Submitted:
08 January 2025
Posted:
09 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
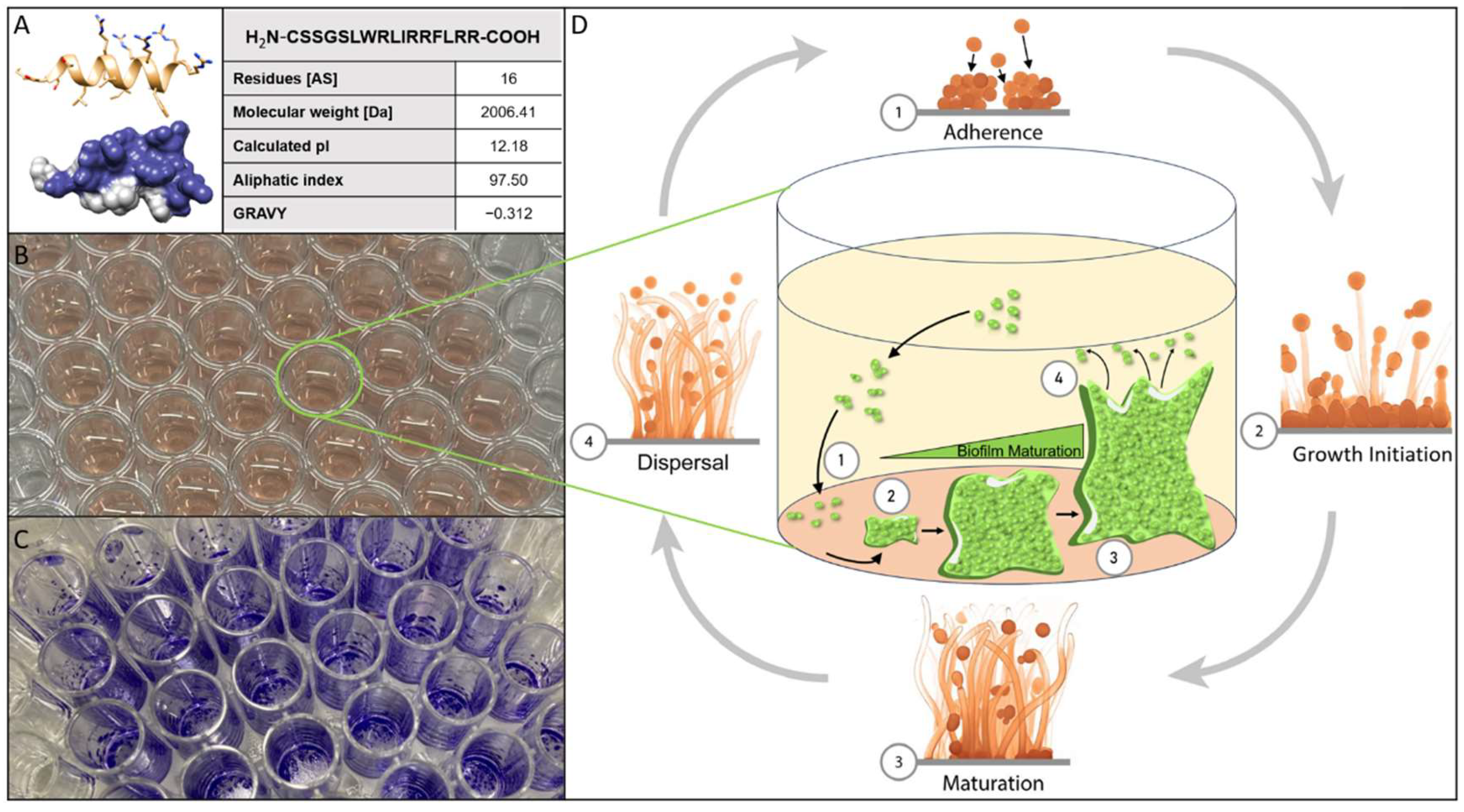
2. Materials and Methods
2.1. Materials
2.2. Candida Cultivation
2.3. Biofilm Kinetic Assay/ Crystal-Violet-Assay
2.4. Resazurin-Reduction-Assay/Viability-Assay
2.5. Permeabilization Assay
2.6. Inhibition of biofilm formation Assay
2.7. Decay of preformed biofilm Assay
3. Results
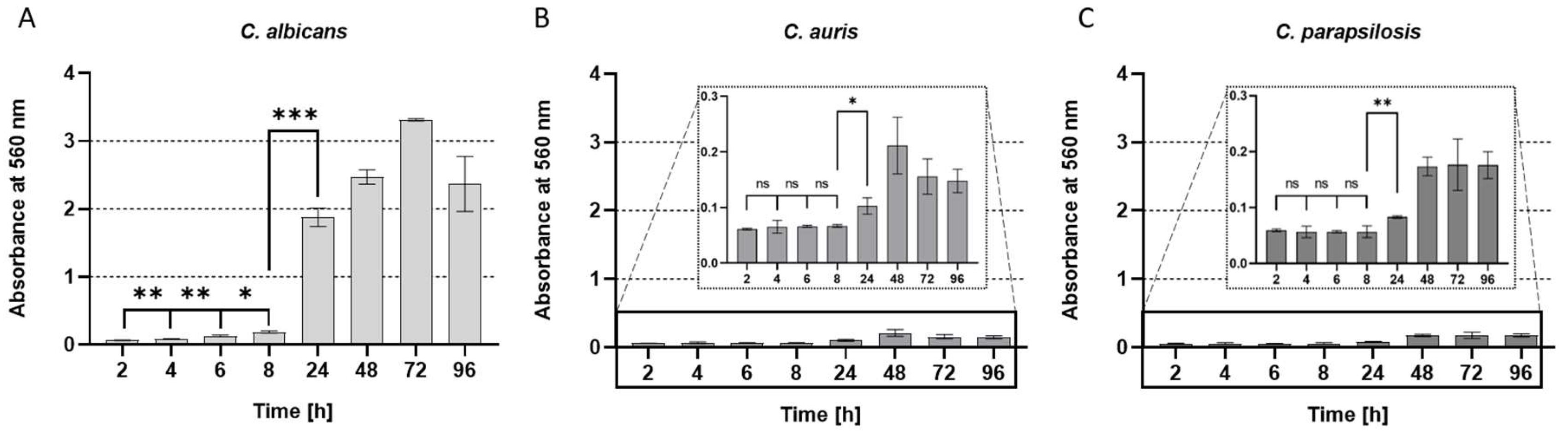
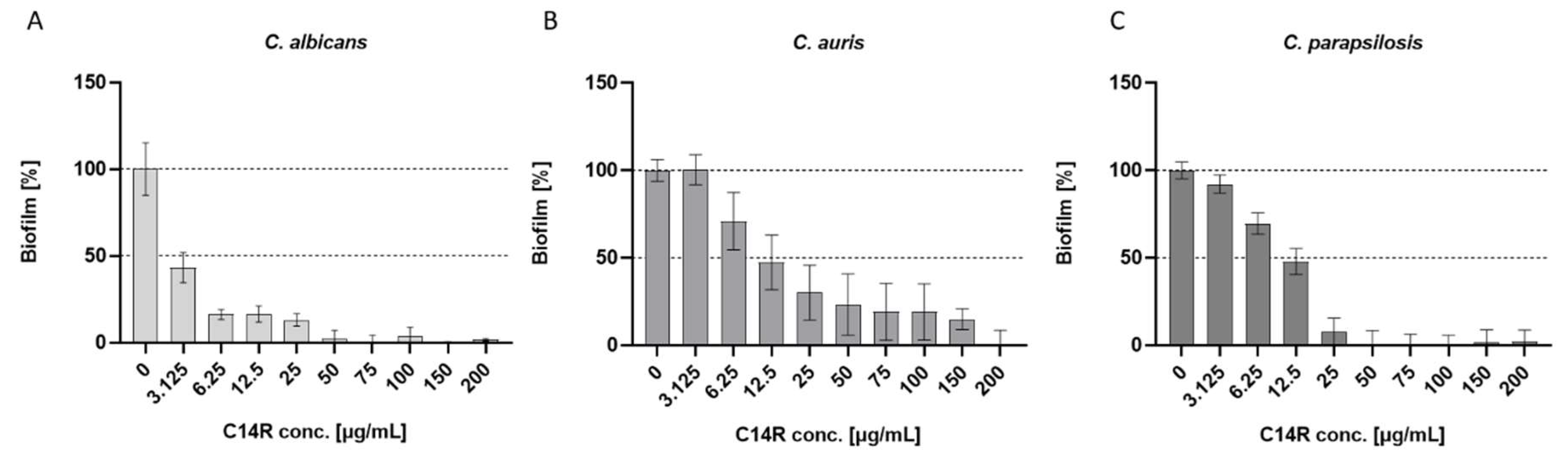
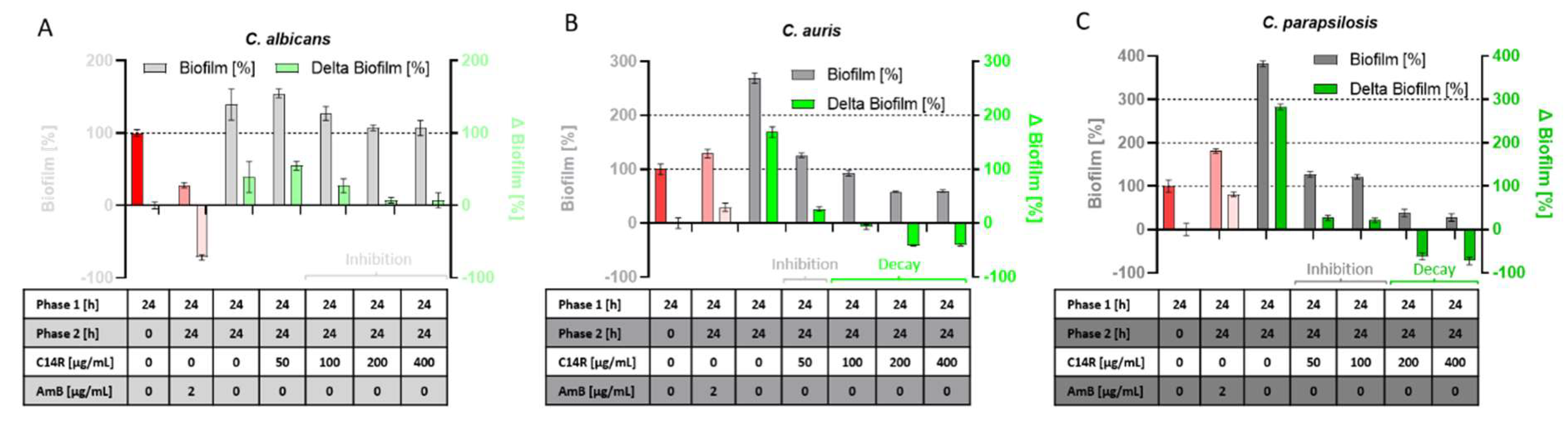
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nadell, C.D.; Bassler, B.L.; Levin, S.A. Observing Bacteria through the Lens of Social Evolution. J Biol 2008, 7, 27. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Davies, D. Understanding Biofilm Resistance to Antibacterial Agents. Nat Rev Drug Discov 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Fox, E.P.; Nobile, C.J. A Sticky Situation: Untangling the Transcriptional Network Controlling Biofilm Development in Candida Albicans. Transcription 2012, 3, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.; Nobile, C. The Role of Candida Albicans Biofilms in Human Disease; Nova Science Publishers, Inc., 2013; ISBN 9781628088823.
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nat Rev Microbiol 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Papon, N.; Courdavault, V.; Clastre, M.; Bennett, R.J. Emerging and Emerged Pathogenic Candida Species: Beyond the Candida Albicans Paradigm. PLoS Pathog 2013, 9, e1003550. [Google Scholar] [CrossRef]
- Sobel, J.D. Vulvovaginal Candidosis. The Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Paramythiotou, E.; Frantzeskaki, F.; Flevari, A.; Armaganidis, A.; Dimopoulos, G. Invasive Fungal Infections in the ICU: How to Approach, How to Treat. Molecules 2014, 19, 1085–1119. [Google Scholar] [CrossRef] [PubMed]
- Tóth, R.; Nosek, J.; Mora-Montes, H.M.; Gabaldon, T.; Bliss, J.M.; Nosanchuk, J.D.; Turner, S.A.; Butler, G.; Vágvölgyi, C.; Gácser, A. Candida Parapsilosis: From Genes to the Bedside. Clin Microbiol Rev 2019, 32. [Google Scholar] [CrossRef]
- Pammi, M.; Holland, L.; Butler, G.; Gacser, A.; Bliss, J.M. Candida Parapsilosis Is a Significant Neonatal Pathogen. Pediatric Infectious Disease Journal 2013, 32, e206–e216. [Google Scholar] [CrossRef] [PubMed]
- Weems, J.J.; Chamberland, M.E.; Ward, J.; Willy, M.; Padhye, A.A.; Solomon, S.L. Candida Parapsilosis Fungemia Associated with Parenteral Nutrition and Contaminated Blood Pressure Transducers. J Clin Microbiol 1987, 25, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.; Kindermann, S.L.; Hou, Q.; Taylor, R.J.; Azie, N.; Horn, D.L. Candidemia and Invasive Candidiasis among Hospitalized Neonates and Pediatric Patients. Curr Med Res Opin 2017, 33, 1803–1812. [Google Scholar] [CrossRef]
- Govender, N.P.; Patel, J.; Magobo, R.E.; Naicker, S.; Wadula, J.; Whitelaw, A.; Coovadia, Y.; Kularatne, R.; Govind, C.; Lockhart, S.R.; et al. Emergence of Azole-Resistant Candida Parapsilosis Causing Bloodstream Infection: Results from Laboratory-Based Sentinel Surveillance in South Africa. Journal of Antimicrobial Chemotherapy 2016, 71, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Cantón, E.; Pemán, J.; Quindós, G.; Eraso, E.; Miranda-Zapico, I.; Álvarez, M.; Merino, P.; Campos-Herrero, I.; Marco, F.; de la Pedrosa, E.G.G.; et al. Prospective Multicenter Study of the Epidemiology, Molecular Identification, and Antifungal Susceptibility of Candida Parapsilosis, Candida Orthopsilosis, and Candida Metapsilosis Isolated from Patients with Candidemia. Antimicrob Agents Chemother 2011, 55, 5590–5596. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Curfs-Breuker, I.; Meis, J.F.; Mouton, J.W. In Vitro Antifungal Susceptibility Testing of Candida Isolates with the EUCAST Methodology, a New Method for ECOFF Determination. Antimicrob Agents Chemother 2017, 61. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Moet, G.J.; Messer, S.A.; Jones, R.N.; Castanheira, M. Candida Bloodstream Infections: Comparison of Species Distributions and Antifungal Resistance Patterns in Community-Onset and Nosocomial Isolates in the SENTRY Antimicrobial Surveillance Program, 2008-2009. Antimicrob Agents Chemother 2011, 55, 561–566. [Google Scholar] [CrossRef]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida Auris Sp. Nov., a Novel Ascomycetous Yeast Isolated from the External Ear Canal of an Inpatient in a Japanese Hospital. Microbiol Immunol 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Morales-López, S.E.; Parra-Giraldo, C.M.; Ceballos-Garzón, A.; Martínez, H.P.; Rodríguez, G.J.; Álvarez-Moreno, C.A.; Rodríguez, J.Y. Invasive Infections with Multidrug-Resistant Yeast Candida Auris, Colombia. Emerg Infect Dis 2017, 23, 162–164. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida Auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clinical Infectious Diseases 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Berman, J.; Novikov, A.; Bash, E.; Shachor-Meyouhas, Y.; Zakin, S.; Maor, Y.; Tarabia, J.; Schechner, V.; Adler, A.; et al. Multidrug-Resistant Candida Haemulonii and C. Auris, Tel Aviv, Israel. Emerg Infect Dis 2017, 23. [Google Scholar] [CrossRef] [PubMed]
- Osei Sekyere, J. Candida Auris: A Systematic Review and Meta-analysis of Current Updates on an Emerging Multidrug-resistant Pathogen. Microbiologyopen 2018, 7. [Google Scholar] [CrossRef]
- A New Fungal Superbug Emerging as a Global Threat. Available online: Https://Www.Forbes.Com/Sites/Judystone/2017/08/24/Candida-Auris-a-New-Fungal-Superbug-Emerging-as-a-Global-Threat/ (accessed on day month year).
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.;
- Kean, R.; Delaney, C.; Sherry, L.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R.; Williams, C.; Ramage, G. Transcriptome Assembly and Profiling of Candida Auris Reveals Novel Insights into Biofilm-Mediated Resistance. mSphere 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Johnson, A.D. Candida Albicans Biofilms and Human Disease. Annu Rev Microbiol 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Mildenberger, V.; Alpízar-Pedraza, D.; Martell-Huguet, E.M.; Krämer, M.; Bolotnikov, G.; Otero-Gonzalez, A.J.; Weil, T.; Rodriguez-Alfonso, A.; Preising, N.; Ständker, L.; et al. The Designed Pore-Forming Antimicrobial Peptide C14R Combines Excellent Activity against the Major Opportunistic Human Pathogen Pseudomonas Aeruginosa with Low Cytotoxicity. Pharmaceuticals 2024, 17, 83. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil Med Res 2021, 8, 48. [Google Scholar] [CrossRef]
- Vélez, N.; Argel, A.; Kissmann, A.-K.; Alpízar-Pedraza, D.; Escandón, P.; Rosenau, F.; Ständker, L.; Firacative, C. Pore-Forming Peptide C14R Exhibits Potent Antifungal Activity against Clinical Isolates of Candida Albicans and Candida Auris. Front Cell Infect Microbiol 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Bodenberger, N.; Kubiczek, D.; Halbgebauer, D.; Rimola, V.; Wiese, S.; Mayer, D.; Rodriguez Alfonso, A.A.; Ständker, L.; Stenger, S.; Rosenau, F. Lectin-Functionalized Composite Hydrogels for “Capture-and-Killing” of Carbapenem-Resistant Pseudomonas Aeruginosa. Biomacromolecules 2018, 19, 2472–2482. [Google Scholar] [CrossRef]
- Bagherabadi, M.; Fleckenstein, M.; Moskalyk, O.; Belluati, A.; Avrutina, O.; Andrieu-Brunsen, A. Grafting and Controlled Release of Antimicrobial Peptides from Mesoporous Silica. J Mater Chem B 2024, 12, 8167–8180. [Google Scholar] [CrossRef]
- Kraemer, M.; Bellion, M.; Kissmann, A.-K.; Herberger, T.; Synatschke, C. V.; Bozdogan, A.; Andersson, J.; Rodriguez, A.; Ständker, L.; Wiese, S.; et al. Aptamers as Novel Binding Molecules on an Antimicrobial Peptide-Armored Composite Hydrogel Wound Dressing for Specific Removal and Efficient Eradication of Pseudomonas Aeruginosa. Int J Mol Sci 2023, 24, 4800. [Google Scholar] [CrossRef] [PubMed]
- Wall, G.; Montelongo-Jauregui, D.; Vidal Bonifacio, B.; Lopez-Ribot, J.L.; Uppuluri, P. Candida Albicans Biofilm Growth and Dispersal: Contributions to Pathogenesis. Curr Opin Microbiol 2019, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. Journal of Visualized Experiments 2011. [Google Scholar] [CrossRef]
- Sherry, L.; Rajendran, R.; Lappin, D.F.; Borghi, E.; Perdoni, F.; Falleni, M.; Tosi, D.; Smith, K.; Williams, C.; Jones, B.; et al. Biofilms Formed by Candida Albicans Bloodstream Isolates Display Phenotypic and Transcriptional Heterogeneity That Are Associated with Resistance and Pathogenicity. BMC Microbiol 2014, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida Auris. Emerg Infect Dis 2017, 23, 328–331. [Google Scholar] [CrossRef]
- Negri, M.; Gonçalves, V.; Silva, S.; Henriques, M.; Azeredo, J.; Oliveira, R. Crystal Violet Staining to Quantify Candida Adhesion to Epithelial Cells. Br J Biomed Sci 2010, 67, 120–125. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Third Edition. CLSI Document M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
- González García, M.; Rodríguez, A.; Alba, A.; Vázquez, A.A.; Morales Vicente, F.E.; Pérez-Erviti, J.; Spellerberg, B.; Stenger, S.; Grieshober, M.; Conzelmann, C.; et al. New Antibacterial Peptides from the Freshwater Mollusk Pomacea Poeyana (Pilsbry, 1927). Biomolecules 2020, 10, 1473. [Google Scholar] [CrossRef]
- Amann, V.; Kissmann, A.-K.; Krämer, M.; Krebs, I.; Perez-Erviti, J.A.; Otero-Gonzalez, A.J.; Morales-Vicente, F.; Rodríguez, A.; Ständker, L.; Weil, T.; et al. Increased Activities against Biofilms of the Pathogenic Yeast Candida Albicans of Optimized Pom-1 Derivatives. Pharmaceutics 2022, 14, 318. [Google Scholar] [CrossRef]
- Häring, M.; Amann, V.; Kissmann, A.-K.; Herberger, T.; Synatschke, C.; Kirsch-Pietz, N.; Perez-Erviti, J.A.; Otero-Gonzalez, A.J.; Morales-Vicente, F.; Andersson, J.; et al. Combination of Six Individual Derivatives of the Pom-1 Antibiofilm Peptide Doubles Their Efficacy against Invasive and Multi-Resistant Clinical Isolates of the Pathogenic Yeast Candida Albicans. Pharmaceutics 2022, 14, 1332. [Google Scholar] [CrossRef]
- Kubiczek, D.; Raber, H.; Gonzalez-García, M.; Morales-Vicente, F.; Staendker, L.; Otero-Gonzalez, A.J.; Rosenau, F. Derivates of the Antifungal Peptide Cm-P5 Inhibit Development of Candida Auris Biofilms In Vitro. Antibiotics 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Raber, H.F.; Sejfijaj, J.; Kissmann, A.-K.; Wittgens, A.; Gonzalez-Garcia, M.; Alba, A.; Vázquez, A.A.; Morales Vicente, F.E.; Erviti, J.P.; Kubiczek, D.; et al. Antimicrobial Peptides Pom-1 and Pom-2 from Pomacea Poeyana Are Active against Candidaauris, C. Parapsilosis and C. Albicans Biofilms. Pathogens 2021, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Amann, V.; Kissmann, A.-K.; Mildenberger, V.; Krebs, I.; Perez-Erviti, J.A.; Martell-Huguet, E.M.; Otero-Gonzalez, A.J.; Morales-Vicente, F.; Rodríguez-Castaño, G.P.; Firacative, C.; et al. Cm-P5 Peptide Dimers Inhibit Biofilms of Candida Albicans Clinical Isolates, C. Parapsilosis and Fluconazole-Resistant Mutants of C. Auris. Int J Mol Sci 2023, 24, 9788. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Santos Fontenelle, R.O.; Brito, E.H.S.; Morais, S.M. Biofilm of Candida Albicans: Formation, Regulation and Resistance. J Appl Microbiol 2021, 131, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Quindós, G.; Marcos-Arias, C.; San-Millán, R.; Mateo, E.; Eraso, E. The Continuous Changes in the Aetiology and Epidemiology of Invasive Candidiasis: From Familiar Candida Albicans to Multiresistant Candida Auris. International Microbiology 2018, 21, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The Changing Epidemiology of Invasive Fungal Infections. In; 2017; pp. 17–65.
- Garcia-Bustos, V. Is Candida Auris the First Multidrug-Resistant Fungal Zoonosis Emerging from Climate Change? mBio 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for Combating Bacterial Biofilm Infections. Int J Oral Sci 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida Biofilm Drug Resistance. Future Microbiol 2013, 8, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.; Khan, T.; Patching, S.G.; Omri, A. Development of Antibiofilm Therapeutics Strategies to Overcome Antimicrobial Drug Resistance. Microorganisms 2022, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Kissmann, A.-K.; Mildenberger, V.; Krämer, M.; Alpízar-Pedraza, D.; Martell-Huguet, E.M.; Perez-Erviti, J.A.; Cetinkaya, A.; Pietrasik, J.; Otero-Gonzalez, A.J.; Firacative, C.; et al. Anti-Biofilm Peptides Can Rescue Fluconazole and Amphotericin B Efficacies against Candida Albicans 2024.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




