Introduction
In the 21st century, serious illnesses from measles, mumps, and rubella (MMR) in the USA are all relatively rare [[1],[2]], all representing mild, short-lived but treatable infectious diseases. Complications are most common in children with comorbidities or generally suffering from poor sanitation, inadequate waste disposal systems and water supply, poverty, and deprivation. Indeed, the CDC declared the elimination of endemic measles in 2000 [[3]] and rubella in 2004 [[4]]. This was confirmed by Papania et al., with reported incidence below 1 case per 1,000,000 for measles since 2001 and 1 case per 10,000,000 for rubella since 2004 [[5]]. Although mumps remain endemic in the USA, Tappe et al. reported 4.54 cases per 100,000 persons in 2019 and 0.67 per 100,000 persons in 2023 [[6]]. However, in 2006, several mumps outbreaks in the USA [[7]] (≈2.2 per 100,000) and Canada [[8]] (≈75.6 per 100,000 for adolescents), were reported in highly vaccinated populations [[9],[10]]. Illness from MMR is of pale significance to the unprecedented rise of chronic and autoimmune disorders in the pediatric population. For example, estimates in the USA indicate 1 in 36 children are diagnosed with autism spectrum disorder (ASD) [[11]], 1 in 10 with attention deficit hyperactivity disorder (ADHD) [[12]], 1 in 12 with asthma [[13]], 1 in 4 with a food allergy [[14]], and 1 in 5 with one or more chronic diseases [[15]].
Vaccines have been hailed as one of the greatest medical advances of the past 160 years [[16]]. Indeed, MMR vaccination has been declared the protagonist for the demise of MMR in the Western world. However, as discussed by Guyer et al. [[17]] “Nearly 90% of the decline in infectious disease mortality among US children occurred before 1940 when few antibiotics or vaccines were available.” Hence, “vaccination does not account for the impressive declines in mortality seen in the first half of the century.” In addition, evidence detailing the durability and longanimity of natural immunity vs. vaccination is resounding [[18],[19],[20]]. This also has further implications for infants whose early protection derives from passive maternal immunity via the placenta or postnatally via breast milk [[21],[22],[23]]. Moreover, natural infections experienced during childhood, such as measles and mumps, can encourage normal immune system development, with reports of protecting effects against Parkinson’s disease [[24]], chronic lymphoid leukemia [[25]], cardiovascular disease [[26]], follicular B-cell non-Hodgkin lymphoma [[27]] and allergies [[28],[29]]. Evidence that surviving measles infection confers beneficial effects beyond lifelong protection from measles disease further indicates the need for policymakers to consider natural immunity as an opportunity cost of vaccination. It also reinforces the necessity for long-term studies comparing a broad range of health outcomes, including all-cause mortality, between fully vaccinated and completely unvaccinated children [[30]].
In 2013, a Cochrane collaboration research review by Demicheli et al. [[31]], reported significant evidence of adverse events from MMR vaccines. Although the scientists did not present statistical confirmation of the existence or a reliable relationship between MMR vaccinations and ASD diagnoses, they did report finding that “problematic internal validity in some included studies and the biases present in the studies (selection, performance, attrition, detection, and reporting) influenced our confidence in their findings”; that “The design and reporting of safety outcomes in MMR vaccine studies, both pre- and post-marketing, are largely inadequate”; and that “The evidence of adverse events following immunization with the MMR vaccine cannot be separated from its role in preventing the target diseases.” Indeed, as discussed by Miller [[32]], MMR vaccination has many documented safety deficits that counteract well-publicized benefits. For example, MMR vaccination has been attributed to the increased risk of emergency hospitalizations, seizures, and thrombocytopenia, a serious bleeding disorder.
The US government and mainstream media routinely propagate the claim that scientific studies have conclusively demonstrated that vaccines cannot cause ASD. The Centers for Disease Control and Prevention (CDC) authoritatively declares on its website that “vaccines do not cause autism” [[33]]. To support its bold proclamation, the agency cites several reports commissioned by the Institute of Medicine (IoM) (now the National Institute of Medicine) in 2004 (“Immunization Safety Review”) [[34]], 2011 (“Effects of Vaccines: Evidence and Causality”) [[35]], and 2013 (“The childhood immunization schedule and safety”) [[36]]. The 2004 report concluded “that the evidence favors rejection of a causal relationship between MMR vaccine and autism.” (p. 7). However, the same IoM report acknowledges “the possibility that MMR could contribute to autism in a small number of children because the epidemiological studies lacked sufficient precision to assess rare occurrences; it was possible, for example, that epidemiological studies would not detect a relationship between autism and MMR vaccination in a subset of the population with a genetic predisposition to autism. The biological models for an association between MMR and autism were not established but not disproved” (p 4). Although the 2004 IoM subtitled the report “Vaccines and Autism” and has erroneously been construed to indemnify all vaccines from the autism epidemic, the committee only ruled on a single vaccine and a single ingredient, the MMR vaccine or the use of thimerosal, respectively. Concerning the MMR vaccine, the. 2004 IoM acknowledged “the possibility that MMR could contribute to autism in a small number of children” (p 4) and that the types of observational studies that had been done “would not detect a relationship between autism and MMR vaccination in a subset of the population with a genetic predisposition to autism” (p 4). The IoM 2011 report further concluded as the 2004 IoM report that the evidence favors a rejection of a causal relationship; however, this conclusion was based principally on four observational studies, each failing to consider the possibility of “genetically susceptible subpopulations.” The 2013 IoM report was an update to earlier reports addressing the safety of the entire infant/child immunization schedule. The committee found that “Studies designed to examine the long-term effects of the cumulative number of vaccines or other aspects of the immunization schedule have not been conducted” (p 5). Consequently, the IoM reviews fail to support the claim for which the CDC cites them.
Wakefield ET AL. 1998
Media, institutional, and public hysteria surrounding the MMR vaccine can be traced back to a case series clinical study of 12 children with regressive developmental disorder, including nine with ASD, by Wakefield et al. in 1998 [[37]]. In 2004, the same year that the IoM issued its report concluding that “no convincing evidence exists for the casual MMR autism”, the Lancet published “retraction of interpretation” for Wakefield et al. [[38]], with a full retraction in 2010. This culminated in what can essentially be described as the “professional castration” of lead author Andrew Wakefield and coauthor John Walker-Smith in 2010 by UK journalist Brian Deer in the British Medical Journal [[39],[40],[41]]. In this case series clinical report, Wakefield et al. describe a pattern of inflammatory disorders of the gut (colitis and ileal-lymphoid-nodular hyperplasia) in children with autism in association with MMR vaccination. Of note, the hypotheses generated by the study, although perceived at the time as almost idiosyncratic, were not new. Work here followed previous speculation from 1994 by Wakefield and coworkers of a causal relationship between the measles virus [[42],[43],[44]] and MMR vaccination [[45]] with Crohn's disease. In the conclusions of their 1998 publication, Wakefield et al. explicitly stated, “We did not prove an association between measles, mumps, and rubella vaccine and the syndrome described. We have identified chronic enterocolitis in children that may be related to neuropsychiatric dysfunction. In most cases, the onset of symptoms was after measles, mumps, and rubella immunization.” Although they do suggest, “Further investigations are needed to examine this syndrome and its possible relation to this vaccine.” To this day, a causal association between ASD and MMR vaccination is still heavily contested. However, nearly 26 years after its publication, as speculated by Wakefield and coworkers in the early '00s [[46],[47]], substantial evidence provides not only a correlational but causal relationship between gut inflammation, gut pathology, and the gut-brain axis in the etiology, pathogenesis, and pathophysiology of ASD [[48]]. Indeed, in 2010, an expert panel of the American Association of Pediatrics (AAP) “an organization of 60,000 pediatricians” [[49]], strongly recommended further investigation into the role of gastrointestinal abnormalities in children with ASD [[50]].
The controversy over MMR and ASD can and should be further traced to a social movement of parents who mobilized around concern over the MMR vaccination, dating from the early 1990s in the UK. Parents reported developmentally normal infancy with sudden regression around the middle of their second or fourth year [[51]]. Children become withdrawn, with symptoms later diagnosed as part of the autistic spectrum, along with severe and painful bowel problems. Reflecting on the timing, many parents came to link developmental regression and autistic symptoms to MMR vaccination. Many parents reported medical gaslighting of their children’s suffering or individual experiences of symptom development. Gaslighting is defined here as “abuse aimed at making victims question their sanity as well as the veracity and legitimacy of their perspectives and feelings” [[52]]. The plight of the patients was further compounded by their experiences of limited governmental and societal recognition of their accounts. Medical gaslighting is not new to MMR and ASD, with growing recognition that the modern allopathic “diagnose-protocol-prescription-paradigm” has created a wider gap between the practitioner and patient [[53]]. Indeed, medical gaslighting appears to be becoming more common, especially for those illnesses reported to be vaccine-induced [[54]] or contested [[55]]. The gaslighting and shared experiences and understanding of parents who reported injury and developmental delays in association with MMR vaccination ultimately led to a “parental-Wakefield alliance.” Their media reportage became a serious concern to scientists and policymakers embroiled in public health and vaccination in the UK, Europe, and the USA. Leach frames this as a contrasting individual or paternal vs. public commitment [[56]]. Parents were primarily concerned about what they saw as the vaccine-damaged health of their children. Government policymakers and their supportive scientific networks had institutional commitments to the continued integrity of a vaccination program with its public obligations and population-level imperatives. Some mention must also be given to pharmaceutical interests [[57]], i.e., “blockbuster” monopoly and profit margin for their shareholders, maximized through millions spent on marketing and lobbying for their products [[58]].
Scientific speculations, controversy, criminal allegations, and nuances [[59],[60]] concerning the case of Wakefield et al., are certainly not the spotlight of this study. However, the vitriol, hysteria, and polarization of perspectives highlighted above give the backdrop, along with a cascade of observational studies investigating the association between MMR vaccination and ASD [[61],[62]]. The debate turns, in part, on the significance attributed to epidemiological as opposed to clinical evidence and on the status attributed to parents’ observations and paternal instincts [[63]], culminating in 2019 with the publication of Hviid et al.
Hviid ET AL. 2019
In 2019, an observational study by Hviid et al. [[64]] was published that was hailed by the mainstream media, especially in the USA, as demonstrating irrevocably that the MMR vaccine cannot cause autism, even among “genetically susceptible children.” The study was published in the journal Annals of Internal Medicine on March 5, 2019, and titled “Measles, Mumps, Rubella Vaccination and Autism: A Nationwide Cohort Study.” It was authored by Anders Hviid, Jørgen Vinsløv Hansen, Morten Frisch, and Mads Melbye. The AAP claimed, “Another study has confirmed children who receive measles, mumps and rubella vaccine (MMR) are not at increased risk of autism”, and that the findings “also held for vaccinated children with a sibling who had been diagnosed with autism. Among girls, the risk of autism was lower in those who were vaccinated” [[65]]. Here are some further illustrative examples of how the US mainstream media reported on the study by Hviid et al.:
A CNN headline declared, “MMR vaccine does not cause autism, another study confirms.” Emphasizing that the “biggest contribution of the study was the inclusion of children at risk of autism”, CNN reported that vaccines do “not increase the risk of autism and does not trigger autism in children who are at risk” [[66]].
A headline from National Public Radio (NPR) similarly declared, “A Large Study Provides More Evidence That MMR Vaccines Don’t Cause Autism.” This article quoted lead author Anders Hviid, conclusively stating that “MMR does not cause autism.” The study, according to NPR, “found no increased risk among subgroups of children who might be unusually susceptible to autism, such as those with a brother or sister with the disorder” [[67]].
The headline of a LiveScience article about the study stated, “Confirmed: No Link Between Autism and Measles Vaccine, even for ‘At Risk’ Kids” [[68]].
A headline in the New York Times trumpeted, “One More Time, With Big Data: Measles Vaccine Doesn’t Cause Autism” [[69]].
“Another Massive Study Finds Measles Vaccine Doesn’t Cause Autism”, said the headline of a Healthline article that quoted coauthor Mads Melbye saying, “It’s time to bury the hypothesis that MMR causes autism” [[70]].
MedicalNewsToday reported, “MMR vaccine does not cause autism, even in those most at risk” [[71]].
“Study Again Confirms No Link Between MMR Vaccine and Autism”, read the headline of a Psychiatry Advisor article claiming that the study showed the vaccine “does not trigger autism in children who are susceptible to the disorder” [[72]].
The New Yorker magazine stated, “The science on this point is settled, to the extent that any science ever is, in the pursuit of proving a negative” [[73]].
The media characterized the study as rejecting the hypothesis of a causal association between MMR vaccine and autism in “susceptible children.” However, as discussed shortly, Hviid et al. excluded children who had any one of several genetic conditions specifically because those conditions are associated with an increased risk of autism. Not one of those media reports relayed this salient fact to readers. Nor, for that matter, was there even the slightest critical examination by the media or the AAP of the study’s methodology, findings, and conclusions. Contrary to what we’ve been told by mainstream media, as discussed in this critical commentary, the study of Hviid et al. cannot conclusively demonstrate that the MMR vaccine does not cause ASD in “susceptible children.” Moreover, it certainly also does not falsify the hypothesis that the MMR vaccine or vaccines administered according to the CDC’s schedule can contribute to the development of ASD in susceptible children. Indeed, there are substantial nuances within the study by Hviid et al., a critical examination of which reveals that it was not faithfully applied to test this hypothesis and therefore cannot possibly have falsified it.
STUDY OVERVIEW
4.1. Aims
A preceding retrospective cohort study (children born in Denmark 1991 – 1998) by Madsen and colleagues, including Hviid and Melbye, was published in 2002 in The New England Journal of Medicine (NEJM) [[74]], and titled, “A population-based study of measles, mumps, and rubella vaccination and autism.” Using analogous methodologies and data sources, Madsen et al. 2002 concluded as Hviid et al. that “This study provides strong evidence against the hypothesis that MMR vaccination causes autism.” Hviid et al. referred to this previous study and stated, “In this study, we aimed to evaluate the association again in a more recent and nonoverlapping cohort of Danish children that has greater statistical power owing to more children, more cases, and longer follow-up”, and “To evaluate whether the MMR vaccine increases the risk for autism in children, subgroups of children, or periods after vaccination.” The authors note the criticism that the earlier study “did not address the concern that MMR vaccination could trigger autism in specific groups of presumably susceptible children.” They claimed that their new study “addresses this concern in detail” by evaluating “the risk for autism after MMR vaccination in subgroups of children defined according to environmental and familial autism risk factors.”
4.2. Study Design, Methodology & Demographic, and Conclusions
The authors analyzed data for 663,236 children born in Denmark to Danish-born mothers from January 1, 1999, through December 31, 2010. Of these children, 5,775 were excluded, resulting in a cohort of 657,461 children. The observation period was from age one until August 31, 2013, so the earliest-born children had reached the age of fourteen by the end of follow-up, whereas the latest-born were still as young as two years. The total number of children who were followed until the end of the study was 650,943, and among these children, 6,517 (1%) had received a diagnosis of autism as of the follow-up end date. The average age of autism diagnosis for their study population was 7.22 years for children born in Denmark from January 1994 – 1999. Parner et al. by contrast, reported in 2008 that the average age of autism diagnosis in Denmark was 5 – 6 years, observing a decrease in the age of diagnosis over the study period [[75]]. Overall, the Hviid et al. study population was about 95% “vaccinated”, with an average vaccination age of 1.34 years (≈ 16 months). Among children with autism, 5,992 (92%), were “vaccinated” and 525 (8%) were “unvaccinated.” Hviid et al. summarize their methodology as follows; “Survival analysis of the time to autism diagnosis with Cox proportional hazards regression was used to estimate hazard ratios of autism according to MMR vaccination status, with adjustment for age, birth year, sex, other childhood vaccines, sibling history of autism, and autism risk factors (based on a disease risk score).”
The Cox regression model has been primarily applied by researchers for time-to-event analysis and allows one to estimate the hazard ratio (HR) of a given endpoint associated with a specific risk factor. Analysis by survival or time-to-event is frequently used in epidemiological and clinical studies [[76]]. As discussed by Anderson et al., time-to-event data typically feature challenges related to, among other things, censored observations and changes over time in the absolute or relative risks, as well as in the values of the predictors. In the context of “rare events” like autism, such approaches can suffer from erratic behavior [[77]]. A fundamental assumption underlying the application of the Cox model is proportional hazards; in other words, the effects of different variables on survival are constant over time and additive over a particular scale [[78]]. The chosen methodology also involved comparing the cumulative incidence of autism for the “vaccinated” and “unvaccinated” cohorts, calculated as the number of new events or cases of a disease divided by the total number of individuals in the population at risk for a specific time interval. Thereby, a child remained “at risk” of developing autism until they received an autism diagnosis or were otherwise “censored” from the study, meaning that they ceased to be included in the population of children under observation and hence ceased contributing to “person-years” at risk.
Children in the cohort contributed person-time to follow-up from 1 year of age to the end of the study on 31 August 2013, until a first diagnosis of autism, or censorship. So, for example, a child born in 1999 who was uncensored from the study until its end without having received an autism diagnosis would have contributed 14 years of “person-time” (or “person-years”) at risk, whereas a child born the same year who received an autism diagnosis in 2004 would have contributed five person-years at risk. The incidence rate among the study population was 129.7 cases of diagnosed autism per 100,000 person-years. Children were excluded due to diagnosis of any of several genetic disorders or conditions or censored due to death, emigration, or disappearance.
The key finding from the main analysis of the study was that “vaccinated” children were not at a higher risk of autism than “unvaccinated” children. As stated in the abstract, “Comparing MMR-vaccinated with MMR-unvaccinated children yielded a fully adjusted autism hazard ratio of 0.93 (95% CI, 0.85 to 1.02).” Figure 3 from Hviid et al. further summarizes HRs, confidence intervals, and p-values of correlation. Except for the reduced association of autism and the MMR vaccination for females (HR = 0.79, 95% CI = 0.64 – 0.97) all p-values were > 0.05, indicating no significant association. On this basis, the authors made bold conclusions that “The study strongly supports that MMR vaccination does not increase the risk for autism, does not trigger autism in susceptible children, and is not associated with clustering of autism cases after vaccination. It adds to previous studies through significant additional statistical power and by addressing hypotheses of susceptible subgroups and clustering of cases.” However, there are numerous reasons why their findings can and should not support these bold conclusions, including major study flaws, numerous discrepancies, and unexplained analysis; salient features, yet all of which remained oblivious to regulators, associations, and mainstream media in the USA.
Study Design Flaws
5.1. Misleading Definition of “Genetic Susceptibility”, Exclusion of Children with High Susceptibility & Inadequate Sample Size
One of the criticisms of the studies cited by the CDC to support its claim that “vaccines do not cause autism” is that they do not consider the possibility of “susceptible subpopulations.” Hviid et al. acknowledge this and state, “Specific definitions of susceptible subgroups have been lacking.” The authors reference and follow the lead of Jain et al. [[79]] in defining “genetic susceptibility” merely as having “a sibling history of autism” at the time of study entrance. Therefore, if a child had an autistic sibling, but the sibling was not diagnosed until after the child had entered the study, then the child would have been misclassified as not “genetically susceptible.” Likewise, if a child had a genetic or environmental susceptibility but had no siblings, the child would have been wrongly classified. 49% of the study population was defined as having no “genetic susceptibility” simply by being an only child, as there were 319,936 children with no siblings out of a study population of 657,461.
The central dogma that autism is a highly heritable genetic disease is under debate. In 2011, Hallmayer et al. [[80]], in the largest twin study to date, reported moderate genetic heritability of 37-38% [[81]]. Later in 2014, using an epidemiological sample from Sweden, Gaugler et al. [[82]] concluded that autism's genetic architecture has a narrow-sense heritability of ≈52.4%, with most due to the common variation and rare de novo mutations. Considering current evidence of moderate autism genetic heritability, if a child had a genetic susceptibility and one or more siblings, but no siblings sharing the genetic trait or environmental trigger, the child would be wrongly classified as not “genetically susceptible.”
Moreover, and counterintuitive to the stated aims of the study, while touting their study as being designed to “address the concern that MMR vaccination could trigger autism in specific groups of presumably susceptible children”, Hviid et al. excluded 620 children who received a diagnosis during the first year of life of any of the following genetic disorders: neurofibromatosis, tuberous sclerosis, Angelman syndrome, Fragile X syndrome, Prader-Willi syndrome, Down syndrome, and DiGeorge syndrome. All these disorders demonstrate a higher rate of comorbidity with autism [[83],[84],[85],[86],[87],[88],[89]]. While they did not explain their rationale for these exclusionary criteria, their working assumption was presumably as follows: if these children were later diagnosed with autism, then it was due to their underlying condition and not vaccination. However, that syllogism is a non sequitur fallacy; the conclusion doesn’t follow from the premise. In effect, Hviid et al. treated all these conditions as competing hypotheses. One would contend they should have treated them as potential risk factors or indicators of epigenetic susceptibilities that might predispose these children to vaccine injury manifesting as symptoms of autism. Therefore, by excluding those children, the authors acted directly contrary to their stated purpose to investigate if “vaccination could trigger autism in specific groups of presumably susceptible children.”
As we have seen, the media touted this study as “large”, quoting a study population of 657,461. However, what the media consistently failed to point out is that only a small number met the authors’ definition of being “genetically susceptible”, with only 838 (0.13%) children meeting the criterion of having a sibling with autism. Following on, the reported HR for “siblings with autism” indicated an HR of 2.69 for autism (95% CI 0.58 – 12.63) among those who received the MMR vaccine compared to those who didn’t. Although this correlation was not statistically significant, one can speculate the result may have been significant if the authors had not excluded the 620 children with genetic disorders. We will never know since the authors have refused to release their underlying data to other scientists to be able to reproduce the authors’ findings.
Apart from genetic factors, Hviid et al. developed an “autism risk score” based on several “environmental autism risk factors”, but these were limited to “maternal age, paternal age, smoking during pregnancy, method of delivery, preterm birth, 5-minute Apgar score, low birth weight, and head circumference.” Although a low 5-minute Apgar score, low birth weight, and large head circumference may be indicative of a developing autism phenotype, it would be incorrect to label them as “risk factors” in the etiology or pathogenesis of ASD. In addition, apart from “smoking during pregnancy”, there is no consideration for assessment of the risk from xenobiotic environmental insults. This includes a long legacy of scientific literature spanning many decades implicating exposure to pharmaceuticals, industrial chemicals, and toxic and heavy metals in the etiology of ASD [[90]]. Future studies should apply a more rigorous risk assessment of exposure to environmental toxins [[91],[92],[93]] and other socioeconomic factors. For example, scientists could develop a risk score for exposure to environmental toxins based on factors including but not restricted to geographical location. Recent work by Palmer et al. [[94]] provides state-of-the-art approaches for rigorous assessment of chemical risk factors and intolerance in children and parents of developing autism and ADHD. This includes a complete evaluation of symptoms, intolerances, and life impacts of chemical, food, and drug exposures.
The second entry for the definition of the verb “lie” in Merriam-Webster’s dictionary is “to create a false or misleading impression” [[95]]. We would infer this is precisely what Hviid et al did when they delivered the public message that their study proved that the MMR vaccine “doesn’t cause autism even in children who are at greater risk of autism” or “genetically susceptible.” Indeed, Hviid et al. defined “genetic susceptibility” and did include children who met their definition. However, as outlined above, such an “ad hoc” definition of “genetic susceptibility” lacks scientific rigor and is inadequate and misleading.
5.2. Failure to control for “healthy user bias”
5.2.1. Jain et al.
A “healthy user bias” has been highlighted in previous studies for vaccination uptake [[96],[97],[98]]. In this scenario, parents of children who show symptoms at an early age or who have an older MMR-vaccinated sibling with autism, developmental delays, or other chronic disease, are more likely to skip the MMR vaccine, thereby biasing correlations in favor of finding no association. This “healthy user bias” was acknowledged by Hviid et al., who reference the study of Jain et al., [[99]] which “identified lower MMR uptake rates in children with affected siblings.” Published in 2015, Jain et al. investigated autism occurrence by MMR vaccine status among US children with older siblings with and without autism. The conclusion drawn by Jain et al. was that “In this large sample of privately insured children with older siblings, receipt of the MMR vaccine was not associated with increased risk of ASD, regardless of whether older siblings had ASD.” Based on the lower vaccine uptake among children who were considered at higher risk of autism due to “genetic factors”, such conclusions would need reevaluation.
To illustrate, as detailed by Jain et al., whereas the MMR vaccination rate for children with unaffected siblings was 84% at age 2 years and 92% at age 5, by contrast, the vaccination rate for children with autistic older siblings was 73% at age 2 and 86% at age 5. This would indicate that parents whose first child is diagnosed with autism after receipt of the MMR vaccine are less likely to get the shot for their second child for fear that it might contribute to the development of autism in the younger sibling. Similarly, parents who notice early developmental delays might skip the MMR vaccine for fear of it contributing to the development of autism. In the authors’ own words, considering that lower relative risk (RR) estimates were observed among children with autistic older siblings versus children with unaffected siblings, “It is possible, for example, that this pattern is driven by selective parental decision-making around MMR immunization, i.e., parents who notice social or communication delays in their children decide to forestall vaccination. Because as a group children with recognized delays are likely to be at higher risk of ASD, such selectivity could result in a tendency for some higher-risk children to be unexposed . . . . It is also plausible that parents of affected older siblings would be especially attentive to developmental delays in their younger children and decide to forestall immunization.” Thus, Jain et al. reasonably hypothesized that families with one child already affected by autism might be particularly concerned about this for any younger siblings, resulting in a lower vaccination rate among “genetically susceptible children.” In addition, although not statistically significant, Jain et al. found a negative correlation between the rate of autism in children with an autistic older sibling and receipt of the MMR vaccine. Rather than indicating some protective effect of the vaccine, we would speculate this would further indicate confounding by “healthy user bias.” This is an inherent risk of confounding in all observational studies, which needs to be accounted for and controlled for.
5.2.2. Hviid et al.
We know that Hviid et al. were aware of “health user selection bias” because they cited Jain et al. and acknowledged their finding of lower vaccine uptake among “susceptible children.” Yet they failed to account for it. Indeed, Hviid et al. affirmed that children who had siblings with autism had 7.32 times greater HR (95% CI 5.29 - 10.12) of autism relative to children who had siblings without autism. They also acknowledged the finding of Jain et al. that children with an autistic older sibling were less likely to receive the MMR vaccine. However, based on their analysis of the Danish study population, they claimed to have observed a vaccination rate of 95.19% and “no appreciable differences in vaccine uptake according to . . . . autism history in siblings.” On closer examination of the data, alternative interpretations are needed. For girls, the authors leaped baselessly to the conclusion that the vaccine is protective, asserting that MMR vaccination “reduced the risk for autism in girls.” We would conjecture that the overall negative (HR = 0.79, 95% CI 0.64 – 0.97) association could instead be due to girls at “higher risk” being less likely to receive the vaccine. The study’s table of population characteristics shows that 838 of the children in the study population had a sibling with autism, among whom 759 (90.6%) were MMR-vaccinated and 79 (9.4%) were not. Thus, whereas in the general study population only 4.8% were “unvaccinated”, the proportion who were unvaccinated among “genetically susceptible” children was nearly double that. Figure 3 from Hviid et al. further shows that among these 838 “genetically susceptible children”, 37 (4.4%, or 1 in 23) were diagnosed with autism. As discussed earlier, the HR shown for this cohort indicates a 2.69 times greater risk of autism among “vaccinated” children compared to “unvaccinated”. Among the 37 children diagnosed with autism, 32 were “vaccinated”, and 5 were “unvaccinated.” Therefore, 4.2% of the susceptible vaccinated children had an autism diagnosis compared to 6.3% of the susceptible unvaccinated children, indicating a possible pooling of children at “higher risk” into the “unvaccinated” group.
To approach the question from yet another angle, 759 of the 625,842 “vaccinated” children had an autistic sibling compared to 79 of the 31,619 “unvaccinated” children. Therefore, 0.12% of “vaccinated” compared to 0.25% of “unvaccinated” children were “genetically susceptible.” The “unvaccinated” were, thus, twice as likely to be “genetically susceptible” according to the author’s definition. In addition, the table shows that 319,936 children in the study had no siblings, among whom 4.2% were “unvaccinated”, and 331,994 had siblings without autism, among whom 5.3% were “unvaccinated.” These proportions contrast with the 9.4% of children with autistic siblings being “unvaccinated.” Children considered “genetically susceptible” were thus 1.8 times more likely than children with non-autistic siblings and 2.3 times more likely than single children to remain MMR-unvaccinated. Looking again at environmental risk factors for autism, Table 1 of the study shows that 3.97% of “very-low risk”, 4.35% of “low risk”, 5.44% of “moderate risk”, and 6.79% of “high-risk” children remained “unvaccinated.” Therefore, “high-risk” children were 1.25 times more likely than “moderate risk”, 1.56 times more likely than “low-risk” and 1.71 times more likely than “very low-risk” children to remain MMR-unvaccinated. This would again indicate potential confounding of “healthy user bias” with environmental and genetic risk factors.
Hviid et al. describe their study as “by far the largest single study to date” and state that it “allows us to conclude from one study that even minute increases in autism risk after MMR vaccination are unlikely, assuming unbiased results.” If their findings instead reflect the same “healthy user bias” identified by Jain et al., then the study by Hviid et al. does not allow us to draw such a conclusion. Strikingly, Hviid et al. acknowledge this limitation: “If the onset of symptoms results in avoidance of vaccination”, they admitted, bias in favor of no association “is possible.” Should they have said, “likely?”
5.3. Failure to Consider All Vaccines Routinely Recommended for Children in Denmark
The study of Hviid et al. focused only on the effect of the MMR vaccine on autism rates and not the complete Danish childhood vaccine schedule. In a secondary analysis, they also considered other routinely administered vaccines as covariables. From this secondary analysis, they concluded that “MMR vaccination did not increase the risk for autism in children characterized by other early childhood vaccinations. . . .” However, this analysis was limited to include only “other childhood vaccinations administered in the first year of life”, with no consideration of vaccinations given to children after twelve months other than the first MMR dose typically given at fifteen months. Other vaccines considered by Hviid et al. included five of the six vaccines recommended by Danish health authorities for routine use in infants under one year of age [[100],[101]]. Hviid et al. described the “mainstays” of the early Danish vaccine schedule as consisting of “MMR and diphtheria, tetanus, acellular pertussis, inactivated polio, and Haemophilus influenzae type b (DTaP-IPV/Hib) combination.” In Denmark, the combination of DTaP, IPV, and Hib vaccines has been recommended for infants, each with a three-dose course. However, Hviid et al. failed to consider that Danish authorities had since October 2007 additionally recommended three doses of the pneumococcal conjugate vaccine (PCV) during the first year of life [[102]]. The introduced formulation was the 7-valent PCV7, replaced with the 13-valent PCV13 starting in April 2010 [[103]]. An additional fourth “booster” dose of DTaP-IPV combination vaccine is also recommended at age 5 [[104]]. A four-dose course of hepatitis B (HepB) vaccine was recommended in 2005 for children born to mothers who are a carrier at birth and then 1, 2, and 3 months of age [[105]], which Hviid et al. further failed to consider. The authors also failed to consider the human papillomavirus vaccine (HPV) [[106],[107]]. According to the World Health Organization (WHO) [[108]], “The primary target group in most of the countries recommending HPV vaccination is young adolescent girls, aged 9-14.” In Denmark, Merck’s [[109]] quadrivalent HPV vaccine Gardasil was introduced in October 2008 as a catch-up program targeting 12-year-old girls, with routine vaccination for girls aged 12 years starting in January 2009. The study’s follow-up period was from January 1, 2000, through August 31, 2013, and girls of this initial birth cohort would have reached the age of thirteen or fourteen. While girls born in subsequent cohorts would have been too young to receive the HPV vaccine (unless administered earlier than the age of 12), girls in this 1999 – 2001 cohort may have received the HPV vaccine starting in 2011.
Thus, while this is not a flaw in the study per se, the choice by Hviid et al. to narrow their focus fails to meaningfully address parents’ concerns about the long-term effects on health outcomes of the complete and extended Danish vaccine schedule [[110]]. However, even if they had done such a study, its findings would not have been generalizable to the US childhood population since Denmark has a different schedule than that recommended in the USA.
5.4. Failure to Account for MMR Formulation Change
According to Hviid et al., the MMR vaccine used in Denmark from 2000 through 2007 contained the Schwarz strain of measles virus, which would have been GlaxoSmithKline’s (GSK’s) “Priorix” vaccine, and a different formulation was used from 2008 through 2013 that contained the Enders’ Edmonton strain, which would have been Merck’s “MMR-II.” This would indicate that children using the Merck formulation were much too young to receive an autism diagnosis as the oldest they would be at the time of study is 6 years of age or younger. On further evaluation, however, the information provided by Hviid et al. is incorrect; they mistakenly reversed the order in which MMR vaccines were used during those periods. According to a 2018 study on the use of the MMR vaccine in Denmark by Sørup et al., [[111]] until 2008, the Danish vaccination program used Merck’s MMR-II, which was marketed in Europe as “Virivac” and contained the Enders Edmonston B strain of measles virus [[112],[113],[114]]. From mid-October 2008, “Virivac” was replaced by GSK’s “Priorix”, which contained the Schwarz strain of measles virus [[115]]. Since mid-June 2013, a new version of Merck’s MMR-II has been used, which is manufactured by Sanofi Pasteur and marketed as “MMRvaxPro”, and which likewise contains the Edmonston strain of measles virus [[116],[117],[118],[119]]. Coauthor Christine Stabell Benn (personal communication, August 19, 2024) [120] corresponded with lead author Signe Sørup to confirm that the information in their paper was correct. GSK’s Priorix was used from 2008 until 2013, not Merck’s MMR-II, as mistakenly reported by Hviid et al. [[121]] (email correspondence is provided in supplementary material, Appendix 1). Following on, the average age of autism diagnosis for their study population was 7.22 years, and the typical age of first MMR vaccination in Denmark is 15 months. Since the study’s follow-up period ended on August 31, 2013, children who received “Priorix” would have been under 5 years of age and thus, on average, too young to receive an autism diagnosis [[122]]. Again, this could bias the study in favor of finding no association between the vaccine administered to the 2008 – 2010 birth cohort and the risk of autism.
5.5. Children Too Young for Autism Diagnosis
Hviid et al., report the average age of the sample as 8.64 years with a standard deviation (SD) of 3.48 years. The average age of autism diagnosis is reported as 7.22 years, with an SD of 2.86 years. If the age of diagnosis follows a normal distribution, 34.2% of the sample (z = -0.408) would be too young to get an autism diagnosis. This could account for as many as 3,387 additional cases not included in the analysis, which would further bias the outcomes to favor acceptance of the null hypothesis and no association between MMR vaccine and autism.
5.6. Failure to Consider a Change of Recommended Age for 2nd MMR Dose
Hviid et al. did not consider the second dose of the MMR vaccine in their primary analysis. In a secondary analysis, they reported “no evidence of a dose-response.” However, they failed to account for a change in the recommended age at which the second dose was administered. The first dose of MMR vaccine is recommended for children in Denmark at 15 months, followed by a second dose at the age of 4 years. However, before April 2008, the second dose was routinely administered at age 12 [[123]]. Therefore, children in the birth cohorts of 1999 – 2001 and 2002 – 2004 would not have received the second dose until years after the average age (7.22 years) of an autism diagnosis for the overall study population. Receiving the second dose of the MMR vaccine earlier in childhood development rather than in early adolescence may be associated with an increased risk of autism. The inclusion in the secondary analysis of those cohorts of older children who did not get both doses during early childhood would again bias the results erroneously in favor of acceptance of the null hypothesis.
5.7. Failure to Consider Maternal Vaccination
Maternal vaccination is another factor that Hviid et al. failed to account for in their study. It is recognized within the scientific community that maternal inflammation is associated with the development of autism in the offspring [[124]]. Vaccines intended to produce an immune response involving inflammation mechanisms could infiltrate the placenta, compromise fetal development, and increase the risk of ASD in offspring [[125],[126],[127]]. In alignment with recommendations by the WHO [[128]], the Danish Health Authority, since 2010, has recommended seasonal trivalent inactivated split influenza virus vaccination for pregnant women with selected high-risk chronic diseases in any trimester; and vaccination is additionally recommended for all pregnant women in the second and third trimesters [[129]]. Mølgaard-Nielsen et al. report up to 10% vaccine uptake by Danish women between 2010 – 2016. This means children born in the last cohort and reaching the age of 3 years by the end of the follow-up period may have been born to mothers vaccinated during pregnancy. Future studies should account for prenatal risk factors, including vaccination and the use of other pharmaceuticals during pregnancy.
5.8. Exclusion of Immigrants
Hviid et al. included only children “born to Danish-born mothers from 1 January 1999 through 31 December 2010” and registered in the Danish Medical Birth Registry, with the exclusion of 1,498 children. Asylum seekers to Europe may come from war-torn countries where health systems have broken down. There is evidence that asylum-seeking children have low coverage of childhood vaccinations in their home countries, as well as high uptake of immunizations in host countries [[130],[131]]. Therefore, immigrant children might receive multiple vaccines, doses, and boosters at once or spaced closer together to “catch up” on ones they may have missed in their home country. This might place immigrant children at higher risk of vaccine injury and developmental disorders such as autism. In addition, children of non-Danish ethnicity may have a higher risk of autism due to one or more epigenetic traits. Their exclusion could further bias the study’s findings.
5.9. Potential Misclassification of Study Subjects
As the authors acknowledge, “A limitation of our study is that we used the date of first diagnosis of autism, which is probably delayed compared with the age at onset of symptoms.” They then suggest this might bias their findings in favor of an association between MMR vaccination and autism by citing a hypothetical example in which “symptoms precede vaccination and diagnosis occurs after vaccination”, resulting in “misclassification of autism cases as vaccinated.” While they focus on the hypothetical scenario of bias favoring an association, they do not account for the misclassification of “vaccinated” children as “unvaccinated.” A study published in 2017 by Holt et al. [[132]], using data from the Danish National Health Service Register, addressed known concerns about the reliability of vaccination coverage data. To that end, the study authors compared MMR vaccination coverage according to medical records from general practitioners with that reported by the national registration database. Researchers report that the national database showed significantly lower vaccine coverage than medical records. Among the practices included in the study, the national database showed vaccine coverage of 86%, whereas the medical records showed coverage of 94%. The study authors state, “More than half of the children who were unvaccinated according to the register-based data (55%) had been vaccinated according to the medical records.” Vaccinated children being misclassified as “unvaccinated” in the study by Hviid et al. would, of course, bias their findings in favor of the null hypothesis and no association.
Discrepancies in Autism Rate in the Study Group vs. Danish Population
In
Figure 1 of their study, Hviid
et al. report 6,517 children with autism out of a population of 650,943, equating to 1%. This includes only subjects followed until the end of the study. If one takes account of the 657,461 children initially included an autism prevalence of 0.99% can be calculated. The prevalence of autism in Denmark in 2016, according to a study published by Schendel and Thorsteinsson [[133]] was 1.65%. Given a study population of 657,461 children, of whom 650,943 were followed until the end of the follow-up period, at a prevalence rate of 1.65%, we should expect there to be between 10,741 and 10,848 children with autism, whereas, in the study population, there were only 6,517. This would indicate an under-ascertainment of between 4,224 and 4,331. However, although the Hviid
et al. study was published in 2019, the observation period for the study population ended on August 31, 2013. Therefore, the prevalence of autism for that year would make the most valid comparison. According to the study of Schendel and Thorsteinsson among children aged 10 years, the prevalence of autism was 1.16% by 2010 (representing the birth cohort of 2000 – 2001), 1.33% by 2012 (birth cohort 2002 – 2003), 1.44% by 2014 (birth cohort 2004 – 2005), and 1.65% by 2016 (birth cohort 2006 – 2007). Given an estimated prevalence by 2012 of 1.33%, we would expect the Hviid
et al. study population to include 8,658 to 8,744 children with autism, indicating that approximately 2,141 to 2,227 autistic children were missing from the study. This suggests either that Schendel and Thorsteinsson's estimated prevalence of autism was grossly inaccurate or that the population under study by Hviid
et al. was not representative of the childhood population of Denmark. The latter explanation is more likely as the study of Schendel and Thorsteinsson, unlike Hviid
et al., was designed to estimate prevalence, and its findings were consistent with CDC data for the US childhood population, with an observed increase in the prevalence of autism for each birth cohort [[134]]. By contrast, when broken down by the age of each birth cohort, the data presented by Hviid
et al. show a decreasing prevalence of autism. Figure 3 from Hviid
et al. summarizes the total number of children with autism for each birth cohort. This enables one to calculate the prevalence of diagnosis based on age for each birth cohort; namely, 1.71% for the 1999 – 2001 cohort, 1.28% for 2002 – 2004, 0.74% for 2005 – 2007, and 0.20% for 2008 – 2010. These discrepancies would indicate methodological flaws in the study of Hviid
et al., that render their study population non-representative. The authors do not acknowledge these discrepancies, much less provide any explanation.
Irreproducible Findings
Reproducibility is an essential aspect of the scientific method [[135]]. It is crucial to advancing scientific knowledge because it ensures that research findings are reliable and not due to error, chance, or bias. Without reproducibility, scientific claims remain unverified and are therefore of questionable reliability. While the data Hviid et al. present show a decreasing rate of autism from one birth cohort to the next, they contradictorily state in their paper that being in the later-born 2008 – 2010 cohort conferred the “highest risk for autism.” However, on inspection of Table 3 in the SI of Hviid et. al, using the 1999 – 2001 cohort for reference, they report an HR of 1.18 for 2002 – 2004, 1.31 for 2005 – 2007, and 1.34 for 2008 – 2010. So, children born in 2009 - 2010 were 1.34 times more likely to be diagnosed with autism than those born in 1999 – 2001, and so on. While this increasing risk is what we would expect to find, as shown, it directly contradicts the data shown in their paper. This puzzling discrepancy was noticed by statistician Elizabeth Clarkson (personal communication, April 4, 2019), who contacted the Annals of Internal Medicine staff and the study’s lead author, Anders Hviid, to inquire about this self-contradiction and to request their raw data (email correspondence is provided in supplementary material, Appendix 2) [136]. In reply to Clarkson’s email inquiry, Hviid confirmed that the trend shown by their HR could not be reproduced from the data they presented in the main paper. However, he said, they could not release their raw data because they were “prohibited from sharing these data by Danish law.” Clarkson then wrote the Annals editors to formally request the authors’ dataset, pointing out that “the results of this sophisticated regression model used for the results reported in Table 3 of the supplemental material is in direct contradiction to the crude associations computed from the data published in the paper itself.” In reply to Elizabeth Clarkson, the journal staff instructed her to direct her request to the study’s authors. Since there is a major self-contradiction between the data reported and their calculated HRs, serious concerns must be raised about their true scientific viability and irreproducibility.
Unexplained Risk of Autism Incidence For Boys and Girls with Genetic Susceptibility
In the abstract, Hviid included the caveat that “no increased risk for autism after MMR vaccination was consistently observed in subgroups of children defined according to sibling history of autism.” One interpretation of the adverb “consistently” would logically imply an increased risk was observed in at least one such subgroup of children. Indeed, for boys who had an autistic sibling, Figure 4 in the SI of Hviid et al. illustrates the cumulative incidence, which represents the male children who met the author’s criterion for having a “genetic susceptibility” to autism. From about age 7 onward, the higher cumulative incidence of autism was among the children who received the MMR vaccine. This increased risk of autism among vaccinated boys was not statistically significant, which may be an artifact of a small subset of boys considered in this analysis.
The same figure illustrates the cumulative incidence of autism among girls with an autistic sibling. From approximately age 4 - 11, among girls with “genetic susceptibility”, a greater cumulative incidence for those who received the MMR vaccine is displayed. However, between the ages of 11 and 12, there is a leap in the cumulative incidence for MMR-unvaccinated girls from approximately 1% to approximately 9%, resulting in a greater incidence of autism among the unvaccinated. Since the study’s follow-up period ended in 2013, the maximum age of 14 on this graph can only represent girls born in 1999. Comparably, only children born in or before 2002 could have reached the age of 11 before the study’s end. One can only speculate on the cause of the sudden increase in the cumulative incidence of autism at about age 11, which would be relevant to the 1999 – 2001 and 2002 – 2004 birth cohorts. The authors do not discuss this sudden increase in cumulative incidence, which is certainly a curiosity for which an explanation is warranted but lacking.
Non-Generalizability to the Us Childhood Population
Taking account of the endorsement of Hviid et al. by the USA´s media, regulators, and professional medical organizations and their claims that the vaccine-autism hypothesis had been falsified, the question arises if the conclusions presented here can be generalized to the US population. While Danish health authorities recommend the HepB vaccine for infants (considered) at risk, the CDC recommends a three-dose regimen of this aluminum-containing vaccine for all infants starting from the first day of birth. Similar to Denmark, during their first year of life, children in the US typically receive three doses each of DTaP, IPV, Hib, and pneumococcal conjugate vaccine (PCV15 or PCV20); but starting at the age of 6 months, American children also receive two or three doses of rotavirus vaccine (RV1 or RV5, respectively) and an inactivated influenza vaccine (IIV), multi-dose formulations of which contain the preservative thimerosal [[137]]. The rotavirus vaccine is not recommended in Denmark, and whereas Danish authorities recommend flu shots only for children aged 2 to 6 years and adults aged 65 or older, the CDC recommends annual flu shots, multi-dose vials of which also contain thimerosal, starting in infancy and continuing throughout an individual’s lifetime. Whereas Danish children receive a booster dose of DTaP and IPV at the age of 5 years, American children receive a fourth dose of IPV at 4 years, and for DTaP, a fourth dose at the age of 15 months, a fifth dose at the age of 4 years, and a booster dose of the adolescent and adult formulation Tdap at the age of 11 years. While in Denmark the first dose of MMR is typically given at 15 months, it is recommended earlier in the US, at 12 months, with a second dose in both countries at 4 years of age. The varicella (VAR) or “chickenpox” vaccine is not on Denmark’s childhood schedule but is recommended by the CDC at the age of 1 year and a second dose at age 4. The hepatitis A (HepA) vaccine is also not on Denmark’s schedule, while the CDC recommends it in a two-dose series spaced six months apart starting at the age of 1 year. The meningococcal (Men) vaccine is another shot not recommended for routine use in Denmark, whereas the CDC recommends it at age 11, with a second dose at age 16. While the HPV vaccine is recommended in Denmark for children aged 12 years, the CDC recommends its two-dose regimen starting at age 11 while okaying its administration for children as young as 9. Additionally, the CDC recommends the aluminum-containing Tdap vaccine, the potentially thimerosal-containing influenza vaccine, and the respiratory syncytial virus (RSV) vaccine for pregnant women [[138]].
In summary, the CDC recommends 73 shots for 17 diseases, with a whopping 28 injections by a neonate’s first birthday. At a two-month “well-childcare visit”, an infant may receive as many as six vaccines for eight pathogens. In comparison, the Danish schedule consists of twelve shots for six pathogens, with only four vaccines by their first birthday (three doses each of DTaP, IPV, Hib, and PCV13). Once again, so many opinion leaders, regulators, media, and professional associations in the USA are oblivious to these salient differences.
10.1. Hviid et al.
Hviid and his three coauthors (Hansen, Frisch, and Melbye), at the time of the study’s publication, were affiliated with the Statens Serum Institut (SSI), which develops vaccines and is “responsible for the purchase and supply of vaccines to the Danish national vaccination programs” [[155]]. Like the CDC, the SSI is a government agency and research institute; its purpose is to “ensure preparedness against infectious diseases and biological threats as well as control of congenital disorders.” For vaccine research, the SSI is “devoted to vaccines against tuberculosis, chlamydia, HIV and novel adjuvants to direct and potentiate the immune responses.” Upon the study’s publication, the SSI issued a press release proclaiming that it “once again invalidates the claim that the MMR vaccine increases children’s risk of developing autism” [[156]].
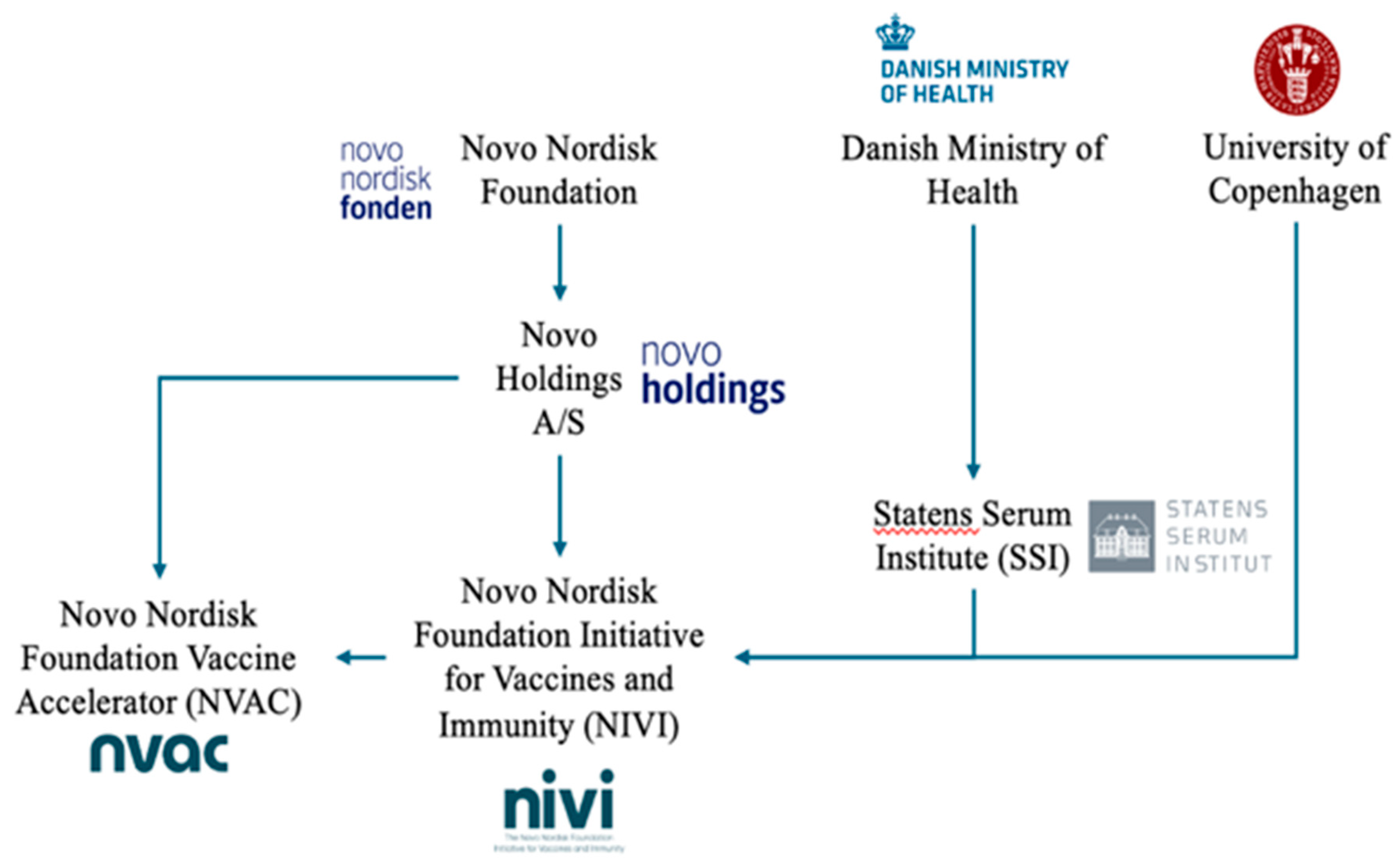
Financial support was provided by the Novo Nordisk Foundation [[157],[158]] and the Danish Ministry of Health [[159]]. The Novo Nordisk Foundation is a charitable foundation that issues funding grants for scientific research while owning the holding company Novo Holdings A/S [[160]], the majority voting shareholder in the Danish pharmaceutical corporation Novo Nordisk [[161]]. Novo Nordisk is a large multinational pharmaceutical company in Denmark with a market capitalization greater than US$497 billion [[162]]. According to their annual report, they anticipate an effective 2019 tax rate of 20-22%. The government of Denmark receives significant tax revenue from Novo Nordisk. Therefore, the Danish Ministry of Health and Novo Nordisk have a vested interest in a study that might influence the demand for the MMR vaccine. Also of note, Novo Holdings A/S investments include vaccine companies [[163]]. For example, in April 2019, the group invested tens of millions of dollars into Oxford Biomedica, which was involved in a consortium to develop and manufacture the AstraZeneca COVID-19 vaccine [[164],[165]]. In December 2023, the Novo Nordisk Foundation Initiative for Vaccines and Immunity (NIVI) was announced, [[166]] which is a partnership between the University of Copenhagen, and the SSI [[167],[168]]. The stated goal of NIVI is “to revolutionize and accelerate vaccine development in Denmark by bridging the gap between academic research and industry innovation.” Simultaneously, the foundation established a limited liability company, the Novo Nordisk Foundation Vaccine Accelerator (NVAC), to “facilitate the translational efforts of NIVI by providing industry-level expertise in vaccine development and conducting the early clinical testing of our vaccine candidates” [[169]]. Predating its vaccine initiative, the Novo Nordisk Foundation had funded numerous researchers in SSI and other institutions active in vaccine-related research [[170],[171],[172],[173],[174],[175]]. The foundation also funded the SSI “Danish National Biobank”, which aims to grant scientists access to data on residents in Denmark from national health registries along with information about biological samples [[176]]. The Danish Ministry of Health and the SSI unquestionably have a stake in preserving their own credibility and existing policies, not unlike the United States Department of Health and Human Services (HHS) and CDC. However, as summarized by Fig. 1 and as is the documented case with American regulatory agencies and professional bodies [[177]], an inherent conflict of interest in researching, marketing, and supplying childhood vaccines becomes apparent, if not explicit.
10.2. Annals of Internal Medicine
Two editors of the Annals of Internal Medicine, Jaya K. Rao (Deputy Editor) and Catharine B. Stack (Deputy Editor for Statistics), disclosed holding stocks in pharmaceutical companies active in vaccine research and manufacture. This includes Eli Lilly, Pfizer, and Johnson and Johnson. Eli Lilly is a former manufacturer of Dr. Jonas Salk’s inactivated polio vaccine and the developer of the mercury-based preservative thimerosal [[178]].
Discussion
In 2019, the AAP and mainstream media in the USA hailed the study by Hviid et al. as additional proof that the MMR vaccine does not increase the risk of ASD, even among “genetically susceptible children.” But the fact is that the study authors excluded children with any one of several genetic conditions placing them at higher risk, with an inadequate definition of “genetic susceptibility.” Based on highlighted methodological flaws, discrepancies, and conflicts of interest, we would venture that the outcomes from Hviid et al. would not indicate evidence of a lack of association between ASD and MMR but, instead, researcher bias to a priori serve the status quo. As an antidote, we would prescribe diligent scientists working in the field to take note and learn from Hviid et al. with a priori consideration of selection bias and risk factors, “healthy user bias”, and data calibration with positive and negative controls [[179]], which would provide paths to much-needed rigor in observation studies; especially when the stakes are so high, with CDC objectives for ubiquitous vaccination in pediatric populations.
Hviid and coworkers have used similar methodologies to assess risks of other adverse events and disorders from MMR vaccination and other pharmaceuticals used in pregnancy or childhood. For example, Hviid and coworkers have reported no association between ASD and thimerosal-containing vaccines [[180]]; no evidence of causality for childhood vaccination and type 1 diabetes [[181]]; an increased rate of febrile seizure following MMR vaccination deemed “small even in high-risk children” [[182]]; no significant association between maternal use of selective serotonin reuptake inhibitors during pregnancy and ASD in their offspring [[183]]; and no association between Ondanse (prescribed for nausea and vomiting) and increased risk of adverse fetal outcomes [[184]]. We would note that in studies for febrile seizure and type I diabetes in pediatric populations, the “genetic susceptibility” evaluation, as for Hviid et al. 2019, was based on family history, i.e., sibling history of adverse events or diabetes diagnosis. Clinical and preclinical evidence of adverse events from these pharmaceuticals is well documented [[185],[186],[187],[188]]. Further critical analysis of these studies, as provided here for Hviid et al. 2019, would be prudent, commended, and much needed.
In a famous case, the government acknowledged that the administration of nine vaccine doses at once to a 19-month-old girl named Hannah Poling “significantly aggravated an underlying mitochondrial disorder, which predisposed her to deficits in cellular energy metabolism and manifested as a regressive encephalopathy with features of autism spectrum disorder” [[189]]. At the time, then CDC director Julie Gerberding admitted on CNN [[190]], “Now, we all know that vaccines can occasionally cause fevers in kids. So, if a child was immunized, got a fever, had other complications from the vaccines, and if you’re predisposed to a mitochondrial disorder, it can certainly set off some damage. Some of the symptoms can be symptoms that have characteristics of autism.” Notably, in 2009 Gerberding left her government job to work for the pharmaceutical giant Merck as president of their vaccine division and later became responsible for “strategic communications” as Chief Patent Officer and Executive Vice President of the Company, Population Health & Sustainability [[191],[192]].
Indeed, a growing body of evidence implicates a strong interplay between environmental insults and epigenetics in the etiology and pathogenesis of ASD [[193]]. Bradstreet argued in a presentation given to the Vaccine Safety Committee in 2004 [[194]] that, “meaningful epidemiological studies should test a priori hypotheses that derive from all clues evident in the clinical histories of affected children . . . .” We would concur and endorse future prospective observational studies that truly account for epigenetic and environmental risk factors as part of the wider “ecological exposome” [[195]]. Although a non-trivial task, the SSI biobank initiative provides Danish researchers access to “25 million biological samples” [[196]], which should be ample and adequate, to define and address the association between genetic susceptibility, MMR, and ASD. In a 2014 interview with journalist Sharyl Attkisson, the CDC’s Director of the Immunization Safety Office, Frank DeStefano, acknowledged that “it’s a possibility” that vaccines could trigger ASD in “genetically susceptible individuals”, but that it is “hard to predict who those children might be”, and trying to determine what underlying conditions put children at greater risk of being injured by vaccines is “very difficult to do” [[197]]. Indeed, researchers at the CDC have acknowledged that no observational study “can definitively establish or disprove the hypothesis that thimerosal exposure increases the risk of ASDs”, which would instead require “a large-scale prospective randomized trial” [[198]].
No golden standard exists to judge if an observed association (or non-association) is genuine [[199]]. Placebo-controlled randomized clinical trials (RCT), although also prone to error [[200]], remain the gold standard for the inference of causality. However, no placebo-controlled RCT has investigated individual vaccines, let alone the overall effects of the vaccine schedule, for long-term health outcomes between vaccinated and unvaccinated children, including all-cause mortality. The common consensus among FDA regulators is that it is unethical to do “vax-unvax” clinical studies with true saline placebos [[201]]. We would agree, inasmuch as puncturing any baby’s skin over the course of a year with 28 intramuscular injections of saline placebo or multiple “biologic pharmaceuticals”, all formulated differently, many in one sitting, as part of any clinical study; would not only be unethical but barbaric. Moreover, this is an exemplary example of the petitio principii fallacy and institutional cognitive dissonance for vaccine research since it presumes a priori that the potential benefits outweigh any possible risks, which is precisely the proposition that should have been determined, as also argued by Bradstreet, through properly designed, rigorous, and robust RCTs. Despite this, a word of caution would be prudent. We would state that no “magic bullet” exists for testing causality, especially for rare and/or long-term serious adverse events. Indeed, questions of causality cannot be answered by RCTs alone because of the inherent low power in such studies [[202]]. Some discourse must also be given to the manufacturing of pharmaceutical biologics. Whereas the chemical synthesis of a small molecule drug may have a dozen steps that must be monitored and controlled, the fermentation process for vaccines may have hundreds [[203]]. Valiant et al. [[204]] and Chooi et al. [[205]] provide ample examples of past and current reports of contamination issues and vaccine recalls. Unquestionably, it is an important risk factor for vaccine injury, as would be the case for any pharmaceutical intended for ubiquitous prophylaxis of pediatric populations [[206]].
The challenges in making inferences from the evidence are no less great (but not particularly greater) when the evidence is based on large observational studies, as per Hviid et al. (or a small clinical case series, as per Wakefield et al.). However, if one truly takes an evidence-based approach, it would be ill-advised to be complacent about immunization and assume its innocence based on observational studies or even systematic reviews of the evidence [[207]]. As early as 2004, Price, Jefferson, and Demicheli highlight methodological issues arising from systematic reviews of vaccine safety, especially for rare and/or long-term serious adverse events [[208]]. Moreover, a growing body of scientists, healthcare professionals, and citizens question the benefits of vaccines, with evidence that child mortality and disease from infectious diseases had already decreased significantly before the widespread use [[209],[210],[211]]. Based on their observations, one can hope that immunization will become redundant through good sanitation, adequate waste disposal systems, clean water, nutritious food supply, wealth, and plenty for all children [[212]]. Nonetheless, the possibility remains that we may be creating long-term damage through vaccination, demonstrated by substantially increasing levels of serious and chronic disease, including autoimmune conditions, especially in populations made vulnerable by acute or chronic anthropogenic xenobiotic insult [[213],[214]].
Hviid et al., provide an example of how studies examining this question concerning the MMR vaccine could be interpreted as having been designed to find no association through design flaws biasing findings. Moreover, to date, no studies have been done that were designed to test the hypothesis that vaccinating according to the CDC’s schedule can contribute to the development of autism, chronic diseases, and all-cause mortality in children with epidemiological susceptibility. Therefore, scientists and medical practitioners must always be on their guard for evidence pointing to vaccine danger, as should be the case for side effects from any pharmaceutical intervention [[215]]. Evidence alone never speaks for itself or conveys the truth because it always requires interpretation. Expert opinion or population-based studies are not a surrogate for evidence-based, first-hand experience or data; science is not about consensus, it’s about the truth [[216],[217]]. Moreover, acquiring the right answers also requires asking the right questions. Barosi and Gale illustrate the point when they state that “Accuracy refers to getting the correct answer, and precision to getting the same answer on repetition regardless of whether it is the correct answer or not. A wrong answer which is reproducible is precise but inaccurate. What we need are accurate, precise answers.” Based on the case presented here, for Hviid et al., nothing could be more vital for the future of vaccine research.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Credit Authorship Contribution Statement
Hammond created the original study conception and first draft. Additional concept development and drafting by Varia and Hooker. Further writing, review, and editing by Varia, Hooker, and Hammond. All authors have read and approved the final manuscript.
Acknowledgments
Consultation, critical insights, and comments for the first draft from JB Handley, James Lyons-Weiler, Stephanie Seneff, and Elizabeth Clarkson.
Competing Interests: The author(s) declare none.
Conflicts of Interest
The stakes in the ASD debate are high. Over half a century, there has been a dramatic increase in ASD rates [[139]]. Identifying causative factors for ASD is already a challenging task for the scientific community, demanding the highest standards of openness and transparency. Any departure from these standards represents a disservice to all. The financial stakes in vaccine research are also high. The global biologics market was US$511.04 billion in 2024 and is expected to reach around US$1,374.51 billion by 2033 [[140]]. The approval and subsequent commercialization of gene therapy candidates are expected to drive growth in the biologics market. The vaccine market worldwide was valued at US$81.06 billion by 2023 and is anticipated to reach around US$152.45 billion by 2033 [[141]]. In the U.S., the COVID-19 vaccine market transitioned to a commercial phase following the depletion of the federal government's purchased stock. The global mumps vaccine market was valued at US$2 billion in 2021 and is projected to reach US$3.5 billion in 2031 [[142]]. When conflicts of interest influence research, the resulting scientific debate on safety and efficiency, etc., can be confounded by misleading information. Indeed, to ensure scientific quality, manuscripts authored by CDC staff are required to undergo an internal review and approval process known as clearance. As part of the domain of ethical standards, “free from conflicts of interests” is explicitly stated [[143]]. Kern et al., [[144]] summarize past and current examples of research conflict of interest and outside influences for tobacco, lead, methylmercury, atrazine, bisphenol A, and olestra. The CDC receives millions of dollars in industry gifts and funding, including substantial support from the pharmaceutical industry [[145],[146]]. Miller and Goldman [[147]] and Hooker [[148],[149]] provide firsthand details of how the CDC suppressed and disallowed deleterious vaccine data from being published and engaged in other acts of questionable scientific integrity. Nissen [[150]] discusses the dependence of professional medical associations on industry funding [[151]].
Physicians and the public rely on journals as unbiased and independent sources of information and to provide leadership to improve trust in medicine and the medical literature. Yet financial conflicts of interest have repeatedly eroded the medical profession's and journals' credibility [[152]]. During the past decade, two former editors-in-chief of the NEJM, Marcia Angell and Arnold Relman, have spoken out about the excessive power of the pharmaceutical industry over medical research, hospitals, and doctors. In a letter to the New York Times on December 28, 2004 [[153]], they pointed out that in the previous year, one drug company had spent 28 percent of its revenues (more than $6 billion) on marketing and administrative expenses. They concluded, “The medical profession should break its dependence on the pharmaceutical industry and educate its own.” In an article in the New York Review of Books on January 15, 2009, Angell wrote, “It is simply no longer possible to believe much of the clinical research that is published, or to rely on the judgment of trusted physicians or authoritative medical guidelines” [[154]].
References
- Centers for Disease Control and Prevention (CDC). Rubella (German Measles, Three-Day Measles). (2024) [cited 2024 Aug 15]. About Rubella. Available from: https://www.cdc.gov/rubella/about/index.
- DeStefano, F. , & Shimabukuro, T. T. ( 6(1), 585–600. [CrossRef]
- Mathis, A. D. (2024). Measles—United States, , 2020–March 28, 2024. MMWR. Morbidity and Mortality Weekly Report, 73. https://www.cdc.gov/mmwr/volumes/73/wr/mm7314a1.htm? 1 January 0951. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2005). Elimination of rubella and congenital rubella syndrome--United States, 1969-2004. MMWR. Morbidity & Mortality Weekly Report, 54(11), 279-282. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5411a5.
- Papania, M. J. , Wallace, G. S., Rota, P. A., Icenogle, J. P., Fiebelkorn, A. P., Armstrong, G. L.,... & Seward, J. F. (2014). Elimination of endemic measles, rubella, and congenital rubella syndrome from the Western hemisphere: The US experience. JAMA Pediatrics, 168(2), 148-155. [CrossRef]
- Tappe, J. , Leung, J., Mathis, A. D., Oliver, S. E., & Masters, N. B. (2024). Characteristics of reported mumps cases in the United States: 2018–2023. Vaccine, 42(25), 126143. [CrossRef]
- Barskey, A. E. , Glasser, J. W., & LeBaron, C. W. (2009). Mumps resurgences in the United States: A historical perspective on unexpected elements. Vaccine, 27(44), 6186-6195. [CrossRef]
- De Serres, G. , Markowski, F., Toth, E., Landry, M., Auger, D., Mercier, M.,... & Skowronski, D. M. (2013). Largest measles epidemic in North America in a decade—Quebec, Canada, 2011: Contribution of susceptibility, serendipity, and superspreading events. The Journal of Infectious Diseases, 207(6), 990-998. [CrossRef]
- Dayan, G. H. , Rubin, S., & Plotkin, S. (2008). Mumps outbreaks in vaccinated populations: Are available mumps vaccines effective enough to prevent outbreaks? Clinical Infectious Diseases, 47(11), 1458-1467. [CrossRef]
- Poland, G. A. , & Jacobson, R. M. (1994). Failure to reach the goal of measles elimination: Apparent paradox of measles infections in immunized persons. Archives of Internal Medicine, 154(16), 1815-1820. [CrossRef]
- Maenner, M. J. , Warren, Z., Williams, A. R., Amoakohene, E., Bakian, A. V., Bilder, D. A.,... & Shaw, K. A. (2023). Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2020. MMWR Surveillance Summaries, 72(2), 1. [CrossRef]
- Li, Y. , Yan, X., Li, Q., Li, Q., Xu, G., Lu, J., & Yang, W. (2023). Prevalence and trends in diagnosed ADHD among us children and adolescents, 2017-2022. JAMA Network Open, 6(10), e2336872-e2336872. /: https. [CrossRef]
- Zahran, H. S. , Bailey, C. M., Damon, S. A., Garbe, P. L., & Breysse, P. N. (2018). Vital signs: Asthma in children—United States, 2001–2016. Morbidity & Mortality Weekly Report, 67(5), 149. [CrossRef]
- Zablotsky, B. , Black, L. I., & Akinbami, L. J. (2023). Diagnosed allergic conditions in children aged 0-17 years: United States, 2021. NCHS Data Brief. No. 459. National Center for Health Statistics. https://www.cdc.gov/nchs/data/databriefs/db459.
- Ullah, F. , & Kaelber, D. C. (2021). Using large aggregated de-identified electronic health record data to determine the prevalence of common chronic diseases in pediatric patients who visited primary care clinics. Academic Pediatrics, 21(6), 1084-1093. [CrossRef]
- Killeen, R. M. (2007). Vaccines—one of the greatest medical advances of modern times. Canadian Pharmacists Journal/Revue des Pharmaciens du Canada, 140(2_suppl), S2-S2. [CrossRef]
- Guyer, B. , Freedman, M. A., Strobino, D. M., & Sondik, E. J. (2000). Annual summary of vital statistics: Tcrends in the health of Americans during the 20th century. Pediatrics, 106(6), 1307-1317. [CrossRef]
- Bianchi, F. P. , Mascipinto, S., Stefanizzi, P., De Nitto, S., Germinario, C., & Tafuri, S. (2021). Long-term immunogenicity after measles vaccine vs. wild infection: An Italian retrospective cohort study. Human Vaccines & Immunotherapeutics, 17(7), 2078-2084. [CrossRef]
- Horstmann, D. M. , Schluederberg, A., Emmons, J. E., Evans, B. K., Randolph, M. F., & Andiman, W. A. (1985). Persistence of vaccine-induced immune responses to rubella: comparison with natural infection. Clinical Infectious Diseases, 7(Supplement_1), S80-S85. [CrossRef]
- Amanna, I. J. , Carlson, N. K. ( 357(19), 1903–1915. [CrossRef]
- Papania, M. , Baughman, A. ( 104(5), e59–e59. [CrossRef] [PubMed]
- Mandomando, I. M. , Naniche, D., Pasetti, M. F., Vallès, X., Cuberos, L., Nhacolo, A.,... & Alonso, P. (2008). Measles-specific neutralizing antibodies in rural Mozambique: Seroprevalence and presence in breast milk. American Journal of Tropical Medicine and Hygiene, 79(5), 787-792. [CrossRef]
- Waaijenborg, S. , Hahné, S. J., Mollema, L., Smits, G. P., Berbers, G. A., van der Klis, F. R.,... & Wallinga, J. (2013). Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. The Journal of Infectious Diseases, 208(1), 10-16. [CrossRef]
- Sasco, A. J. , & Paffenbarger JR, R. S. (1985). Measles infection and Parkinson's disease. American Journal of Epidemiology, 122(6), 1017-1031. [CrossRef]
- Parodi, S. , Crosignani, P. ( 133(8), 1892–1899. [CrossRef] [PubMed]
- Kubota, Y. , Iso, H., Tamakoshi, A., & JACC Study Group. (2015). Association of measles and mumps with cardiovascular disease: The Japan Collaborative Cohort (JACC) study. Atherosclerosis, 241(2), 682-686. [CrossRef]
- Montella, M. , Dal Maso, L., Crispo, A., Talamini, R., Bidoli, E., Grimaldi, M.,... & Franceschi, S. (2006). Do childhood diseases affect NHL and HL risk? A case-control study from northern and southern Italy. Leukemia Research, 30(8), 917-922. [CrossRef]
- Rosenlund, H. , Bergström, A. ( 123(3), 771–778. [CrossRef]
- Shaheen, S. O. , Barker, D. J. P., Heyes, C. B., Shiell, A. W., Aaby, P., Hall, A. J., & Goudiaby, A. (1996). Measles and atopy in Guinea-Bissau. The Lancet, 347(9018), 1792-1796. [CrossRef]
- Benn, C. S. , Amenyogbe, N., Björkman, A., Domínguez-Andrés, J., Fish, E. N., Flanagan, K. L.,... & Aaby, P. (2023). Implications of non-specific effects for testing, approving, and regulating vaccines. Drug Safety, 46(5), 439-448. [CrossRef]
- Demicheli, V. , Rivetti, A., Debalini, M. G., & Di Pietrantonj, C. (2013). Vaccines for measles, mumps and rubella in children. Evidence-Based Child Health: A Cochrane Review Journal, 8(6), 2076-2238. [CrossRef]
- Miller, N. Z. (2021). Vaccines and sudden infant death: An analysis of the VAERS database 1990–2019 and review of the medical literature. Toxicology Reports, 8, 1324-1335. [CrossRef]
- Centers for Disease Control and Prevention (CDC). CDC vaccine safety. 2024 [cited 2024 Jul 14]. Autism and Vaccines. Available from: https://www.cdc.gov/vaccine-safety/about/autism.html?CDC_AAref_Val=https://www.cdc.gov/vaccinesafety/concerns/autism.
- Board on Health Promotion, Disease Prevention, & Immunization Safety Review Committee. (2004). Immunization safety review: Vaccines and autism. N: Washington (DC). [CrossRef]
- Committee to Review Adverse Effects of, Vaccines; Institute of Medicine. Adverse effects of vaccines: Evidence and causality. Clayton, E. W. Committee to Review Adverse Effects of Vaccines; Institute of Medicine. Adverse effects of vaccines: Evidence and causality. Clayton, E. W., Rusch, E., Ford, A., & Stratton, K. (Eds.). (2012). Adverse effects of vaccines: Evidence and causality. Washington (DC): National Academies Press (US). [CrossRef]
- Board on Population Health, Public Health Practice, & Committee on the Assessment of Studies of Health Outcomes Related to the Recommended Childhood Immunization Schedule. (2013). The childhood immunization schedule and safety: Stakeholder concerns, scientific evidence, and future studies. Washington (DC): National Academies Press (US). [CrossRef]
- Wakefield, A. J. , Murch, S. H., Anthony, A., Linnell, J., Casson, D. M., Malik, M.,... & Walker-Smith, J. A. (1998). RETRACTED: Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. The Lancet, 351(9103), 637-641. [CrossRef]
- Murch, S. H. , Anthony, A. A. ( 363(9411), 750. [CrossRef]
- Deer, B. (2010). Wakefield’s “autistic enterocolitis” under the microscope. BMJ, 340. [CrossRef]
- Deer, B. (2011). How the vaccine crisis was meant to make money. BMJ, 342. [CrossRef]
- Deer, B. (2011). How the case against the MMR vaccine was fixed. BMJ, 342. [CrossRef]
- Ekbom, A. , Adami, H. O., Wakefield, A., & Zack, M. J. T. L. (1994). Perinatal measles infection and subsequent Crohn's disease. The Lancet, 344(8921), 508-510. [CrossRef]
- Wakefield, A. J. , Ekbom, A., Dhillon, A. P., Pittilo, R. M., & Pounder, R. E. (1995). Crohn's disease: Pathogenesis and persistent measles virus infection. Gastroenterology, 108(3), 911-916. [CrossRef]
- Ekbom, A. , Daszak, P., Kraaz, W., & Wakefield, A. J. (1996). Crohn's disease after in-utero measles virus exposure. The Lancet, 348(9026), 515-517. [CrossRef]
- Thompson, N. P. , Pounder, R. E., Wakefield, A. J., & Montgomery, S. M. (1995). Is measles vaccination a risk factor for inflammatory bowel disease? The Lancet, 345(8957), 1071-1074. [CrossRef]
- Wakefield, A. J. , Puleston, J. M., Montgomery, S. M., Anthony, A., O'leary, J. J., & Murch, S. H. (2002). The concept of entero-colonic encephalopathy, autism and opioid receptor ligands. Alimentary Pharmacology & Therapeutics, 16(4), 663-674. [CrossRef]
- Wakefield, A. J. , Ashwood, P., & Collins, I. (2005). The gut-brain axis in childhood developmental disorders: Viruses and vaccines. Neuropsychiatric Disorders & Infection, 198. [CrossRef]
- Varia, J. , Herbert, M., & Hooker, B. (2024). The neuroimmunology of autism. [CrossRef]
- Turville, C. , & Golden, I. (2015). Autism and vaccination: The value of the evidence base of a recent meta-analysis. Vaccine, 33(42), 5494-5496. [CrossRef]
- Buie, T. , Campbell, D. B., Fuchs III, G. J., Furuta, G. T., Levy, J., VandeWater, J.,... & Winter, H. (2010). Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASD: A consensus report. Pediatrics, 125(Supplement_1), S1-S18. [CrossRef]
- Casiday, R. E. (2007). Children's health and the social theory of risk: Insights from the British measles, mumps and rubella (MMR) controversy. Social Science & Medicine, 65(5), 1059-1070. [CrossRef]
- Shapiro, D. , & Hayburn, A. (2024). Medical gaslighting as a mechanism for medical trauma: Case studies and analysis. Current Psychology, 1-14. [CrossRef]
- Williams, S. J. , & Calnan, M. (1996). The ‘limits’ of medicalization? Modern medicine and the lay populace in ‘late’ modernity. Social Science & Medicine, 42(12), 1609-1620. [CrossRef]
- Bennett, P. , Celik, F., Winstanley, J., Hunt, B. J., & Pavord, S. (2023). Living with vaccine-induced immune thrombocytopenia and thrombosis: A qualitative study. BMJ Open, 13(7), e072658. [CrossRef]
- Fagen, J. L. , Shelton, J. A., & Luché-Thayer, J. (2023, December). Medical gaslighting and Lyme disease: The patient experience. In Healthcare (Vol. 12, No. 1, p. 78). MDPI. [CrossRef]
- Leach, M. (2005). MMR mobilisation: Citizens and science in a British vaccine controversy. The Institute of Development Studies & Partner Organizations. https://hdl.handle.net/20.500. 1241. [Google Scholar]
- Angell, M. (2009). Drug companies & doctors: A story of corruption. The New York Review of Books, 56(1), 8-12. http://www.nybooks. 2223. [Google Scholar]
- Mello, M. M. , Abiola, S., & Colgrove, J. (2012). Pharmaceutical companies’ role in state vaccination policymaking: The case of human papillomavirus vaccination. American Journal of Public Health, 102(5), 893-898. [CrossRef]
- Lewis, D. L. (2012). Apparent egregious ethical misconduct by British Medical Journal, Brian Deer. UK Research Integrity Office (UKRIO), Reference, (2011-060). https://www.junkscience.com/wp-content/uploads/2012/01/david-lewis-bmj.
- Sharav, V. (2017). L’Affaire Wakefield: Shades of Dreyfus and BMJ’s descent into tabloid science. Alliance for Human Research Protection. https://ahrp.org/wp-content/uploads/2017/10/LAffaire-Wakefield-Shades-of-Dreyfus-BMJs-Descent-to-Tabloid-Journalism-Vera-Sharav-2017.
- Mohammed, S. A. , Rajashekar, S., Ravindran, S. G., Kakarla, M., Gambo, M. A., Salama, M. Y.,... & Hamid, P. (2022). Does vaccination increase the risk of autism spectrum disorder? Cureus, 14(8). [CrossRef]
- Turville, C. , & Golden, I. (2015). Autism and vaccination: The value of the evidence base of a recent meta-analysis. Vaccine, 33(42), 5494-5496. [CrossRef]
- Poltorak, M. , Leach, M., Fairhead, J., & Cassell, J. (2005). ‘MMR talk’ and vaccination choices: An ethnographic study in Brighton. Social Science & Medicine, 61(3), 709-719. [CrossRef]
- Hviid, A. , Hansen, J. V., Frisch, M., & Melbye, M. (2019). Measles, mumps, rubella vaccination and autism: a nationwide cohort study. Annals of Internal Medicine, 170(8), 513-520. [CrossRef]
- Jenco, M. AAP News. (2024) [cited 2024 Sep 9]. Study: MMR vaccine not linked to increased autism risk. Available from: https://publications.aap. 1392. [Google Scholar]
- Bracho-Sanchez, E. CNN. (2019) [cited 2024 Aug 1]. MMR vaccine does not cause autism, another study confirms. Available from: https://www.cnn.com/2019/03/04/health/mmr-vaccine-autism-study/index.
- Stein, R. NPR. (2019) [cited 2024 Aug 15]. A large study provides more evidence that MMR vaccines don’t cause autism. Available from: https://www.npr. 2019. [Google Scholar]
- Geggel, L. Live Science. (2019) [cited 2024 Aug 15]. Confirmed: No link between autism and measles vaccine, even for “at risk” kids. Available from: https://www.livescience.com/64909-measles-vaccine-not-linked-to-autism.
- Hoffman, J. New York Times. (2019) [cited 2024 Aug 1]. One more time, with big data: Measles vaccine doesn’t cause autism. Available from: https://www.nytimes.com/2019/03/05/health/measles-vaccine-autism.
- Mammoser, G. Healthline. (2024) [cited 2024 Aug 10]. Another massive study finds measles vaccine doesn’t cause autism. Available from: https://web.archive.org/web/20190307053507/https://www.healthline.
- MedicalNewsToday. Transcripts. (2019) [cited 2024 Aug 1]. MMR vaccine does not cause autism, even in those most at risk. Available from: https://www.medicalnewstoday. 3246.
- May, B. Psychiatry Advisor. (2019) [cited 2024 Aug 1]. Study again confirms no link between MMR vaccine and autism. Available from: https://www.psychiatryadvisor.
- Paumgarten, N. The New Yorker. (2019) [cited 2024 Aug 1]. The message of measles. Available from: https://www.newyorker. 2019. [Google Scholar]
- Madsen, K. M. , Hviid, A., Vestergaard, M., Schendel, D., Wohlfahrt, J., Thorsen, P.,... & Melbye, M. (2002). A population-based study of measles, mumps, and rubella vaccination and autism. New England Journal of Medicine, 347(19), 1477-1482. [CrossRef]
- Parner, E. T. , Schendel, D. E., & Thorsen, P. (2008). Autism prevalence trends over time in Denmark: Changes in prevalence and age at diagnosis. Archives of Pediatrics & Adolescent Medicine, 162(12), 1150-1156. [CrossRef]
- Kragh Andersen, P. , Pohar Perme, M., van Houwelingen, H. C., Cook, R. J., Joly, P., Martinussen, T.,... & Therneau, T. M. (2021). Analysis of time-to-event for observational studies: Guidance to the use of intensity models. Statistics in Medicine, 40(1), 185-211. [CrossRef]
- Benkeser, D. , Carone, M. B. ( 37(2), 280–293. [CrossRef]
- Abd ElHafeez, S. , D’Arrigo, G., Leonardis, D., Fusaro, M., Tripepi, G., & Roumeliotis, S. (2021). Methods to analyze time-to-event data: The cox regression analysis. Oxidative Medicine & Cellular Longevity, 2021(1), 1302811. [CrossRef]
- Jain, A. , Marshall, J. J. ( 313(15), 1534–1540. [CrossRef]
- Betancur, C. (2011). Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Research, 1380, 42-77. [CrossRef]
- Hallmayer, J. , Cleveland, S., Torres, A., Phillips, J., Cohen, B., Torigoe, T.,... & Risch, N. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry, 68(11), 1095-1102. /: https. [CrossRef]
- Gaugler, T. , Klei, L. D. ( 46(8), 881–885. [CrossRef]
- Hocking, M. C. , Albee, M. V., Kim, M., Berman, J. I., Fisher, M. J., Roberts, T. P. L., & Blaskey, L. (2024). Social challenges, autism spectrum disorder, and attention deficit/hyperactivity disorder in youth with neurofibromatosis type I. Applied Neuropsychology: Child, 1-9. [CrossRef]
- Wiznitzer, M. (2004). Autism and tuberous sclerosis. [CrossRef]
- Peters, S. U. , Beaudet, A. L., Madduri, N., & Bacino, C. A. (2004). Autism in Angelman syndrome: Implications for autism research. Clinical Genetics, 66(6), 530-536. [CrossRef]
- Abbeduto, L. , McDuffie, A., & Thurman, A. J. (2014). The fragile X syndrome–autism comorbidity: What do we really know? Frontiers in Genetics, 5, 355. [CrossRef]
- Bennett, J. A. , Germani, T., Haqq, A. M., & Zwaigenbaum, L. (2015). Autism spectrum disorder in Prader–Willi syndrome: A systematic review. American Journal of Medical Genetics Part A, 167(12), 2936-2944. [CrossRef]
- Reilly, C. (2009). Autism spectrum disorders in Down syndrome: A review. Research in Autism Spectrum Disorders, 3(4), 829-839. [CrossRef]
- Vorstman, J. A. , Morcus, M. E., Duijff, S. N., Klaassen, P. W., Beemer, F. A., Swaab, H.,... & van Engeland, Herman. (2006). The 22q11. 2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. Journal of the American Academy of Child & Adolescent Psychiatry, 45(9), 1104-1113. [CrossRef]
- Bjørklund, G. , Mkhitaryan, M., Sahakyan, E., Fereshetyan, K., Meguid, N. A., Hemimi, M.,... & Yenkoyan, K. (2024). Linking environmental chemicals to neuroinflammation and autism spectrum disorder: Mechanisms and implications for prevention. Molecular Neurobiology, 1-13. [CrossRef]
- Park, S. K. , Tao, Y., Meeker, J. D., Harlow, S. D., & Mukherjee, B. (2014). Environmental risk score as a new tool to examine multi-pollutants in epidemiologic research: an example from the NHANES study using serum lipid levels. PloS One, 9(6), e98632. [CrossRef]
- Liu, J. , & Lewis, G. (2014). Environmental toxicity and poor cognitive outcomes in children and adults. Journal of Environmental Health, 76(6), 130. https://pubmed.ncbi.nlm.nih. 2464. [Google Scholar]
- Maitre, L. , Bustamante, M., Hernández-Ferrer, C., Thiel, D., Lau, C. H. E., Siskos, A. P.,... & Vrijheid, M. (2022). Multi-omics signatures of the human early life exposome. Nature Communications, 13(1), 7024. [CrossRef]
- Palmer, R. F. , Kattari, D. S. ( 14(1), 350–367. [CrossRef]
- Merriam-Webster. Definition of the verb “lie”, meaning number two. (2024) [cited 2014 Aug 19]. Available from: https://www.merriam-webster.
- Glickman, G. , Harrison, E. ( 377(11), 1099–1101. [CrossRef] [PubMed]
- Zerbo, O. , Modaressi, S. P. ( 172(5), 469–475. [CrossRef]
- Kuwaik, G. A. , Roberts, W. ( 18(2), 148–155. [CrossRef] [PubMed]
- Jain, A. , Marshall, J. J. ( 313(15), 1534–1540. [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). ECDC vaccine scheduler. (2024) [cited 2024 Jul 12]. Denmark: Recommended vaccinations. Available from: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByCountry?
- Statens Serum Institut. Danish childhood vaccination program vaccination offers protection. 13th ed. Sundhedsstyrelsen. (2024) [cited 2024 Jul 2]. Available from: https://www.sst.dk/-/media/Udgivelser/2023/Boernevaccination/Pjecer/Boernevaccinationsprogrammet-i-Danmark-2022-Engelsk.
- Ingels, H. , Rasmussen, J. ( 30(26), 3944–3950. [CrossRef]
- Harboe, Z. B. , Dalby, T., Weinberger, D. M., Benfield, T., Mølbak, K., Slotved, H. C.,... & Valentiner-Branth, P. (2014). Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clinical Infectious Diseases, 59(8), 1066-1073. [CrossRef]
- Wójcik, O. P. , Simonsen, J., Mølbak, K., & Valentiner-Branth, P. (2013). Validation of the 5-year tetanus, diphtheria, pertussis and polio booster vaccination in the Danish childhood vaccination database. Vaccine, 31(6), 955-959. [CrossRef]
- Harder, K. M. , Cowan, S., Eriksen, M. B., Krarup, H. B., & Christensen, P. B. (2011). Universal screening for hepatitis B among pregnant women led to 96% vaccination coverage among newborns of HBsAg positive mothers in Denmark. Vaccine, 29(50), 9303-9307. [CrossRef]
- Centers for Disease Control and Prevention (CDC). CDC Vaccines & immunizations. (2013). Recommended immunization schedule for persons age 0 through 18 years. Available from: https://web.archive.org/web/20130203110629/https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.
- U.S. Food & Drug Administration (FDA). (2023) [cited 2024 Sep 15]. Gardasil. Available from: https://www.fda.
- World Health Organization (WHO). WHO International. (2018) [cited 2024 Mar 18]. Human papillomavirus (HPV). Available from: https://web.archive.org/web/20190724085659/https://www.ssi.
- Statens Serum Institut. (2018) [cited 2024 Aug 19]. Vaccination against cervical cancer (HPV). Available from: https://www.ssi.
- Turville, C. , & Golden, I. (2015). Autism and vaccination: The value of the evidence base of a recent meta-analysis. Vaccine, 33(42), 5494-5496. [CrossRef]
- Sørup, S. , Jensen, A. K., Aaby, P., & Benn, C. S. (2019). Revaccination with measles-mumps-rubella vaccine and infectious disease morbidity: A Danish register-based cohort study. Clinical Infectious Diseases, 68(2), 282-290. [CrossRef]
- Davidkin, I. , Peltola, H., Leinikki, P., & Valle, M. (2000). Duration of rubella immunity induced by two-dose measles, mumps and rubella (MMR) vaccination. A 15-year follow-up in Finland. Vaccine, 18(27), 3106-3112. [CrossRef]
- Paunio, M. , Heinonen, O. P., Virtanen, M., Leinikki, P., Patja, A., & Peltola, H. (2000). Measles history and atopic diseases: A population-based cross-sectional study. JAMA, 283(3), 343-346. [CrossRef]
- U.S. Food & Drug Administration (FDA). U.S. Food & Drug Administration (FDA). (2022) [cited 2024 Sep 15]. GlaxoSmithKline Biologicals SA, Priorix Package Insert. Available from: https://web.archive.org/web/20240713033807/https://www.fda. 1517.
- Sørup, S. , Jensen, A. K., Aaby, P., & Benn, C. S. (2019). Revaccination with measles-mumps-rubella vaccine and infectious disease morbidity: A Danish register-based cohort study. Clinical Infectious Diseases, 68(2), 282-290. [CrossRef]
- Gillet, Y. , Habermehl, P., Thomas, S., Eymin, C., & Fiquet, A. (2009). Immunogenicity and safety of concomitant administration of a measles, mumps and rubella vaccine (MM-RvaxPro®) and a varicella vaccine (VARIVAX®) by intramuscular or subcutaneous routes at separate injection sites: A randomised clinical trial. BMC Medicine, 7, 1-11. [CrossRef]
- European Commission. Sanofi Pasteur, M-M-RVAXPRO Package Insert. (2024) [cited 2024 Aug 19]. Available from: https://ec.europa.eu/health/documents/community-register/2010/2010090688568/anx_88568_en.
- Statens Serum Institut. (2024) [cited 2024 Aug 19]. Uge 24 – 2013. Available from: https://www.ssi. 2013.
- Benn, C. S. (MD, PhD) Chair of Health Sciences, Department of Clinical Research, Danish Institute for Advanced Study (DIAS) University of Southern Denmark (personal communication, , 2024). 19 August.
- Statens Serum Institut. (2008) [cited 2024 Aug 19]. Pneumokokvaccine I Det Danske Børnevaccinationsprogram. Available from: https://www.ssi.dk/-/media/arkiv/dk/aktuelt/nyhedsbreve/epi-nyt/2008/2008-pdf/epi-nyt---2008---uge-38.
- Parner, E. T. , Schendel, D. E., & Thorsen, P. (2008). Autism prevalence trends over time in Denmark: Changes in prevalence and age at diagnosis. Archives of Pediatrics & Adolescent Medicine, 162(12), 1150-1156. [CrossRef]
- Statens Serum Institut. (2008) [cited 2024 Aug 19]. MMR 2 Vaccination Advanced to 4 Years. Available from: https://web.archive.org/web/20240816180533/https://en.ssi.dk/-/media/arkiv/uk/news/epi-news/2008/pdf/epi-news---2008---no-9.
- Knuesel, I. , Chicha, L. P. ( 10(11), 643–660. [CrossRef]
- Wu, W. L. , Hsiao, E. Y., Yan, Z., Mazmanian, S. K., & Patterson, P. H. (2017). The placental interleukin-6 signaling controls fetal brain development and behavior. Brain, Behavior, & Immunity, 62, 11-23. [CrossRef]
- Zerbo, O. , Qian, Y. A. ( 171(1), e163609–e163609. [CrossRef]
- Hooker, B. S. (2017). Influenza vaccination in the first trimester of pregnancy and risk of autism spectrum disorder. JAMA Pediatrics, 171(6), 600-600. [CrossRef]
- Jorgensen, P. , Mereckiene, J., Cotter, S., Johansen, K., Tsolova, S., & Brown, C. (2018). How close are countries of the WHO European Region to achieving the goal of vaccinating 75% of key risk groups against influenza? Results from national surveys on seasonal influenza vaccination programmes, 2008/2009 to 2014/2015. Vaccine, 36(4), 442-452. [CrossRef]
- Mølgaard-Nielsen, D. , Fischer, T. ( 286(4), 469–480. [CrossRef]
- Nakken, C. S. , Skovdal, M., Nellums, L. B., Friedland, J. S., Hargreaves, S., & Norredam, M. (2018). Vaccination status and needs of asylum-seeking children in Denmark: A retrospective data analysis. Public Health, 158, 110-116. [CrossRef]
- Cherri, Z. , Lau, K., Nellums, L. B., Himmels, J., Deal, A., McGuire, E.,... & Hargreaves, S. (2024). The immune status of migrant populations in Europe and implications for vaccine-preventable disease control: A systematic review and meta-analysis. Journal of Travel Medicine, taae033. [CrossRef]
- Holt, N. , Mygind, A., & Bro, F. (2017). Danish MMR vaccination coverage is considerably higher than reported. Danish Medical Journal, 64(2), A5345. https://ugeskriftet.
- Schendel, D. E. , & Thorsteinsson, E. (2018). Cumulative incidence of autism into adulthood for birth cohorts in Denmark, 1980-2012. JAMA, 320(17), 1811-1813. [CrossRef]
- Centers for Disease Control and Prevention (CDC). About Autism Spectrum Disorder (ASD). (2024) [cited 2024 Jul 15]. Data and Statistics on Autism Spectrum Disorder. Available from: https://www.cdc.gov/autism/data-research/index.
- Munafò, M. R. , Nosek, B. A., Bishop, D. V., Button, K. S., Chambers, C. D., Percie du Sert, N.,... & Ioannidis, J. (2017). A manifesto for reproducible science. Nature Human Behavior, 1(1), 1-9. [CrossRef]
- Clarkson, E (PhD), Chief Statistician at the National Institute for Aviation Research (NIAR) and Senior Research Engineer and Chief Statistician for the National Center for Advanced Materials Performance (NCAMP). Wichita State University (personal communication, , 2019). 4 April.
- Centers for Disease Control and Prevention (CDC). CDC Vaccines & Immunizations. 2023. Child and Adolescent Immunization Schedule by Age. Available from: https://www.cdc.gov/vaccines/hcp/imz-schedules/child-adolescent-age.
- Centers for Disease Control and Prevention (CDC). CDC Pregnancy and Vaccination. 2024 [cited 2024 Aug 15]. Vaccine recommendations before, during, and after pregnancy. Available from: https://www.cdc.gov/vaccines-pregnancy/recommended-vaccines/index.
- Bennett, J. (2019) Shortcomings in the latest MMR vaccination and autism study: A healthcare administrator’s response. Science, Public Health Policy & the Law. 1, 2019-2024. https://publichealthpolicyjournal.
- BioSpace. (2024) [cited 2024 Nov 2]. Biologics market size to hit around USD 1. 37 trillion by 2033. Available from: https://www.biospace. 2033.
- Precedence Research. (2024) [cited 2024 Nov 2]. Vaccines market size, share, and trends 2024 to 2034. Available from: https://www.precedenceresearch.
- Allied Market Research. (2022) [cited 2024 Nov 2]. Mumps vaccine market size, share, competitive landscape and trend analysis report, by age group, by distribution channel: Global opportunity analysis and Industry Forecast, 2021-2031. Available from: https://www.alliedmarketresearch. 1540.
- Parker, E. M. , Zhu, B. P., Li, Z., Puddy, R. W., Kelly, M. A., Scott, C.,... & Stephens, J. W. (2024). Domains of excellence: A CDC framework for developing high-quality, impact-driven public health science publications. Journal of Public Health Management & Practice, 30(1), 72-78. [CrossRef]
- Kern, J. K. , Geier, D. A., Deth, R. C., Sykes, L. K., Hooker, B. S., Love, J. M.,... & Geier, M. R. (2017). Systematic assessment of research on autism spectrum disorder (ASD) and mercury reveals conflicts of interest and the need for transparency in autism research. Science & Engineering Ethics, 23, 1691-1718. [CrossRef]
- Lenzer, J. (2015). Centers for Disease Control and Prevention: Protecting the private good? BMJ, 350. [CrossRef]
- Huntoon, L. R. (2020). CDC: Bias and disturbing conflicts of interest. Journal of American Physicians & Surgeons, 23, 66-69. https://www.jpands.org/vol25no3/huntoon.
- Miller, N. Z. , & Goldman, G. S. (2022). Case study: Varicella vaccine and the suppression of data. Journal of American Physicians and Surgeons, 27(1). https://jpands.org/vol27no1/miller.
- Hooker, B. S. (2018). Reanalysis of CDC data on autism incidence and time of first MMR vaccination. Journal of American Physicians & Surgeons, 23(4), 105-110. https://www.jpands.org/vol23no4/hooker.
- Hooker, B. S. (2017). CDC data manipulation exposed: Four years later. Journal of American Physicians & Surgeons, 22(4), 119-121. https://www.jpands.org/vol22no4/hooker.
- Nissen, S. E. (2017). Conflicts of interest and professional medical associations: Progress and remaining challenges. JAMA, 317(17), 1737-1738. [CrossRef]
- Deirdre, I. Fox News. (2010) [cited 2024 Nov 2]. Conflict of children’s interest inside the American academy of pediatrics. Available from: https://www.foxnews.
- Steinbrook, R. , Kassirer, J. P., & Angell, M. (2015). Justifying conflicts of interest in medical journals: A very bad idea. BMJ, 350. [CrossRef]
- Angell M, Relman A. S. New York Times. (2004) [cited 2024 Nov 1]. Physicians, educate yourselves. Available from: https://www.nytimes.com/2004/12/30/opinion/physicians-educate-yourselves-017566.
- Angell, M. (2009). Drug companies & doctors: A story of corruption. The New York Review of Books, 56(1), 8-12. https://www.nybooks.com/articles/2009/01/15/drug-companies-doctorsa-story-of-corruption/.
- Statens Serum Institut. (2022) [cited 2024 Jul 2]. Vaccine research. Available from: https://en.ssi.
- Statens Serum Institut. (2019) [cited 2024 Aug 19]. No Association between MMR vaccine and autism. Available from: https://en.ssi. 2019.
- Novo Nordisk Foundation. (2024) [cited 2024 Aug 2]. Who are we? Available from: https://novonordiskfonden.
- International Committee of Medical Journal Editors. ICMJE Form for disclosure of potential conflicts of interest. (2019) [cited 2024 Aug 1]. Available from: https://rmed.acponline.org/authors/conflictFormServlet/M18-2101/ICMJE/M18-2101-Conflicts.
- Indenrigs-OG Sundhedsministeriet (2022) [cited 2024 Aug 1]. Minister for the interior and health of Denmark. Available from: https://www.ism.
- Novo Nordisk Foundation. (2022) [cited 2024 Aug 21]. Ownership. Available from: https://novonordiskfonden.
- Novo Nordisk A/S. (2024) [cited 2024 Aug 2]. Corporate governance. Available from: https://www.novonordisk.com/about/corporate-governance.
- CompaniesMarketCap. Global ranking. (2024) [cited 2024 Aug 1]. Market capitalization of Novo Nordisk (NVO). Available from: https://companiesmarketcap.
- Novo Holdings. (2024) [cited 2024 Aug 21]. Corporate governance. Available from: https://www.novonordisk.com/about/corporate-governance.
- Novo Holdings. (2021) [cited 2024 Aug 21]. Investments. Available from: https://novoholdings.
- Novo Holdings. (2021) [cited 2024 Aug 21]. Joining the fight against COVID-19 while running a life-saving business. Available from: https://novoholdings.
- Novo Nordisk Foundation Initiative for Vaccines and Immunity (NIVI). (2023) [cited 2024 Aug 21]. Projekter og initiativer. Available from: https://novonordiskfonden.
- University of Copenhagen. (2023) [cited 2024 Aug 21]. New initiative puts Danish vaccine science on the global map. Available from: https://healthsciences.ku. 2023.
- NIVI Novo Nordisk Foundation Initiative for Vaccines and Immunity. (2024) [cited 2024 Aug 23]. About the initiative. Available from: https://nivi.ku.
- Novo Nordisk Foundation. (2024) [cited 2024 Aug 2]. The Novo Nordisk Foundation Vaccine Accelerator (NVAC). Available from: https://novonordiskfonden.
- Statens Serum Institut. (2018) [cited 2024 Aug 19]. Vaccination against cervical cancer (HPV). Available from: https://www.ssi.
- Olsen, S. F. , Halldorsson, T. I., Thorne-Lyman, A. L., Strøm, M., Gørtz, S., Granstrøm, C.,... & Zhou, W. (2018). Plasma concentrations of long chain N-3 fatty acids in early and mid-pregnancy and risk of early preterm birth. EBioMedicine, 35, 325-333. [CrossRef]
- Novo Nordisk Foundation. (2019) [cited 2024 Aug 2]. The Novo Nordisk Foundation awards grants to 12 excellent research leaders within bioscience and basic biomedicine. Available from: https://researchleaderprogramme.
- Statens Serum Institut. (2023) [cited 2024 Aug 19]. New research sheds light on genetics of placenta growth. Available from: https://en.ssi. 2023.
- Statens Serum Institut. (2024) [cited 2024 Jul 2]. SSI researcher receives multi-million kroner grant from the Novo Nordisk Foundation. Available from: https://en.ssi. 2019.
- Statens Serum Institut. (2024) [cited 2024 Jul 2]. Babies’ own genes influence when they are born. Available from: https://en.ssi. 2019.
- Statens Serum Institut. (2023) [cited 2024 Aug 2]. The Danish national biobank. Available from: https://en.ssi.
- Angell, M. , & Relman, A. S. (2002). Patents, profits & American medicine: Conflicts of interest in the testing & marketing of new drugs. Daedalus, 131(2), 102-111. https://www.jstor. 2002. [Google Scholar]
- Kennedy Jr, R. F. (2015). Thimerosal: Let the science speak: The evidence supporting the immediate removal of mercury—A known neurotoxin—From vaccines. Simon & Schuster.
- Schuemie, M. J. , Hripcsak, G., Ryan, P. B., Madigan, D., & Suchard, M. A. (2018). Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data. Proceedings of the National Academy of Sciences, 115(11), 2571-2577. [CrossRef]
- Hviid, A. , Stellfeld, M., Wohlfahrt, J., & Melbye, M. (2003). Association between thimerosal-containing vaccine and autism. JAMA, 290(13), 1763-1766. [CrossRef]
- Hviid, A. , Stellfeld, M. ( 350(14), 1398–1404. [CrossRef]
- Vestergaard, M. , Hviid, A., Madsen, K. M., Wohlfahrt, J., Thorsen, P., Schendel, D.,... & Olsen, J. (2004). MMR vaccination and febrile seizures: Evaluation of susceptible subgroups and long-term prognosis. JAMA, 292(3), 351-357. [CrossRef]
- Hviid, A. , Melbye, M. ( 369(25), 2406–2415. [CrossRef]
- Pasternak, B. , Svanström, H. ( 368(9), 814–823. [CrossRef] [PubMed]
- Geier, D. A. , King, P. G., Hooker, B. S., Dórea, J. G., Kern, J. K., Sykes, L. K., & Geier, M. R. (2015). Thimerosal: Clinical, epidemiologic and biochemical studies. Clinica Chimica acta, 444, 212-220. [CrossRef]
- Kern, J. , Geier, D. ( 72(2), 113–153. [CrossRef] [PubMed]
- Mezzacappa, A. , Lasica, P. A., Gianfagna, F., Cazas, O., Hardy, P., Falissard, B.,... & Gressier, F. (2017). Risk for autism spectrum disorders according to period of prenatal antidepressant exposure: A systematic review and meta-analysis. JAMA Pediatrics, 171(6), 555-563. [CrossRef]
- Kennedy, D. (2016). Ondansetron and pregnancy: Understanding the data. Obstetric Medicine, 9(1), 28-33. [CrossRef]
- Kirby, D. (2008). The vaccine-autism court document every American should read. Huffington Post. https://www.jeremyrhammond.com/wp-content/uploads/2019/10/080226-Vaccine-Autism-Court-Document-Kirby-HuffPost.
- Transcripts. (2008) [cited 2024 Aug 1]. Unraveling the mystery of autism; talking with the CDC director; stories of children with autism; aging with autism. Available from: http://transcripts.cnn.com/TRANSCRIPTS/0803/29/hcsg.01.
- Reuters. Reuters. (2009) [cited 2024 Aug 21]. Former CDC head lands vaccine job at Merck. Available from: https://www.reuters. 5200.
- Merck. (2022) [cited 2024 Aug 21]. Dr. Julie L. Gerberding to Retire from Merck. Available from: https://www.merck.
- Hallmayer, J. , Cleveland, S. ( 68(11), 1095–1102. [CrossRef]
- Bradstreet, J. (2004). Biological evidence of significant vaccine related side-effects resulting in neurodevelopmental disorders. Presentation to the Vaccine Safety Committee of the Institute of Medicine, The National Academies of Science, 9. https://www.amalgam-informationen.de /dokument/Bradstreet.
- Maitre, L. , Bustamante, M., Hernández-Ferrer, C., Thiel, D., Lau, C. H. E., Siskos, A. P.,... & Vrijheid, M. (2022). Multi-omics signatures of the human early life exposome. Nature Communications, 13(1), 7024. [CrossRef]
- Laugesen, K. , Mengel-From, J., Christensen, K., Olsen, J., Hougaard, D. M., Boding, L.,... & Sørensen, H. T. (2023). A review of major Danish biobanks: Advantages and possibilities of health research in Denmark. Clinical Epidemiology, 213-239. [CrossRef]
- Attkisson, S. (2018) [cited 2024 Aug 13]. CDC: “Possibility” that vaccines rarely trigger autism. Available from: https://sharylattkisson. 2018. [Google Scholar]
- Price, C. S. , Thompson, W. W., Goodson, B., Weintraub, E. S., Croen, L. A., Hinrichsen, V. L.,... & DeStefano, F. (2010). Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics, 126(4), 656-664. [CrossRef]
- Janiaud, P. , Agarwal, A., Tzoulaki, I., Theodoratou, E., Tsilidis, K. K., Evangelou, E., & Ioannidis, J. P. (2021). Validity of observational evidence on putative risk and protective factors: appraisal of 3744 meta-analyses on 57 topics. BMC Medicine, 19, 1-17. [CrossRef]
- Ioannidis, J. P. (2016). Why most clinical research is not useful. PLoS medicine, 13(6), e1002049. [CrossRef]
- Kennedy. R. F, Hooker B. (2023) Vax-Unvax: Let the science speak. Skyhorse Publishing.
- Chandler, J. , Cumpston, M. J. H. W. ( 2019). Cochrane handbook for systematic reviews of interventions. Hoboken, Wiley. [CrossRef]
- Barnes, H. J. , Ragnarsson, G., & Alván, G. (2009). Quality and safety considerations for recombinant biological medicines: A regulatory perspective. International Journal of Risk & Safety in Medicine, 21(1-2), 13-22. [CrossRef]
- Valiant, W. G. , Cai, K. M. ( 80, 6–17. [CrossRef]
- Chooi, W. H. , Ng, P. W., Hussain, Z., Ming, L. C., Ibrahim, B., & Koh, D. (2022). Vaccine contamination: Causes and control. Vaccine, 40(12), 1699. [CrossRef]
- Aranha, H. , & Solutions, C. P. (2011). Virus safety of biopharmaceuticals. Contract Pharma, 13. https://www.contractpharma.
- English, J. M. (1995). The rights and wrongs of measles vaccination. British Homoeopathic Journal, 84(3), 156-163. [CrossRef]
- Price, D. , Jefferson, T., & Demicheli, V. (2004). Methodological issues arising from systematic reviews of the evidence of safety of vaccines. Vaccine, 22(15-16), 2080-2084. [CrossRef]
- Halvorsen, R. (2007). The Truth About Vaccines: How we are used as guinea pigs without knowing it. Gibson Square.
- McKinlay, J. B. , & McKinlay, S. M. (1977). The questionable contribution of medical measures to the decline of mortality in the United States in the twentieth century. The Milbank Memorial Fund Quarterly. Health & Society, 405-428. http://ftp.columbia.edu/itc/hs/pubhealth/rosner/g8965/client_edit/readings/week_2/mckinlay.
- Armstrong, G. L. , Conn, L. W. ( 281(1), 61–66. [CrossRef]
- Beck, M. A. (1996). The role of nutrition in viral disease. The Journal of Nutritional Biochemistry, 7(12), 683-690. [CrossRef]
- Hooker, B. S. , & Miller, N. Z. (2020). Analysis of health outcomes in vaccinated and unvaccinated children: Developmental delays, asthma, ear infections and gastrointestinal disorders. SAGE Open Medicine, 8, 2050312120925344. [CrossRef]
- Miller, N. Z. , & Goldman, G. S. (2023). Neonatal, infant, and under age five vaccine doses routinely given in developed nations and their association with mortality rates. Cureus, 15(7). [CrossRef]
- Light, D. W. , Lexchin, J., & Darrow, J. J. (2013). Institutional corruption of pharmaceuticals and the myth of safe and effective drugs. Journal of Law, Medicine & Ethics, 41(3), 590-600. [CrossRef]
- Barosi, G. , & Gale, R. P. (2018). Is there expert consensus on expert consensus? Bone Marrow Transplantation, 53(8), 1055-1060. [CrossRef]
- Djulbegovic, B. , & Guyatt, G. ( 322(8), 725–726. [CrossRef]
- Bragazzi, N. L. , Watad, A., Amital, H., & Shoenfeld, Y. (2017). Debate on vaccines and autoimmunity: Do not attack the author, yet discuss it methodologically. Vaccine, 35(42), 5522-5526. [CrossRef]
- Martin, B. (2015). On the suppression of vaccination dissent. Science & Engineering Ethics, 21, 143-157. [CrossRef]
- Elisha, E. , Guetzkow, J., Shir-Raz, Y., & Ronel, N. (2024). Suppressing scientific discourse on vaccines? Self-perceptions of researchers and practitioners. In Hec Forum (Vol. 36, No. 1, pp. 71-89). Dordrecht: Springer Netherlands. [CrossRef]
- Kempner, J. (2008). The chilling effect: how do researchers react to controversy? PLoS Medicine, 5(11), e222. [CrossRef]
- Martin, B. (1981). The scientific straightjacket: The power structure of science and the suppression of environmental scholarship. Ecologist, 11(1), 33-43. https://documents.uow.edu.au/~bmartin/pubs/81ecol.
- Larsson, A. , Oxman, A. D., Carling, C., & Herrin, J. (2003). Medical messages in the media–barriers and solutions to improving medical journalism. Health Expectations, 6(4), 323-331. [CrossRef]
- Shuchman, M. , & Wilkes, M. S. (1997). Medical scientists and health news reporting: A case of miscommunication. Annals of Internal Medicine, 126(12), 976-982. [CrossRef]
- Carroll, A. E. New York Times. (2018) [cited 2024 Aug 1]. Why it’s still worth getting a flu shot. Available from: https://www.nytimes.com/2018/01/11/upshot/flu-shot-risks-benefits-strain.
- Carroll, A. E. New York Times. (2017) [cited 2024 Aug 1]. A link between alcohol and cancer? It’s not nearly as scary as it seems. Available from: https://www.nytimes.com/2017/11/10/upshot/health-alcohol-cancer-research.
- Lipworth, W. , Kerridge, I. ( 38(12), 768–770. [CrossRef]
- Wiley, K. E. , Leask, J., Attwell, K., Helps, C., Barclay, L., Ward, P. R., & Carter, S. M. (2021). Stigmatized for standing up for my child: A qualitative study of non-vaccinating parents in Australia. SSM-population Health, 16, 100926. [CrossRef]
- Kennedy Jr, R. F. (2022). Profiles of the vaccine-injured: "A lifetime price to pay." Simon & Schuster.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).