Submitted:
08 January 2025
Posted:
08 January 2025
You are already at the latest version
Abstract
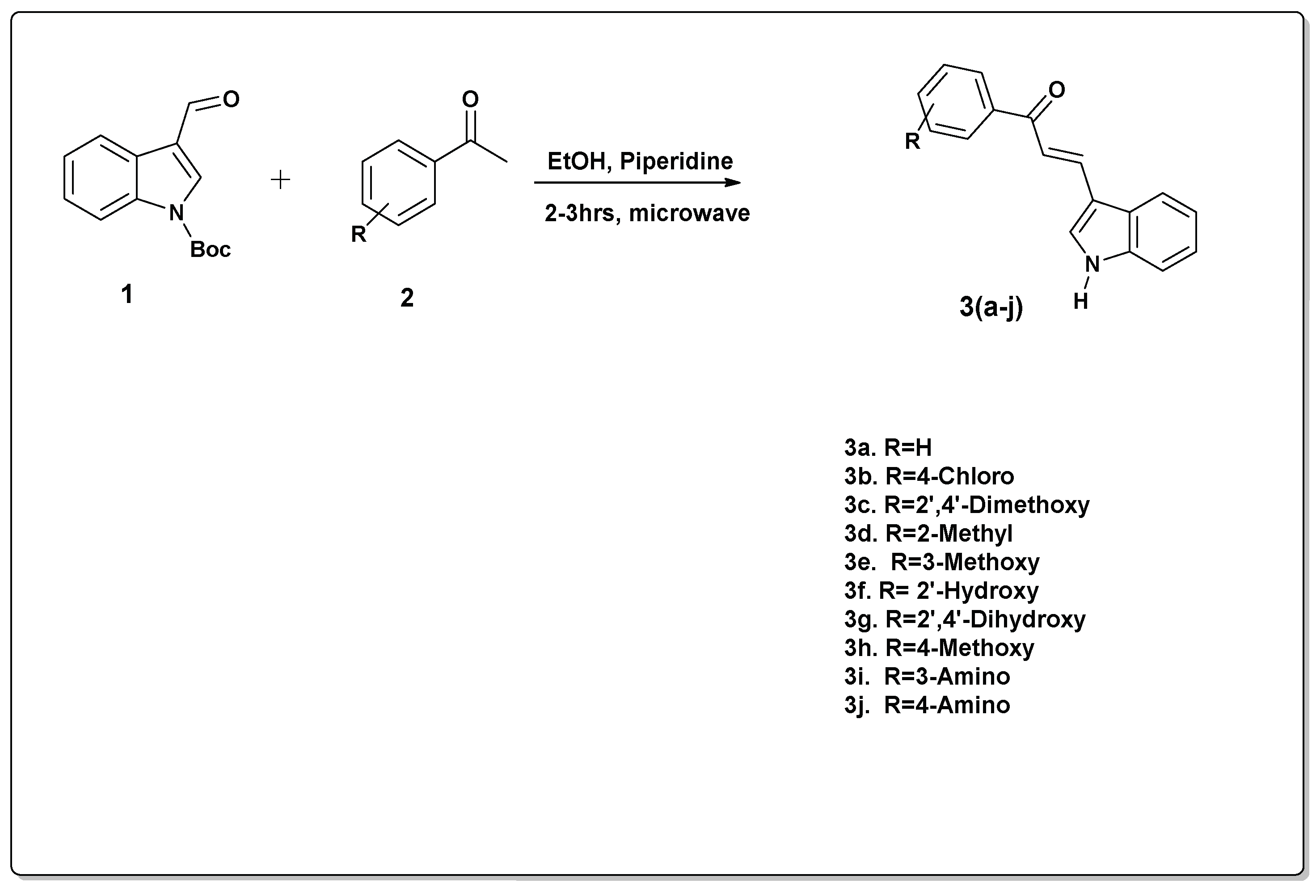
Indole-chalcone hybrids are a large group of compounds known for their excellent biological properties against diverse pathogens. The current research describes a rapid synthetic pathway for the synthesis of ten (10) indole-chalcone hybrids 3(a-j), from 1-Boc-3-formylindole (1) and acetophenone derivatives (2), in a one-pot approach. The Boc was deprotected during the reaction and occurred automatically at the end of the reaction. 1H-NMR, 13C-NMR, and MS were used to elucidate the structures of the compounds. Contrary to previous methods for the synthesis of indole-chalcone hybrids, this novel synthetic method, which involves using a Boc-protected indole via microwave-assisted synthesis, is advantageous because it is a one-pot approach making it facile, and rapid.
Keywords:
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.1.1. Unsuccessful Synthetic Approaches
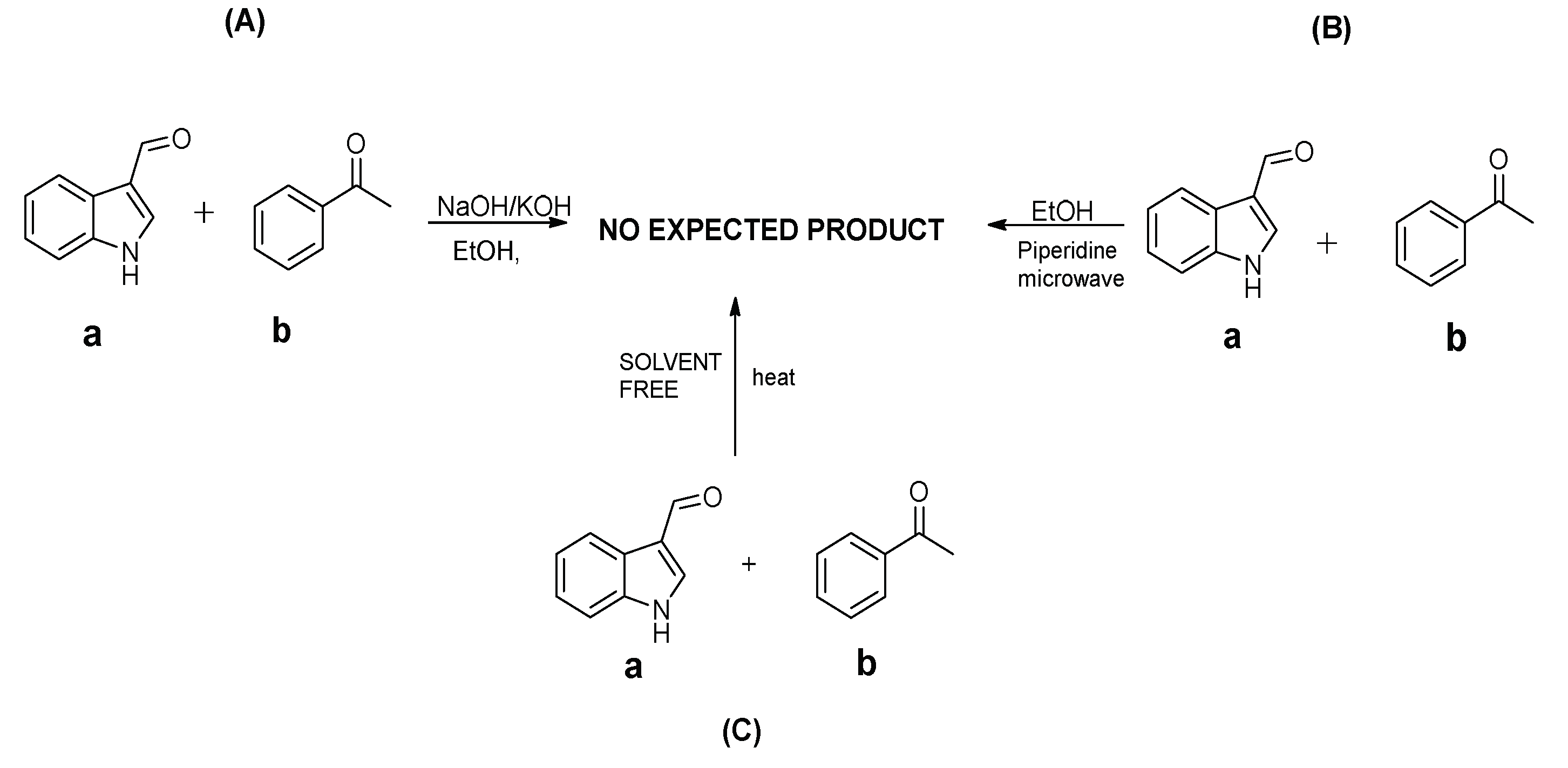
2.1.2. The Successful Synthetic Approach
3. Material and Methods
3.1. General Experimental Information
3.2. Experimental Procedures and Characterization of Compounds
3.2.1. Synthesis of (E)-3-(1H-indol-3-yl)-1-phenylprop-2-en-1-one (S1-01)
3.2.2. Synthesis of (E)-1-(4-chlorophenyl)-3-(1H-indol-3-yl)prop-2-en-1-one (S1-03)
3.2.3. Synthesis of (E)-1-(2,4-dimethoxyphenyl)-3-(1H-indol-3-yl)prop-2-en-1-one (S1-04)
3.2.4. Synthesis of (E)-3-(1H-indol-3-yl)-1-(o-tolyl)prop-2-en-1-one (S1-05)
3.2.5. Synthesis of (E)-3-(1H-indol-3-yl)-1-(3′-Methoxyphenyl)prop-2-en-1-one (S1-06)
3.2.6. Synthesis of (E)-1-(4-hydroxyphenyl)-3-(1H-indol-3-yl)prop-2-en-1-one (S1-07)
3.2.7. Synthesis of (E)-1-(2,4-dihydroxyphenyl)-3-(1H-indol-3-yl)prop-2-en-1-one (S1-08)
3.2.8. Synthesis of (E)-3-(1H-indol-3-yl)-1-(4-methoxyphenyl)prop-2-en-1-one (S1-09)
3.2.9. Synthesis of (E)-1-(3-aminophenyl)-3-(1H-indol-3-yl)prop-2-en-1-one (S1-10)
3.2.10. Synthesis of (E)-1-(4-aminophenyl)-3-(1H-indol-3-yl)prop-2-en-1-one (S1-11)
Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rammohan, A.; Reddy, J. S.; Sravya, G.; Rao, C. N.; and Zyryanov, G. V. Chalcone Synthesis, Properties and Medicinal Applications: A Review. Environ. Chem. Lett. 2020 18(2), 433-458. [CrossRef]
- Urbonavičius, A.; Fortunato, G.; Ambrazaitytė, E.; Plytninkienė, E.; Bieliauskas, A.; Milišiūnaitė, V.; Luisi, R.; Arbačiauskienė, E.; Krikštolaitytė, S.; and Šačkus, A. Synthesis and Characterization of Novel Heterocyclic Chalcones from 1-Phenyl-1H-pyrazol-3-ol. Molecules. 2022 27(12), 3752. [CrossRef]
- Mukhtar, S. S.; Morsy, N. M.; Hassan, A. S.; Hafez, T. S.; Hassaneen, H. M.; and Saleh, F. M. A Review of Chalcones: Synthesis, Reactions, and Biological Importance. Egypt. J. Chem. 2022 65(8), 379-395. [CrossRef]
- Shalaby, M.A.; Rizk, S. A.; and Fahim, A.M. Synthesis, Reactions and Application of Chalcones: A Systematic Review. Org. Biomol. Chem. 2023 21(26), 5317-5346. [CrossRef]
- Jasim, H. A.; Nahar, L.; Jasim, M. A.; Moore, S. A.; Ritchie, K. J.; and Sarker, S. D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules. 2021 11(8), 1203. [CrossRef]
- Goyal, K.; Kaur, R.; Goyal, A.; and Awasthi, R. Chalcones: A Review on Synthesis and Pharmacological Activities. J. Appl. Pharm. Sci. 2021 11(1), 001-014. [CrossRef]
- Vijayakumar, B. G.; Ramesh, D.; Joji, A.; Jayachandra P. J.; and Kannan, T. In Silico Pharmacokinetic and Molecular Docking Studies of Natural Flavonoids and Synthetic Indole Chalcones against Essential Proteins of SARS-CoV-2. Eur. J. Pharmacol. 2020 886, 173448. [CrossRef]
- Bułakowska, A.; Sławiński, J.; Hering, A.; Gucwa, M.; Ochocka, J. R.; Hałasa, R.; Balewski, Ł.; and Stefanowicz-Hajduk, J. New Chalcone Derivatives Containing 2,4-Dichlorobenzenesulfonamide Moiety with Anticancer and Antioxidant Properties. Int J Mol Sci. 2023 25(1), 274. [CrossRef]
- Ugwu, D. I.; Ezema, B. E.; Okoro, U. C.; Eze, F. U.; Ekoh, O. C.; Egbujor, M. C.; and Ugwuja, D. I. Synthesis and Pharmacological Applications of Chalcones: A Review. Int. J. Chem. Sci. 2015 13(1), 459-500.
- Pereira, R.; Silva, A. M. S.; Ribeiro, D.; Silva, V. L. M.; and Fernandes, E. Bis-chalcones: A Review of Synthetic Methodologies and Anti-Inflammatory Effects. Eur J. Med. Chem. 2023 252, 115280. [CrossRef]
- Thapa, P.; Upadhyay, S. P.; Suo, W. Z.; Singh, V.; Gurung, P.; Lee, E. S.; Sharma, R.; and Sharma, M. Chalcone and its Analogs: Therapeutic and Diagnostic Applications in Alzheimer’s Disease. Bioorg. Chem. 2021 108, 104681. [CrossRef]
- Rozmer, Z.; and Perjési, P. Naturally occurring chalcones and their biological activities. Phytochemistry Reviews. 2016 15(1), 87-120. [CrossRef]
- Kudličková, Z.; Michalková, R.; Salayová, A.; Ksiažek, M.; Vilková, M.; Bekešová, S.; and Mojžiš, J. Design, Synthesis, and Evaluation of Novel Indole Hybrid Chalcones and Their Antiproliferative and Antioxidant Activity. Molecules. 2023 28(18), 6583. [CrossRef]
- Yan, J.; Chen, J.; Zhang, S.; Hu, J.; Huang, L.; and Li, X. Synthesis, Evaluation, and Mechanism Study of Novel Indole-Chalcone Derivatives Exerting Effective Antitumor Activity Through Microtubule Destabilization in Vitro and in Vivo. J Med. Chem. 2016 59(11), 5264-5283. [CrossRef]
- Badria, F. A.; Soliman, S. M.; Atef, S.; Islam, M. S.; Al-Majid, A. M.; Dege, N.; Ghabbour, H. A.; Ali, M.; El-Senduny, F. F.; and Barakat, A. Anticancer Indole-Based Chalcones: A Structural and Theoretical Analysis. Molecules. 2019 24(20), 3728. [CrossRef]
- Sayed, M.; Kamal El-Dean, A. M.; Ahmed, M.; and Hassanien, R. Synthesis, Characterization, and Screening for Anti-inflammatory and Antimicrobial Activity of Novel Indolyl Chalcone Derivatives. J. Het. Chem. 2018 55(5), 1166-1175. [CrossRef]
- Sunil, T.; Samson, M.; Ofentse, P.; Shivaji, T.; Pravin, K.; and Rajandra, P. Biological Role of Chalcones in Medicinal Chemistry, In Vector Borne Diseases. In C. David, B. Sujit, & R. Syamal (Eds.). 2020 (pp. Ch. 9). IntechOpen. [CrossRef]
- Chen, X.; Li, H.; Wang, M.; Sun, D.; Lu, J.; Zhu, T.; Chen, H.; Chen, L.; and Liu, S. Discovery of Chalcone Derivatives as Bifunctional Molecules with Anti-SARS-CoV-2 and Anti-inflammatory Activities. Journal of Natural Products. 2024 87(12), 2680-2694. [CrossRef]
- Tyagi, R.; Yadav, K.; Khanna, A.; Mishra, S. K.; and Sagar, R. Efficient synthesis of indole-chalcones based glycohybrids and their anticancer activity. Bioorganic & Medicinal Chemistry, 2024 109, 117778. [CrossRef]
- Karaman, B.; Alhalabi, Z.; Swyter, S.; Mihigo, S. O.; Andrae-Marobela, K.; Jung, M.; Sippl, W.; and Ntie-Kang, F. Identification of Bichalcones as Sirtuin Inhibitors by Virtual Screening and In Vitro Testing. Molecules. 2018 23(2), 416. [CrossRef]
- Robinson, M. W.; Overmeyer, J. H.; Young, A. M.; Erhardt, P. W.; and Maltese, W. A. Synthesis and evaluation of indole-based chalcones as inducers of methuosis, a novel type of nonapoptotic cell death. J Med Chem. 2012 55(5), 1940-1956. [CrossRef]
- Ramesh, D.; Joji, A.; Vijayakumar, B. G.; Sethumadhavan, A.; Mani, M.; and Kannan, T. Indole chalcones: Design, synthesis, in vitro and in silico evaluation against Mycobacterium tuberculosis. Eur J. Med. Chem. 2020 198, 112358. [CrossRef]
- Elkanzi, N. A. A.; Hrichi, H.; Alolayan, R. A.; Derafa, W.; Zahou, F. M.; and Bakr, R. B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega, 2022 7(32), 27769-27786. [CrossRef]
- Chauhan, R.; Dwivedi, J.; Siddiqi Anees, A.A.; and Kishore, D. Synthesis and antimicrobial activity of chalcone derivatives of indole nucleus. Pharm. Chem. J. 2011 44, 542–550. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




