Vitro Characterization of Circulating Endothelial Progenitor Cells Isolated from Patients with Acute Coronary Syndrome. PLOS ONE 2013, 8, e56377. [Google Scholar] [CrossRef]
- Kourek, C.; Dimopoulos, S.; Alshamari, M.; Zouganeli, V.; Psarra, K.; Mitsiou, G.; Ntalianis, A.; Pittaras, T.; Nanas, S.; Karatzanos, E. A Cardiac Rehabilitation Program Increases the Acute Response of Endothelial Progenitor Cells to Maximal Exercise in Heart Failure Patients. 2022, 38, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Appleby, S.L.; Cockshell, M.P.; Pippal, J.B.; Thompson, E.J.; Barrett, J.M.; Tooley, K.; Sen, S.; Sun, W.Y.; Grose, R.; Nicholson, I.; et al. Characterization of a Distinct Population of Circulating Human Non-Adherent Endothelial Forming Cells and Their Recruitment via Intercellular Adhesion Molecule-3. PLOS ONE 2012, 7, e46996. [Google Scholar] [CrossRef] [PubMed]
- Van Craenenbroeck, E.M.; Van Craenenbroeck, A.H.; van Ierssel, S.; Bruyndonckx, L.; Hoymans, V.Y.; Vrints, C.J.; Conraads, V.M. Quantification of circulating CD34+/KDR+/CD45dim endothelial progenitor cells: Analytical considerations. Int. J. Cardiol. 2013, 167, 1688–1695. [Google Scholar] [CrossRef]
- Kalender, G.; Kornberger, A.; Lisy, M.; Beiras-Fernandez, A.; Stock, U. Kinetics of circulating endothelial progenitor cells in patients undergoing carotid artery surgery. Ther. Clin. Risk Manag. 2016, ume 12, 1841–1847. [Google Scholar] [CrossRef]
- Delorme, B.; Basire, A.; Gentile, C.; Sabatier, F.; Monsonis, F.; Desouches, C.; Blot-Chabaud, M.; Uzan, G.; Sampol, J.; Dignat-George, F. Presence of endothelial progenitor cells, distinct from mature endothelial cells, within human CD146+ blood cells. Thromb. Haemost. 2005, 94, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, L.; Etemadifar, M.; Ganji, H.; Soleimani, R.; Talebi, M.; Eskandari, N.; Samani, F.S.; Meamar, R. Evaluation of the circulating CD34 +, CD309 +, and endothelial progenitor cells in patients with first attack of optic neuritis. Adv. Biomed. Res. 2015, 4, 151. [Google Scholar] [CrossRef]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.-Q.; Broussy, S.; Han, B.; Fang, H. Recent advances of anti-angiogenic inhibitors targeting VEGF/VEGFR axis. Front. Pharmacol. 2024, 14, 1307860. [Google Scholar] [CrossRef] [PubMed]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, Z.I.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef]
- Duetz, C.; Van Gassen, S.; Westers, T.M.; van Spronsen, M.F.; Bachas, C.; Saeys, Y.; van de Loosdrecht, A.A. Computational flow cytometry as a diagnostic tool in suspected-myelodysplastic syndromes. Cytom. Part A 2021, 99, 814–824. [Google Scholar] [CrossRef] [PubMed]
- O'Neill, K.; Aghaeepour, N.; Špidlen, J.; Brinkman, R. Flow Cytometry Bioinformatics. PLOS Comput. Biol. 2013, 9, e1003365. [Google Scholar] [CrossRef] [PubMed]
- Sandstedt, J.; Jonsson, M.; Lindahl, A.; Jeppsson, A.; Asp, J. C-kit+ CD45− cells found in the adult human heart represent a population of endothelial progenitor cells. Basic Res. Cardiol. 2010, 105, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, K.L.; Sills, T.M.; Coskun, S.; Vasavada, H.; Sanglikar, S.; Goldie, L.C.; Hirschi, K.K. Hemogenic Endothelial Cell Specification Requires c-Kit, Notch Signaling, and p27-Mediated Cell-Cycle Control. Dev. Cell 2013, 27, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Suzuki, S.; Fujino, N.; Ota, C.; Yamada, M.; Suzuki, T.; Yamaya, M.; Kondo, T.; Kubo, H. c-Kit immunoexpression delineates a putative endothelial progenitor cell population in developing human lungs. Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L855–L865. [Google Scholar] [CrossRef] [PubMed]
- Guerin, C.L.; Guyonnet, L.; Goudot, G.; Revets, D.; Konstantinou, M.; Chipont, A.; Chocron, R.; Blandinieres, A.; Khider, L.; Rancic, J.; et al. Multidimensional Proteomic Approach of Endothelial Progenitors Demonstrate Expression of KDR Restricted to CD19 Cells. Stem Cell Rev. Rep. 2021, 17, 639–651. [Google Scholar] [CrossRef]
- Ran, S.; E Thorpe, P. Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int. J. Radiat. Oncol. 2002, 54, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, A.H.; Arledge, C.A.; Xing, F.; Chan, M.D.; Brekken, R.A.; Habib, A.A.; Zhao, D. Exposed Phosphatidylserine as a Biomarker for Clear Identification of Breast Cancer Brain Metastases in Mouse Models. Cancers 2024, 16, 3088. [Google Scholar] [CrossRef] [PubMed]
- Pontejo, S.M.; Murphy, P.M. Chemokines act as phosphatidylserine-bound “find-me” signals in apoptotic cell clearance. PLOS Biol. 2021, 19, e3001259. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, Y.-Z.; Zhang, Y.; Wang, X.; Zhao, X.; Godfroy, J.I.; Liang, Q.; Zhang, M.; Zhang, T.; Yuan, Q.; et al. A lysine-rich motif in the phosphatidylserine receptor PSR-1 mediates recognition and removal of apoptotic cells. Nat. Commun. 2015, 6, 5717–5717. [Google Scholar] [CrossRef] [PubMed]
- Timeus, F.; Crescenzio, N.; Valle, P.; Pistamiglio, P.; Piglione, M.; Garelli, E.; Ricotti, E.; Rocchi, P.; Strippoli, P.; Di Montezemolo, L.C.; et al. Stem cell factor suppresses apoptosis in neuroblastoma cell lines. 1997, 25, 1253–1260. [Google Scholar]
- de la Puente, P.; Muz, B.; Azab, F.; Azab, A.K. Cell Trafficking of Endothelial Progenitor Cells in Tumor Progression. Clin. Cancer Res. 2013, 19, 3360–3368. [Google Scholar] [CrossRef]
- Sun, L.; Hui, A.-M.; Su, Q.; Vortmeyer, A.; Kotliarov, Y.; Pastorino, S.; Passaniti, A.; Menon, J.; Walling, J.; Bailey, R.; et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 2006, 9, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Raspollini, M.R.; Amunni, G.; Villanucci, A.; Baroni, G.; Taddei, A.; Taddei, G.L. c-KIT expression and correlation with chemotherapy resistance in ovarian carcinoma: an immunocytochemical study. Ann. Oncol. 2004, 15, 594–597. [Google Scholar] [CrossRef]
- Kashiwagi, S.; Yashiro, M.; Takashima, T.; Aomatsu, N.; Kawajiri, H.; Ogawa, Y.; Onoda, N.; Ishikawa, T.; Wakasa, K.; Hirakawa, K. c-Kit expression as a prognostic molecular marker in patients with basal-like breast cancer. Br. J. Surg. 2013, 100, 490–496. [Google Scholar] [CrossRef]
- Das Roy, L.; Curry, J.M.; Sahraei, M.; Besmer, D.M.; Kidiyoor, A.; E Gruber, H.; Mukherjee, P. Arthritis augments breast cancer metastasis: role of mast cells and SCF/c-Kit signaling. Breast Cancer Res. 2013, 15, R32–R32. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, H.; Qian, C. c-Kit-Positive Adipose Tissue-Derived Mesenchymal Stem Cells Promote the Growth and Angiogenesis of Breast Cancer. BioMed Res. Int. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).