Submitted:
02 January 2025
Posted:
07 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Diagnostic Criteria of Sarcopenia
3. Molecular Mechanisms and Pathophysiology for Sarcopenia in CKD
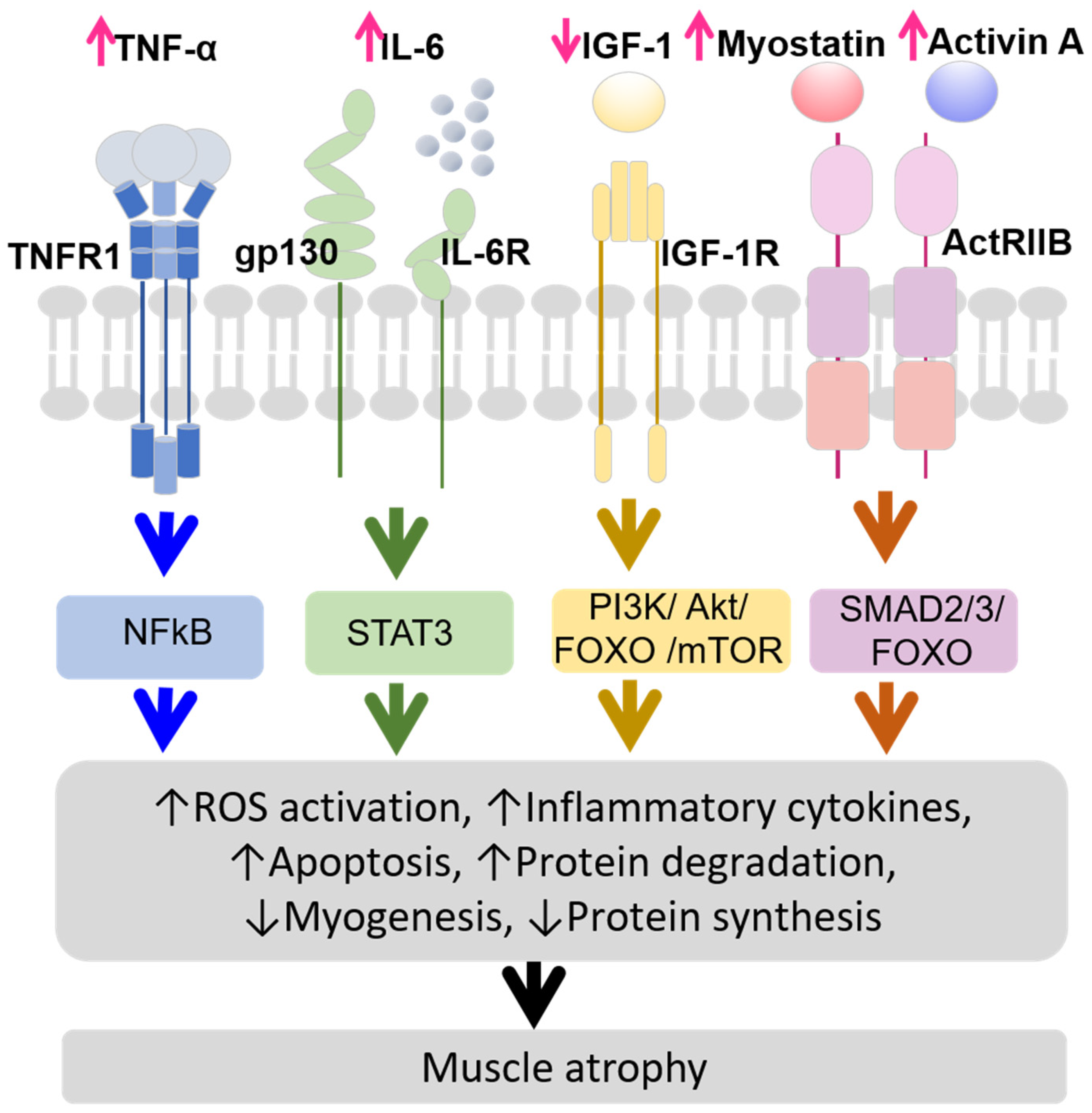
- TNF-α (tumor necrosis factor-alpha) stimulates the ubiquitin-proteasome system (UPS) [20], which is crucial for regulating the signaling pathways activated by TNF-α binding to TNF type 1 receptor (TNFR1). When TNF-α binds to TNFR1 on myofibers, it activates nuclear factor κB (NFκB) and reactive oxygen species (ROS) production. This activation promotes pro-inflammatory gene programs, including the secretion of interleukin-6 (IL-6) and Interleukin-1 beta (IL-1β) [21,22,23,24,25,26];
- IL-6 levels are also elevated in CKD [27,28], and its increase or overexpression reduces muscle mass and protein metabolism [29,30,31]. IL-6 binds to glycoprotein 130 (gp130) and IL-6 receptor (IL-6R) on myofibers, activating signal transducer and activator of transcription 3 (STAT3) signaling and inducing suppressor of cytokine signaling 3 (SOCS3), which inhibits IGF-1 effects, leading to protein degradation and muscle atrophy [32,33];
- IGF-1 is a key growth mediator that promotes muscle health by binding to its receptor, insulin-like growth factor-1 receptor (IGF-1R), stimulating protein synthesis, and inhibiting protein degradation [34]. In CKD, IGF-1 levels decrease, leading to impaired muscle protein synthesis and increased protein degradation. This process contributes to muscle atrophy through several pathways, including ROS activation, myogenesis, apoptosis, increased protein degradation via the PI3K/Akt/FOXO (Phosphoinositide 3-kinase/ Protein Kinase B/ Forkhead box protein O) pathway, and decreased protein synthesis due to disrupted PI3K/Akt/mTOR (mammalian target of ramamycin) pathway [35,36,37];
- Myostatin and Activin A are transforming growth factor-beta (TGF-β) family members that play significant roles in muscle atrophy in CKD. Myostatin production is induced by inflammatory cytokines linking inflammation to muscle atrophy [32]. Myostatin and Activin A bind to Activin receptor type IIB (ActRIIB) and contribute to muscle atrophy by activating the mothers against decapentaplegic homolog 2/3 (SMAD2/3) and FOXO pathways, leading to increased protein degradation and inhibited muscle growth [35,38,39,40].
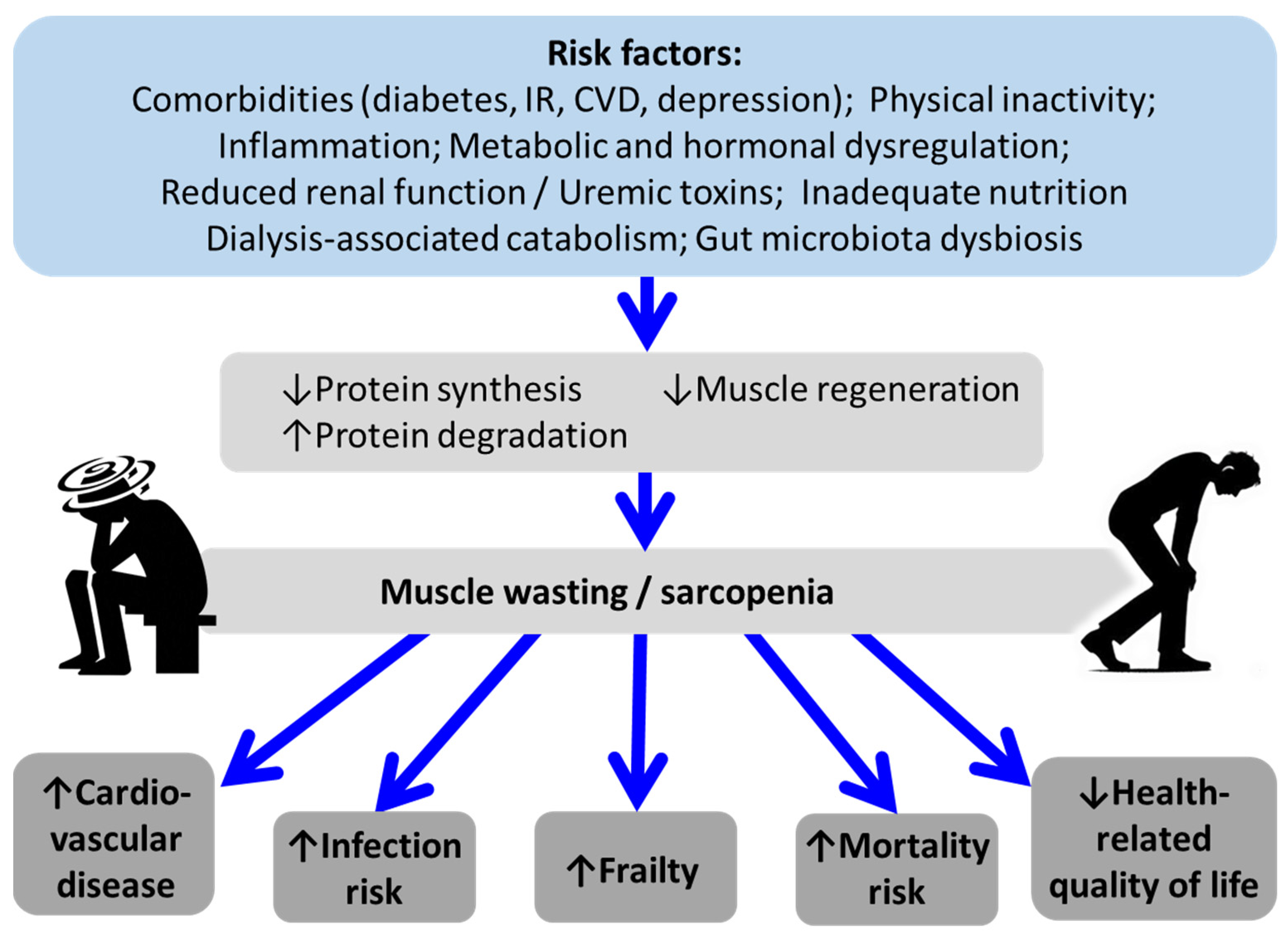
4. Factors Associated with Sarcopenia in CKD
4.1. Inflammation
4.2. Metabolic and Hormonal Dysregulation
4.3. Inadequate Nutritional Status
4.4. Physical Inactivity
4.5. Gut Microbiota Dysbiosis and the Metabolites
4.6. MicroRNA
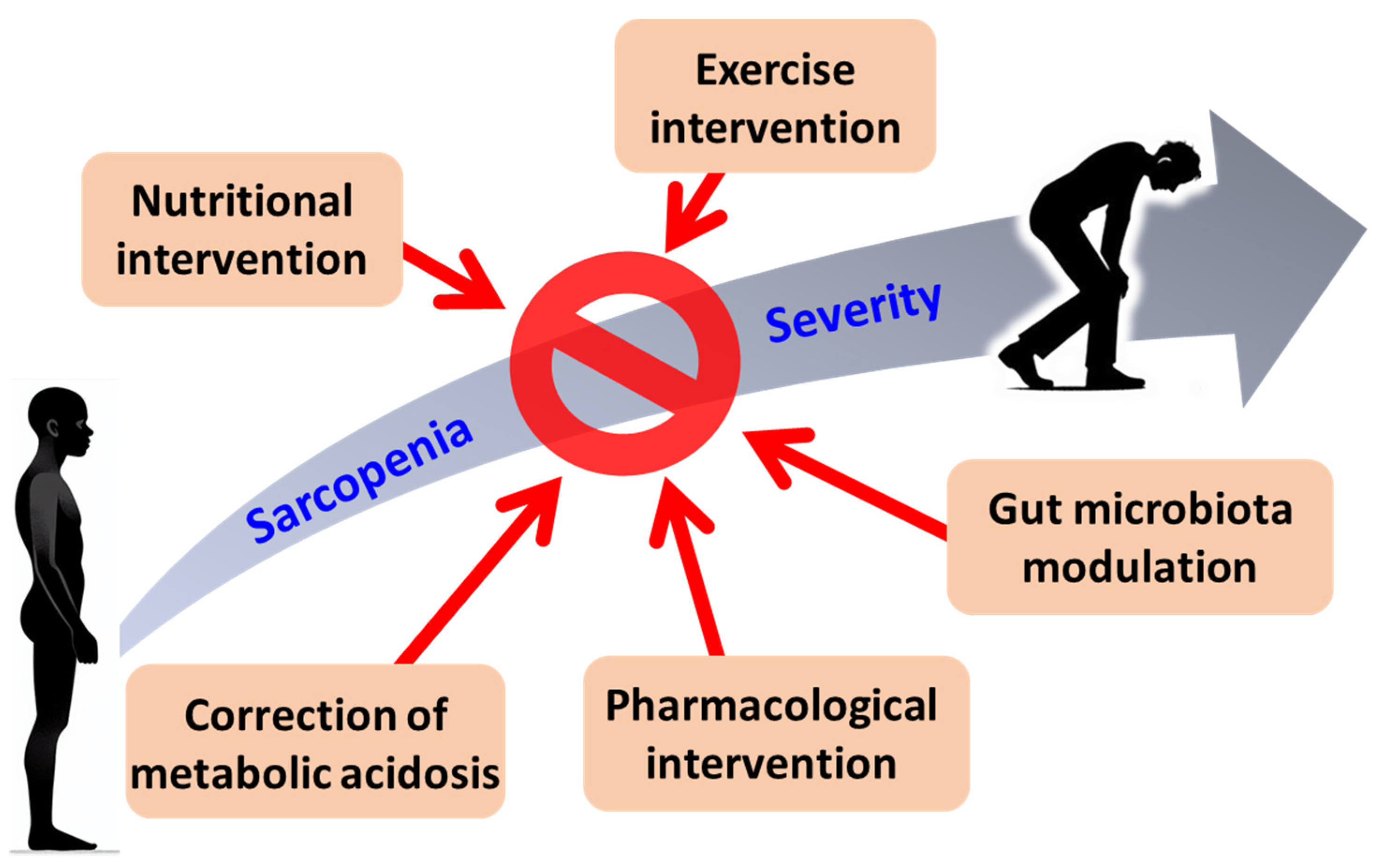
5. Specific Therapeutic Approaches for Sarcopenia in CKD
5.1. Nutritional Interventions
5.2. Exercise Interventions
5.3. Correction of MA
5.4. Gut Microbiota Modulation
5.5. Pharmacological Interventions
6. Future Perspectives
6. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6MWT | 6-minute walking test |
| ActRIIB | Activin receptor type IIB |
| AGE | Advanced glycation end products |
| Akt | Protein Kinase B |
| AMPK | AMP-activated protein kinase |
| ASM | Appendicular skeletal muscle mass |
| ASMI | Appendicular skeletal muscle mass index |
| AST-120 | An oral adsorbent |
| AWGS | Asian Working Group for Sarcopenia |
| BIA | Bioelectrical impedance analysis |
| CKD | Chronic kidney disease |
| COPD | Chronic obstructive pulmonary disease |
| CRP | C-reactive protein |
| CT | Computed tomography |
| CVD | Cardiovascular disease |
| DM | Diabetes mellitus |
| DXA | Dual-energy X-ray absorptiometry |
| eGFR | Estimated glomerular filtration rate |
| ESRD | End-stage renal disease |
| EWGSOP2 | European Working Group on Sarcopenia in Older People 2 |
| FOXO1 | Forkhead box protein O1 |
| FOXO | Forkhead box protein O |
| GH | Growth hormone |
| HPT | Hyperparathyroidism |
| HRQoL | Health-related quality of life |
| IGF-1 | Insulin-like growth factor-1 |
| IGF1-R | Insulin-like growth factor-1 receptor |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-6R | Interleukin-6 receptor |
| IR | Insulin resistance |
| IS | Indoxyl sulfate |
| JAK | Janus kinase |
| LBM | Lean body mass |
| MA | Metabolic acidosis |
| MAMC | Mid-arm muscle circumference |
| miRNA | MicroRNA |
| MRI | Magnetic resonance imaging |
| mTOR | Mammalian target of rapamycin |
| NFκB | Nuclear factor kappa B |
| PCS | p-Cresyl sulfate |
| PI3K | Phosphoinositide 3-kinase |
| PPI | Proton pump inhibitor |
| PTEN | Phosphatase and tensin homolog |
| QoL | Quality of life |
| RCT | Randomized controlled trial |
| ROS | Reactive oxygen species |
| SARC-F | Sarcopenia Assessment Tool Questionnaire |
| SARC-F/Calf | SARC-F Questionnaire with Calf Circumference |
| SARM | Selective androgen receptor modulator |
| SCFA | Short-chain fatty acid |
| SGA | Subjective global assessment |
| SOCS3 | Suppressor of cytokine signaling 3 |
| SMAD 2/3 | Mothers against decapentaplegic homolog 2/3 |
| SPPB | Short physical performance battery |
| STAT3 | Signal transducer and activator of transcription 3 |
| STAT | Signal transducer and activator of transcription |
| TGFβ | Transforming growth factor-beta |
| TNF-α | Tumor necrosis factor-alpha |
| TUG | Timed up and go test |
| UPS | Ubiquitin-proteasome system |
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O'Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Heitman, K.; Alexander, M.S.; Faul, C. Skeletal Muscle Injury in Chronic Kidney Disease-From Histologic Changes to Molecular Mechanisms and to Novel Therapies. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Cuppari, L.; Stenvinkel, P.; Lindholm, B.; Avesani, C.M. Sarcopenia in chronic kidney disease: what have we learned so far? J Nephrol 2021, 34, 1347–1372. [Google Scholar] [CrossRef] [PubMed]
- Chatzipetrou, V.; Begin, M.J.; Hars, M.; Trombetti, A. Sarcopenia in Chronic Kidney Disease: A Scoping Review of Prevalence, Risk Factors, Association with Outcomes, and Treatment. Calcif Tissue Int 2022, 110, 1–31. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Duarte, M.P.; Almeida, L.S.; Neri, S.G.R.; Oliveira, J.S.; Wilkinson, T.J.; Ribeiro, H.S.; Lima, R.M. Prevalence of sarcopenia in patients with chronic kidney disease: a global systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2024, 15, 501–512. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Trombetti, A.; Reid, K.F.; Hars, M.; Herrmann, F.R.; Pasha, E.; Phillips, E.M.; Fielding, R.A. Age-associated declines in muscle mass, strength, power, and physical performance: impact on fear of falling and quality of life. Osteoporos Int 2016, 27, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020, 21, 300–307 e302. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D'Angelo, E.; Pahor, M.; Bernabei, R.; et al. Sarcopenia: an overview. Aging Clin Exp Res 2017, 29, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Cordeiro, A.C.; Avesani, C.M.; Carrero, J.J.; Lindholm, B.; Amparo, F.C.; Amodeo, C.; Cuppari, L.; Kamimura, M.A. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant 2015, 30, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord 2017, 16, 21. [Google Scholar] [CrossRef]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant 2018, 33, iii35–iii40. [Google Scholar] [CrossRef]
- Meuwese, C.L.; Carrero, J.J.; Stenvinkel, P. Recent insights in inflammation-associated wasting in patients with chronic kidney disease. Contrib Nephrol 2011, 171, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A. Nutrition, inflammation and chronic kidney disease. Curr Opin Nephrol Hypertens 2008, 17, 162–167. [Google Scholar] [CrossRef]
- Kaizu, Y.; Ohkawa, S.; Odamaki, M.; Ikegaya, N.; Hibi, I.; Miyaji, K.; Kumagai, H. Association between inflammatory mediators and muscle mass in long-term hemodialysis patients. Am J Kidney Dis 2003, 42, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Kaysen, G.A.; Young, B.S.; Hung, A.M.; da Silva, M.; Chertow, G.M. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr 2003, 77, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Du, J.; Bailey, J.L.; Price, S.R. Mechanisms causing muscle proteolysis in uremia: the influence of insulin and cytokines. Miner Electrolyte Metab 1999, 25, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Langen, R.C.; Schols, A.M.; Kelders, M.C.; Wouters, E.F.; Janssen-Heininger, Y.M. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J 2001, 15, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Schwartz, R.J. TNF-alpha regulates early differentiation of C2C12 myoblasts in an autocrine fashion. FASEB J 2001, 15, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Llovera, M.; Garcia-Martinez, C.; Lopez-Soriano, J.; Carbo, N.; Agell, N.; Lopez-Soriano, F.J.; Argiles, J.M. Role of TNF receptor 1 in protein turnover during cancer cachexia using gene knockout mice. Mol Cell Endocrinol 1998, 142, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Reid, M.B. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am J Physiol Regul Integr Comp Physiol 2000, 279, R1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B.; Li, Y.P. Tumor necrosis factor-alpha and muscle wasting: a cellular perspective. Respir Res 2001, 2, 269–272. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Panguluri, S.K.; Gupta, S.K.; Dahiya, S.; Lundy, R.F.; Kumar, A. Tumor necrosis factor-alpha regulates distinct molecular pathways and gene networks in cultured skeletal muscle cells. PLoS One 2010, 5, e13262. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Heimburger, O.; Barany, P.; Suliman, M.; Fehrman-Ekholm, I.; Lindholm, B.; Stenvinkel, P. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis 2003, 41, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Ikizler, A.T.; Kovesdy, C.P.; Raj, D.S.; Stenvinkel, P.; Kalantar-Zadeh, K. Wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle 2011, 2, 9–25. [Google Scholar] [CrossRef]
- Pelosi, M.; De Rossi, M.; Barberi, L.; Musaro, A. IL-6 impairs myogenic differentiation by downmodulation of p90RSK/eEF2 and mTOR/p70S6K axes, without affecting AKT activity. Biomed Res Int 2014, 2014, 206026. [Google Scholar] [CrossRef]
- Pelosi, L.; Berardinelli, M.G.; Forcina, L.; Ascenzi, F.; Rizzuto, E.; Sandri, M.; De Benedetti, F.; Scicchitano, B.M.; Musaro, A. Sustained Systemic Levels of IL-6 Impinge Early Muscle Growth and Induce Muscle Atrophy and Wasting in Adulthood. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.; Baltgalvis, K.A. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev 2010, 38, 168–176. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, J.; Dong, Y.; Tweardy, D.J.; Dong, Y.; Garibotto, G.; Mitch, W.E. Stat3 activation links a C/EBPdelta to myostatin pathway to stimulate loss of muscle mass. Cell Metab 2013, 18, 368–379. [Google Scholar] [CrossRef]
- Wang, X.H.; Mitch, W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 2014, 10, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Colak, T.; Bayraktar, N.; Sezer, S. Evaluation of Dynapenia and Sarcopenia and Their Associations With Serum Insulin-Like Growth Factor-1 Levels in Renal Transplant Recipients. J Ren Nutr 2022, 32, 354–362. [Google Scholar] [CrossRef]
- Kir, S.; Komaba, H.; Garcia, A.P.; Economopoulos, K.P.; Liu, W.; Lanske, B.; Hodin, R.A.; Spiegelman, B.M. PTH/PTHrP Receptor Mediates Cachexia in Models of Kidney Failure and Cancer. Cell Metab 2016, 23, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L.; Zheng, B.; Hu, Z.; Price, S.R.; Mitch, W.E. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J Am Soc Nephrol 2006, 17, 1388–1394. [Google Scholar] [CrossRef]
- Ding, H.; Gao, X.L.; Hirschberg, R.; Vadgama, J.V.; Kopple, J.D. Impaired actions of insulin-like growth factor 1 on protein Synthesis and degradation in skeletal muscle of rats with chronic renal failure. Evidence for a postreceptor defect. J Clin Invest 1996, 97, 1064–1075. [Google Scholar] [CrossRef]
- Verzola, D.; Barisione, C.; Picciotto, D.; Garibotto, G.; Koppe, L. Emerging role of myostatin and its inhibition in the setting of chronic kidney disease. Kidney Int 2019, 95, 506–517. [Google Scholar] [CrossRef]
- Bataille, S.; Dou, L.; Bartoli, M.; Sallee, M.; Aniort, J.; Ferkak, B.; Chermiti, R.; McKay, N.; Da Silva, N.; Burtey, S.; et al. Mechanisms of myostatin and activin A accumulation in chronic kidney disease. Nephrol Dial Transplant 2022, 37, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Solagna, F.; Tezze, C.; Lindenmeyer, M.T.; Lu, S.; Wu, G.; Liu, S.; Zhao, Y.; Mitchell, R.; Meyer, C.; Omairi, S.; et al. Pro-cachectic factors link experimental and human chronic kidney disease to skeletal muscle wasting programs. J Clin Invest 2021, 131. [Google Scholar] [CrossRef]
- Bakinowska, E.; Olejnik-Wojciechowska, J.; Kielbowski, K.; Skoryk, A.; Pawlik, A. Pathogenesis of Sarcopenia in Chronic Kidney Disease-The Role of Inflammation, Metabolic Dysregulation, Gut Dysbiosis, and microRNA. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Fox, E.R.; Benjamin, E.J.; Sarpong, D.F.; Nagarajarao, H.; Taylor, J.K.; Steffes, M.W.; Salahudeen, A.K.; Flessner, M.F.; Akylbekova, E.L.; Fox, C.S.; et al. The relation of C--reactive protein to chronic kidney disease in African Americans: the Jackson Heart Study. BMC Nephrol 2010, 11, 1. [Google Scholar] [CrossRef]
- Adejumo, O.A.; Okaka, E.I.; Okwuonu, C.G.; Iyawe, I.O.; Odujoko, O.O. Serum C-reactive protein levels in pre-dialysis chronic kidney disease patientsin southern Nigeria. Ghana Med J 2016, 50, 31–38. [Google Scholar] [CrossRef]
- Spoto, B.; Leonardis, D.; Parlongo, R.M.; Pizzini, P.; Pisano, A.; Cutrupi, S.; Testa, A.; Tripepi, G.; Zoccali, C.; Mallamaci, F. Plasma cytokines, glomerular filtration rate and adipose tissue cytokines gene expression in chronic kidney disease (CKD) patients. Nutr Metab Cardiovasc Dis 2012, 22, 981–988. [Google Scholar] [CrossRef]
- Herbelin, A.; Urena, P.; Nguyen, A.T.; Zingraff, J.; Descamps-Latscha, B. Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int 1991, 39, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Bonanni, A.; Sofia, A.; Montecucco, F.; D'Amato, E.; Cademartori, V.; Parodi, E.L.; Viazzi, F.; Venturelli, C.; Brunori, G.; et al. Toll-like receptor 4 signalling mediates inflammation in skeletal muscle of patients with chronic kidney disease. J Cachexia Sarcopenia Muscle 2017, 8, 131–144. [Google Scholar] [CrossRef]
- Tuttle, C.S.L.; Thang, L.A.N.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res Rev 2020, 64, 101185. [Google Scholar] [CrossRef]
- Watanabe, H.; Enoki, Y.; Maruyama, T. Sarcopenia in Chronic Kidney Disease: Factors, Mechanisms, and Therapeutic Interventions. Biol Pharm Bull 2019, 42, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.A.; Oliveira, D.; Barbosa, S.R.; Correa, J.; Colugnati, F.A.B.; Mansur, H.N.; Fernandes, N.; Bastos, M.G. Sarcopenia in patients with chronic kidney disease not yet on dialysis: Analysis of the prevalence and associated factors. PLoS One 2017, 12, e0176230. [Google Scholar] [CrossRef]
- Wahlin-Larsson, B.; Wilkinson, D.J.; Strandberg, E.; Hosford-Donovan, A.; Atherton, P.J.; Kadi, F. Mechanistic Links Underlying the Impact of C-Reactive Protein on Muscle Mass in Elderly. Cell Physiol Biochem 2017, 44, 267–278. [Google Scholar] [CrossRef]
- Rose-John, S. Therapeutic targeting of IL-6 trans-signaling. Cytokine 2021, 144, 155577. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 signalling in health and disease. F1000Res 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Zanders, L.; Kny, M.; Hahn, A.; Schmidt, S.; Wundersitz, S.; Todiras, M.; Lahmann, I.; Bandyopadhyay, A.; Wollersheim, T.; Kaderali, L.; et al. Sepsis induces interleukin 6, gp130/JAK2/STAT3, and muscle wasting. J Cachexia Sarcopenia Muscle 2022, 13, 713–727. [Google Scholar] [CrossRef]
- Lin, Y.L.; Wang, C.H.; Chang, I.C.; Hsu, B.G. A Novel Application of Serum Creatinine and Cystatin C to Predict Sarcopenia in Advanced CKD. Front Nutr 2022, 9, 828880. [Google Scholar] [CrossRef]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12. [Google Scholar] [CrossRef]
- Raj, D.S.; Dominic, E.A.; Wolfe, R.; Shah, V.O.; Bankhurst, A.; Zager, P.G.; Ferrando, A. Coordinated increase in albumin, fibrinogen, and muscle protein synthesis during hemodialysis: role of cytokines. Am J Physiol Endocrinol Metab 2004, 286, E658–664. [Google Scholar] [CrossRef]
- Chalupsky, M.; Goodson, D.A.; Gamboa, J.L.; Roshanravan, B. New insights into muscle function in chronic kidney disease and metabolic acidosis. Curr Opin Nephrol Hypertens 2021, 30, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.L. Metabolic Acidosis and Subclinical Metabolic Acidosis in CKD. J Am Soc Nephrol 2018, 29, 376–382. [Google Scholar] [CrossRef]
- Enoki, Y.; Watanabe, H.; Arake, R.; Sugimoto, R.; Imafuku, T.; Tominaga, Y.; Ishima, Y.; Kotani, S.; Nakajima, M.; Tanaka, M.; et al. Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1. Sci Rep 2016, 6, 32084. [Google Scholar] [CrossRef]
- Sato, E.; Mori, T.; Mishima, E.; Suzuki, A.; Sugawara, S.; Kurasawa, N.; Saigusa, D.; Miura, D.; Morikawa-Ichinose, T.; Saito, R.; et al. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci Rep 2016, 6, 36618. [Google Scholar] [CrossRef]
- Thome, T.; Salyers, Z.R.; Kumar, R.A.; Hahn, D.; Berru, F.N.; Ferreira, L.F.; Scali, S.T.; Ryan, T.E. Uremic metabolites impair skeletal muscle mitochondrial energetics through disruption of the electron transport system and matrix dehydrogenase activity. Am J Physiol Cell Physiol 2019, 317, C701–C713. [Google Scholar] [CrossRef] [PubMed]
- Enoki, Y.; Watanabe, H.; Arake, R.; Fujimura, R.; Ishiodori, K.; Imafuku, T.; Nishida, K.; Sugimoto, R.; Nagao, S.; Miyamura, S.; et al. Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction. J Cachexia Sarcopenia Muscle 2017, 8, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Pillon, N.J.; Vella, R.E.; Croze, M.L.; Pelletier, C.C.; Chambert, S.; Massy, Z.; Glorieux, G.; Vanholder, R.; Dugenet, Y.; et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol 2013, 24, 88–99. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Eguchi, Y.; Toyoguchi, T.; Inage, K.; Fujimoto, K.; Orita, S.; Suzuki, M.; Kanamoto, H.; Abe, K.; Norimoto, M.; Umimura, T.; et al. Advanced glycation end products are associated with sarcopenia in older women: aging marker dynamics. J Women Aging 2021, 33, 328–340. [Google Scholar] [CrossRef]
- Mori, H.; Kuroda, A.; Ishizu, M.; Ohishi, M.; Takashi, Y.; Otsuka, Y.; Taniguchi, S.; Tamaki, M.; Kurahashi, K.; Yoshida, S.; et al. Association of accumulated advanced glycation end-products with a high prevalence of sarcopenia and dynapenia in patients with type 2 diabetes. J Diabetes Investig 2019, 10, 1332–1340. [Google Scholar] [CrossRef]
- Gungor, O.; Ulu, S.; Hasbal, N.B.; Anker, S.D.; Kalantar-Zadeh, K. Effects of hormonal changes on sarcopenia in chronic kidney disease: where are we now and what can we do? J Cachexia Sarcopenia Muscle 2021, 12, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Mitch, W.E.; Price, S.R. Pathophysiological mechanisms leading to muscle loss in chronic kidney disease. Nat Rev Nephrol 2022, 18, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Carrero, J.J.; von Walden, F.; Ikizler, T.A.; Nader, G.A. Muscle wasting in end-stage renal disease promulgates premature death: established, emerging and potential novel treatment strategies. Nephrol Dial Transplant 2016, 31, 1070–1077. [Google Scholar] [CrossRef]
- Cheema, B.; Abas, H.; Smith, B.; O'Sullivan, A.J.; Chan, M.; Patwardhan, A.; Kelly, J.; Gillin, A.; Pang, G.; Lloyd, B.; et al. Investigation of skeletal muscle quantity and quality in end-stage renal disease. Nephrology (Carlton) 2010, 15, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Alvestrand, A.; Bergstrom, J.; Furst, P.; Germanis, G.; Widstam, U. Effect of essential amino acid supplementation on muscle and plasma free amino acids in chronic uremia. Kidney Int 1978, 14, 323–329. [Google Scholar] [CrossRef]
- Bergstrom, J.; Furst, P.; Noree, L.O. Treatment of chronic uremic patients with protein-poor diet and oral supply of essential amino acids. I. Nitrogen balance studies. Clin Nephrol 1975, 3, 187–194. [Google Scholar]
- Zhang, F.; Yin, X.; Huang, L.; Zhang, H. The "adult inactivity triad" in patients with chronic kidney disease: A review. Front Med (Lausanne) 2023, 10, 1160450. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Y.; Lv, W.; Fu, P.; Yuan, H. Prevalence and severity of sarcopenia in patients on maintenance hemodialysis: a cross-sectional study. BMC Nephrol 2024, 25, 385. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, B.; Hassounah, F.; Price, S.R.; Klein, J.; Mohamed, T.M.A.; Wang, Y.; Park, J.; Cai, H.; Zhang, X.; et al. The impact of senescence on muscle wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle 2023, 14, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Hu, Q.; Huang, Z.; Lai, Z.; Wang, X.; Cai, M.; Lin, H. Sarcopenia and mild kidney dysfunction and risk of all-cause and cause-specific mortality in older adults. Nephrol Dial Transplant 2024, 39, 989–999. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Zhao, Y.Y.; Pahl, M.V. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant 2016, 31, 737–746. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 2014, 39, 230–237. [Google Scholar] [CrossRef]
- Lau, W.L.; Savoj, J.; Nakata, M.B.; Vaziri, N.D. Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Clin Sci (Lond) 2018, 132, 509–522. [Google Scholar] [CrossRef]
- van Krimpen, S.J.; Jansen, F.A.C.; Ottenheim, V.L.; Belzer, C.; van der Ende, M.; van Norren, K. The Effects of Pro-, Pre-, and Synbiotics on Muscle Wasting, a Systematic Review-Gut Permeability as Potential Treatment Target. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.M.; Coombes, J.S.; Forbes, J.M.; Szeto, C.C.; McWhinney, B.C.; Ungerer, J.P.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin J Am Soc Nephrol 2016, 11, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes, C.K.D.M.B.D.U.W.G. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (2011) 2017, 7, 1–59. [Google Scholar] [CrossRef]
- Ammirati, A.L. Chronic Kidney Disease. Rev Assoc Med Bras (1992) 2020, 66Suppl 1, s03–s09. [Google Scholar] [CrossRef]
- Wang, X.H.; Hu, Z.; Klein, J.D.; Zhang, L.; Fang, F.; Mitch, W.E. Decreased miR-29 suppresses myogenesis in CKD. J Am Soc Nephrol 2011, 22, 2068–2076. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, A.; Wang, H.; Klein, J.D.; Tan, L.; Wang, Z.M.; Du, J.; Naqvi, N.; Liu, B.C.; Wang, X.H. miR-26a Limits Muscle Wasting and Cardiac Fibrosis through Exosome-Mediated microRNA Transfer in Chronic Kidney Disease. Theranostics 2019, 9, 1864–1877. [Google Scholar] [CrossRef]
- Xu, J.; Li, R.; Workeneh, B.; Dong, Y.; Wang, X.; Hu, Z. Transcription factor FoxO1, the dominant mediator of muscle wasting in chronic kidney disease, is inhibited by microRNA-486. Kidney Int 2012, 82, 401–411. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, C.; Zhang, A.; Cai, H.; Price, S.R.; Wang, X.H. MicroRNA-23a and MicroRNA-27a Mimic Exercise by Ameliorating CKD-Induced Muscle Atrophy. J Am Soc Nephrol 2017, 28, 2631–2640. [Google Scholar] [CrossRef]
- Zhang, A.; Li, M.; Wang, B.; Klein, J.D.; Price, S.R.; Wang, X.H. miRNA-23a/27a attenuates muscle atrophy and renal fibrosis through muscle-kidney crosstalk. J Cachexia Sarcopenia Muscle 2018, 9, 755–770. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.; Chen, Z.; Wang, Y.; Gamboa, J.L.; Ikizler, T.A.; Garibotto, G.; Mitch, W.E. Mechanisms Regulating Muscle Protein Synthesis in CKD. J Am Soc Nephrol 2020, 31, 2573–2587. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Sayer, A.A.; Cooper, R.; Robinson, S.M. Nutrition in the prevention and treatment of skeletal muscle ageing and sarcopenia: a single nutrient, a whole food and a whole diet approach. Proc Nutr Soc 2024, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Giacosa, A.; Barrile, G.C.; Mansueto, F.; Rondanelli, M. The nutritional support to prevent sarcopenia in the elderly. Front Nutr 2024, 11, 1379814. [Google Scholar] [CrossRef]
- Kuwabara, A.; Matsumoto, M.; Hatamoto, Y.; Fujita, S. Vitamin D and muscle health: insights from recent studies. Curr Opin Clin Nutr Metab Care 2024, 27, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Kressel, H.; Matsakas, A. Current Research on Vitamin D Supplementation against Sarcopenia: A Review of Clinical Trials. Int J Sports Med 2023, 44, 843–856. [Google Scholar] [CrossRef]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyere, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [PubMed]
- Fenercioglu, A.K. The Anti-Inflammatory Roles of Vitamin D for Improving Human Health. Curr Issues Mol Biol 2024, 46, 13514–13525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, W. Vitamin D and Sarcopenia in the Senior People: A Review of Mechanisms and Comprehensive Prevention and Treatment Strategies. Ther Clin Risk Manag 2024, 20, 577–595. [Google Scholar] [CrossRef]
- Hendriks, F.K.; Smeets, J.S.J.; van der Sande, F.M.; Kooman, J.P.; van Loon, L.J.C. Dietary Protein and Physical Activity Interventions to Support Muscle Maintenance in End-Stage Renal Disease Patients on Hemodialysis. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Beckwee, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J Nutr Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Hurst, C.; Robinson, S.M.; Witham, M.D.; Dodds, R.M.; Granic, A.; Buckland, C.; De Biase, S.; Finnegan, S.; Rochester, L.; Skelton, D.A.; et al. Resistance exercise as a treatment for sarcopenia: prescription and delivery. Age Ageing 2022, 51. [Google Scholar] [CrossRef]
- Battaglia, Y.; Baciga, F.; Bulighin, F.; Amicone, M.; Mosconi, G.; Storari, A.; Brugnano, R.; Pozzato, M.; Motta, D.; D'Alessandro, C.; et al. Physical activity and exercise in chronic kidney disease: consensus statements from the Physical Exercise Working Group of the Italian Society of Nephrology. J Nephrol 2024, 37, 1735–1765. [Google Scholar] [CrossRef]
- Dubey, A.K.; Sahoo, J.; Vairappan, B.; Haridasan, S.; Parameswaran, S.; Priyamvada, P.S. Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: a randomized controlled trial. Nephrol Dial Transplant 2020, 35, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Lin, H.M.; Wang, H.Y.; Chuang, M.H.; Hsieh, C.C.; Tsai, K.T.; Chen, J.Y. Sodium Bicarbonate Treatment and Clinical Outcomes in Chronic Kidney Disease with Metabolic Acidosis: A Meta-Analysis. Clin J Am Soc Nephrol 2024, 19, 959–969. [Google Scholar] [CrossRef]
- Zheng, G.; Cao, J.; Wang, X.H.; He, W.; Wang, B. The gut microbiome, chronic kidney disease, and sarcopenia. Cell Commun Signal 2024, 22, 558. [Google Scholar] [CrossRef]
- Nishikawa, M.; Ishimori, N.; Takada, S.; Saito, A.; Kadoguchi, T.; Furihata, T.; Fukushima, A.; Matsushima, S.; Yokota, T.; Kinugawa, S.; et al. AST-120 ameliorates lowered exercise capacity and mitochondrial biogenesis in the skeletal muscle from mice with chronic kidney disease via reducing oxidative stress. Nephrol Dial Transplant 2015, 30, 934–942. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 2004, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.M.; Gonzalez, M.; Poueymirou, W.T.; Kline, W.O.; Na, E.; Zlotchenko, E.; Stitt, T.N.; Economides, A.N.; Yancopoulos, G.D.; Glass, D.J. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 2004, 24, 9295–9304. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Cha, R.H. Pharmacologic therapeutics in sarcopenia with chronic kidney disease. Kidney Res Clin Pract 2024, 43, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Kwon, K.S. Pharmacological Interventions for Treatment of Sarcopenia: Current Status of Drug Development for Sarcopenia. Ann Geriatr Med Res 2019, 23, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Basualto-Alarcon, C.; Varela, D.; Duran, J.; Maass, R.; Estrada, M. Sarcopenia and Androgens: A Link between Pathology and Treatment. Front Endocrinol (Lausanne) 2014, 5, 217. [Google Scholar] [CrossRef]
- Therdyothin, A.; Phiphopthatsanee, N.; Isanejad, M. The Effect of Omega-3 Fatty Acids on Sarcopenia: Mechanism of Action and Potential Efficacy. Mar Drugs 2023, 21. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A. Optimal nutrition in hemodialysis patients. Adv Chronic Kidney Dis 2013, 20, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Carrero, J.J.; Lindholm, B. Causes of poor appetite in patients on peritoneal dialysis. J Ren Nutr 2011, 21, 12–15. [Google Scholar] [CrossRef]
| EWGSOP2 | AWGS | |
| Measurement | ||
| Case findings for further sarcopenia evaluation | - Clinical suspicion or SARC-F ≥ 4 [9] | - With any of the following: clinical conditions (e.g., functional decline, unintentional weight loss, depressive mood, etc.) or comorbidities (e.g., heart failure, COPD, DM, CKD, etc.) - Calf circumference (< 34 cm for men, < 33 cm for women) or SARC-F ≥ 4, or SARC-F/Calf ≥ 11 - DXA < 7.0 kg/m2 (M), <5.4 kg/m2 (F) - Bioimpedance < 7.0 kg/m2 (M), <5.7 kg/m2 (F) |
| Cutt-off points | ||
| Muscle strength | - HGS:< 27 kg (M), < 16 kg (F) or 5 times chair stand test: > 15s | - HGS:< 28 kg (M), < 18 kg (F) |
| Muscle quantity or quality | - ASM: < 20 kg (M), < 15 kg (F) or - ASMI: < 7.0 kg/m2 (M), < 5.5 kg/m2 (F)- |
- ASM: < 7.0 kg/m2 (M), or < 5.4 kg/m2 (F, by DXA), or < 5.7 kg/m2 (F, by BIA) |
| Physical performance | - Gait speed ≤ 0.8 m/s - SPPB: ≤ 8 points - TUG: ≥ 20 s |
- 6MWT < 1.0 m/s or - SPPB ≤ 9 points or - 5-time Chair stand test ≥ 12s |
| Classification | ||
| Probable sarcopenia | Low muscle strength | N/A |
| Confirmed sarcopenia | Low muscle strength + Low muscle quantity or quality | Low ASM + low muscle strength or low physical performance |
| Severe sarcopenia | Low muscle strength + low muscle quantity or quality + low Physical performance | Low ASM + low muscle strength or low physical performance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





