Submitted:
08 January 2025
Posted:
08 January 2025
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Reactor Setup
2.2. Wastewater and Inoculum
2.3. Analytical Methods
3. Results and Discussion
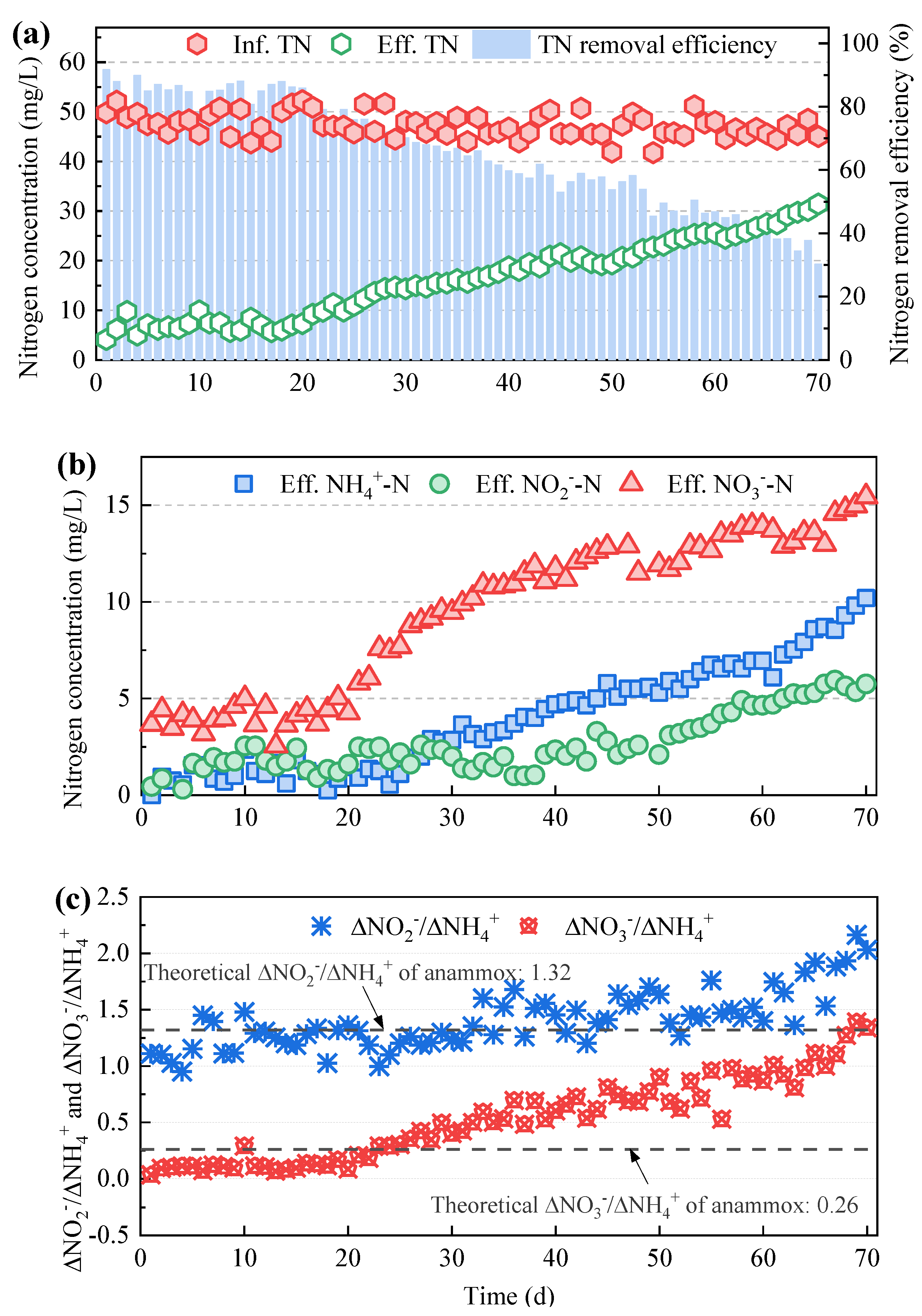
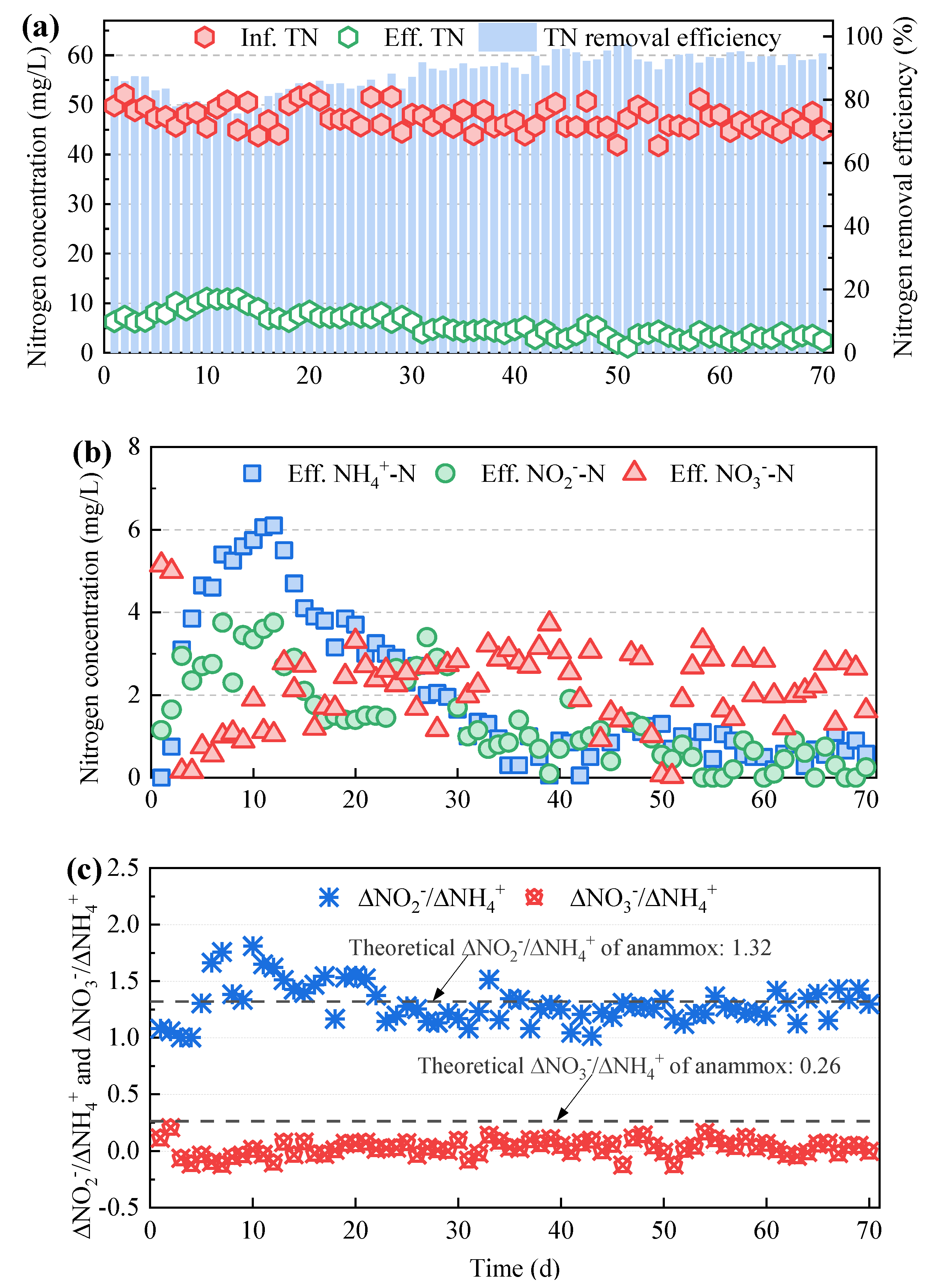
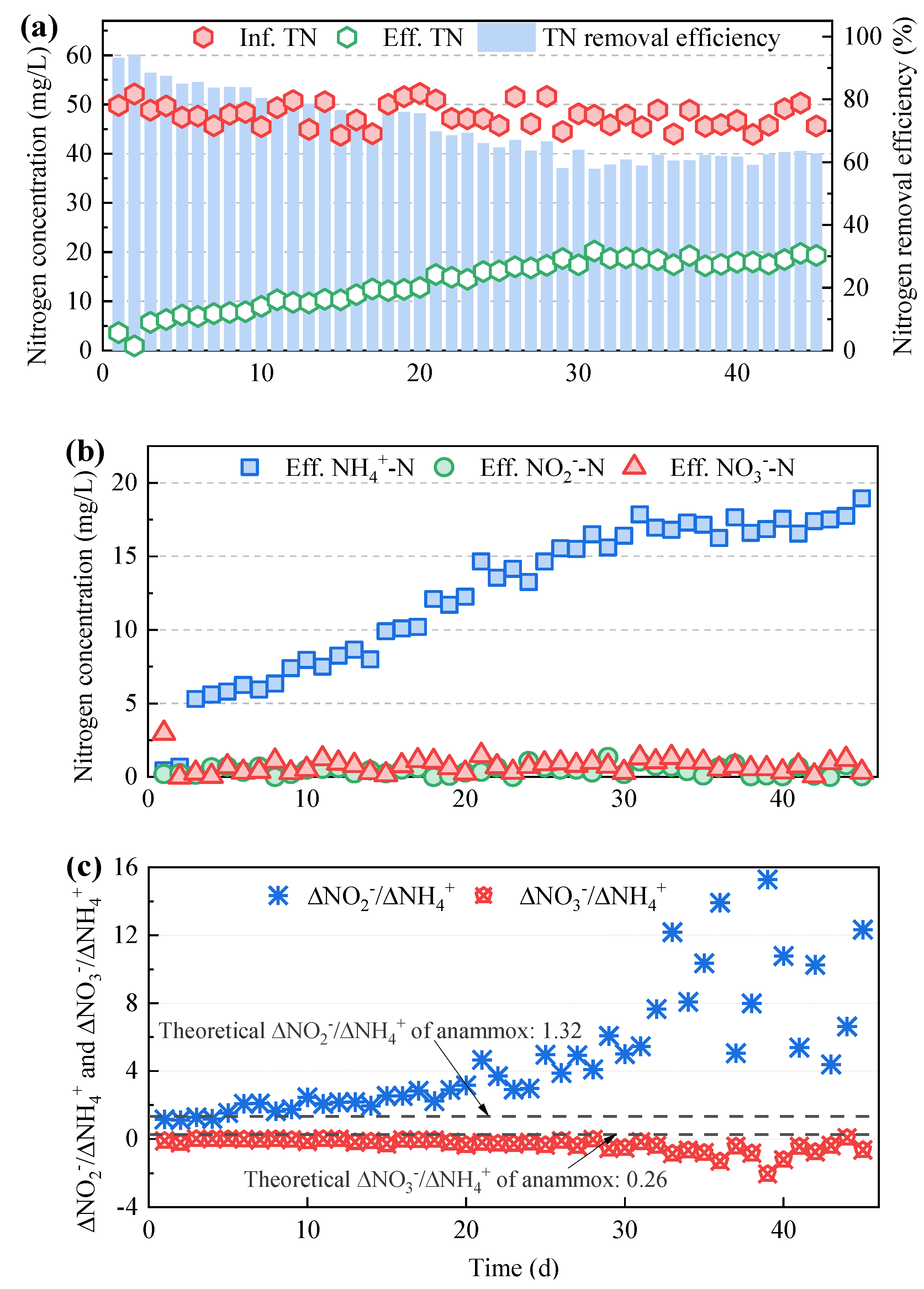
3.1. Nitrogen Removal Performance of the Mainstream Anammox Reactors at Various C/N Ratios
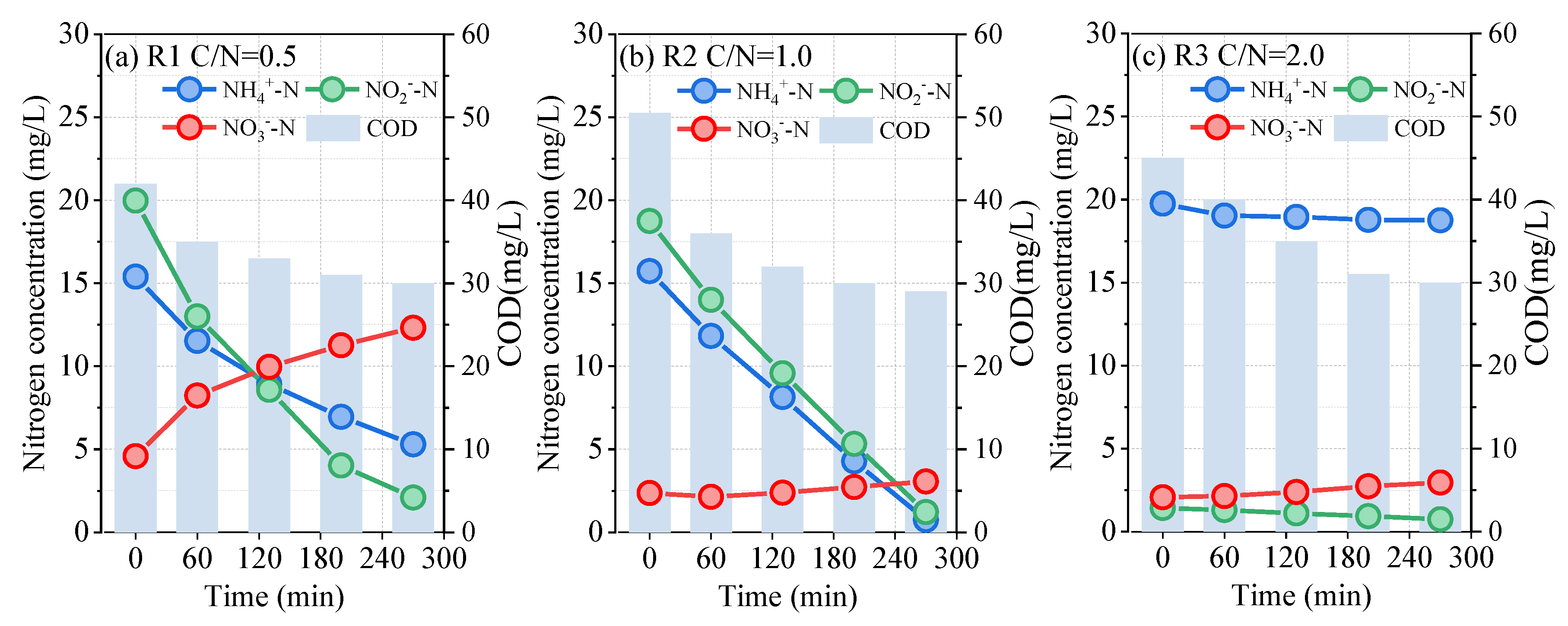
3.2. Nitrogen Transformation in the Typical Cycles
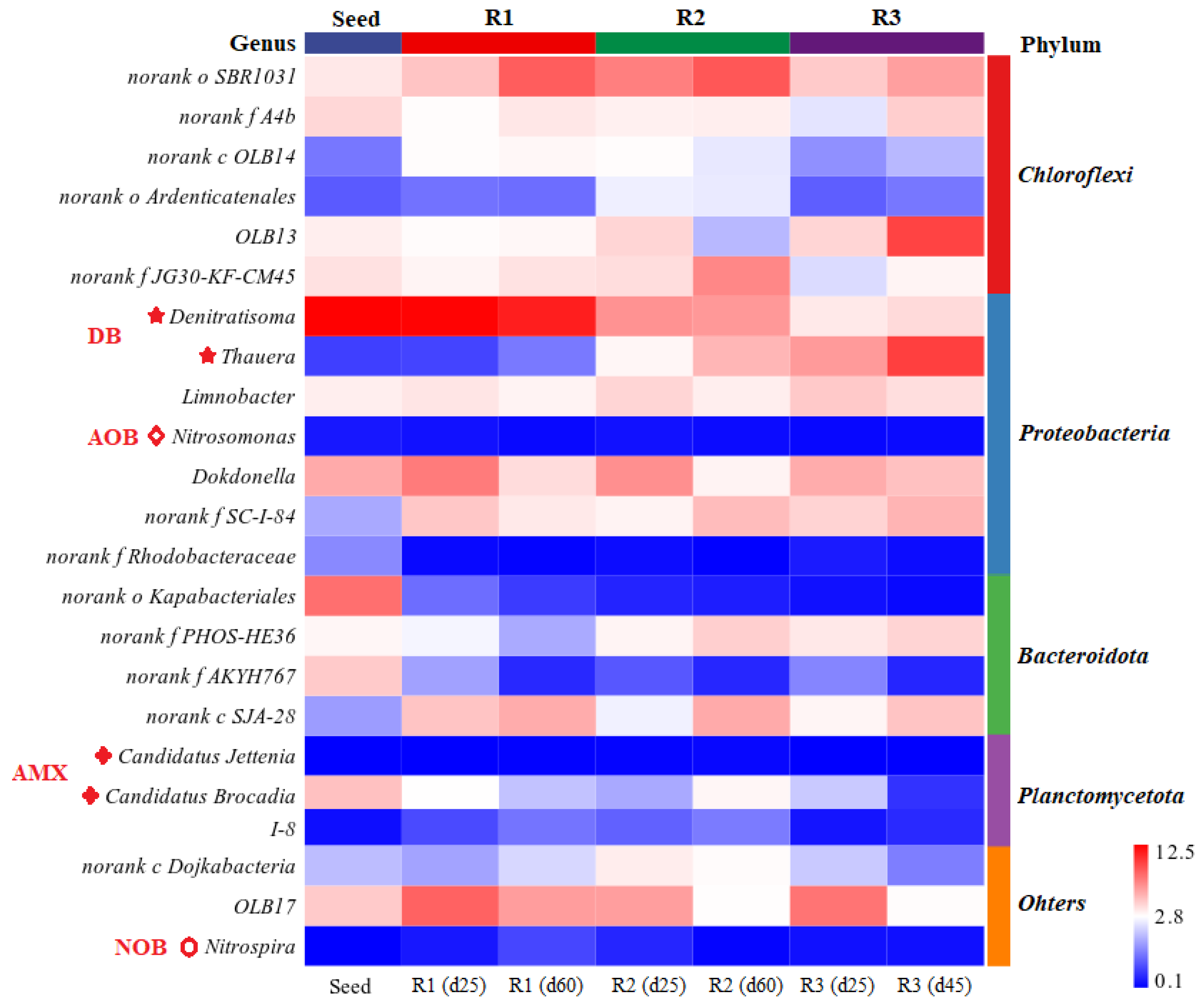
3.3. Microbial Dynamics at Various Influent C/N Ratios
3.4. Implication of This Work
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kartal, B.; Kuenen, J.G.; van Loosdrecht, M.C.M. Sewage treatment with anammox. Science 2010, 328, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, S.Y.; Cao, S.B.; Miao, Y.Y.; Jia, F.X.; Du, R.; Peng, Y.Z. Biological nitrogen removal from sewage via anammox: Recent advances. Bioresour. Technol. 2016, 200, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, L.; Zeng, W.; Li, J.; Zhang, Q.; Li, X.; Peng, Y. A loading rate switch strategy for stable nitritation in mainstream municipal wastewater. Nat. Sustainability 2024, 7, 305–314. [Google Scholar] [CrossRef]
- Regmi, P.; Miller, M.W.; Holgate, B.; Bunce, R.; Park, H.; Chandran, K.; Wett, B.; Murthy, S.; Bott, C.B. Control of aeration, aerobic SRT and COD input for mainstream nitritation/denitritation. Water Res. 2014, 57, 162–171. [Google Scholar] [CrossRef]
- Isanta, E.; Reino, C.; Carrera, J.; Perez, J. Stable partial nitritation for low-strength wastewater at low temperature in an aerobic granular reactor. Water Res. 2015, 80, 149–158. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.J.; Gan, Y.P.; Peng, Y.Z. Bio-augmentation to rapid realize partial nitrification of real sewage. Chemosphere 2012, 88, 1097–1102. [Google Scholar] [CrossRef]
- Cao, Y.; van Loosdrecht, M.C.M.; Daigger, G.T. Mainstream partial nitritation–anammox in municipal wastewater treatment: status, bottlenecks, and further studies. Appl. Microbiol. Biotechnol. 2017, 101, 1365–1383. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, S.J.; Zhang, L.; Yi, P.; Wang, J.M.; Wang, S.Y.; Peng, Y.Z. The feasibility of using a two-stage autotrophic nitrogen removal process to treat sewage. Bioresour. Technol. 2011, 102, 8331–8334. [Google Scholar] [CrossRef]
- Yang, Y.; Long, Y.; Xu, J.; Liu, S.; Liu, L.; Liu, C.; Tian, Y. Achieving robust and highly efficient nitrogen removal in a mainstream anammox reactor by introducing low concentrations of readily biodegradable organics. Front. Microbiol. 2023, 14, 1186819. [Google Scholar] [CrossRef]
- Díaz, C.; Belmonte, M.; Campos, J.L.; Franchi, O.; Faúndez, M.; Vidal, G.; Argiz, L.; Pedrouso, A.; Val del Rio, A.; Mosquera-Corral, A. Limits of the anammox process in granular systems to remove nitrogen at low temperature and nitrogen concentration. Process Saf. Environ. Prot. 2020, 138, 349–355. [Google Scholar] [CrossRef]
- Li, W.; Zhuang, J.L.; Zhou, Y.Y.; Meng, F.G.; Kang, D.; Zheng, P.; Shapleigh, J.P.; Liu, Y.D. Metagenomics reveals microbial community differences lead to differential nitrate production in anammox reactors with differing nitrogen loading rates. Water Res. 2020, 169, 115279. [Google Scholar] [CrossRef] [PubMed]
- Guillén, J.S.; Vazquez, C.L.; de Oliveira Cruz, L.; Brdjanovic, D.; van Lier, J. Long-term performance of the Anammox process under low nitrogen sludge loading rate and moderate to low temperature. Biochem. Eng. J. 2016, 110, 95–106. [Google Scholar] [CrossRef]
- Strous, M.; Kuenen, J.G.; Jetten, M.S.M. Key physiology of anaerobic ammonium oxidation. Appl. Environ. Microbiol. 1999, 65, 3248–3250. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.-L.; Sun, X.; Zhao, W.-Q.; Zhang, X.; Zhou, J.-J.; Ni, B.-J.; Liu, Y.-D.; Shapleigh, J.P.; Li, W. The anammox coupled partial-denitrification process in an integrated granular sludge and fixed-biofilm reactor developed for mainstream wastewater treatment: Performance and community structure. Water Res. 2022, 210, 117964. [Google Scholar] [CrossRef]
- Ji, J.; Peng, Y.; Li, X.; Zhang, Q.; Liu, X. A novel partial nitrification-synchronous anammox and endogenous partial denitrification (PN-SAEPD) process for advanced nitrogen removal from municipal wastewater at ambient temperatures. Water Res. 2020, 175, 115690. [Google Scholar] [CrossRef]
- Bi, Z.; Takekawa, M.; Park, G.; Soda, S.; Qiao, S.; Ike, M. Effects of the C/N ratio and bacterial populations on nitrogen removal in the simultaneous anammox and heterotrophic denitrification process: Mathematic modeling and batch experiments. Chem. Eng. J. 2015, 280, 606–613. [Google Scholar] [CrossRef]
- Xu, G.J.; Zhou, Y.; Yang, Q.; Lee, Z.M.P.; Gu, J.; Lay, W.S.; Cao, Y.S.; Liu, Y. The challenges of mainstream deammonification process for municipal used water treatment. Appl. Microbiol. Biotechnol. 2015, 99, 2485–2490. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, L.; Zhang, Q.; Li, X.; Peng, Y. Enrichment of comammox bacteria in anammox-dominated low-strength wastewater treatment system within microaerobic conditions: Cooperative effect driving enhanced nitrogen removal. Chem. Eng. J. 2023, 453, 139851. [Google Scholar] [CrossRef]
- APHA. Standard methods for examination of water and wastewater, 21st Edition ed; American Public Health Association: Washington, 2005. [Google Scholar]
- Yang, Y.; Jiang, Y.; Long, Y.; Xu, J.; Liu, C.; Zhang, L.; Peng, Y. Insights into the mechanism of the deterioration of mainstream partial nitritation/anammox under low residual ammonium. J. Environ. Sci. 2023, 126, 29–39. [Google Scholar] [CrossRef]
- Sliekers, A.O.; Derwort, N.; Gomez, J.L.C.; Strous, M.; Kuenen, J.G.; Jetten, M.S.M. Completely autotrophic nitrogen removal over nitrite in one single reactor. Water Res. 2002, 36, 2475–2482. [Google Scholar] [CrossRef]
- Du, R.; Cao, S.; Li, B.; Niu, M.; Wang, S.; Peng, Y. Performance and microbial community analysis of a novel DEAMOX based on partial-denitrification and anammox treating ammonia and nitrate wastewaters. Water Res. 2017, 108, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.A.; Ahmad, S.; Gao, L.; Ismail, S.; Wang, Z.; El-Baz, A.; Ni, S.-Q. Multi-omics analysis revealed the selective enrichment of partial denitrifying bacteria for the stable coupling of partial-denitrification and anammox process under the influence of low strength magnetic field. Water Res. 2023, 245, 120619. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Mao, Y.; Bergaust, L.; Bakken, L.R.; Frostegård, Å. Strains in the genus Thauera exhibit remarkably different denitrification regulatory phenotypes. Environ. Microbiol. 2013, 15, 2816–2828. [Google Scholar] [CrossRef] [PubMed]
- Suri, N.; Zhang, Y.; Gieg, L.M.; Ryan, M.C. Denitrification biokinetics: Towards optimization for industrial applications. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Ren, T.; Chi, Y.; Wang, Y.; Shi, X.; Jin, X.; Jin, P. Diversified metabolism makes novel Thauera strain highly competitive in low carbon wastewater treatment. Water Res. 2021, 206, 117742. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J. Long-term low DO enriches and shifts nitrifier community in activated sludge. Environ. Sci. Technol. 2013, 47, 5109–5117. [Google Scholar] [CrossRef]
- Ali, P.; Zalivina, N.; Le, T.; Riffat, R.; Ergas, S.; Wett, B.; Murthy, S.; Al-Omari, A.; deBarbadillo, C.; Bott, C.; et al. Primary sludge fermentate as carbon source for mainstream partial denitrification–anammox (PdNA). Water Environ. Res. 2021, 93, 1044–1059. [Google Scholar] [CrossRef]
- Cao, S.; Wang, S.; Peng, Y.; Wu, C.; Du, R.; Gong, L.; Ma, B. Achieving partial denitrification with sludge fermentation liquid as carbon source: the effect of seeding sludge. Bioresour. Technol. 2013, 149, 570–574. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




