Submitted:
08 January 2025
Posted:
08 January 2025
You are already at the latest version
Abstract
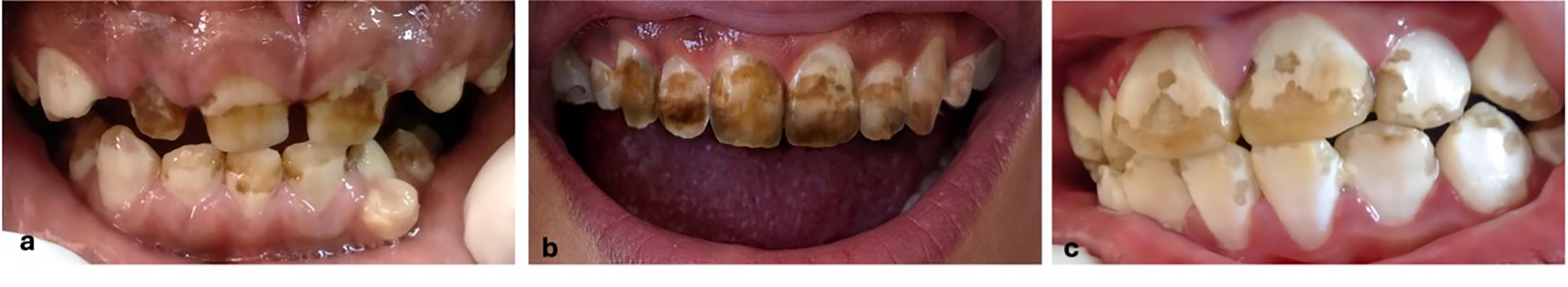
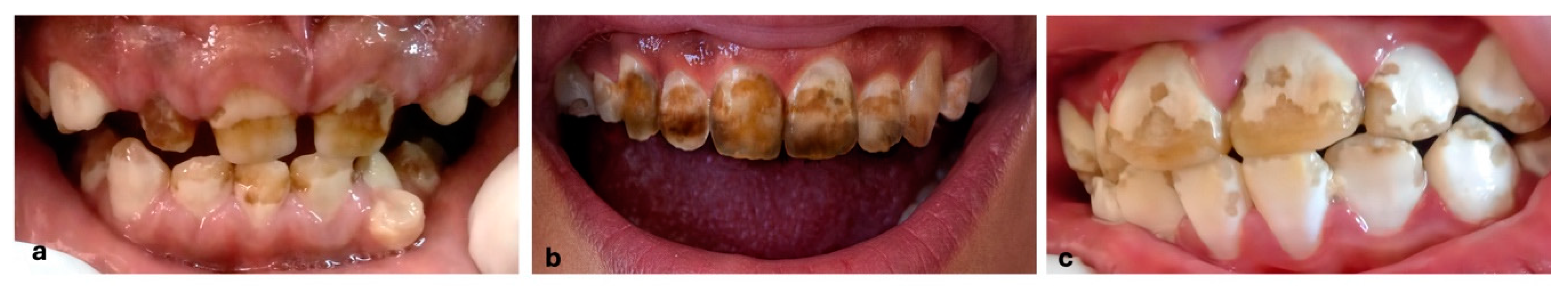
Keywords:
1. Introduction
2. Fluoride Toxicity
3. Diet-Related Toxicity
4. Toxicity Related to Cellular Damage, Organs, and Tissues
5. Biomarkers in Fluorosis
6. Acute Biomarkers in Fluorosis
7. Chronic Biomarkers in Fluorosis
8. Discussion
9. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pramanik, S.; Saha, D. The Genetic Influence in Fluorosis. Environ. Toxicol. Pharmacol. 2017, 56, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Pang, S.; Sun, D. The Pathogenesis of Endemic Fluorosis: Research Progress in the Last 5 Years. J. Cell. Mol. Med. 2019, 23, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, A.; Verma, K.; Paliwal, S.; Sharma, S.; Dwivedi, J. Fluoride: A Review of Pre-clinical and Clinical Studies. Environ. Toxicol. Pharmacol. 2017, 56, 297–313. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, 2011; p. 340. [Google Scholar] [CrossRef]
- Kanduti, D.; Sterbenk, P.; Artnik, B. Fluoride: A Review of Use and Effects on Health. Mater. Socio Med. 2016, 28, 133. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, M.; Rout, K.; Gupta, S.K.; Singh, P.; Anand, S.; Mishra, B.K. Facile Synthesis of Additive-Assisted Nano Goethite Powder and Its Application for Fluoride Remediation. J. Nanopart. Res. 2010, 12, 681–686. [Google Scholar] [CrossRef]
- Halder, A.; Singh, S.; Adhikari, A.; Singh, P.; Sarkar, P.K.; Pal, U.; Ghosh, R.; Shikha, D.; Solanki, Y.S.; Agarwal, M.; Gupta, A.B.; Chakraborty, R.; Saha-Dasgupta, T.; Das, R.; Pal, S.K. Selective and Fast Responsive Sensitized Micelle for Detection of Fluoride Level in Drinking Water. ACS Sustain. Chem. Eng. 2019, 7, 16355–16363. [Google Scholar] [CrossRef]
- Ayoob, S.; Gupta, A.K. Fluoride in Drinking Water: A Review on the Status and Stress Effects. Crit. Rev. Environ. Sci. Technol. 2006, 36, 433–487. [Google Scholar] [CrossRef]
- Paudyal, H.; Inoue, K.; Kawakita, H.; Ohto, K. Removal of Fluoride by Effectively Using Spent Cation Exchange Resin. J. Mater. Cycles Waste Manag. 2018, 20, 975–984. [Google Scholar] [CrossRef]
- Dhar, V.; Bhatnagar, M. Physiology and Toxicity of Fluoride. Indian J. Dent. Res. 2009, 20, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Lavalle-Carrasco, J.; Vergara-Onofre, M.; González-González, R.; Bologna-Molina, R.; Isiordia-Espinoza, M.A.; Gaona, E.; Molina-Frechero, N. A Systematic Review and Meta-Analysis of the Relationship Between the Severity of Dental Fluorosis and Fluoride Biomarkers in Endemic Areas. Biol. Trace Elem. Res. 2023, 201, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, M.R.; Dodamani, A.S.; Jadhav, H.C.; Naik, R.G.; Deshmukh, M.A. Mitigation of Fluorosis—A Review. J. Clin. Diagn. Res. 2015, 9, ZE05–ZE09. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.M.; Lakhkar, B.B.; Patil, S.S. Curse of Fluorosis. Indian J. Pediatr. 2018, 85, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Wasana, H.M.; Perera, G.D.; Gunawardena, P.S.; Fernando, P.S.; Bandara, J. WHO Water Quality Standards vs Synergic Effects of Fluoride, Heavy Metals, and Hardness in Drinking Water on Kidney Tissues. Sci. Rep. 2017, 14, 42516. [Google Scholar] [CrossRef]
- Malin, J.A.; Lesseur, C.; Busgang, S.A.; Curtin, P.; Wright, R.O.; Sanders, A.P. Fluoride Exposure and Kidney and Liver Function Among Adolescents in the United States: NHANES, 2013–2016. Environ. Int. 2019, 132, 105012. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, M.; Li, Y.; Liu, H.; Hou, C.; Zeng, Q.; Li, P.; Zhao, Q.; Dong, L.; Yu, X.; Liu, L.; Zhang, S.; Wang, A. Low-to-Moderate Fluoride Exposure in Relation to Overweight and Obesity Among School-Age Children in China. Ecotoxicol. Environ. Saf. 2019, 15, 109558. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Amador, D.; Navarro, M.E.; Carrizales, L.; Morales, R.; Calderón, J. Decreased Intelligence in Children and Exposure to Fluoride and Arsenic in Drinking Water. Cad. Saúde Pública 2007, 23, S579–S587. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Yousefi, M.; Yaseri, M.; Jalilzadeh, M.; Mahvi, A.H. Skeletal Fluorosis in Relation to Drinking Water in Rural Areas of West Azerbaijan, Iran. Sci. Rep. 2017, 7, 17300. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Mondal, N.K. Dental Fluorosis and Urinary Fluoride Concentration as a Reflection of Fluoride Exposure and Its Impact on IQ Level and BMI of Children of Laxmisagar, Simlapal Block of Bankura District, W.B., India. Environ. Monit. Assess. 2016, 188, 218. [Google Scholar] [CrossRef]
- Kanduti, D.; Sterbenk, P.; Artnik, A. Fluoride: A Review of Use and Effects on Health. Mat. Soc. Med. 2016, 28, 133. [Google Scholar] [CrossRef]
- Valdez, J.L.; Calderón, H.J.; Córdova, A.R.I.; Sandoval, A.S.Y.; Alegría, T.J.A.; Costilla, S.R.; Rocha, A.D. Level of Exposure to Fluorides by the Consumption of Different Types of Milk in Residents from an Area of Mexico with Endemic Hydrofluorosis. An. Pediatr. (Engl. Ed.) 2019, 90, 342–348. [Google Scholar] [CrossRef]
- Bashash, M.; Thomas, D.; Hu, H.; Martinez-Mier, E.A.; Sanchez, B.N.; Basu, N.; Peterson, K.E.; Ettinger, A.S.; Wright, R.; Zhang, Z.; Liu, Y.; Schnaas, L.; Mercado-García, A.; Téllez-Rojo, M.M.; Hernández-Ávila, M. Prenatal Fluoride Exposure and Cognitive Outcomes in Children at 4 and 6–12 Years of Age in Mexico. Environ. Health Perspect. 2017, 125, 097017. [Google Scholar] [CrossRef] [PubMed]
- Valdez, J.L.; López Guzmán, O.D.; Cervantes, F.M.; Costilla-Salazar, R.; Calderón, H.J.; Alcaraz, C.Y.; Rocha-Amador, D.O. In Utero Exposure to Fluoride and Cognitive Development Delay in Infants. Neurotoxicology 2017, 59, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Barbier, O.; Arreola, L.; Del Razo, L. Molecular Mechanisms of Fluoride Toxicity. Chem. Biol. Interact. 2010, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Lennon, M.A.; Whelton, H.; O’Mullane, D.; Ekstrand, J. Fluoride. Rolling Revision of the WHO Guidelines for Drinking-Water Quality. World Health Organization, Switzerland, 2004.
- Nanayakkara, S.; Senevirathna, S.T.M.L.D.; Harada, K.H.; Chandrajith, R.; Nanayakkara, N.; Koizumi, A. The Influence of Fluoride on Chronic Kidney Disease of Uncertain Aetiology (CKDu) in Sri Lanka. Chemosphere 2020, 257, 127186. [Google Scholar] [CrossRef] [PubMed]
- McLure, F.J. Ingestion of Fluoride and Dental Caries Quantitative Relations Based on Food and Water Requirements of Children One to Twelve Years Old. Am. J. Dis. Child. 1943, 66, 362–369. [Google Scholar] [CrossRef]
- Yadav, K.K.; Kumar, S.; Pham, Q.B.; Gupta, N.; Rezania, S.; Kamyab, H.; et al. Fluoride Contamination, Health Problems and Remediation Methods in Asian Groundwater: A Comprehensive Review. Ecotoxicol. Environ. Saf. 2019, 182, 109362. [Google Scholar] [CrossRef]
- Castiblanco-Rubio, G.A.; Baston, M.; Hernandez-F, M.; Martinez-Mier, E.A.; Cantoral, A. Associations Between Dietary Patterns, Fluoride Intake and Excretion in Women Exposed to Fluoridated Salt: A Preliminary Study. Nutrients 2024, 16, 3404. [Google Scholar] [CrossRef]
- Li, M.; Cao, J.; Zhao, Y.; Wu, P.; Li, X.; Khodaei, F.; Han, Y.; Wang, J. Fluoride Impairs Ovary Development by Affecting Oogenesis and Inducing Oxidative Stress and Apoptosis in Female Zebrafish (Danio rerio). Chemosphere 2020, 256, 127105. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Wu, P.; Manthari, R.K.; Zhao, Y.; Li, W.; Wang, J. Self-Recovery Study of the Adverse Effects of Fluoride on Small Intestine: Involvement of Pyroptosis Induced Inflammation. Sci. Total Environ. 2020, 742, 140533. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Manna, P.; Sil, P.C. Aqueous Extract of the Bark of Terminalia arjuna Plays a Protective Role Against Sodium-Fluoride-Induced Hepatic and Renal Oxidative Stress. J. Nat. Med.-Tokyo 2007, 61, 251–260. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Xu, G.; Wei, J.; Chang, K.; Tian, X.; Liu, M.; Yan, X.; Huo, M.; Song, G. Selenium Attenuates Apoptosis and p-AMPK Expressions in Fluoride-Induced NRK-52E Cells. Environ. Sci. Pollut. Res. 2019, 26, 15685–15697. [Google Scholar] [CrossRef] [PubMed]
- Burgos Aceves, M.A.; Migliaccio, V.; Lepretti, M.; Paolella, G.; Di Gregorio, I.; Penna, S.; Faggio, C.; Lionetti, L. Dose-Dependent Response to the Environmental Pollutant Dichlorodiphenylethylene (DDE) in HepG2 Cells: Focus on Cell Viability and Mitochondrial Fusion/Fission Proteins. Toxics 2021, 9, 270. [Google Scholar] [CrossRef]
- Sang, H.; Zhang, L.; Li, J. Anti-Benzopyrene-7,8-Diol-9,10-Epoxide Induces Apoptosis via Mitochondrial Pathway in Human Bronchiolar Epithelium Cells Independent of the Mitochondrial Permeability Transition Pore. Food Chem. Toxicol. 2012, 50, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Ol, M.S.; Nawaz, M.; Ahsan, H. Role of Bcl-2 Family Proteins and Caspases in the Regulation of Apoptosis. Mol. Cell. Biochem. 2011, 351, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chiang, W.; Sumpter, R.; Mishra, P.; Levine, B. Prohibitin 2 is an Inner Mitochondrial Membrane Mitophagy Receptor. Cell 2017, 168, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Yi, J.; Li, Y.; Yang, B.; Shang, P.; Mehmood, K.; Bilal, R.M.; Zhang, H.; Chang, Y.; Tang, Z.; Wang, Y.; Li, Y. The Potential Risks of Chronic Fluoride Exposure on Nephrotoxicity via Altering Glucolipid Metabolism and Activating Autophagy and Apoptosis in Ducks. Toxicology 2021, 461, 152906. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, Y.; Deng, C. Protein and mRNA Expression of Shh, Smo, and Gli 1 and Inhibition by Cyclopamine in Hepatocytes of Rats with Chronic Fluorosis. Toxicol. Lett. 2014, 225, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Barbier, O.; Arreola-Mendoza, L.; Del Razo, L.M. Molecular Mechanisms of Fluoride Toxicity. Chem. Biol. Interact. 2010, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. A Mini Review of Fluoride-Induced Apoptotic Pathways. Environ. Sci. Pollut. Res. Int. 2018, 25, 33926–33935. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Luo, Q.; Liu, H.; Chen, L.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. The Mitochondrial Pathway is Involved in Sodium Fluoride (NaF)-Induced Renal Apoptosis in Mice. Toxicol. Res. 2018, 7, 792–808. [Google Scholar] [CrossRef]
- Wang, J.; Yue, B.; Zhang, X.; Guo, X.; Sun, Z.; Niu, R. Effect of Exercise on Microglial Activation and Transcriptome of Hippocampus in Fluorosis Mice. Sci. Total Environ. 2021, 760, 143376. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, B.; Zhang, S.; Li, J.; Qi, J.; Bai, C.; Zhang, J.; Liang, S. Glycine Alleviates Fluoride-Induced Oxidative Stress, Apoptosis, and Senescence in a Porcine Testicular Sertoli Cell Line. Reprod. Domest. Anim. 2021, 56, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Liu, J.; Zhao, W.P.; Zhang, Z.H.; Li, S.Q.; Li, S.H.; Zhu, S.Q.; Zhou, B.H. Effect of Fluoride on Small Intestine Morphology and Serum Cytokine Contents in Rats. Biol. Trace Elem. Res. 2019, 189, 511–518.46. [Google Scholar] [CrossRef]
- Moyson, S.; Liew, H.J.; Fazio, A.; Van Dooren, N.; Delcroix, A.; Faggio, C.; Blust, R.; De Boeck, G. Kidney activity increases in copper exposed goldfish(Carassius auratus auratus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 190, 32–37. [Google Scholar] [CrossRef]
- Akinrinde, A.S.; Tijani, M.; Awodele, O.A.; Oyagbemi, A.A. Fluoride-induced hepatotoxicity is prevented by L-Arginine supplementation via suppressionof oxidative stress and stimulation of nitric oxide production in rats. Toxicology and Environmental Health Sciences 2021, 13, 57–64. [Google Scholar] [CrossRef]
- Sinha, M.; Manna, P.; Sil, P.C. Aqueous extract of the bark of Terminalia arjuna plays a protective role against sodium-fluoride-induced hepatic and renal oxidative stress. J NAT MED 2007, 61, 251–260. [Google Scholar] [CrossRef]
- Thylstrup, A.; Fejerskov, O. Clinical Appearance of Dental Fluorosis in Permanent Teeth in Relation to Histologic Changes. Community Dent. Oral Epidemiol. 1978, 6, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Abanto Alvarez, J.; Rezende, K.M.; Marocho, S.M.; Alves, F.B.; Celiberti, P.; Ciamponi, A.L. Dental Fluorosis: Exposure, Prevention, and Management. Med. Oral Patol. Oral Cir. Bucal. 2009, 14, E103–E107. [Google Scholar] [PubMed]
- Oliveira Chagas, F.; Rocha Valadas, L.A.; Sorazabal, A.; Dayo, A.; Botelho Dantas, T.C.F.; Squassi, A. Fluoride in Drinking Groundwater and Prevalence of Fluorosis in Children and Adolescents: A Systematic Review. Acta Odontol. Latinoam. 2023, 36, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Rompante, P. Mecanismos Preventivos do Fluór e Cárie Dentária. Acta Pediatr. Port. 2009, 40, 223–228. [Google Scholar] [CrossRef]
- Mellberg, J.R.; Ripa, L.W. Fluoride Metabolism. In Fluorides in Preventive Dentistry: Theory and Clinical Applications; Quintessence Publishing Co Limited: 1983; pp. 81–102.
- Majumdar, K.K. Health Impact of Supplying Safe Drinking Water Containing Fluoride Below Permissible Level on Fluorosis Patients in a Fluoride-Endemic Rural Area of West Bengal. Indian J. Public Health 2011, 55, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Flora, S.J.S. Fluoride in Drinking Water and Skeletal Fluorosis: A Review of the Global Impact. Curr. Environ. Health Rep. 2020, 7, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, M.S. Chronic Fluorosis: The Disease and Its Anaesthetic Implications. Indian J. Anaesth. 2016, 60, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Yousefi, M.; Yaseri, M.; Jalilzadeh, M.; Mahvi, A.H. Skeletal Fluorosis in Relation to Drinking Water in Rural Areas of West Azerbaijan, Iran. Sci. Rep. 2017, 7, 17300. [Google Scholar] [CrossRef] [PubMed]
- Sellami, M.; Riahi, H.; Maatallah, K.; Ferjani, H.; Bouaziz, M.C.; Ladeb, M.F. Skeletal Fluorosis: Don’t Miss the Diagnosis! Skeletal Radiol. 2020, 49, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Spira, L. Mottled Nails. An Early Sign of Fluorosis. J. Hyg. 1943, 43, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Buzalaf, M.A.; Rodrigues, M.H.; Pessan, J.P.; Leite, A.L.; Arana, A.; Villena, R.S.; et al. Biomarkers of Fluoride in Children Exposed to Different Sources of Systemic Fluoride. J. Dent. Res. 2011, 90, 215–219. [Google Scholar] [CrossRef]
- Whitford, G.M. Monitoring Fluoride Exposure with Fingernail Clippings. Schweiz. Monatsschr. Zahnmed. 2005, 115, 685–689. [Google Scholar] [PubMed]
- Joshi, N.A.; Ajithkrishnan, C.G. Scalp Hair as Biomarker for Chronic Fluoride Exposure among Fluoride Endemic and Low-Fluoride Areas: A Comparative Study. Int. J. Trichol. 2018, 10, 71–75. [Google Scholar] [CrossRef]
- Pessan, J.P.; Buzalaf, M.R. Historical and Recent Biological Markers of Exposure to Fluoride. Monogr. Oral Sci. 2011, 22, 52–65. [Google Scholar]
- Rugg-Gunn, A.J.; Villa, A.E.; Buzalaf, M.R. Contemporary Biological Markers of Exposure to Fluoride. Monogr. Oral Sci. 2011, 22, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Levy, F.M.; Bastos, J.R.; Buzalaf, M.A. Nails as Biomarkers of Fluoride in Children of Fluoridated Communities. J. Dent. Child. 2004, 71, 121–125. [Google Scholar]
- Villa, A.; Anabalon, M.; Zohouri, V.; Maguire, A.; Franco, A.M.; Rugg-Gunn, A. Relationships Between Fluoride Intake, Urinary Fluoride Excretion, and Fluoride Retention in Children and Adults: An Analysis of Available Data. Caries Res. 2010, 44, 60–68. [Google Scholar] [CrossRef]
- Thomas, D.B.; Basu, N.; Martinez-Mier, E.A.; Sánchez, B.N.; Zhang, Z.; Liu, Y.; et al. Urinary and Plasma Fluoride Levels in Pregnant Women from Mexico City. Environ. Res. 2016, 150, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Zipkin, I.; Likins, R.C.; McClure, F.; Steere, A.C. Urinary Fluoride Levels Associated with Use of Fluoridated Drinking Waters. Public Health Rep. 1956, 71, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Idowu, O.S.; Duckworth, R.M.; Valentine, R.A.; Zohoori, F.V. Biomarkers for the Assessment of Exposure to Fluoride in Adults. Caries Res. 2021, 55, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Rango, T.; Jeuland, M.; Manthrithilake, H.; McCornick, P. Nephrotoxic Contaminants in Drinking Water and Urine, and Chronic Kidney Disease in Rural Sri Lanka. Sci. Total Environ. 2015, 518–519, 574–585. [Google Scholar] [CrossRef]
- Buzalaf, M.A.; Whitford, G.M. Fluoride Metabolism. Monogr. Oral Sci. 2011, 22, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Schamschula, R.G.; Sugár, E.; Un, P.S.; Tóth, K.; Barmes, D.E.; Adkins, B.L. Physiological Indicators of Fluoride Exposure and Utilization: An Epidemiological Study. Community Dent. Oral Epidemiol. 1985, 13, 104–107. [Google Scholar] [CrossRef]
- Hulka, B.S. Overview of Biological Markers. In Biological Markers in Epidemiology; Hulka, B.S., Wilcosky, T.C., Griffith, J.D., Eds.; Oxford University Press: New York, 1990; pp. 3–15. [Google Scholar]
- Czarnowski, W.; Krechniak, J. Fluoride in the Urine, Hair, and Nails of Phosphate Fertiliser Workers. Br. J. Ind. Med. 1990, 47, 349–351. [Google Scholar] [CrossRef]
- Boivin, G.; Chapuy, M.C.; Baud, C.A.; Meunier, P.J. Fluoride Content in Human Iliac Bone: Results in Controls, Patients with Fluorosis, and Osteoporotic Patients Treated with Fluoride. J. Bone Miner. Res. 1988, 3, 497–502. [Google Scholar] [CrossRef]
- Kokot, Z.; Drzewiecki, D. Fluoride Levels in Hair of Exposed and Unexposed Populations in Poland. Fluoride 2000, 33, 196–204. [Google Scholar]
- Elekdag-Turk, S.; Almuzian, M.; Turk, T.; Buzalaf, M.A.R.; Alnuaimi, A.; Dalci, O.; Darendeliler, M.A. Big Toenail and Hair Samples as Biomarkers for Fluoride Exposure: A Pilot Study. BMC Oral Health 2019, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.A.; Ajithkrishnan, C.G. Scalp Hair as Biomarker for Chronic Fluoride Exposure among Fluoride Endemic and Low Fluoride Areas: A Comparative Study. Int. J. Trichol. 2018, 10, 71–75. [Google Scholar] [CrossRef]
- Mehta, A. Biomarkers of Fluoride Exposure in the Human Body. Indian J. Dent. 2013, 4, 207–210. [Google Scholar] [CrossRef]
- Czarnowski, W.; Krechniak, J. Fluoride in the Urine, Hair, and Nails of Phosphate Fertiliser Workers. Br. J. Ind. Med. 1990, 47, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, F.C. Biomarkers of Exposure to Fluorides. J. Appl. Oral Sci. 2006, 14, 41–43. [Google Scholar]
- Kotecha, P.V.; Patel, S.V.; Bhalani, K.D.; Shah, D.; Shah, V.S.; Mehta, K.G.; et al. Prevalence of Dental Fluorosis and Dental Caries in Association with High Levels of Drinking Water Fluoride Content in a District of Gujarat, India. Indian J. Med. Res. 2012, 135, 873–877. [Google Scholar]
- Eskandari, F.; Kumah, E.A.; Azevedo, L.; Stephenson, J.; John, S.; Zohoori, F.V. Fluoride Exposure in Community Prevention Programmes for Oral Health Using Nail Clippings and Spot Urine Samples: A Systematic Review and Meta-Analysis. Caries Res. 2023, 57, 197–210. [Google Scholar] [CrossRef]
- Kumah, E.A.; Eskandari, F.; Azevedo, L.B.; John, S.; Zohoori, F.V. Mapping the Evidence for Monitoring Fluoride Exposure in Community Prevention Programmes for Oral Health Using Nail Clippings and Spot Urine Samples: A Scoping Review. BMC Oral Health 2022, 22, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Chagas, F.; Rocha Valadas, L.A.; Sorazabal, A.; Dayo, A.; Botelho Dantas, T.C.F.; Squassi, A. Fluoride in Drinking Groundwater and Prevalence of Fluorosis in Children and Adolescents: A Systematic Review. Acta Odontol. Latinoam. 2023, 36, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Lavalle-Carrasco, J.; Molina-Frechero, N.; Nevárez-Rascón, M.; Sánchez-Pérez, L.; Hamdan-Partida, A.; González-González, R.; Cassi, D.; Isiordia-Espinoza, M.A.; Bologna-Molina, R. Recent Biomarkers for Monitoring the Systemic Fluoride Levels in Exposed Populations: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 317. [Google Scholar] [CrossRef]
- Buzalaf, M.A.; Massaro, C.S.; Rodrigues, M.H.; Fukushima, R.; Pessan, J.P.; Whitford, G.M.; et al. Validation of Fingernail Fluoride Concentration as a Predictor of Risk for Dental Fluorosis. Caries Res. 2012, 46, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Corrêa Rodrigues, M.H.; de Magalhães Bastos, J.R.; Rabelo Buzalaf, M.A. Fingernails and Toenails as Biomarkers of Subchronic Exposure to Fluoride from Dentifrice in 2- to 3-Year-Old Children. Caries Res. 2004, 38, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, R.; Rigolizzo, D.S.; Maia, L.P.; Sampaio, F.C.; Lauris, J.R.; Buzalaf, M.A. Environmental and Individual Factors Associated with Nail Fluoride Concentration. Caries Res. 2009, 43, 147–154. [Google Scholar] [CrossRef] [PubMed]
| Symptoms | Definition | Risk Factors |
|---|---|---|
| Ostecondensation | Increase in bone density, observed radiographically | Excessive fluoride deposition in bone matrix |
| Cortical thickening: | Increased thickness of cortical bone | Prolonged fluoride exposure |
| Ligament and interosseous ossification | Ossification of ligaments and interosseous membranes | Fluoride accumulation |
| Diffuse skeletal pain | Structural changes and inflammation, associated with toxicity | Symptom linked to chronic fluoride exposure |
| Limited mobility | Decreased joint movement due to stiffness and ossification of surrounding tissues | Related to chronic exposure |
| Skeletal deformity | Abnormal changes in the shape or structure bone | Related to chronic exposure |
| Manifestation | Description |
|---|---|
| Nail Mottling | Appears as opaque spots and transverse bands |
| Longitudinal striations | Vertical lines running along the length of the nail |
| Abnormal curvatures | Curved or claw-like nail shapes |
| Color Changes | Ranges from dark gray to brown |
| Onychia | Nail inflammation can affect both the growth and appearance of the nails |
| Onycholysis | Separation of the nail from its bed, which can lead to nail loss |
| Onychomadesis | Shedding of the nail, which can be partial or complete |
| Nails fragility | Nails that break or crack easily due to their weakness |
| Development of pitting on the nails. | Formation of small depressions on the surface of the nails |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





