Submitted:
07 January 2025
Posted:
08 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation for GPQT Sampling
2.2.2. Completion of the GPQT Referral Form
2.2.3. GastroPanel® Quick Test (GPQT)
2.2.4. Sample Collection for GPQT
2.2.5. Sample Processing for GPQT
2.2.6. Interpreting the GPQT Results
2.2.7. Gastroscopy and Biopsies
2.2.8. Biopsy Protocols
2.2.9. Interpretation of the Gastric Biopsies
2.2.10. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | Gastric cancer |
| AG | Atrophic gastritis |
| Hp | Helicobacter pylori |
| GPQT | GastroPanel® Quick test |
| POC | Point-of-care |
| DA | Diagnostic accuracy |
| USS | Updated Sydney System |
| AGA | Atrophic antrum gastritis |
| AGC | Atrophic corpus gastritis |
| AGP | Atrophic pan-gastritis |
| AUC | Area under ROC curve |
| ROC | Receiver operating characteristics |
| PGI | Pepsinogen I |
| PGII | Pepsinogen II |
| G-17 | Gastrin-17; G-17b (basal), G-17s (stimulated) |
| ELISA | Enzyme-linked immunosorbent assay |
| IARC | International Agency of Research on Cancer |
| EGD | Esophago-gastro-duodenoscopy |
| SE | Sensitivity |
| SP | Specificity |
| PPV | Positive predictive value |
| NPV | Negative predictive values |
| K-T | Kimura-Takemoto classification |
| ICC | Intra-class correlation coefficient |
| LR+ | Positive likelihood ratio |
| LR- | Negative likelihood ratio |
| kw | Weighted kappa test |
| OA | Overall agreement |
| PPI | Proton pump inhibitor |
| NSAID | Non-steroidal anti-inflammatory drugs |
| AGA2+ | Moderate/Severe atrophic antrum gastritis |
| AGC2+ | Moderate/Severe atrophic corpus gastritis |
| GP | GastroPanel® |
| H2 | Histamin-2 receptor |
| OLGA | Operative link to gastric atrophy |
| OLGIM | Operative link to gastric intestinal metaplasia |
| G-cells | Gastrin-secreting cells |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Correa, P.; Haenszel, W.; Cuello, C.; Zavala, D.; Fontham, E.; Zarama, G.; Tannenbaum, S.; Collazos, T.; Ruiz, B. Gastric precancerous process in a high-risk population: cohort follow-up. Cancer Res 1990, 50, 4737–4740. [Google Scholar] [PubMed]
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef]
- Sipponen, P.; Marshall, B.J. Gastritis and gastric cancer. Western countries. Gastroenterol Clin North Am 2000, 29, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M. Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc Jpn Acad Ser B Phys Biol Sci 2017, 93, 196–219. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Sipponen, P.; Naumann, M. H. pylori-Gastric Cancer Task Force. Helicobacter pylori eradication has the potential to prevent gastric cancer: a state-of-the-art critique. Am J Gastroenterol 2005, 100, 2100–2115. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O'Morain, C.; Rugge, M.; Suerbaum, S.; Tilg, H.; Sugano, K.; El-Omar, E.M. European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut Online ahead of print. 2022. [Google Scholar] [CrossRef]
- Plummer, M.; Franceschi, S.; Munoz, N. Epidemiology of gastric cancer. IARC Sci Publ 2004, 157, 311–326. [Google Scholar] [PubMed]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Moayyedi, P.; Talley, N.J.; Fennerty, M.B.; Vakil, N. Can the clinical history distinguish between organic and functional dyspepsia? JAMA 2006, 295, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Sipponen, P.; Price, A.B. The Sydney system for classification of gastritis 20 years ago. J Gastroenterol Hepatol 2011, 26 (Suppl. 1), 31–34. [Google Scholar] [CrossRef] [PubMed]
- Agréus, L.; Kuipers, E.J.; Kupcinskas, L.; Malfertheiner, P.; Di Mario, F.; Leja, M.; Mahachai, V.; Yaron, N.; van Oijen, M.; Perez- Perez, G.; Rugge, M.; Ronkainen, J.; Salaspuro, M.; Sipponen, P.; Sugano, K.; Sung, J. Rationale in diagnosis and screening of atrophic gastritis with stomach-specific plasma biomarkers. Scand J Gastroenterol 2012, 47, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Ichinose, M.; Shimizu, A.; Huang, S.C.; Oka, H.; Furihata, C.; Matsushima, T.; Takahashi, K. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn 1987, 22, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Samloff, I.M.; Varis, K.; Ihamäki, T.; Siurala, M.; Rotter, J.I. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterol 1982, 83, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Biohit HealthCare. GastroPanel. Available at: https://www.gastropanel.com/healthcare-professionals-and-laboratories/forms-and-instructions (Last access on October 17, 2024).
- Syrjänen, K.; Eskelinen, M.; Peetsalu, A.; Sillakivi, T.; Sipponen, P.; Härkönen, M.; Paloheimo, L.; Mäki, M.; Tiusanen, T.; Suovaniemi, O.; DiMario, F.; Fan, Z.P. GastroPanel® Biomarker Panel: The most comprehensive test for Helicobacter pylori infection and its clinical sequelae. A critical review. Anticancer Res 2019, 39, 1091–1104. [Google Scholar] [CrossRef]
- Iijima, K.; Abe, Y.; Kikuchi, R.; Koike, T.; Ohara, T.; Sipponen, P.; Shimosegawa, T. Serum biomarker tests are useful in delineating between patients with gastric atrophy and normal, healthy stomach. World J Gastroenterol 2009, 15, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, F.; Moussa, A.M.; Caruana, P.; Merli, R.; Cavallaro, L.G.; Cavestro, G.M.; Dal Bo, N.; Iori, V.; Pilotto, A.; Leandro, G.; Franzé, A.; Rugge, M. Serological biopsy in first-degree relatives of patients with gastric cancer affected by Helicobacter pylori infection. Scand J Gastroenterol 2003, 38, 1223–1227. [Google Scholar] [CrossRef]
- Väänänen, H.; Vauhkonen, M.; Helske, T.; Kääriäinen, I.; Rasmussen, M.; Tunturi-Hihnala, H.; Koskenpato, J.; Sotka, M.; Turunen, M.; Sandström, R.; Ristikankare, M.; Jussila, A.; Sipponen, P. Non-endoscopic diagnosis of atrophic gastritis with a blood test. Correlation between gastric histology and serum levels of gastrin-17 and pepsinogen I: a multi-centre study. Eur J Gastroenterol Hepatol 2003, 15, 885–891. [Google Scholar] [CrossRef]
- Hartleb, M.; Wandzel, P.; Waluga, M.; Matyszczyk, B.; Bołdys, H.; Romanczyk, T. Non-endoscopic diagnosis of multifocal atrophic gastritis; efficacy of serum gastrin-17, pepsinogens and Helicobacter pylori antibodies. Acta Gastroenterol Belg 2004, 67, 320–326. [Google Scholar] [PubMed]
- Pasechnikov, V.D.; Chukov, S.Z.; Kotelevets, S.M.; Mostovov, A.N.; Mernova, V.P.; Polyakova, M.B. Possibility of non-invasive diagnosis of gastric mucosal precancerous changes. World J Gastroenterol 2004, 10, 3146–3150. [Google Scholar] [CrossRef] [PubMed]
- Pasechnikov, V.D.; Chukov, S.Z.; Kotelevets, S.M.; Mostovov, A.N.; Mernova, V.P.; Polyakova, M.B. Invasive and non-invasive diagnosis of Helicobacter pylori-associated atrophic gastritis: a comparative study. Scand J Gastroenterol 2005, 40, 297–301. [Google Scholar] [CrossRef]
- Nardone, G.; Rocco, A.; Staibano, S.; Mezza, E.; Autiero, G.; Compare, D.; De Rosa, G.; Budillon, G. Diagnostic accuracy of the serum profile of gastric mucosa in relation to histological and morphometric diagnosis of atrophy. Aliment Pharmacol Ther 2005, 22, 1139–1146. [Google Scholar] [CrossRef]
- Graham, D.Y.; Nurgalieva, Z.Z.; El-Zimaity, H.M.; Opekun, A.R.; Campos, A.; Guerrero, L. Noninvasive versus histologic detection of gastric atrophy in a Hispanic population in North America. Clin Gastroenterol Hepatol 2006, 4, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Storskrubb, T.; Aro, P.; Ronkainen, J.; Sipponen, P.; Nyhlin, H.; Talley, N.J. Serum biomarkers provide an accurate method for diagnosis of atrophic gastritis in a general population: the Kalixanda study. Scand J Gastroenterol 2008, 43, 1448–1455. [Google Scholar] [CrossRef]
- Koivurova, O.-P.; Ukkola, O.; Koivikko, M.; Ebeling, T.; Yliaska, I.; Koskela, R.; Blomster, T.; Ala-Rämi, A.; Kettunen, O.; Karttunen, T.J.; Mäkinen, M.; Ronkainen, J.; Syrjänen, K. Screening of the patients with autoimmune thyroid disease (AITD) and type 1 diabetes mellitus (DM1) for atrophic gastritis (AG) by serological biomarker testing (GastroPanel®). EC Gastroenterol Digest Syst 2020, 7, 181–195. [Google Scholar]
- Mäki, M.; Söderström, D.; Paloheimo, L.; Hendolin, P.; Suovaniemi, O.; Syrjänen, K. Helicobacter pylori (Hp) IgG ELISA of the new-generation GastroPanel® is highly accurate in diagnosis of Hp-Infection in gastroscopy referral patients. Anticancer Res 2020, 40, 6387–6398. [Google Scholar] [CrossRef]
- Sanchez-Lopez, J.Y.; Diaz-Herrera, L.C. Pepsinogen I, pepsinogen II, gastrin-17, and Helicobacter pylori serological biomarkers in the diagnosis of precursor lesions of gastric cancer. Arch Med Sci 2024, 20, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, N.; Petryszyn, P.; Blin, J.; Leroy, M.; Le Berre-Scoul, C.; Jirka, I. A panel of stomach-specific biomarkers (GastroPanel®) for the diagnosis of atrophic gastritis: A prospective, multicenter study in a low gastric cancer incidence area. Helicobacter 2020, 25, e12727. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, K. A Panel of serum biomarkers (GastroPanel®) in non-invasive diagnosis of atrophic gastritis. Systematic review and meta-analysis. Anticancer Res 2016, 36, 5133–5144. [Google Scholar] [CrossRef]
- Zagari, R.M.; Rabitti, S.; Greenwood, D.C.; Eusebi, L.H.; Vestito, A.; Bazzoli, F. Systematic review with meta-analysis: Diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment Pharmacol Ther 2017, 46, 1–11. [Google Scholar] [CrossRef]
- Syrjänen, K. Accuracy of serum biomarker panel (GastroPanel®) in diagnosis of atrophic gastritis of the corpus (AGC). Systematic review and meta-analysis. Anticancer Res 2022, 42, 1679–1696. [Google Scholar] [CrossRef] [PubMed]
- Koivurova, O.P. ; Koskela, R:, Blomster, T.; Ala-Rämi, A.; Lumme, H.; Kettunen, O.; Hukkanen, J.; Karttunen, T.J.; Mäkinen, M.; Ronkainen, J.; Syrjänen, K. Serological biomarker panel in diagnosis of atrophic gastritis and Helicobacter pylori infection in gastroscopy referral patients. Clinical validation of the new-generation GastroPanel® test. Anticancer Res 2021, 41, 5527–5537. [Google Scholar] [CrossRef] [PubMed]
- Biohit HealthCare. GastroPanel. Available at: GastroPanel® ELISA - Biohit (biohithealthcare.com) Last accessed on October 23, 2024].
- GastroPanel quick test NT. Available online: https://www.biohithealthcare.com/en/products/gastropanel-quick-test-nt/.
- https://www.biohithealthcare.com/wp-content/uploads/2023/12/GPQTNT_402230en_2.1_UG_FINAL_20231219.
- Kimura, K.; Takemoto, T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969, 1, 87–97. [Google Scholar] [CrossRef]
- Seed, P.T.; Tobias, A. Summary statistics for diagnostic tests. Stata Techn Bull 2001, 59, 9-12. https://www.researchgate.net/publication/24137572_Summary_statistics_for_diagnostic_tests.
- Fagan, T.J. Letter: nomogram for Bayes theorem. N Engl J Med 1975, 293, 257. [Google Scholar] [CrossRef]
- Ebule, A.I.; Ndze, V.N.; Thierry, N.K.; Etienne, G.; Ornella, M.M.M.; Aurelien, M.A.; Dominique, N.N.; Mäki, M.; Syrjänen, K. Association of Helicobacter pylori infection and atrophic gastritis with chronic renal insufficiency in Yaounde Cameroon, using GastroPanel® serological biomarker panel (Pepsinogen I; Pepsinogen II; Gastrin-17; Helicobacter pylori IgG). JAMB 2021, 21, 62–67. [Google Scholar] [CrossRef]
- Bakulina, N.; Tikhonov, S.; Malkov, V.; Vorobyev, S.; Belyakov, I.; Peshkova, N.; Belko, E.; Syrjänen, K. Non-invasive screening of autoimmune atrophic gastritis (AAG) in asymptomatic subjects by serological biomarker (GastroPanel®) test. Anticancer Res 2022, 42, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Roman, L.D.; Lukyanchuk, R.; Sablin, O.A.; Araslanova, E.I.; Eklund, C.; Hendolin, P.; Paloheimo, L.; Syrjänen, K. Prevalence of infection and atrophic gastritis in a population-based screening with serum biomarker panel (GastroPanel®) in St. Petersburg. Anticancer Res 2016, 36, 4129–4138. [Google Scholar] [PubMed]
- Benberin, V.; Bektayeva, R.; Karabayeva, R.; Lebedev, A.; Akemeyeva, K.; Paloheimo, L.; Syrjänen, K. Prevalence of H.pylori infection and atrophic gastritis among asymptomatic and dyspeptic adults in Kazakhstan. A Hospital-Based screening with a panel of serum biomarkers. Anticancer Res 2013, 33, 4595–4602. [Google Scholar] [PubMed]
- Sivandzadeh, G.R.; Zadeh Fard, S.A.; Zahmatkesh, A.; Anbardar, M.H.; Lankarani, K.B. Value of serological biomarker panel in diagnosis of atrophic gastritis and Helicobacter pylori Infection. Middle East J Dig Dis 2023, 15, 37–44. [Google Scholar] [CrossRef]
- Paloheimo, L.; Tiusanen, T.; Suovaniemi, O.; Syrjänen, K. Serological biomarker test (GastroPanel®) in diagnosis of functional gastric disorders, Helicobacter pylori and atrophic gastritis in a random sample of patients referred for testing due to dyspeptic symptoms. Anticancer Res 2021, 41, 811–891. [Google Scholar] [CrossRef]
- Rugge, M.; Genta, R.M.; Fassan, M.; Valentini, E.; Coati, I.; Guzzinati, S.; Savarino, E.; Zorzi, M.; Farinati, F.; Malfertheiner, P. OLGA gastritis staging for the prediction of gastric cancer risk: A Long-term follow-up study of 7436 patients. Am J Gastroenterol 2018, 113, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2022, 22, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, K.J.; Sipponen, P.; Härkönen, M.; Peetsalu, A.; Korpela, S. Accuracy of GastroPanel testing in detection of atrophic gastritis. Eur J Gastroenterol Hepatol 2015, 27, 102–104. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Number of Patients n=266 |
Per Cent of Total 100% |
|---|---|---|
| Gender: | ||
| Women | 135 | 50.8 |
| Men | 131 | 49.2 |
| Age (Median; Range) | 52.0 years | (18-92 years) |
| History recorded in GastroPanel®(GP) referral form: | ||
| 1.Helicobacter diagnosed: | ||
| Hp diagnosed in GP testing | 15 | 5.6 |
| Hp diagnosed withing one year | 25 | 9.4 |
| Hp never diagnosed | 223 | 83.8 |
| Responder does not know | 3 | 1.2 |
| 2.Helicobacter eradication, if done: | ||
| Yes, eradication successful | 12 | 38.7 |
| Eradication not successful | 12 | 38.7 |
| Responder does not know | 7 | 22.6 |
| 3.Use of PPI-medication: | ||
| No PPI-medication | 68 | 25.6 |
| Continuous use of PPI-medication | 197 | 74.1 |
| Responder does not recall | 1 | 0.3 |
| 4.Symptoms of high acidity (heartburn) | ||
| No symptoms of high acidity | 6 | 2.3 |
| Continuous symptoms of high acidity | 259 | 97.4 |
| Data missing | 1 | 0.3 |
| GPQT Diagnosis | No. of Cases | PGI (M±SD) | PGII (M±SD) | PGI/PGII (M±SD) | G-17b (M±SD) | HpAb (M±SD) |
|---|---|---|---|---|---|---|
| Normal | 136 | 165.6(80.2) | 17.1(12.0) | 12.3(6.5) | 23.2(21.9) | 10.8(14.7) |
| Hp-gastritis | 106 | 175.8(79.8) | 24.6(16.5) | 9.5(6.9) | 25.4(21.1) | 119.8(51.3) |
| AGA | 2 | 160.0(22.6) | 20.4(2.9) | 7.8(0.3) | 2.3(1.2) | 134.5(40.3) |
| AGC | 19 | 20.1(9.7) | 14.4(9.7) | 1.8(1.0) | 50.6(13.2) | 16.4(35.7) |
| AGP | 2 | 30.9(6.9) | 6.5(5.0) | 6.1(3.6) | 0.9(0.0) | 10.1(5.8) |
| Total Series | 265* | 158.2(86.4) | 19.9(14.3) | 10.4(6.9) | 25.8(22.1) | 55.7(64.1) |
|
USS Grade |
No of Cases |
PGI (M±SD) |
PGII (M±SD) | PGI/PGII (M±SD) | G-17b (M±SD) |
HpAb (M±SD) |
| Normal | 146 | 170.5(82.9) | 18.2(12.7) | 12.1(7.1) | 23.3(21.8) | 30.0(45.6) |
| Hp-gastritis | 65 | 158.7(78.5) | 21.9(15.6) | 9.6(6.5) | 23.0(20.5) | 106.9(62.2) |
| AGA | 21 | 180.1(88.0) | 27.0(16.5) | 8.0(4.9) | 25.3(22.3) | 103.0(69.5) |
| AGC | 15 | 28.0(40.9) | 14.0(10.8) | 2.2(2.0) | 53.0(11.6) | 28.7(54.7) |
| AGP | 2 | 90.5(77.2) | 23.8(19.4) | 3.7(0.2) | 28.5(38.9) | 19.2(18.7) |
| Total Series | 249* | 159.0(86.9) | 19.7(14.0) | 10.4(7.0) | 25.3(22.1) | 56.0(63.9) |
| GPQT Diagnosis |
The Updated Sydney System (USS) |
|||||
|---|---|---|---|---|---|---|
| Normal | Hp-gastritis | AGA | AGC | AGP | Total | |
| Normal | 109 | 13 | 4 | 0 | 1 | 127 |
| Hp-gastritis | 35 | 52 | 13 | 1 | 0 | 101 |
| AGA | 0 | 0 | 2 | 0 | 0 | 2 |
| AGC | 2 | 0 | 1 | 14 | 0 | 17 |
| AGP | 0 | 0 | 1 | 0 | 1 | 2 |
| Total | 146 | 65 | 21 | 15 | 2 | 249 |
| Overall agreement (OA): 178/249; 0.714 (95%CI 0.654-0.770); | ||||||
| *Weighted kappa (w): ICC=0.823 (95%CI 0.773-0.862); | ||||||
| GPQT Profile/ USS Endpoint |
Sensitivity |
Specificity |
PPV |
NPV |
AUC |
|---|---|---|---|---|---|
| @AGA Profile: | |||||
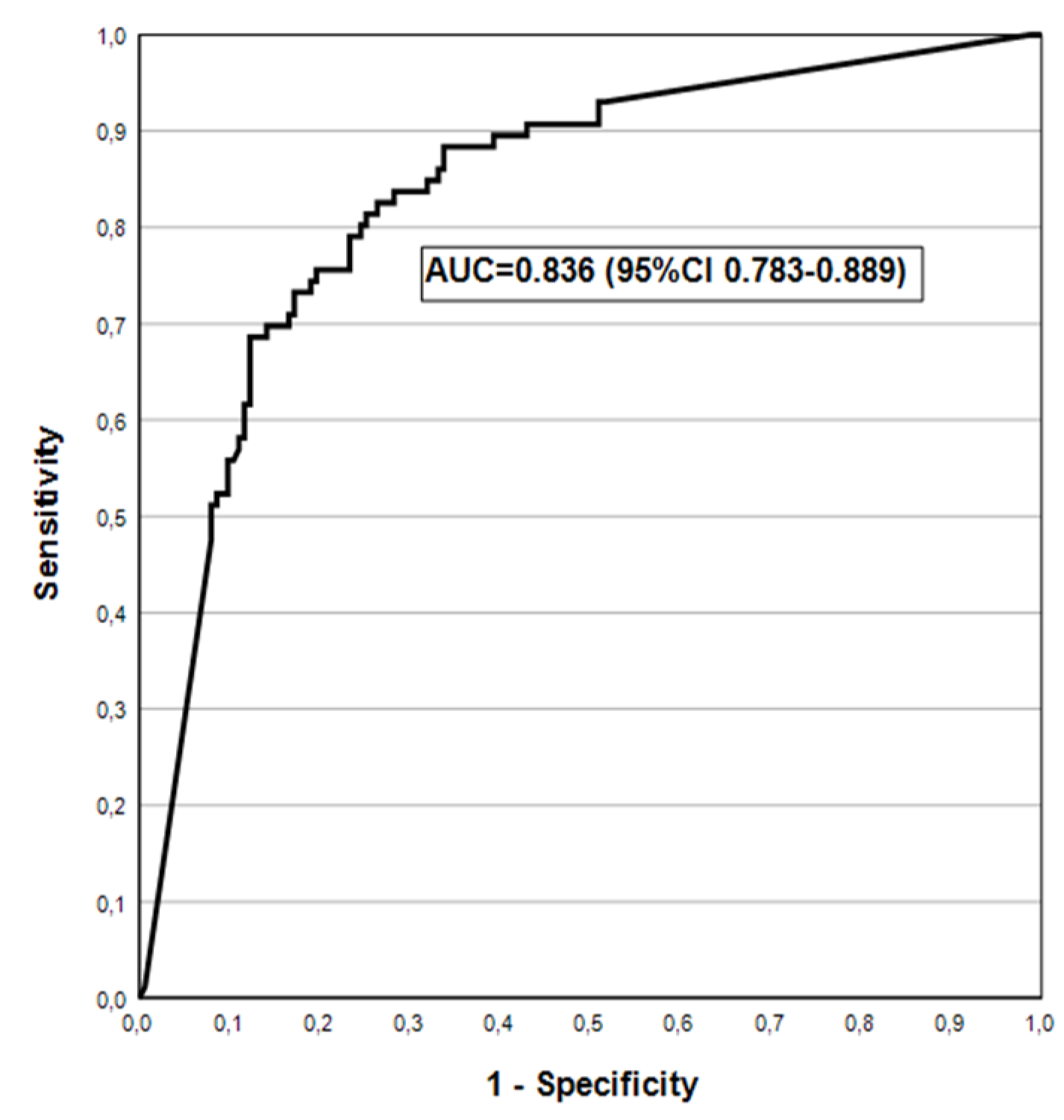
| *AGA | 14.3(3.0-36.3) | 99.6(97.6-100.0) | 75.0(19.4-99.4) | 92.7(88.6-95.6) | 0.569(0.492-0.646) |
| AGA2+ | 30.0(6.6-65.2) | 99.6(97.7-100.0) | 75.0(19.4-99.4) | 97.1(94.2-98.8) | 0.648(0.498-0.798) |
| @AGC Profile: | |||||
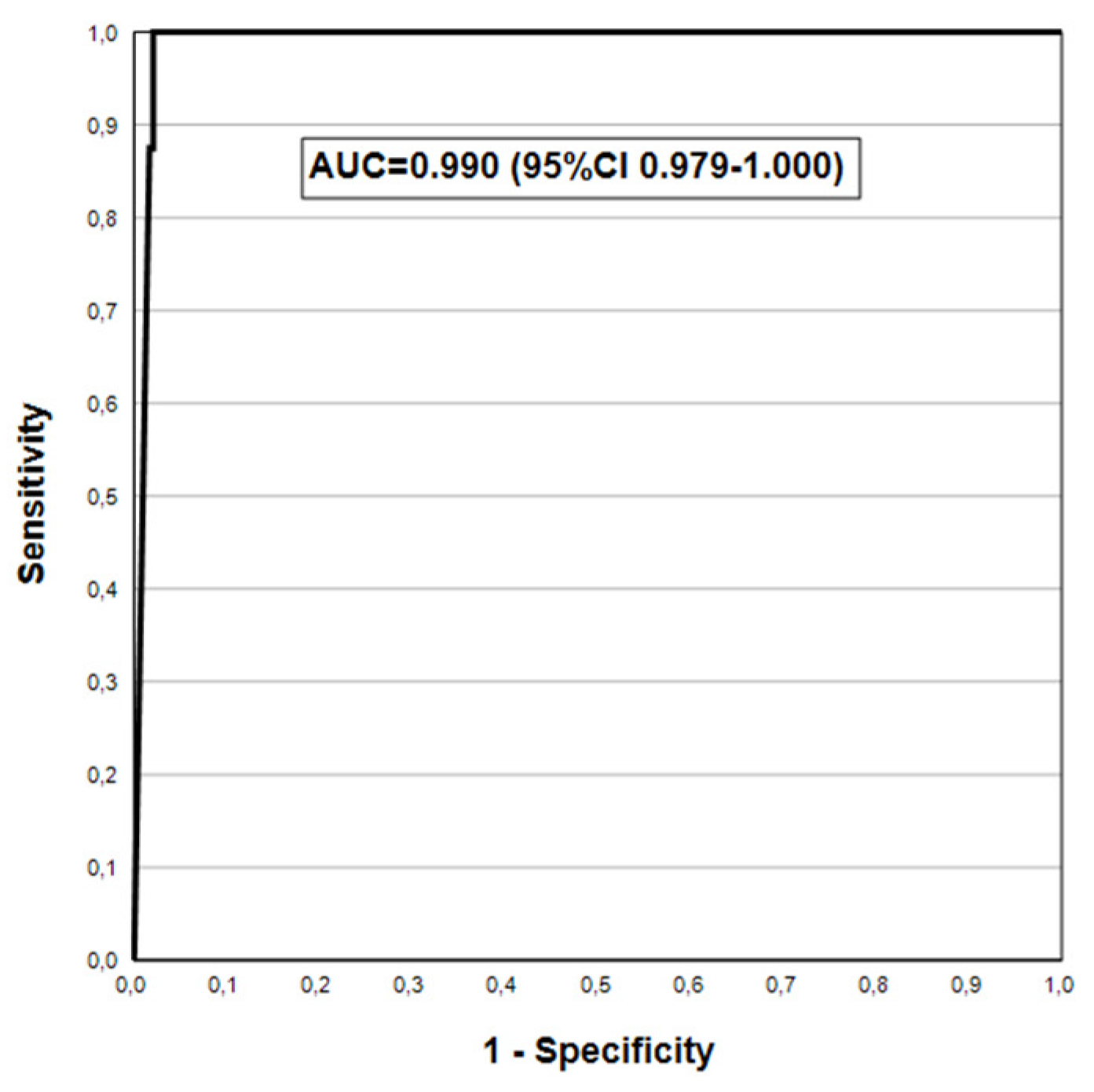
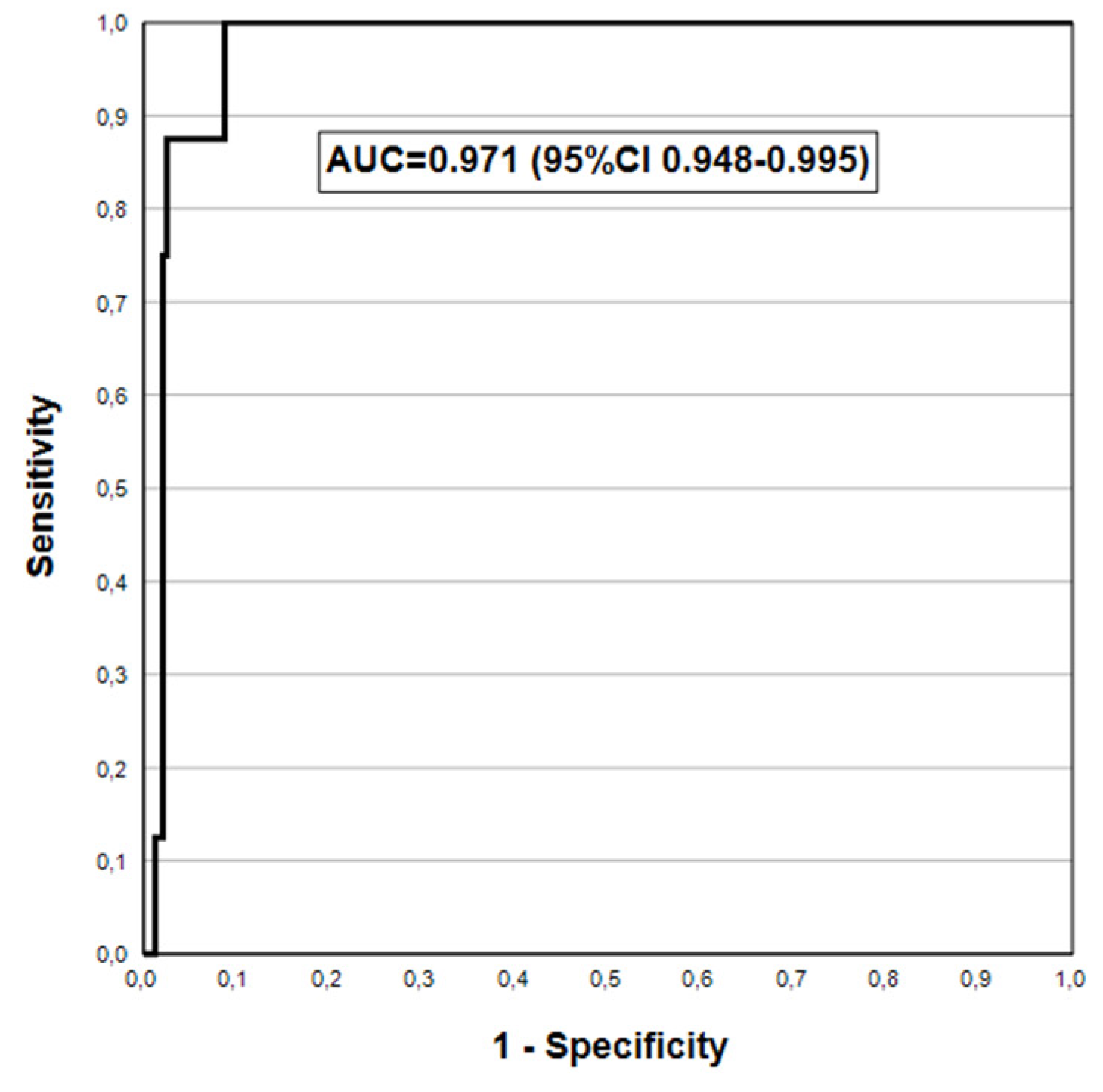
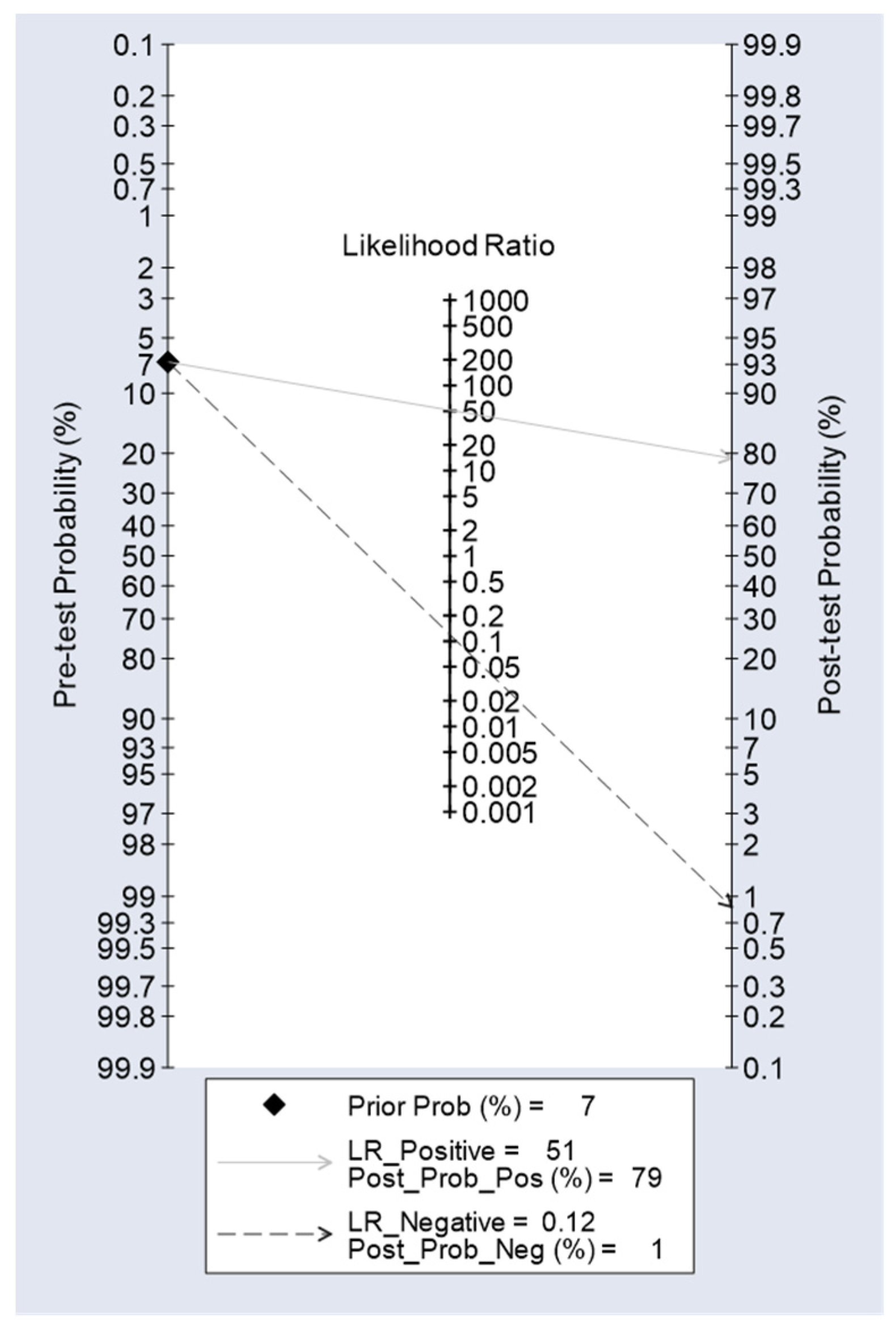
| **AGC | 88.2(63.6-98.5) | 98.3(95.6-99.5) | 78.9(54.4-93.9) | 99.1(96.9-99.9) | 0.933(0.853-1.000) |
| AGC2+ | 100(63.1-100) | 95.4(92.0-97.7) | 42.1(20.3-66.5) | 100(98.4-100) | 0.977(0.964-0.990) |
| Endpoint | Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|
| G-17b: | |||||
| *AGA | 9.5(1.1-30.4) | 96.9(93.8-98.8) | 22.2(2.8-60.0) | 92.1(87.9-95.2) | 0.532(0.467-0.598) |
| AGA2+ | 20.0(2.5-55.6) | 97.1(94.1-98.8) | 22.2(2.8-60.0) | 96.7(93.5-98.6) | 0.585(0.454-0.716) |
| PGI (15): | |||||
| **AGC | 52.9(27.8-77.0) | 99.1(96.9-99.9) | 81.8(48.2-97.7) | 96.6(93.5-98.5) | 0.760(0.638-0.883) |
| AGC2+ | 87.5(47.3-97.7) | 98.3(95.8-99.5) | 63.6(30.8-89.1) | 99.6(97.7-100) | 0.929(0.806-1.000) |
| PGI (30): | |||||
| **AGC | 76.5(50.1-93.2) | 98.7(96.3-99.7) | 81.3(54.4-96.0) | 98.3(95.7-99.5) | 0.876(0.772-0.980) |
| AGC2+ | 100(63.1-100) | 96.7(93.6-98.6) | 50.0(24.7-75.3) | 100(98.4-100) | 0.983(0.972-0.995) |
| PGI/PGII: | |||||
| **AGC | 64.7(38.3-85.8) | 97.4(94.5-99.0) | 64.7(38.3-85.8) | 97.4(94.5-99.0) | 0.811(0.693-0.928) |
| AGC2+ | 87.5(47.3-99.7) | 95.9(92.5-98.0) | 41.2(18.4-67.1) | 99.6(97.6-100) | 0.917(0.794-1.000) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





