1. Introduction
Infectious diseases caused by pathogenic agents have been a major problem to humans which account for the death of millions of people annually worldwide (Reygaert, 2018). Some of these diseases threaten the health of millions people especially where no cure or vaccine exists (Ade et al., 2020)). Recently, infectious disease caused by corona virus (covid-19) became one of the top cause of death across the globe (Facts, 2015).
Antimicrobial resistance (AMR) possess urgent public health problem and economic challenge across the world (WHO, 2023). AMR occurs when pathogens change over time and no longer respond to antimicrobial drugs making the infection hard to treat (WHO, 2023). Misuse and overdose of antimicrobial drugs in humans result to antimicrobial drug resistance (Alberta et al., 2020).
Hydrazone are class of organic compounds formed by replacement of oxygen of carbonyl compound to carbon nitrogen double (R-CH=N-), (Ii et al., 2021). Hydrazone Schiff base compounds and their complexes have also drowned great attention last two decades due to their high level pharmaceutical potentials such as, antimicrobial, antibacterial, antifungal, anti-inflammatory etc. (Nesterkina et al., 2020).
2. Materials and Methods
Most of the glassware used for this experiments were of Pyrex product and the glassware were cleaned and rinsed with organic solvent and dried in an oven before use to avoid contamination of the products. Some of these equipment were sourced from the Department of Pharmaceutical and Medicinal Chemistry and pharmaceutical microbiology, Ahmadu Bello University (ABU), Zaria.
2.10. Reagents, Solvents and Standard Drug
The reagents were purchased from Sigma-Aldrich, Germany. All the solvents and reagents used for this research are of analytical grade and used without further purification such as, Phenyl-hydrazine, 4-bromoacetophenone, 4nitrophenylhydrazine, Sodium hydroxide (20%), substituted Acetophenone, Hydrazine hydrate, 2,4diphenylhydrazine, glacial acetic acid, distilled water, and some organic solvents such as methanol (Merck), ethanol absolute (Merck), n-hexane, ethyl acetate (EtOAc), chloroform (CHCl3), and dichloromethane (DCM).
2.20. Chemistry
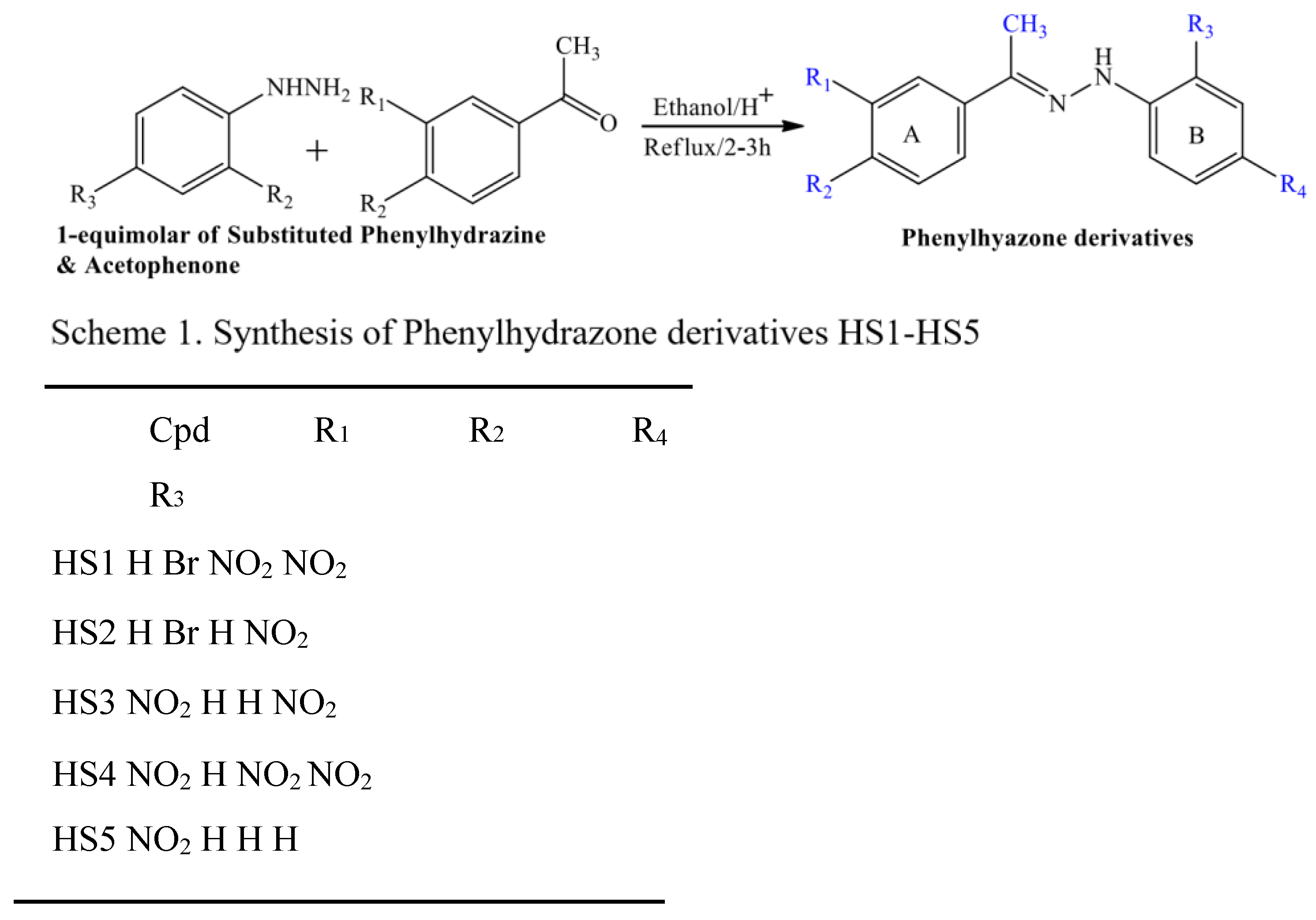
In this current research (E)-1-phenyl-2-(1-phenylethylidene) hydrazone derivatives (1-5) were obtained by following the synthetic protocols as mentioned in Scheme.1 below where an equimolar of substituted both acetophenone and phenylhydrazone were dissolved in 100mL round bottle flask contained (30ml) of absolute ethanol with few drops glacial acetic acid as catalyst, under refluxed for 3hrs at 65oC (scheme 1) (Arshad et al., 2019). The progress of the reactions monitored by thin layer chromatography (TLC) and the reaction mixture was filtered off using vacuum machine filtration, and dried to obtained the desire product (Moussa et al., 2020), (Fen et al., 2023). The purity of the compounds was checked by TLC using the solvent ratio of hexane: ethyl acetate (7:3) and their structures were confirm using spectral data (IR, 1H NMR) and elemental analysis.
3.0. Antimicrobial Activity
3.1. Antimicrobial Evaluation of the Synthesized Compounds
Synthesized compounds were screened for antimicrobial evaluation by MIC (Minimum Inhibitory Concentration) method as follows:
3.2. Source of Test Organisms
The clinical strains obtained from Ahmadu Bello University Teaching Hospital (ABUTH) Shika and pure cultures of Staphylococcus aureus (SA), Escherichia coli (EC), Streptococcus pneumoniae (SP), Bacillus subtilis (BS), Salmonella typhi (ST), Aspergillus niger (AN) and Pseudomonas aeruginosa (PS) from Department of Pharmaceutical microbiology Laboratory, Faculty of Pharmaceutical Sciences, at Ahmadu Bello University (ABU) in Zaria.
3.3. Procedure Preparation of Inoculum:
Three Gram positive (S.aureus, S.pneumonia and Bacillus subtilis), three Gram negative (E. coli, P. aeruginosa and S. typhi) and one fungal (A.niger) cultures were taken for the study where the microbial cultures were periodically sub-cultured in Nutrient broth for bacterial cultures while potato dextrose broth for fungal cultures.
For the study the cultures were grown overnight in the respective medium (Agrawal & Jeyabalan, 2017).
3.4. Preparation of Test Sample:
All the glassware used for the experiments were sterilized using (AUTOCLAVE) and dry off at 60 °C for 30min in an oven before used (Al-otibi et al., 2021). Different concentrations of the five (5) test samples were dissolved in dimethyl sulphoxide (DMSO). Various concentrations of the test solutions were made in DMSO, such as 50 µg/mg, 25 µg/mg, 12.5 µg/mg and 6.25 µg/mg for zone of inhibition and 12.5µg/mg, 6.25µg/mg, 3.125µg/mg and 1.5625µg/mg were subsequently were used for determination of MIC
3.5. Standard References
The antibiotic Ciprofloxacin was used as standard reference in the case of gram negative and gram positive bacteria where Terbinafine was used as standard reference in the case of fungi (Elhady et al., 2017).
3.6. Determination of Antimicrobial Activity (Zone of Inhibition)
The synthesized compound were evaluated in testing antibacterial activity and antifungal by measuring the zone of inhibition for each bacterial culture by agar well diffusion method, where the bacterial strains were cultured on the Mueller–Hinton Agar (MHA) and fungus strains on the potato dextrose agar (PDA) media plate (Al-otibi et al., 2021).
A sterile cork borer of 6mm diameter was used to make wells on the medium and 1ml of the synthetic compound was dropped into the well and the experiment was repeated in triplicate and the plates were then incubated at 370C for 24h. After incubation period, the diameters with no growth of inoculated microorganisms (inhibition zones) around the well were measured with transparent ruler in millimeter, averaged and the mean values were tabulated (Thi et al., 2015). The reference drugs ciprofloxacin and Terbinafine used as control for antibacterial and antifungal were completely inhibited the growth of the test organism
3.7. Determination of Minimum Inhibitory Concentration (MIC)
Minimum inhibitory concentration (MIC) is the least concentration of an antimicrobial agent that would inhibit the visible growth of the pathogen after 24h (bacteria) and 48h (fungi) incubation (You et al., 2021) (Ade et al., 2020).
MIC values of the synthesized compounds were determined using agar dilution technique and where the four concentration of different five samples, twenty eight test tubes for each compounds were sterilized and labelled appropriately. 2ml of sterile Mueller hinton broth (MHB) for bacterial and potato dextrose broth (PDA-broth) for fungal were added to each test tubes followed by dilution of 1 ml from my synthetic sample and was inoculated with the test microorganism and one tube was set as a control with 2 ml of medium and 1ml of 0.5 Mc Farland standard solution where all the inoculums and All tested tubes were incubated at 37°C for 24hrs (antibacterial) and 30°C (antifungal) for 3 days. The test tubes were then checked after the incubation period for growth of microorganisms, where the presence of turbidity indicates the growth while those resemble to control indicate the inhibitory effect of the test compound against the tested microbes this method was developed using (Agrawal & Jeyabalan, 2017).
4.0. Results and Discusion
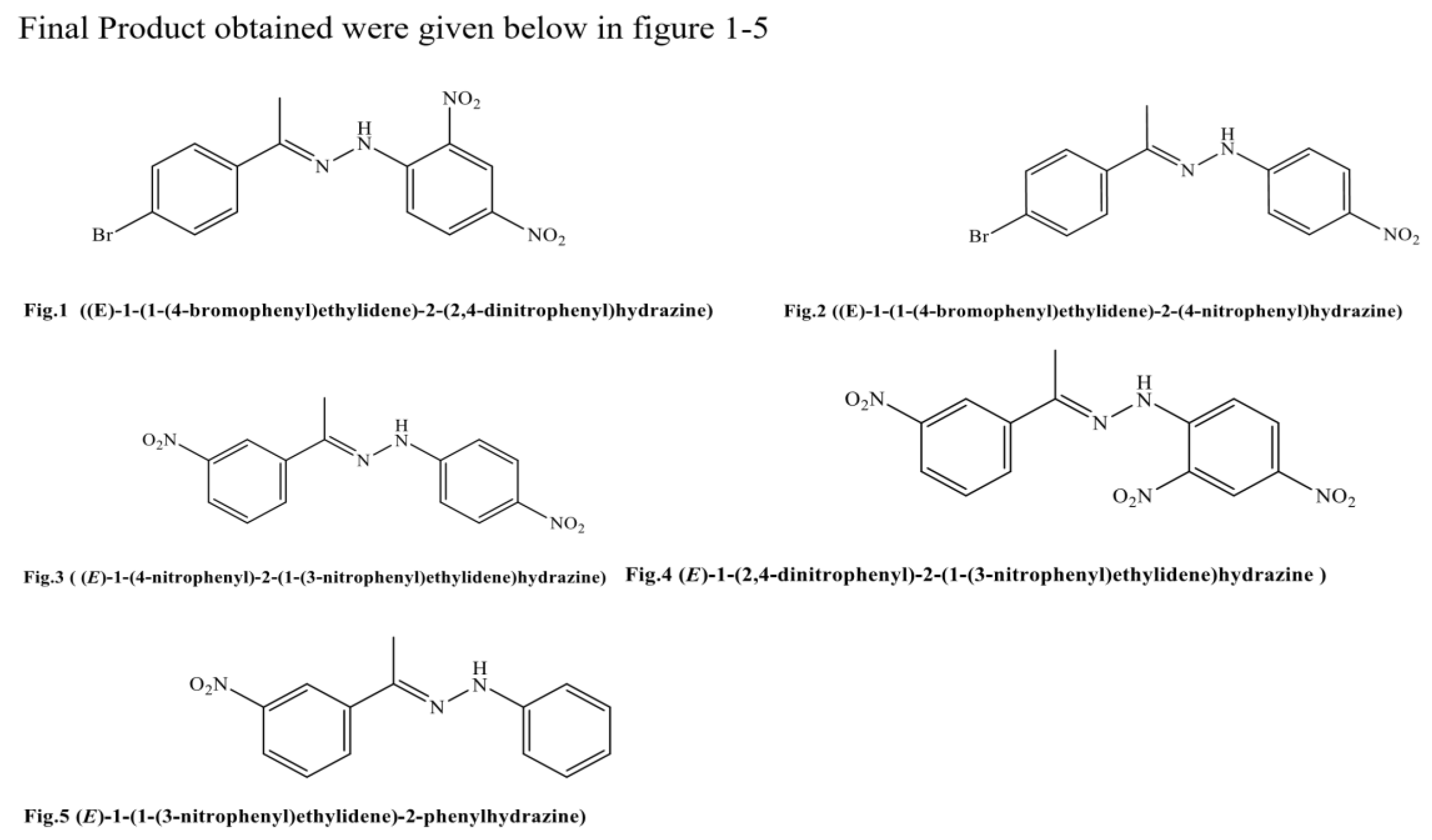
Table 1.
Zone of inhibition using (Agar well diffusion method).
Table 1.
Zone of inhibition using (Agar well diffusion method).
Cpd
Code
|
Conc.
(µg/ml)
|
Inhibition zone represented by (mm) |
|
| Gram-positive bacteria |
Gram- negative bacteria |
Fungi |
| S. aureus |
B. subtilis |
S.pneumonia |
E. coli |
S. typhi |
P. aeruginosa |
A. niger |
| HS1 |
50 |
20 |
22 |
NA |
18 |
20 |
12 |
15 |
| |
25 |
18 |
18 |
NA |
15 |
18 |
NA |
13 |
| |
12.5 |
16 |
NA |
NA |
NA |
15 |
NA |
12 |
| |
6.25 |
16 |
NA |
NA |
NA |
NA |
NA |
NA |
| HS2 |
50 |
19 |
15 |
15 |
15 |
18 |
17 |
25 |
| |
25 |
17 |
13 |
12 |
12 |
15 |
13 |
20 |
| |
12.5 |
15 |
12 |
11 |
11 |
13 |
12 |
17 |
| |
6.25 |
14 |
11 |
NA |
NA |
NA |
NA |
15 |
| HS3 |
50 |
17 |
17 |
20 |
17 |
22 |
14 |
20 |
| |
25 |
15 |
18 |
18 |
13 |
20 |
13 |
18 |
| |
12.5 |
14 |
15 |
15 |
12 |
19 |
12 |
15 |
| |
6.25 |
12 |
NA |
NA |
NA |
18 |
NA |
NA |
| HS4 |
50 |
19 |
20 |
15 |
15 |
20 |
15 |
20 |
| |
25 |
16 |
17 |
NA |
13 |
19 |
R |
17 |
| |
12.5 |
15 |
15 |
NA |
12 |
16 |
R |
15 |
| |
6.25 |
13 |
12 |
NA |
NA |
NA |
NA |
12 |
| HS5 |
50 |
20 |
11 |
18 |
22 |
20 |
22 |
18 |
| |
25 |
17 |
NA |
15 |
19 |
19 |
18 |
16 |
| |
12.5 |
15 |
NA |
12 |
16 |
16 |
16 |
13 |
| |
6.25 |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
| CF |
10 |
- |
- |
- |
- |
- |
- |
- |
| TN |
30 |
- |
- |
- |
- |
- |
- |
- |
Table 2.
Minimal inhibitory concentrations (MIC) (mg/ml) of hydrazones Derivatives Against seven Pathogenic Microbes.
Table 2.
Minimal inhibitory concentrations (MIC) (mg/ml) of hydrazones Derivatives Against seven Pathogenic Microbes.
| |
Test
Name
|
Gram-positive bacteria |
Gram- negative bacteria |
Fungi |
| S. aureus |
B. subtilis |
S.pneumonia |
E. coli |
S. typhi |
P. aeruginosa |
A. niger |
| HS1 |
MIC |
12.5 |
3.125 |
NA |
1.56 |
12.5 |
3.125 |
12.5 |
| HS2 |
MIC |
12.5 |
12.5 |
6.25 |
12.5 |
12.5 |
6.25 |
12.5 |
| HS3 |
MIC |
6.25 |
3.125 |
3.125 |
1.56 |
6.25 |
6.25 |
12.5 |
| HS4 |
MIC |
12.5 |
3.125 |
6.25 |
3.125 |
6.25 |
6.25 |
12.5 |
| HS5 |
MIC |
3.125 |
6.25 |
12.5 |
6.25 |
12.5 |
3.125 |
6.25 |
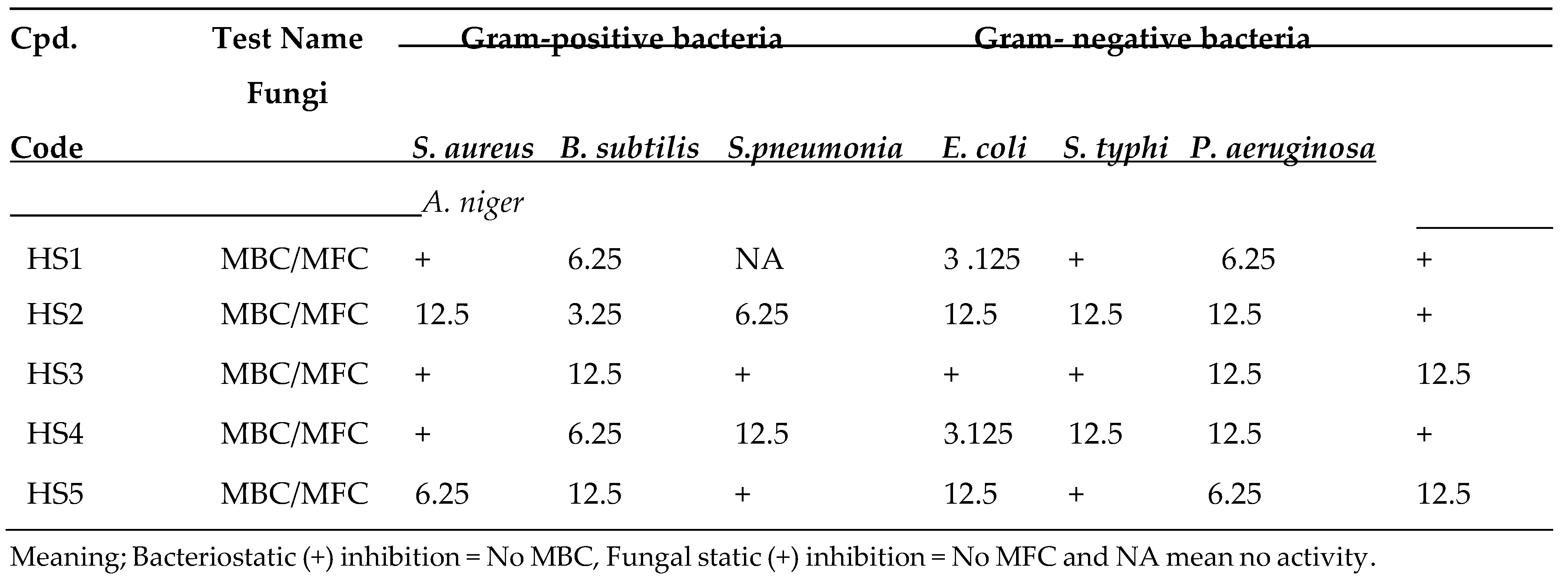
Table 3.
Minimum Bactericidal/Fungicidal Concentrations (MBC/MFC) (mg/ml) of synthetic hydrazones Derivatives Against seven isolate.
Table 3.
Minimum Bactericidal/Fungicidal Concentrations (MBC/MFC) (mg/ml) of synthetic hydrazones Derivatives Against seven isolate.
Table 4.
Phenylhydrazone compounds and their characteristics. .
Table 4.
Phenylhydrazone compounds and their characteristics. .
| Code |
Mol. Formula |
Mol.Wt. g/mol |
Color |
M.P. (0C) |
Rf Value |
Yield (%) |
| HS1 |
C14H11BrN4O4 |
379.17 |
Pink |
213-215 |
0.91 |
40.00 |
| HS2 |
C14H12BrN3O2 |
334.17 |
Brown |
220-222 |
0.85 |
67.00 |
| HS3 |
C14H12N4O4 |
300.27 |
Sandy- brown |
213-215 |
0.83 |
67.00 |
| HS4 |
C14H11N5O6 |
345.27 |
Yellow |
210-212 |
0.87 |
89.00 |
| HS5 |
C14H13N3O2 |
255.27 |
Reddish Brown |
110-112 |
0.89 |
81.00 |
5.0. Conclusion
Five (5) new compounds of (E)-Substituted-N-(phenylhydrazones) derivatives were synthesized and obtained by cyclization reaction with an excellent yield of (40–89%) and all compounds were structurally characterized and confirmed by spectral analysis.
The compounds were screen out for their antimicrobial activity and found to have shown promising antimicrobial activity in vitro compared to reference standard drugs.
Acknowledgments
I would like to express my sincere appreciation to the supervisors and entire staff of the department of pharmaceutical and medicinal chemistry, Pharmaceutical Microbiology both from faculty of Pharmaceutical Sciences, Ahmadu Bello University Zaria for their support towards the success of this research.
Abbreviations
❖ MIC: Minimal inhibitory concentration, ZOI; Zone of inhibition, MBC; Minimum Bactericidal concentration, MFC; Minimum Fungicidal concentration, FTIR; Fourier transform infrared, NMR; nuclear magnetic resonance, 1D; one-dimensional, 2D; two dimensional and HS1-5: synthetic hydrazone (symbolic code number)
References
- Ade, A. , Amengor, C. D. K., Brobbey, A., Ayensu, I., Harley, B. K., & Boakye, Y. D. (2020). Synthesis and Antimicrobial Resistant Modulatory Activity of 2, 4-Dinitrophenylhydrazone Derivatives as Agents against Some ESKAPE Human Pathogens. 2020.
- Agrawal, P., & Jeyabalan, G. Indian Journal of Pharmaceutical and Biological Research ( IJPBR ) Synthesis and antimicrobial activity of some newer semicarbazone analogues. 2017, 5, 12–17.
- Al-otibi, F. , Alkhudhair, S. K., Alharbi, R. I., Al-askar, A. A., Aljowaie, R. M., & Al-shehri, S. (2021). The Antimicrobial Activities of Silver Nanoparticles from and Fungi. Molecules, 26(6081).
- Arshad, M. , Shoeb, M., Shahab, K., Asghar, A., & Dabeer, N. (2019). benzeneamine : synthesis, characterization, antibacterial, and MTT assessment. SN Applied Sciences, 1(6), 1–8.
- https://doi.org/10.1007/s42452-019-0571-8. [CrossRef]
- Elhady, H. A., Al-nathali, H. S., & El-sayed, R. (2017). ISSN : 2320-5407 Manuscript Info Abstract.
- Introduction : - ISSN : 2320-5407 Results and discussion : -. 5(10), 1716–1725.
- https://doi.org/10.21474/IJAR01/5694. [CrossRef]
- Facts, K. E. Y. (2015). WHO, “Antimicrobials: Handle with Care” in 2020. 1–6.
- Fen, K., Dergisi, B., & Derivatives, H. (2023). Karadeniz Fen Bilimleri Dergisi. 13(1), 135–152.
- https://doi.org/10.31466/kfbd.1184337. [CrossRef]
- Ii, Z. N. , Al-qadsy, I., Saeed, W. S., Alrabie, A., & Al-adhreai, A. (2021). Synthesis, Characterization, SingleCrystal X-ray Structure and Biological Activities of [(Z)-N -(4-Methoxybenzylidene)benzohydrazide– Nickel(II)] Complex. Ii, 1–16.
- Moussa, Z., Al-mamary, M., Al-juhani, S., & Ahmed, S. A. (2020). Heliyon Preparation and biological assessment of some aromatic hydrazones derived from hydrazides of phenolic acids and aromatic aldehydes. Heliyon, 6(April), e05019. [CrossRef]
- Nesterkina, M. , Barbalat, D., & Kravchenko, I. (2020). Design, synthesis and pharmacological pro fi le of ( − ) - verbenone hydrazones. 943–950.
- Thi, N. , Thoa, L., Nam, P. C., & Nhat, D. M. (2015). Antibacterial Activities of The Extracts of Mimosa pudica L. An in-vitro Study. 5(5), 358–361.
- You, A., Be, M. A. Y., & In, I. (2021). Microwave-assisted synthesis and antioxidant activity of an imine , ( E ) -1- ( 3- bromobenzylidene ) -2-phenylhydrazine Microwave-Assisted Synthesis and Antioxidant Activity of 040041(June 2020).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).