1. Introduction
Stroke remains a leading cause of disability in adults, with hemiparesis of the upper limb being the most common motor disorder [
1]. Almost 2/3 of people after stroke have an unfavorable natural prognosis. Motor loss often persists in the chronic phase of stroke, limiting daily activities. Although significant advances have been made in understanding recovery mechanisms, optimizing rehabilitation strategies remains challenging.
The severity of the initial motor impairment is a major determinant of functional outcome. Studies have shown that the initial severity of the motor deficit is an important predictor of long-term motor outcomes [
2]. Upper limb motor recovery is relatively predictable in people with mild impairment, but the patterns of motor recovery are highly variable in those with moderate to severe initial impairment [
3]. Krakauer et al [
4] pointed out that if the trajectory of motor recovery is predictable, then the efficacy of current motor rehabilitation is questionable. Motor rehabilitation is based on activity-based training and aims to reduce motor deficits and improve functional abilities through learning- and use-dependent mechanisms [
5], making it a crucial process for improving individuals’ quality of life.
Although the principles of motor learning have been clearly established (repetition, difficulty, goal-directed task exercises [
6,
7,
8,
9]), the required amount of training to stimulate brain plasticity is unclear. Current doses of motor retraining are not sufficient to exceed spontaneous recovery trajectories. Although motor rehabilitation is essential, the relationship between treatment dose and motor recovery is not yet fully understood. Understanding this relationship is particularly important for people with severe motor deficits, where the recovery profile seems less predictable than that of people with mild or moderate impairment [
3].
Robotic motor rehabilitation offers repetitive, reproducible and quantifiable training for the paretic limb. The device records the content of training sessions in real-time so that at the end of each session [
10,
11,
12,
13] precise data are available on the number of repetitions, the actual time spent in the activity and the type of effort made (i.e., modality used, from assistance to resistance [
14]).
Doses provided in post-stroke motor rehabilitation are both a crucial issue and a challenge facing clinicians and researchers alike. Indeed, treatment dose is a complex concept in which the quantity of active ingredients, their frequency and duration must be taken into consideration to achieve the best outcome. Hayward et al [
15] conceptualized a novel framework to provide an accurate description of dose, taking into account its multidimensional nature. This framework enables precise description, implementation, monitoring and reporting of the dose administered as part of a non-pharmacological intervention.
To date, studies quantifying the treatment administered to people after stroke have not described dose in relation to the severity of the motor deficit, despite the fact the initial severity can affect outcomes. Such information would be useful for clinicians to determine the treatment plan according to the severity of the motor deficit and follow changes in motor function.
The aim of this work was to describe the dose of robotic motor rehabilitation using the framework described by Hayward et al [
15] in a sample of people with upper limb hemiparesis in the subacute phase after stroke. We described the dose both in terms of initial motor severity and in terms of recovery: “good” and “poor” recoverers.
2. Materials and Methods
2.1. Participants
We conducted a retrospective study of data from a cohort of thirty-six people with hemiparesis who underwent an upper limb rehabilitation program combining robotic therapy and conventional occupational therapy in the subacute phase of stroke. The study was carried out in accordance with current French legislation, and data processing was carried out within the framework of reference methodology No. 004 (MR004) [
16]. All precautions to preserve the safety of the personal data processed (confidentiality, integrity and availability) were rigorously respected.
2.2. Training Intervention
All participants underwent an upper limb rehabilitation program involving robotic therapy and occupational therapy. In conventional occupational therapy, interventions involved passive muscle stretches performed by a clinician, active reaching movements and specific grasping and releasing tasks performed by the participant. In robotic therapy, participants performed many repeated movements with the paretic arm using an end-effector type robotic device. This involved performing goal-directed shoulder and elbow movements in the horizontal plane while holding the handle of the robotic arm with the distal part of the upper limb. The circular pointing task consisted of repeated movements from a rest position (center) towards eight visual targets located around a circle. The distance to travel between the center and each target could be chosen from three options (3, 9 or 14 cm i.e., the maximum distance).
The combined robotic and occupational therapy was provided for 4 weeks, 4 to 5 days a week, with one 60-minute session per day for each. During robotic therapy, the difficulty, i.e., the effort required by the participant to reach the targets, could be modulated according to their motor performance using different physical modalities. The therapist tailors the treatment plan according to the participant's motor performance and behavior. Thus, the participant could perform movements with robotic assistance (assisted), without assistance (unassisted) and against resistance (resisted) from the robot.
2.3. Outcome Measures
All participants were assessed before and after the upper limb rehabilitation program using the FMA [
17] for the upper limb and robot-derived kinematic parameters. The FMA quantifies motor deficiencies in the joint segments of the arm. This outcome measure is reliable, sensitive to change and has been validated for use in spastic paresis in the sub-acute phase of stroke [
18,
19]. The maximum score is 66 points, with higher scores indicating lower levels of impairment.
Kinematic analysis was performed on data measured and recorded by the robot during unassisted, planar movements from the center of the circle to one of 8 targets placed equidistantly on the circle 14 cm from the center. A total of 80 movements were performed. To optimize the analysis, trajectories in only three directions (front and side), classically considered the most difficult for the paretic upper limb, were considered in the calculation of kinematic parameters. Four parameters were computed:
temporal parameter defined by mean velocity (cm/s);
smoothness parameter defined by the number of peaks in the velocity profile;
reaching distance parameter was defined as the distance between the center of the 14 cm diameter circle and the orthogonal projection of the position of the end-effector at the end of the movement (cm);
path accuracy parameter defined the root mean square error (cm) from the straight line.
All kinematic parameters were normalized by values from healthy individuals and expressed as a percentage of those values.
2.4. Data Analysis
The cohort was initially stratified by motor severity-based categories using the stratification of Woodbury et al [
20]: severe (0<FM score≤19 pts), moderate (20≤FM score≤47 pts), and mild (FM score≥48 pts) [
20]. A change in motor severity category between the start and the end of the upper limb rehabilitation program separated the cohort into good and poor recoverers. This method was used to express a clinically important change in motor function. Dose articulation was described in robotic therapy using the framework of Hayward et al (
Table 1).
2.5. Statistical Analysis
To analyze the treatment effects on the clinical scores and on kinematic parameters, t-tests compared data pre- and post-combined rehabilitation program (N=36). Wilcoxon tests were then used to compare pre- and post-intervention treatment effects between those with severe, moderate and mild impairments. Finally, Mann-Whitney tests were used to compare poor vs good recoverers between the pre- and post-intervention treatment effects; in cases where the p-value was <0.05, Wilcoxon tests were used as post hoc tests.
Correlations between the initial motor impairment severity (FMA) and all pre-treatment kinematic outcomes were analyzed using Spearman's rho coefficient. Correlations between the initial motor impairment severity and time spent in each robot physical modality were explored using Spearman's rho coefficient.
Two-way repeated measures Anova was performed only on the severe impairment group, on poor and good recoverers, to determine the difference in physical modality use over five robotic sessions (S1, S4, S8, S12, S16). If the Anova result was significant, multiple comparisons with Bonferroni corrections between sessions were performed for each modality. For all statistical tests, significance was set to p < 0.05.
3. Results
3.1. Baseline Characteristics
Thirty-six individuals were included in this study, twenty-three of whom had severe initial impairment (mean (SD) initial FMA score of 12 (6) points), eight moderate impairment (initial FMA score of 37 (8) points and five mild impairment (initial FMA score of 53 (2) points). In the severely impaired group, twelve were good recoverers with an initial FMA score of 15 (5) points (
vs 9 (6) points for poor recoverers (n=11)). In the moderate impairment group, three participants were classed as good recoverers, with an initial FMA score of 41 (12) points (
vs 34 (3) points for poor recoverers (N=5). Participants’ baseline characteristics are presented in
Table 2.
3.2. Clinical Outcomes
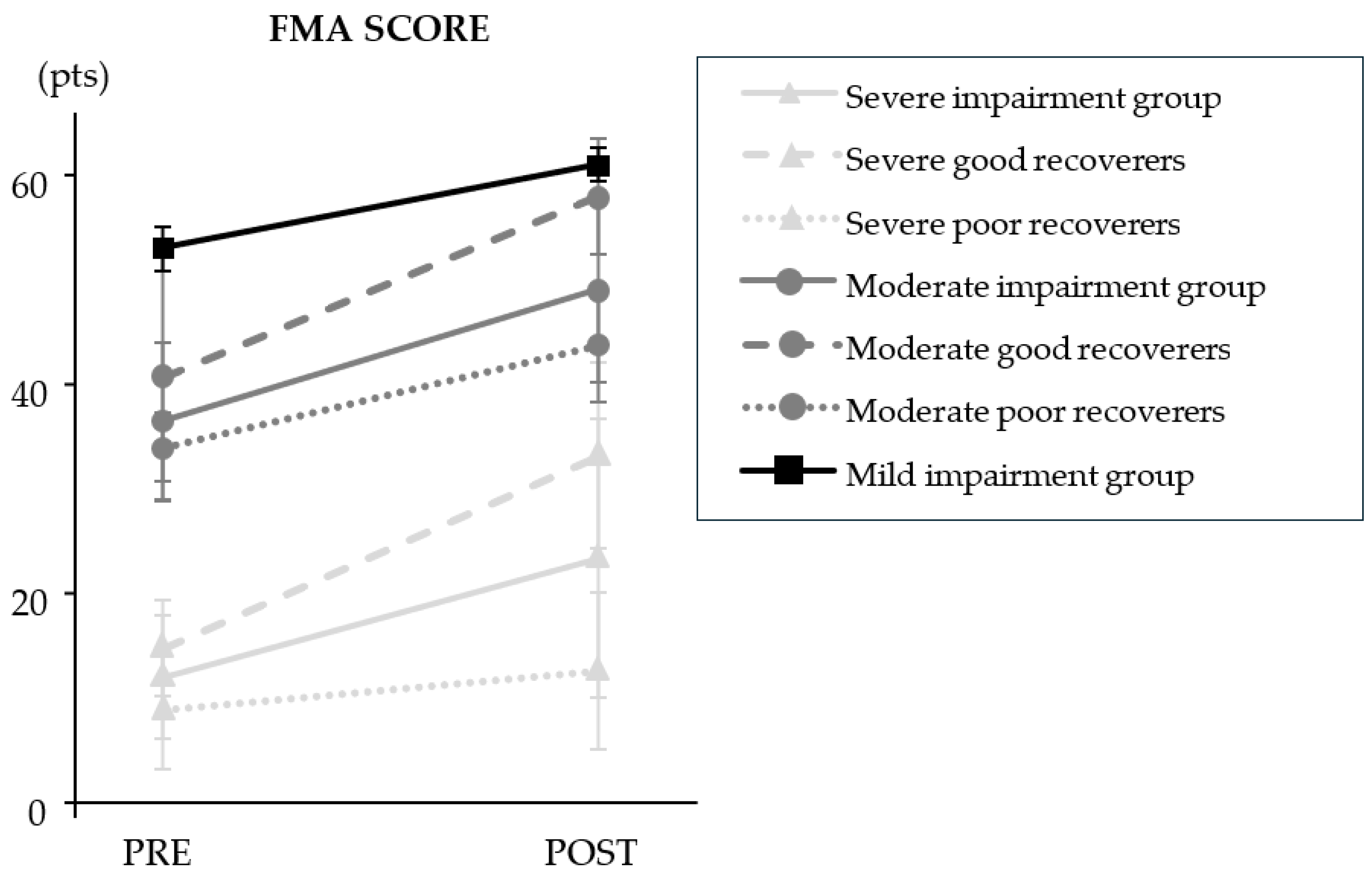
The clinical outcomes are presented in
Figure 1 and
Table 3. Overall, the results showed a statistically significant mean increase in total FMA score of 11 (10) points after the combined program (
p=4.12E
-8). The stratified results showed that FMA scores changed from pre- to post-intervention in participants with severe (+ 11 (12) points;
p=6.90E
-5) and moderate (+ 13 (6) points;
p=7.81E
-3) impairment. Improvement in poor and good recoverers only differed significantly in the severely impaired group (
p=4.92E
-4); poor recoverers improved by 4 (4) points and good recoverers improved by 18 (12) points. FMA score did not improve after the combined program in the mild impairment group (+ 8 (2) points; NS).
3.3. Kinematic Parameter Outcomes
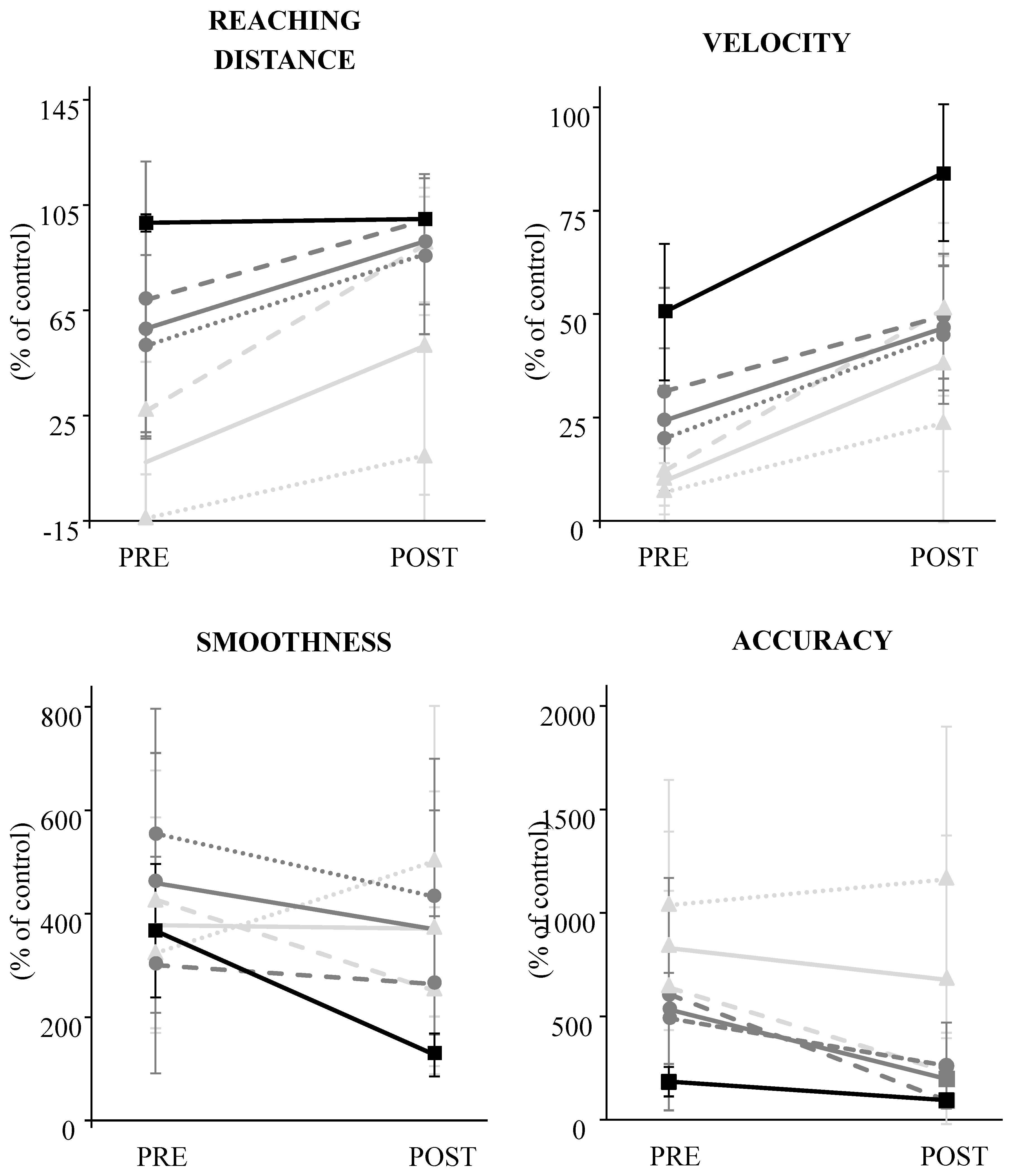
The kinematic outcomes are summarized in
Figure 2. The results showed that mean reaching distance (+36 (47) %; p=5.02E-4) and velocity (+28 (22) %, p=4.92E-4) improved at the end of the combined rehabilitation program.
The stratified results showed that reaching distance (+44 (51) %; p=3.76E-4) and velocity (+28 (23) %, p=6.09E-6) improved in participants with severe impairment, and velocity (+22 (20) %; p=1.53E-2) and accuracy (-336 (360) %, p=3.37E-2) improved in those with moderate impairment. In the mild impairment group, velocity (34 (20) %, p=1.80E-2), accuracy (-89 (44) %, p=1.08E-2) and smoothness (-240 (119) %, p=1.08E-2) improved.
The difference between poor and good recoverers was only significant in the severe impairment group for velocity (p=1.06E-2) and smoothness (p=5.09E-3). In poor recoverers, velocity improved by 17 (20) % but smoothness deteriorated by 177 (293) %. In good recoverers, velocity improved by 39 (21) % and smoothness improved by -176 (270) %.
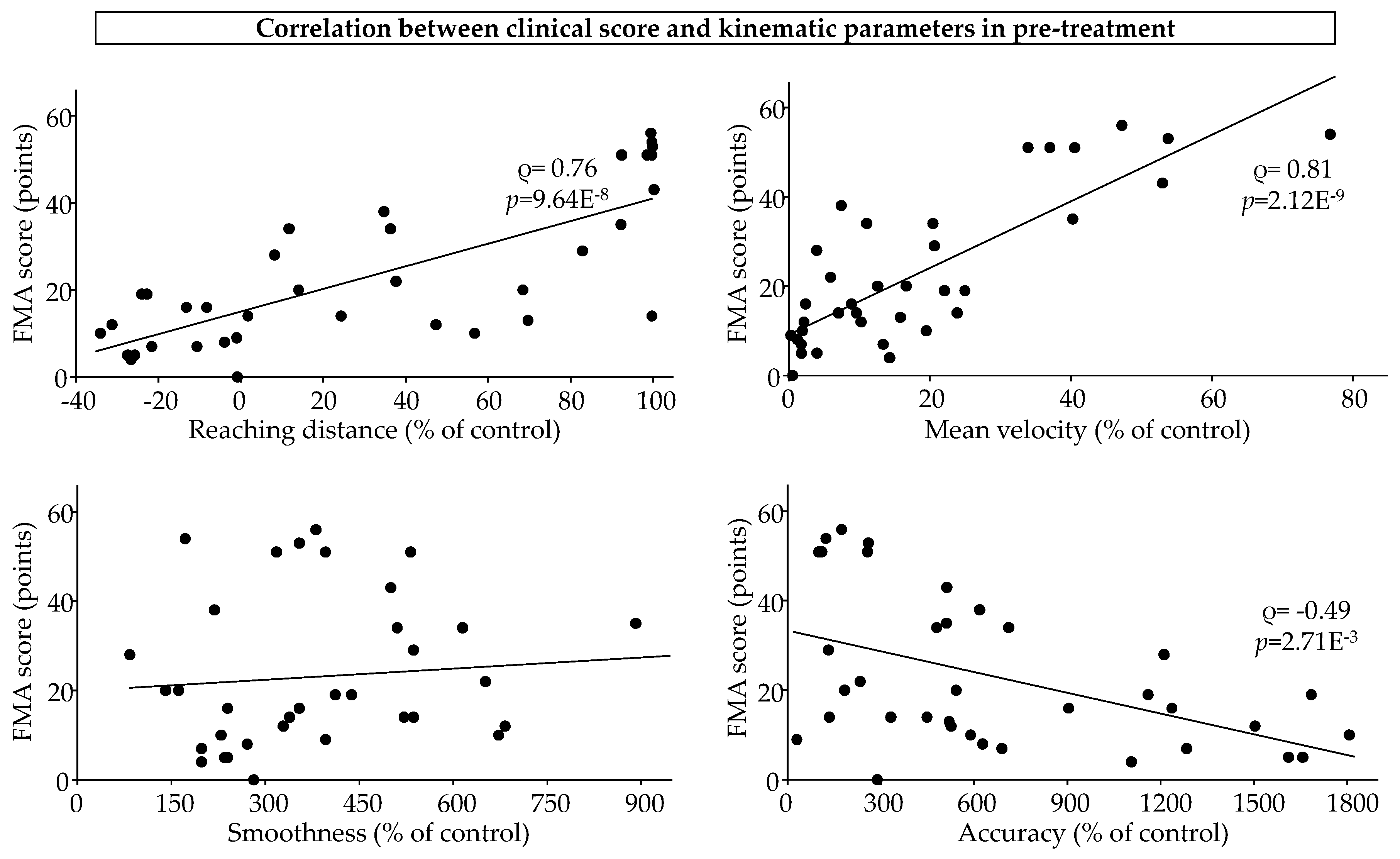
The correlation results are shown in
Figure 3. Pre-treatment, FMA scores were strongly correlated with reaching distance (Spearman’s rho = 0.76;
p=9.64E
-8) and mean velocity (Spearman’s rho = 0.81;
p=2.12E
-9) and moderately negatively correlated with accuracy (Spearman’s rho = -0.49;
p=2.71E
-3).
3.4. Dose of Robotic Training
The dose of robotic training described using Hayward’s framework is reported in
Table 4. Overall, this description showed that severely impaired participants performed 76% of assisted movements, 17% of unassisted movements and 7% of resisted movements. Good recoverers performed 63% of assisted movements, 25% of unassisted movements and 12% of resisted movements whereas poor recoverers performed 94% of assisted movements.
The use of robot modalities was similar between participants with moderate impairment and good recoverers with severe impairment: 58% of movements were assisted, 26% of movements were unassisted and 17% of movements were resisted. The use of each modality was relatively evenly distributed for the good recoverers, with 43% of assisted movements, 26% of unassisted movements and 30% of resisted movements. The use of each modality for the poor recoverers was similar to that of the good recoverers with severe impairment (i.e., 64% of movements were assisted, 26% of movements were assisted and 9% of movements were resisted).
Participants with mild impairment were more likely to use the unassisted modality (40% of movements) and the resisted modality (39%) and the assisted modality was only used for 18% of movements.
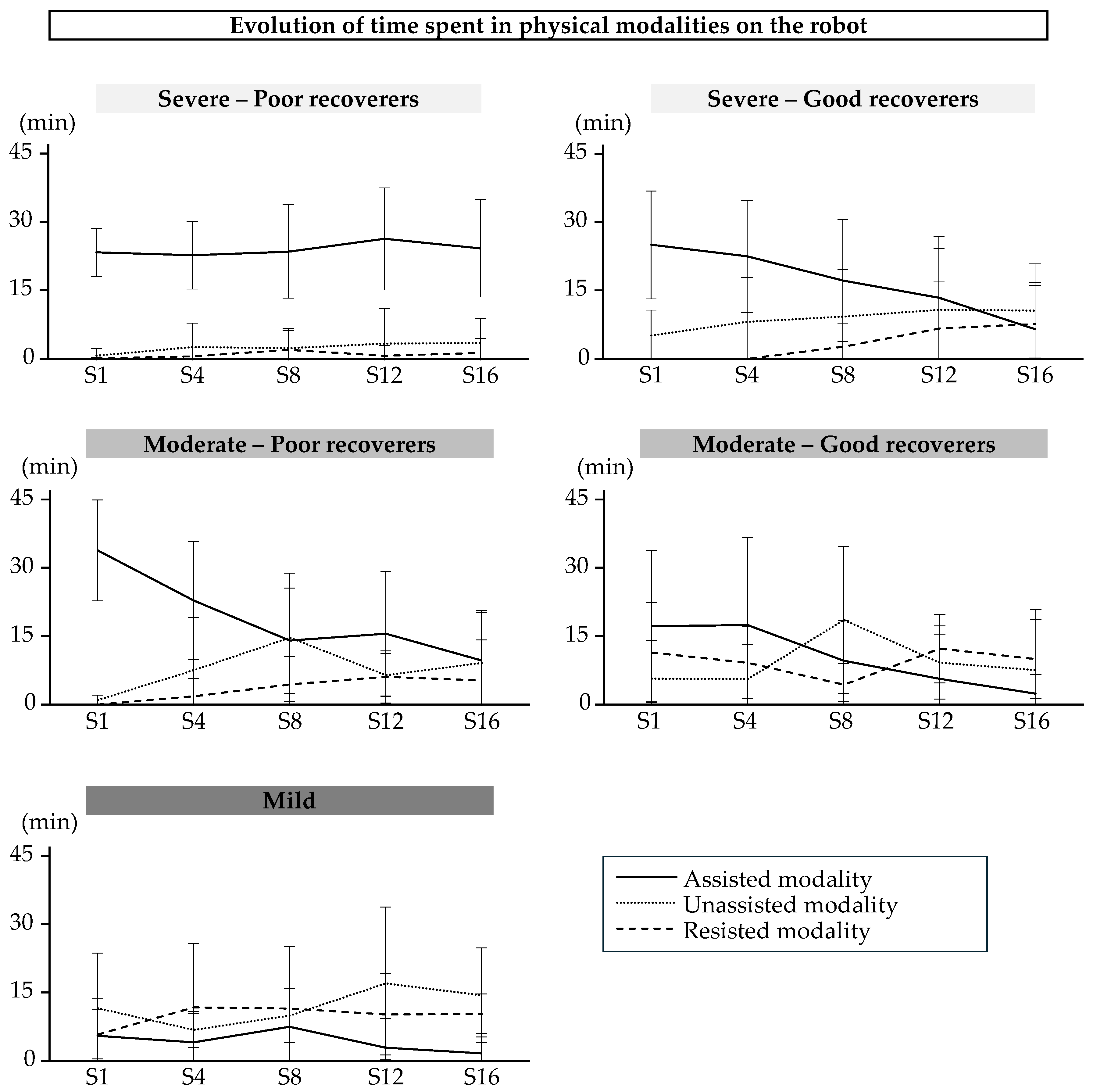
The patterns of use of the physical modalities during training sessions with the robot are shown in
Figure 4. The two-way repeated measures Anova indicated a significant modality x session interaction only in good recoverers with severe impairment (p=1.56E-06). The time spent in the assisted modality decreased over time for these participants (Session 1 vs Session 16, p=2.11E-4; Session 4 vs Session 12, p=2.98E-3; Session 4 vs Session 16, p=2.23E-4).
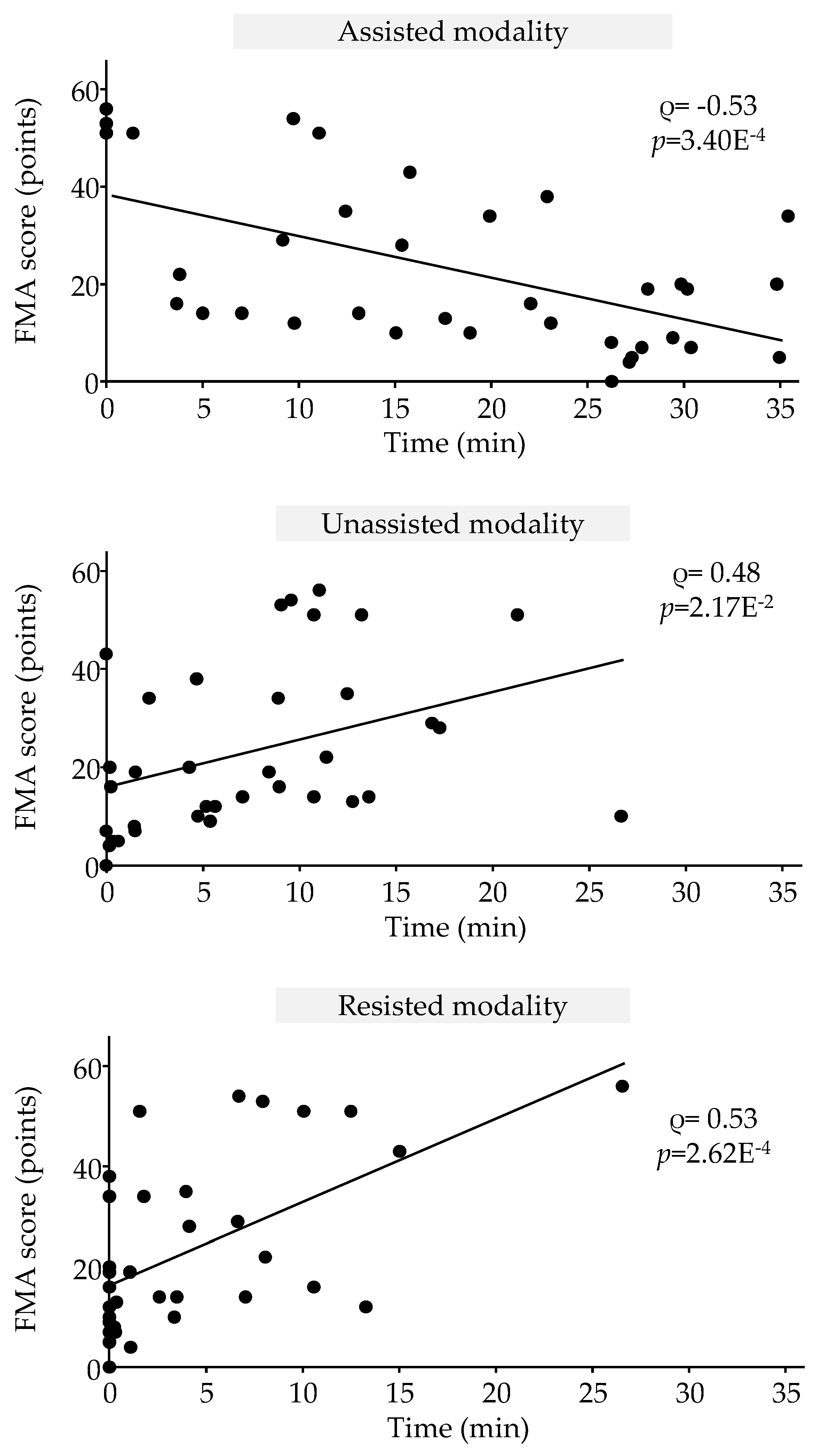
The correlation results are shown in
Figure 5. Initial FMA scores were moderately negatively correlated with mean time spent in assisted modality (Spearman’s rho = -0.53; p = 3.40E-4) and moderately correlated with mean time spent in unassisted modality (Spearman’s rho = 0.48; p = 2.17E-2) and with mean time spent in resisted modality (Spearman’s rho = 0.53; p = 2.62E-4).
4. Discussion
The aim of this retrospective study was to accurately describe and monitor the dose of upper limb motor training using a robotic device administered to a cohort of people after stroke with subacute paresis. The robotic training was combined with conventional upper limb care. The originality of this work lies in the double stratification: 1) the initial severity of the motor deficit, and 2) a change in motor severity category, which defined good and poor recoverers.
The results of this study showed that clinical scores improved in all participants, regardless of the initial motor deficit level. However, the sub-cohort of participants with initial severe impairment clearly showed two recovery profiles despite the similar initial deficit. FMA score improved by 18 points in good recoverers after the combined program, whereas it only improved by 4 points in poor recoverers. The main finding of this study indicates that the use of physical modalities is dependent on the level of initial motor impairment but may also be a critical determinant of motor outcomes.
Participants with the most severe impairment, particularly those with FMA<10 pts, predominantly used the assisted modality to perform repeated movements, which may be insufficiently challenging to promote cortical stimulation [
21]; conversely, those with less severe impairment used unassisted and resisted modalities.
4.1. Relevance of Parameters to Monitor Paretic Upper Limb Training After Stroke
In the literature, the dose of upper limb rehabilitation is often measured by the scheduled duration of therapy, a variable considered relevant for quantifying physical training [
22]. The results of a meta-analysis showed a positive relationship between the scheduled duration of therapy and motor outcomes; thus, higher doses of therapy could lead to clinically significant improvements [
7]. In the present study, all participants received 60 minutes of scheduled robotic therapy, but the associated motor outcomes differed between groups and subgroups, showing that this parameter is not sufficient to describe the dose and then interpret the results according to changes in motor impairment. The parameter of time spent in active intervention seems more relevant, as it objectively quantifies the number of minutes the participant is active on the task; this study showed that participants were only active for 57% of the scheduled session time (60 minutes), which is far shorter than the scheduled time in actual practice. These results are consistent with the literature [
23,
24], but again, this parameter alone cannot describe practice dosage [
25,
26] since the amount of rehabilitation time was the same for all participants.
Similarly, counting the number of movement repetitions does not describe the dose of practice. This parameter is interesting, but its use independently of the effort associated with the movement is insufficient to quantify practice intensity [
27].
Hayward et al [
15] dissected each motor task performance episode into the combined description of duration (in minutes), intensity (repetitions/second) and difficulty (physical effort). Using this methodological framework, the results of the present study showed that the cohort performed 457 assisted movements (53% of total active time), 174 unassisted movements (22% of total active time) and 118 movements against resistance (12% of total active time). This more detailed description provides an accurate representation of what people do during robotic therapy. Dose quantification can also stimulate the individual by providing feedback on motor performance and managing the difficulty of the movements performed.
4.2. Tailoring Exercise Difficulty: The Impact of Manipulating Physical Treatment Modalities in Robotic Therapy
In this study, the difficulty of motor training was related to the physical effort requirement and probably to the participant’s intention to be engaged in performing repeated movements. In robotic rehabilitation, this difficulty is modulated by manipulating the training modalities and the physical interactions between the user and the robotic arm. In this form of physical therapy, the effort required for each physical modality can be ranked in an orderly fashion; a movement against resistance will require greater effort than an unassisted movement, which in turn will require greater effort than an assisted movement. It is therefore not surprising to observe in this study that the participants with more severe motor deficits spent more time performing assisted than unassisted or resisted movements. Conversely, those with milder motor deficits spent more time performing resisted movements.
It is difficult to compare this observation with the literature as few studies have investigated the change in time spent using each modality over time as a function of the initial motor deficit. According to the literature, the assisted modality is predominantly used [
14]. In this study, the participants - whether mild, moderate or severe - were active for the same amount of time (34 min on average) and performed a comparable number of movements, averaging between 600 and 900 movements per session. However, describing these parameters in terms of the modality used demonstrates how therapists managed the effort demand on the participants.
4.3. Identification of Physical Modality Use Patterns
The results of this study demonstrated that there are four therapeutic profiles in the use of robotic modalities depending on the evolution of motor performance over the course of the program. In order of difficulty, they are:
Profile A: Use of assisted modality (A)
Profile B: Integration of unassisted modality (A+B)
Profile C: Integration of the resisted modality (B+C)
Profile D: Leaving assisted modality (B-A+C)
For the participants with very severe impairments (FMA<10 pts), the therapist decided to use the assisted modality for the duration of the program (profile A). Those whose motor performance was deemed sufficient by the therapist were able to benefit from the unassisted modality in addition to the assisted modality (i.e. simultaneous use of assisted and unassisted modalities; profile B). For participants with the most favorable evolution pattern, the therapist decided to integrate the third physical modality available on the robot, the resisted modality (profile C). Finally, a last therapeutic profile appears for a small proportion of participants for whom the therapist has decided to stop using assisted modality and use only two physical modalities, unassisted and resisted (profile D).
4.4. Therapist's Role in Adjusting Robotic Therapy Training Parameters for Individuals with the Most Severe Impairment
Although robotic therapy seems appropriate for people with minimal upper-limb motor function, the literature highlights the potential slacking effect (i.e., individuals allow themselves to be carried along by the assistance, with minimal participation [
28,
29]. The results of the present study showed that a sub-group of individuals with severe and moderate impairment can generate sufficient motor activity on the robot to achieve a favorable outcome. Analysis of the physical training modalities used by these sub-groups enables them to be identified at mid-treatment (S8); these participants benefited from all physical modalities at this stage, with partial release from assistance. This result is consistent with one of the fundamental principles governing motor learning after stroke, “use it and improve it or lose it” [
30].
In individuals with severe and moderate motor impairments, the use of robotic assistance should be as short as possible to encourage the most active behavior possible. Robotic algorithms are programmed to adapt to the individual's motor behavior and, therefore, to their engagement level in the task and not actually to their motor capacity. If the robot performs the movement for the individual, or conversely, if the individual performs the movement with assistance too easily, they will perform many movements without generating effort. In both situations, the therapist's supervision is critical to adapt the program to the individual’s needs, even during the session if fatigue occurs. The therapist can also use performance and assistance indicators to adjust the difficulty, either modifying the exercise parameters or proposing a higher-level physical modality, such as the unassisted or resisted modality. A study showed the importance of considering the severity of upper-limb paresis when deciding on the level of assistance [
31]. The authors suggested increasing the level of robotic assistance for individuals with severe to moderate paresis (FMA<30) and reducing assistance for those with mild paresis. The results of the present study are consistent with these recommendations. However, it is strongly recommended that assistance be used for as short a time as possible to promote repetition of unassisted movements [
21].
4.5. Is Robotic Therapy Inappropriate for People with Very Severe Impairment?
Considering the progression of kinematic parameters in “poor recoverers”, the changes were discrete, with improvements in range and speed of active movement. This suggests that, despite the use of assistance, the movements produced by these participants were at least partially active. These individuals likely need more prolonged therapy to achieve more significant motor outcomes. Furthermore, analysis of kinematic parameters showed different improvement strategies depending on the initial severity of the motor deficit. At the end of the combined program, those with severe impairment could use the robot to reach targets faster and further - a more quantitative strategy; those with moderate impairment were also able to reach targets faster but more accurately; and those with mild impairment could reach targets faster, more accurately and more smoothly - a more qualitative strategy. Using this analysis to complement clinical analysis provides more insight into recovery processes and can help define which therapy should be administered to whom and when. Although the relevance of kinematic analysis seems obvious, the question of its true significance has yet to be answered. It is unclear whether improvements in kinematic parameters represent true recovery or an adaptation to the task.
4.6. Limitations
This retrospective study has several limitations. The sample size was relatively small, particularly for the subgroups with moderate and mild motor impairments, limiting the generalizability of the findings. The retrospective nature of this study also limits the generalizability of the results. The study focused on a limited set of kinematic parameters, potentially neglecting other important aspects of motor recovery, such as muscle activation patterns and cortical plasticity. Neuroradiological and/or neurophysiological evaluation of the cortico-spinal tract would help to explore underlying lesional profiles in people with severe impairment. Furthermore, the kinematic parameter assessment involved a similar motor task as that used in rehabilitation; thus, improvement may also reflect a task-learning effect rather than a true improvement in motor function.
5. Conclusions
This study highlights the importance of tailoring robotic therapy to the individual needs of people after a stroke. By stratifying individuals according to their initial motor severity and motor outcomes, we can identify distinct recovery patterns that could help optimize treatment strategies. A multidimensional approach to quantifying dose, considering factors such as physical modality, intensity, and duration, provides valuable insights into the treatment dose-related motor outcome. Although robotic therapy shows promise for improving motor function, future research should explore its long-term effects and investigate the neural mechanisms underlying recovery. Larger, prospective studies are needed to validate these findings and refine clinical practice.
Author Contributions
Conceptualization, O.P. and C.D.; methodology, O.P. and C.D..; software, O.P.; validation, O.P. and C.D.; formal analysis, O.P. and C.D.; investigation, O.P. and C.D.; writing—original draft preparation, O.P. and C.D.; writing—review and editing, O.P. and C.D.; supervision, O.P. and C.D.; project administration, O.P. and C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, performed in accordance with current French legislation (reference N◦004 (MR004)), and granted approval by our internal ethics committee in line with the Data Protection Act.
Informed Consent Statement
Written informed consent was obtained from the participants to publish this paper.
Data Availability Statement
All data are available in electronic format at the Centre de Réadaptation Fonctionnelle (CRF) Les Trois Soleils.
Acknowledgments
The authors would like to thank all participants for their contribution to the study. Furthermore, the authors would like to thank the therapists at Les Trois Soleils rehabilitation center for their daily involvement in the robot-assisted program, without which this study would not have been possible, and would also like to thank Johanna Robertson for English language improvement.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FMA |
Fugl-meyer assessement |
References
- Lawrence, E.S.; Coshall, C.; Dundas, R.; Stewart, J.; Rudd, A.G.; Howard, R.; Wolfe, C.D.A. Estimates of the Prevalence of Acute Stroke Impairments and Disability in a Multiethnic Population. Stroke 2001, 32, 1279–1284. [CrossRef]
- Puig, J.; Blasco, G.; Schlaug, G.; Stinear, C.M.; Daunis-i-Estadella, P.; Biarnes, C.; Figueras, J.; Serena, J.; Hernández-Pérez, M.; Alberich-Bayarri, A.; et al. Diffusion Tensor Imaging as a Prognostic Biomarker for Motor Recovery and Rehabilitation after Stroke. Neuroradiology 2017, 59, 343–351. [CrossRef]
- Prabhakaran, S.; Zarahn, E.; Riley, C.; Speizer, A.; Chong, J.Y.; Lazar, R.M.; Marshall, R.S.; Krakauer, J.W. Inter-Individual Variability in the Capacity for Motor Recovery after Ischemic Stroke. 2008, 22, 64–71.
- Krakauer, J.W.; Carmichael, S.T.; Corbett, D.; Wittenberg, G.F. Getting Neurorehabilitation Right: What Can Be Learned from Animal Models? Neurorehabil. Neural Repair 2012, 26, 923–931. [CrossRef]
- Kwakkel, G.; Stinear, C.; Essers, B.; Munoz-Novoa, M.; Branscheidt, M.; Cabanas-Valdés, R.; Lakičević, S.; Lampropoulou, S.; Luft, A.R.; Marque, P.; et al. Motor Rehabilitation after Stroke: European Stroke Organisation (ESO) Consensus-Based Definition and Guiding Framework. Eur. Stroke J. 2023, 8, 880–894. [CrossRef]
- Kwakkel, G.; Wagenaar, R.C.; Twisk, J.W.R.; Lankhorst, G.J.; Koetsier, J.C. Intensity of Leg and Arm Training after Primary Middle-Cerebral-Artery Stroke: A Randomised Trial. Lancet 1999, 354, 191–196. [CrossRef]
- Kwakkel, G.; Peppen, R. Van; Wagenaar, R.C.; Dauphinee, S.W.; Richards, C.; Ashburn, A.; Miller, K.; Lincoln, N.; Partridge, C.; Wellwood, I.; et al. Effects of Augmented Exercise Therapy Time After Stroke : A Meta-Analysis. Stroke 2004, 35, 2529–2539. [CrossRef]
- Bütefisch, C.; Hummelsheim, H.; Denzler, P.; Mauritz, K.H. Repetitive Training of Isolated Movements Improves the Outcome of Motor Rehabilitation of the Centrally Paretic Hand. J. Neurol. Sci. 1995, 130, 59–68. [CrossRef]
- Feys, H.; De Weerdt, W.; Verbeke, G.; Steck, G.C.; Capiau, C.; Kiekens, C.; Dejaeger, E.; Van Hoydonck, G.; Vermeersch, G.; Cras, P. Early and Repetitive Stimulation of the Arm Can Substantially Improve the Long-Term Outcome after Stroke: A 5-Year Follow-up Study of a Randomized Trial. Stroke 2004, 35, 924–929. [CrossRef]
- Lum, P.S.; Burgar, C.G.; Shor, P.C.; Majmundar, M.; Van der Loos, M. Robot-Assisted Movement Training Compared with Conventional Therapy Techniques for the Rehabilitation of Upper-Limb Motor Function after Stroke. Arch. Phys. Med. Rehabil. 2002, 83, 952–959. [CrossRef]
- Masiero, S.; Armani, M.; Ferlini, G.; Rosati, G.; Rossi, A. Randomized Trial of a Robotic Assistive Device for the Upper Extremity during Early Inpatient Stroke Rehabilitation. Neurorehabil. Neural Repair 2014, 28, 377–386. [CrossRef]
- Volpe, B.T.; Krebs, H.I.; Hogan, N.; Edelstein, L.; Diels, C.; Aisen, M. A Novel Approach to Stroke Rehabilitation: Robot-Aided Sensorimotor Stimulation. Neurology 2000, 54, 1938–1944. [CrossRef]
- Lo, A.C.; Guarino, P.D.; Richards, L.G.; Haselkorn, J.K.; Wittenberg, G.F.; Federman, D.G.; Ringer, R.J.; Wagner, T.H.; Krebs, H.I.; Volpe, B.T.; et al. Robot-Assisted Therapy for Long-Term Upper-Limb Impairment after Stroke. N. Engl. J. Med. 2010, 362, 1772–1783. [CrossRef]
- Basteris, A.; Nijenhuis, S.M.; Stienen, A.H.A.; Buurke, J.H.; Prange, G.B. Training Modalities in Robot-Mediated Upper Limb Rehabilitation in Stroke : A Framework for Classification Based on a Systematic Review. J Neuroeng Rehabil. 2014, 11, 1–15. [CrossRef]
- Hayward, K.S.; Churilov, L.; Dalton, E.J.; Brodtmann, A.; Campbell, B.C.V.; Copland, D.; Dancause, N.; Godecke, E.; Hoffmann, T.C.; Lannin, N.A.; et al. Advancing Stroke Recovery Through Improved Articulation of Nonpharmacological Intervention Dose. Stroke 2021, 52, 761–769. [CrossRef]
- Gorphe, P.; Jannin, C. Regulatory Aspects of Prospective and Retrospective Clinical Research in France in 2018. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2019, 136, 103–108. [CrossRef]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer Assessment of Motor Recovery after Stroke: A Critical Review of Its Measurement Properties. Neurorehabil. Neural Repair 2002, 16, 232–240. [CrossRef]
- Sanford, J.; Moreland, J.; Swanson, L.R.; Stratford, P.W.; Gowland, C. Reliability of the Fugl-Meyer Assessment for Testing Motor Performance in Patients Following Stroke. Phys. Ther. 1993, 73, 447–454. [CrossRef]
- Duncan, P.W.; Propst, M.; Nelson, S.G. Reliability of the Fugl-Meyer Assessment of Sensorimotor Recovery Following Cerebrovascular Accident. Phys. Ther. 1983, 63, 1606–1610. [CrossRef]
- Woodbury, M.L.; Velozo, C.A.; Richards, L.G.; Duncan, P.W. Rasch Analysis Staging Methodology to Classify Upper Extremity Movement Impairment after Stroke. Arch. Phys. Med. Rehabil. 2013, 94, 1527–1533. [CrossRef]
- Bo Nielsen, J.; Willerslev-Olsen, M.; Christiansen, L.; Lundbye-Jensen, J.; Lorentzen, J. Science-Based Neurorehabilitation: Recommendations for Neurorehabilitation from Basic Science. J. Mot. Behav. 2015, 47, 7–17. [CrossRef]
- Lohse, K.R.; Lang, C.E.; Boyd, L.A. Is More Better? Using Metadata to Explore Dose−Response Relationships in Stroke Rehabilitation. Stroke 2014, 45, 2053–2058. [CrossRef]
- James, J.; Mcglinchey, M.P. How Active Are Stroke Patients in Physiotherapy Sessions and Is This Associated with Stroke Severity? 2021. [CrossRef]
- English, C.; Hillier, S.; Kaur, G.; Hundertmark, L. People with Stroke Spend More Time in Active Task Practice, but Similar Time in Walking Practice, When Physiotherapy Rehabilitation Is Provided in Circuit Classes Compared to Individual Therapy Sessions: An Observational Study. J. Physiother. 2014, 60, 50–54. [CrossRef]
- Kaur, G.; English, C.; Hillier, S. How Physically Active Are People with Stroke in Physiotherapy Sessions Aimed at Improving Motor Function? A Systematic Review. Stroke Res. Treat. 2012, 2012, 820673. [CrossRef]
- Clark, B.; Whitall, J.; Kwakkel, G.; Mehrholz, J.; Ewings, S.; Burridge, J. The Effect of Time Spent in Rehabilitation on Activity Limitation and Impairment after Stroke. Cochrane Database Syst. Rev. 2021, 2021. [CrossRef]
- Scrivener, K.; Sherrington, C.; Schurr, K. Exercise Dose and Mobility Outcome in a Comprehensive Stroke Unit : Description and Prediction from a Prospective Cohort Study. J. Rehabil. Med. 2012, 44, 824–829. [CrossRef]
- Emken, J.L.; Bobrow, J.E.; Reinkensmeyer, D.J. Robotic Movement Training as an Optimization Problem: Designing a Controller That Assists Only as Needed. 9th Int. Conf. Rehabil. Robot. 2005. ICORR 2005. 2005, 307–312.
- Marchal-Crespo, L.; Reinkensmeyer, D.J. Review of Control Strategies for Robotic Movement Training after Neurologic Injury. J. Neuroeng. Rehabil. 2009, 6, 20.
- Kleim, J.A.; Jones, T.A. Principles of Experience-Dependent Neural Plasticity: Implications for Rehabilitation after Brain Damage. J. Speech, Lang. Hear. Res. 2008, 51, 225–239. [CrossRef]
- Takebayashi, T.; Takahashi, K.; Okita, Y.; Kubo, H.; Hachisuka, K.; Domen, K. Impact of the Robotic-Assistance Level on Upper Extremity Function in Stroke Patients Receiving Adjunct Robotic Rehabilitation: Sub-Analysis of a Randomized Clinical Trial. J. Neuroeng. Rehabil. 2022, 19. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).