Submitted:
02 January 2025
Posted:
03 January 2025
You are already at the latest version
Abstract
Keywords:
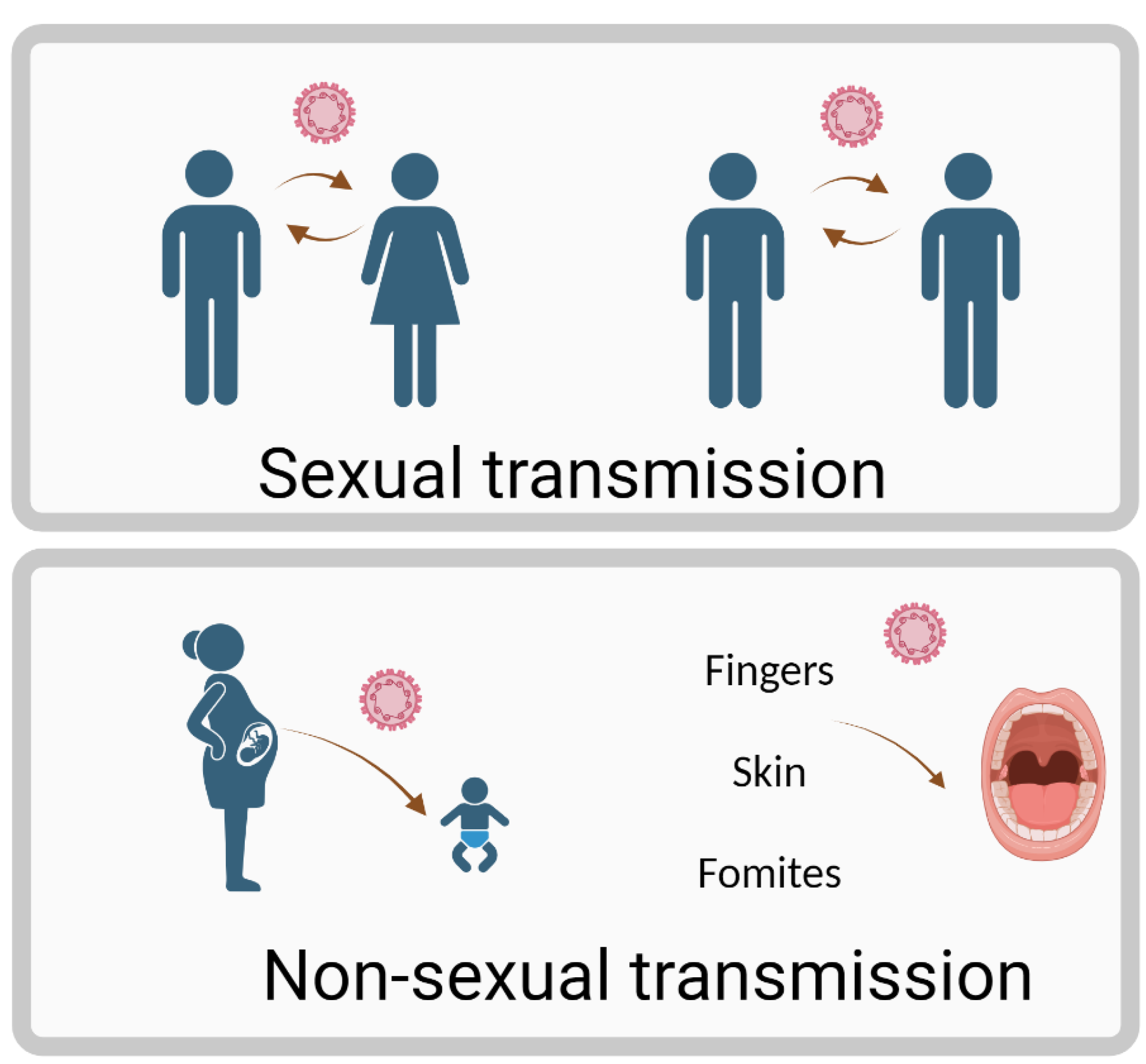
1. Introduction
2. Overview of HPV and Oral Infections
3. The Oral Microbiota
4. HPV and Oral Microbiota Interaction
5. Oral Microbiota as a Biomarker for HPV-Related Cancers
6. Therapeutic Implications and Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barsouk A, Aluru JS, Rawla P, Saginala K, Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med Sci (Basel). 2023 Jun 13;11(2):42. [CrossRef] [PubMed] [PubMed Central]
- Riva G, Albano C, Gugliesi F, Pasquero S, Pacheco SFC, Pecorari G, Landolfo S, Biolatti M, Dell'Oste V. HPV Meets APOBEC: New Players in Head and Neck Cancer. Int J Mol Sci. 2021 Jan 30;22(3):1402. [CrossRef] [PubMed] [PubMed Central]
- Gao L, Xu T, Huang G, Jiang S, Gu Y, Chen F. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell. 2018 May;9(5):488-500. Epub 2018 May 7. [CrossRef] [PubMed] [PubMed Central]
- Peng X, Cheng L, You Y, Tang C, Ren B, Li Y, Xu X, Zhou X. Oral microbiota in human systematic diseases. Int J Oral Sci. 2022 Mar 2;14(1):14. [CrossRef] [PubMed] [PubMed Central]
- Sun J, Tang Q, Yu S, Xie M, Xie Y, Chen G, Chen L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020 Sep;9(17):6306-6321. Epub 2020 Jul 7. [CrossRef] [PubMed] [PubMed Central]
- Tuominen H, Rautava J. Oral Microbiota and Cancer Development. Pathobiology. 2021;88(2):116-126. Epub 2020 Nov 11. [CrossRef] [PubMed]
- Wakabayashi R, Nakahama Y, Nguyen V, Espinoza JL. The Host-Microbe Interplay in Human Papillomavirus-Induced Carcinogenesis. Microorganisms. 2019 Jul 13;7(7):199. [CrossRef] [PubMed] [PubMed Central]
- Upadhyay R, Dhakal A, Wheeler C, Hoyd R, Jagjit Singh M, Karivedu V, Bhateja P, Bonomi M, Valentin S, Gamez ME, Konieczkowski DJ, Baliga S, Grecula JC, Blakaj DM, Gogineni E, Mitchell DL, Denko NC, Spakowicz D, Jhawar SR. Comparative analysis of the tumor microbiome, molecular profiles, and immune cell abundances by HPV status in mucosal head and neck cancers and their impact on survival. Cancer Biol Ther. 2024 Dec 31;25(1):2350249. Epub 2024 May 9. [CrossRef] [PubMed] [PubMed Central]
- Zhang Y, D'Souza G, Fakhry C, Bigelow EO, Usyk M, Burk RD, Zhao N. Oral Human Papillomavirus Associated With Differences in Oral Microbiota Beta Diversity and Microbiota Abundance. J Infect Dis. 2022 Sep 21;226(6):1098-1108. [CrossRef] [PubMed] [PubMed Central]
- Di Spirito F, Di Palo MP, Folliero V, Cannatà D, Franci G, Martina S, Amato M. Oral Bacteria, Virus and Fungi in Saliva and Tissue Samples from Adult Subjects with Oral Squamous Cell Carcinoma: An Umbrella Review. Cancers (Basel). 2023 Nov 22;15(23):5540. [CrossRef] [PubMed] [PubMed Central]
- Kumar P, Gupta S, Das BC. Saliva as a potential non-invasive liquid biopsy for early and easy diagnosis/prognosis of head and neck cancer. Transl Oncol. 2024 Feb;40:101827. Epub 2023 Dec 2. [CrossRef] [PubMed] [PubMed Central]
- Lim Y, Fukuma N, Totsika M, Kenny L, Morrison M, Punyadeera C. The Performance of an Oral Microbiome Biomarker Panel in Predicting Oral Cavity and Oropharyngeal Cancers. Front Cell Infect Microbiol. 2018 Aug 3;8:267. [CrossRef] [PubMed] [PubMed Central]
- Zhang Y, D'Souza G, Fakhry C, Bigelow EO, Usyk M, Burk RD, Zhao N. Oral Human Papillomavirus Associated With Differences in Oral Microbiota Beta Diversity and Microbiota Abundance. J Infect Dis. 2022 Sep 21;226(6):1098-1108. [CrossRef] [PubMed] [PubMed Central]
- Kroon SJ, Ravel J, Huston WM. Cervicovaginal microbiota, women's health, and reproductive outcomes. Fertil Steril. 2018 Aug;110(3):327-336. [CrossRef] [PubMed]
- Harper A, Vijayakumar V, Ouwehand AC, Ter Haar J, Obis D, Espadaler J, Binda S, Desiraju S, Day R. Viral Infections, the Microbiome, and Probiotics. Front Cell Infect Microbiol. 2021 Feb 12;10:596166. [CrossRef] [PubMed] [PubMed Central]
- Zhang Z, Ma Q, Zhang L, Ma L, Wang D, Yang Y, Jia P, Wu Y, Wang F. Human papillomavirus and cervical cancer in the microbial world: exploring the vaginal microecology. Front Cell Infect Microbiol. 2024 Jan 25;14:1325500. [CrossRef] [PubMed] [PubMed Central]
- Bravo IG, Félez-Sánchez M. Papillomaviruses: Viral evolution, cancer and evolutionary medicine. Evol Med Public Health. 2015 Jan 28;2015(1):32-51. [CrossRef] [PubMed] [PubMed Central]
- Gravitt PE, Winer RL. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses. 2017 Sep 21;9(10):267. [CrossRef] [PubMed] [PubMed Central]
- Michaud DS, Langevin SM, Eliot M, Nelson HH, Pawlita M, McClean MD, Kelsey KT. High-risk HPV types and head and neck cancer. Int J Cancer. 2014 Oct 1;135(7):1653-61. Epub 2014 Mar 7. [CrossRef] [PubMed] [PubMed Central]
- Del Río-Ospina L, Soto-DE León SC, Camargo M, Sánchez R, Moreno-Pérez DA, Pérez-Prados A, Patarroyo ME, Patarroyo MA. Multiple high-risk HPV genotypes are grouped by type and are associated with viral load and risk factors. Epidemiol Infect. 2017 May;145(7):1479-1490. Epub 2017 Feb 10. [CrossRef] [PubMed] [PubMed Central]
- Cogliano V., Baan R., Straif K., Grosse Y., Secretan B., El Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. [CrossRef]
- Oyouni AAA. Human papillomavirus in cancer: Infection, disease transmission, and progress in vaccines. J Infect Public Health. 2023 Apr;16(4):626-631. Epub 2023 Feb 21. [CrossRef] [PubMed]
- Ong KJ, Checchi M, Burns L, Pavitt C, Postma MJ, Jit M. Systematic review and evidence synthesis of non-cervical human papillomavirus-related disease health system costs and quality of life estimates. Sex Transm Infect. 2019 Feb;95(1):28-35. Epub 2018 Sep 21. [CrossRef] [PubMed]
- Roman BR, Aragones A. Epidemiology and incidence of HPV-related cancers of the head and neck. J Surg Oncol. 2021 Nov;124(6):920-922. Epub 2021 Sep 23. [CrossRef] [PubMed] [PubMed Central]
- Jain M, Yadav D, Jarouliya U, Chavda V, Yadav AK, Chaurasia B, Song M. Epidemiology, Molecular Pathogenesis, Immuno-Pathogenesis, Immune Escape Mechanisms and Vaccine Evaluation for HPV-Associated Carcinogenesis. Pathogens. 2023 Nov 23;12(12):1380. [CrossRef] [PubMed] [PubMed Central]
- Steinbach A, Riemer AB. Immune evasion mechanisms of human papillomavirus: An update. Int J Cancer. 2018 Jan 15;142(2):224-229. Epub 2017 Sep 13. [CrossRef] [PubMed]
- Shen-Gunther J, Xia Q, Stacey W, Asusta HB. Molecular Pap Smear: Validation of HPV Genotype and Host Methylation Profiles of ADCY8, CDH8, and ZNF582 as a Predictor of Cervical Cytopathology. Front Microbiol. 2020 Oct 15;11:595902. [CrossRef] [PubMed] [PubMed Central]
- Litwin TR, Clarke MA, Dean M, Wentzensen N. Somatic Host Cell Alterations in HPV Carcinogenesis. Viruses. 2017 Aug 3;9(8):206. [CrossRef] [PubMed] [PubMed Central]
- Xu S, Shi C, Zhou R, Han Y, Li N, Qu C, Xia R, Zhang C, Hu Y, Tian Z, Liu S, Wang L, Li J, Zhang Z. Mapping the landscape of HPV integration and characterising virus and host genome interactions in HPV-positive oropharyngeal squamous cell carcinoma. Clin Transl Med. 2024 Jan;14(1):e1556. [CrossRef] [PubMed] [PubMed Central]
- Guo C, Dai W, Zhou Q, Gui L, Cai H, Wu D, Hou J, Li C, Li S, Du H, Wu R. Cervicovaginal microbiota significantly changed for HPV-positive women with high-grade squamous intraepithelial lesion. Front Cell Infect Microbiol. 2022 Aug 4;12:973875. [CrossRef] [PubMed] [PubMed Central]
- Osazuwa-Peters N, Simpson MC, Massa ST, Adjei Boakye E, Antisdel JL, Varvares MA. 40-year incidence trends for oropharyngeal squamous cell carcinoma in the United States. Oral Oncol. 2017 Nov;74:90-97. Epub 2017 Oct 2. Epub 2017 Oct 2. [CrossRef] [PubMed]
- Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008 Feb 1;26(4):612-9. [CrossRef] [PubMed]
- Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005 Feb;14(2):467-75. [CrossRef] [PubMed]
- Sonawane K, Suk R, Chiao EY, Chhatwal J, Qiu P, Wilkin T, Nyitray AG, Sikora AG, Deshmukh AA. Oral Human Papillomavirus Infection: Differences in Prevalence Between Sexes and Concordance With Genital Human Papillomavirus Infection, NHANES 2011 to 2014. Ann Intern Med. 2017 Nov 21;167(10):714-724. Epub 2017 Oct 17. [CrossRef] [PubMed] [PubMed Central]
- Ghanem AS, Memon HA, Nagy AC. Evolving trends in oral cancer burden in Europe: a systematic review. Front Oncol. 2024 Oct 18;14:1444326. [CrossRef] [PubMed] [PubMed Central]
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010 Jul 1;363(1):24-35. Epub 2010 Jun 7. [CrossRef] [PubMed] [PubMed Central]
- Taberna M, Mena M, Pavón MA, Alemany L, Gillison ML, Mesía R. Human papillomavirus-related oropharyngeal cancer. Ann Oncol. 2017 Oct 1;28(10):2386-2398. [CrossRef] [PubMed]
- Hu M, Coleman S, Fadlullah MZH, Spakowicz D, Chung CH, Tan AC. Deciphering the Tumor-Immune-Microbe Interactions in HPV-Negative Head and Neck Cancer. Genes (Basel). 2023 Aug 8;14(8):1599. [CrossRef] [PubMed] [PubMed Central]
- Mahal BA, Catalano PJ, Haddad RI, Hanna GJ, Kass JI, Schoenfeld JD, Tishler RB, Margalit DN. Incidence and Demographic Burden of HPV-Associated Oropharyngeal Head and Neck Cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2019 Oct;28(10):1660-1667. Epub 2019 Jul 29. [CrossRef] [PubMed]
- Fullerton ZH, Butler SS, Mahal BA, Muralidhar V, Schoenfeld JD, Tishler RB, Margalit DN. Short-term mortality risks among patients with oropharynx cancer by human papillomavirus status. Cancer. 2020 Apr 1;126(7):1424-1433. Epub 2020 Jan 13. [CrossRef] [PubMed]
- Christianto S, Li KY, Huang TH, Su YX. The Prognostic Value of Human Papilloma Virus Infection in Oral Cavity Squamous Cell Carcinoma: A Meta-Analysis. Laryngoscope. 2022 Sep;132(9):1760-1770. Epub 2021 Dec 25. [CrossRef] [PubMed]
- Mehanna H, Bryant TS, Babrah J, Louie K, Bryant JL, Spruce RJ, Batis N, Olaleye O, Jones J, Struijk L, Molijn A, Vorsters A, Rosillon D, Taylor S, D'Souza G. Human Papillomavirus (HPV) Vaccine Effectiveness and Potential Herd Immunity for Reducing Oncogenic Oropharyngeal HPV-16 Prevalence in the United Kingdom: A Cross-sectional Study. Clin Infect Dis. 2019 Sep 27;69(8):1296-1302. [CrossRef] [PubMed] [PubMed Central]
- Chaturvedi AK, Graubard BI, Broutian T, Pickard RKL, Tong ZY, Xiao W, Kahle L, Gillison ML. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J Clin Oncol. 2018 Jan 20;36(3):262-267. Epub 2017 Nov 28. [CrossRef] [PubMed] [PubMed Central]
- Meites E, Winer RL, Newcomb ME, Gorbach PM, Querec TD, Rudd J, Collins T, Lin J, Moore J, Remble T, Swanson F, Franz J, Bolan RK, Golden MR, Mustanski B, Crosby RA, Unger ER, Markowitz LE. Vaccine Effectiveness Against Prevalent Anal and Oral Human Papillomavirus Infection Among Men Who Have Sex With Men-United States, 2016-2018. J Infect Dis. 2020 Nov 13;222(12):2052-2060. [CrossRef] [PubMed] [PubMed Central]
- National Institute of Health. (2024). Human oral Microbiome database. https://www.homd.
- Baker JL, Mark Welch JL, Kauffman KM, McLean JS, He X. The oral microbiome: diversity, biogeography and human health. Nat Rev Microbiol. 2024 Feb;22(2):89-104. Epub 2023 Sep 12. [CrossRef] [PubMed] [PubMed Central]
- Caselli E, Fabbri C, D'Accolti M, Soffritti I, Bassi C, Mazzacane S, Franchi M. Defining the oral microbiome by whole-genome sequencing and resistome analysis: the complexity of the healthy picture. BMC Microbiol. 2020 May 18;20(1):120. [CrossRef] [PubMed] [PubMed Central]
- Yamashita Y, Takeshita T. The oral microbiome and human health. J Oral Sci. 2017;59(2):201-206. [CrossRef] [PubMed]
- Butler RR 3rd, Soomer-James JTA, Frenette M, Pombert JF. Complete Genome Sequences of Two Human Oral Microbiome Commensals, Streptococcus salivarius ATCC 25975 and S. salivarius ATCC 27945. Genome Announc. 2017 Jun 15;5(24):e00536-17. [CrossRef] [PubMed] [PubMed Central]
- Ahn J, Yang L, Paster BJ, Ganly I, Morris L, Pei Z, Hayes RB. Oral microbiome profiles: 16S rRNA pyrosequencing and microarray assay comparison. PLoS One. 2011;6(7):e22788. Epub 2011 Jul 29. [CrossRef] [PubMed] [PubMed Central]
- Wang B, Yao M, Lv L, Ling Z, Li L. The human microbiota in health and disease. Engineering. 2017 Feb; 3(1): 71–82.
- Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018 Dec;16(12):745-759. [CrossRef] [PubMed] [PubMed Central]
- Damgaard C, Reinholdt J, Enevold C, Fiehn NE, Nielsen CH, Holmstrup P. Immunoglobulin G antibodies against Porphyromonas gingivalis or Aggregatibacter actinomycetemcomitans in cardiovascular disease and periodontitis. J Oral Microbiol. 2017 Sep 10;9(1):1374154. Erratum in: J Oral Microbiol. 2018 Jan 31;10(1):1433122. [CrossRef] [PubMed] [PubMed Central]
- Koziel J, Mydel P, Potempa J. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep. 2014 Mar;16(3):408. [CrossRef] [PubMed] [PubMed Central]
- Dibello V, Lozupone M, Manfredini D, Dibello A, Zupo R, Sardone R, Daniele A, Lobbezoo F, Panza F. Oral frailty and neurodegeneration in Alzheimer's disease. Neural Regen Res. 2021 Nov;16(11):2149-2153. [CrossRef] [PubMed] [PubMed Central]
- Leng Y, Hu Q, Ling Q, Yao X, Liu M, Chen J, Yan Z, Dai Q. Periodontal disease is associated with the risk of cardiovascular disease independent of sex: A meta-analysis. Front Cardiovasc Med. 2023 Feb 27;10:1114927. [CrossRef] [PubMed] [PubMed Central]
- Petersen, PE. Oral cancer prevention and control--the approach of the World Health Organization. Oral Oncol. 2009 Apr-May;45(4-5):454-60. Epub 2008 Sep 18. [CrossRef] [PubMed]
- Shin JM, Kamarajan P, Fenno JC, Rickard AH, Kapila YL. Metabolomics of Head and Neck Cancer: A Mini-Review. Front Physiol. 2016 Nov 8;7:526. [CrossRef] [PubMed] [PubMed Central]
- Zhang W, Yin Y, Jiang Y, Yang Y, Wang W, Wang X, Ge Y, Liu B, Yao L. Relationship between vaginal and oral microbiome in patients of human papillomavirus (HPV) infection and cervical cancer. J Transl Med. 2024 Apr 29;22(1):396. [CrossRef] [PubMed] [PubMed Central]
- Bertolini M, Costa RC, Barão VAR, Cunha Villar C, Retamal-Valdes B, Feres M, Silva Souza JG. Oral Microorganisms and Biofilms: New Insights to Defeat the Main Etiologic Factor of Oral Diseases. Microorganisms. 2022 Dec 6;10(12):2413. [CrossRef] [PubMed] [PubMed Central]
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010 Sep;8(9):623-33. Epub 2010 Aug 2. [CrossRef] [PubMed]
- Bowen WH, Burne RA, Wu H, Koo H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018 Mar;26(3):229-242. Epub 2017 Oct 30. [CrossRef] [PubMed] [PubMed Central]
- Lebeaux D, Ghigo JM, Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. 2014 Sep;78(3):510-43. [CrossRef] [PubMed] [PubMed Central]
- Gillison, ML. Human papillomavirus-related diseases: oropharynx cancers and potential implications for adolescent HPV vaccination. J Adolesc Health. 2008 Oct;43(4 Suppl):S52-60. [CrossRef] [PubMed] [PubMed Central]
- Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010 Aug;11(8):781-9. Epub 2010 May 5. [CrossRef] [PubMed] [PubMed Central]
- Jayaprakash V, Reid M, Hatton E, Merzianu M, Rigual N, Marshall J, Gill S, Frustino J, Wilding G, Loree T, Popat S, Sullivan M. Human papillomavirus types 16 and 18 in epithelial dysplasia of oral cavity and oropharynx: a meta-analysis, 1985-2010. Oral Oncol. 2011 Nov;47(11):1048-54. Epub 2011 Aug 3. [CrossRef] [PubMed] [PubMed Central]
- Hübbers CU, Akgül B. HPV and cancer of the oral cavity. Virulence. 2015;6(3):244-8. [CrossRef] [PubMed] [PubMed Central]
- Pyeon D, Pearce SM, Lank SM, Ahlquist P, Lambert PF. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog. 2009 Feb;5(2):e1000318. Epub 2009 Feb 27. [CrossRef] [PubMed] [PubMed Central]
- Schiffman M, Doorbar J, Wentzensen N, de Sanjosé S, Fakhry C, Monk BJ, Stanley MA, Franceschi S. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016 Dec 1;2:16086. [CrossRef] [PubMed]
- Constantin M, Chifiriuc MC, Mihaescu G, Vrancianu CO, Dobre EG, Cristian RE, Bleotu C, Bertesteanu SV, Grigore R, Serban B, Cirstoiu C. Implications of oral dysbiosis and HPV infection in head and neck cancer: from molecular and cellular mechanisms to early diagnosis and therapy. Front Oncol. 2023 Dec 18;13:1273516. [CrossRef] [PubMed] [PubMed Central]
- Hajishengallis, G. Immune evasion strategies of Porphyromonas gingivalis. J Oral Biosci. 2011;53(3):233-240. [CrossRef] [PubMed] [PubMed Central]
- Kim HS, Kim CG, Kim WK, Kim KA, Yoo J, Min BS, Paik S, Shin SJ, Lee H, Lee K, Kim H, Shin EC, Kim TM, Ahn JB. Fusobacterium nucleatum induces a tumor microenvironment with diminished adaptive immunity against colorectal cancers. Front Cell Infect Microbiol. 2023 Mar 7;13:1101291. [CrossRef] [PubMed] [PubMed Central]
- Oliva M, Schneeberger PHH, Rey V, Cho M, Taylor R, Hansen AR, Taylor K, Hosni A, Bayley A, Hope AJ, Bratman SV, Ringash J, Singh S, Weinreb I, Perez-Ordoñez B, Chepeha D, Waldron J, Xu W, Guttman D, Siu LL, Coburn B, Spreafico A. Transitions in oral and gut microbiome of HPV+ oropharyngeal squamous cell carcinoma following definitive chemoradiotherapy (ROMA LA-OPSCC study). Br J Cancer. 2021 Apr;124(9):1543-1551. Epub 2021 Mar 10. [CrossRef] [PubMed] [PubMed Central]
- Elnaggar JH, Huynh VO, Lin D, Hillman RT, Abana CO, El Alam MB, Tomasic KC, Karpinets TV, Kouzy R, Phan JL, Wargo J, Holliday EB, Das P, Mezzari MP, Ajami NJ, Lynn EJ, Minsky BD, Morris VK, Milbourne A, Messick CA, Klopp AH, Futreal PA, Taniguchi CM, Schmeler KM, Colbert LE. HPV-related anal cancer is associated with changes in the anorectal microbiome during cancer development. Front Immunol. 2023 Mar 29;14:1051431. [CrossRef] [PubMed] [PubMed Central]
- Shiao SL, Kershaw KM, Limon JJ, You S, Yoon J, Ko EY, Guarnerio J, Potdar AA, McGovern DPB, Bose S, Dar TB, Noe P, Lee J, Kubota Y, Maymi VI, Davis MJ, Henson RM, Choi RY, Yang W, Tang J, Gargus M, Prince AD, Zumsteg ZS, Underhill DM. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell. 2021 Sep 13;39(9):1202-1213.e6. Epub 2021 Jul 29. [CrossRef] [PubMed] [PubMed Central]
- Rieth KKS, Gill SR, Lott-Limbach AA, Merkley MA, Botero N, Allen PD, Miller MC. Prevalence of High-Risk Human Papillomavirus in Tonsil Tissue in Healthy Adults and Colocalization in Biofilm of Tonsillar Crypts. JAMA Otolaryngol Head Neck Surg. 2018 Mar 1;144(3):231-237. [CrossRef] [PubMed] [PubMed Central]
- Hinten F, Hilbrands LB, Meeuwis KAP, IntHout J, Quint WGV, Hoitsma AJ, Massuger LFAG, Melchers WJG, de Hullu JA. Reactivation of Latent HPV Infections After Renal Transplantation. Am J Transplant. 2017 Jun;17(6):1563-1573. Epub 2017 Feb 2. [CrossRef] [PubMed]
- Liu X, Ma X, Lei Z, Feng H, Wang S, Cen X, Gao S, Jiang Y, Jiang J, Chen Q, Tang Y, Tang Y, Liang X. Chronic Inflammation-Related HPV: A Driving Force Speeds Oropharyngeal Carcinogenesis. PLoS One. 2015 Jul 20;10(7):e0133681. [CrossRef] [PubMed] [PubMed Central]
- Williams VM, Filippova M, Soto U, Duerksen-Hughes PJ. HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Future Virol. 2011 Jan 1;6(1):45-57. [CrossRef] [PubMed] [PubMed Central]
- Georgescu SR, Mitran CI, Mitran MI, Caruntu C, Sarbu MI, Matei C, Nicolae I, Tocut SM, Popa MI, Tampa M. New Insights in the Pathogenesis of HPV Infection and the Associated Carcinogenic Processes: The Role of Chronic Inflammation and Oxidative Stress. J Immunol Res. 2018 Aug 27;2018:5315816. [CrossRef] [PubMed] [PubMed Central]
- Lee GJ, Chung HW, Lee KH, Ahn HS. Antioxidant vitamins and lipid peroxidation in patients with cervical intraepithelial neoplasia. J Korean Med Sci. 2005 Apr;20(2):267-72. [CrossRef] [PubMed] [PubMed Central]
- Du Q, Ren B, He J, Peng X, Guo Q, Zheng L, Li J, Dai H, Chen V, Zhang L, Zhou X, Xu X. Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. ISME J. 2021 Mar;15(3):894-908. Epub 2020 Nov 4. [CrossRef] [PubMed] [PubMed Central]
- Gainza-Cirauqui ML, Nieminen MT, Novak Frazer L, Aguirre-Urizar JM, Moragues MD, Rautemaa R. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. J Oral Pathol Med. 2013 Mar;42(3):243-9. Epub 2012 Aug 22. [CrossRef] [PubMed]
- Muzio LL, Ballini A, Cantore S, Bottalico L, Charitos IA, Ambrosino M, Nocini R, Malcangi A, Dioguardi M, Cazzolla AP, Brauner E, Santacroce L, Cosola MD. Overview of Candida albicans and Human Papillomavirus (HPV) Infection Agents and their Biomolecular Mechanisms in Promoting Oral Cancer in Pediatric Patients. Biomed Res Int. 2021 Nov 2;2021:7312611. [CrossRef] [PubMed] [PubMed Central]
- McKeon MG, Gallant JN, Kim YJ, Das SR. It Takes Two to Tango: A Review of Oncogenic Virus and Host Microbiome Associated Inflammation in Head and Neck Cancer. Cancers (Basel). 2022 Jun 25;14(13):3120. [CrossRef] [PubMed] [PubMed Central]
- Ortiz AP, González D, Vivaldi-Oliver J, Castañeda M, Rivera V, Díaz E, Centeno H, Muñoz C, Palefsky J, Joshipura K, Pérez CM. Periodontitis and oral human papillomavirus infection among Hispanic adults. Papillomavirus Res. 2018 Jun;5:128-133. Epub 2018 Mar 16. [CrossRef] [PubMed] [PubMed Central]
- Mazul AL, Taylor JM, Divaris K, Weissler MC, Brennan P, Anantharaman D, Abedi-Ardekani B, Olshan AF, Zevallos JP. Oral health and human papillomavirus-associated head and neck squamous cell carcinoma. Cancer. 2017 Jan 1;123(1):71-80. Epub 2016 Aug 29. [CrossRef] [PubMed] [PubMed Central]
- NHS inform Mouth cancer Mouth cancer | NHS inform.
- Yang J, Guo K, Zhang A, Zhu Y, Li W, Yu J, Wang P. Survival analysis of age-related oral squamous cell carcinoma: a population study based on SEER. Eur J Med Res. 2023 Oct 10;28(1):413. [CrossRef] [PubMed] [PubMed Central]
- Tajmirriahi N, Razavi SM, Shirani S, Homayooni S, Gasemzadeh G. Evaluation of metastasis and 5-year survival in oral squamous cell carcinoma patients in Isfahan (2001-2015). Dent Res J (Isfahan). 2019 Mar-Apr;16(2):117-121. [PubMed] [PubMed Central]
- Bramati C, Abati S, Bondi S, Lissoni A, Arrigoni G, Filipello F, Trimarchi M. Early diagnosis of oral squamous cell carcinoma may ensure better prognosis: A case series. Clin Case Rep. 2021 Oct 25;9(10):e05004. [CrossRef] [PubMed] [PubMed Central]
- Colevas, AD. HPV DNA as a Biomarker in Oropharyngeal Cancer: A Step in the Right Direction. Clin Cancer Res. 2022 Oct 3;28(19):4171-4172. [CrossRef] [PubMed]
- Ekanayake Weeramange C, Liu Z, Hartel G, Li Y, Vasani S, Langton-Lockton J, Kenny L, Morris L, Frazer I, Tang KD, Punyadeera C. Salivary High-Risk Human Papillomavirus (HPV) DNA as a Biomarker for HPV-Driven Head and Neck Cancers. J Mol Diagn. 2021 Oct;23(10):1334-1342. Epub 2021 Jul 27. [CrossRef] [PubMed] [PubMed Central]
- Wolf A, Moissl-Eichinger C, Perras A, Koskinen K, Tomazic PV, Thurnher D. The salivary microbiome as an indicator of carcinogenesis in patients with oropharyngeal squamous cell carcinoma: A pilot study. Sci Rep. 2017 Jul 19;7(1):5867. [CrossRef] [PubMed] [PubMed Central]
- Guerrero-Preston R, Godoy-Vitorino F, Jedlicka A, Rodríguez-Hilario A, González H, Bondy J, Lawson F, Folawiyo O, Michailidi C, Dziedzic A, Thangavel R, Hadar T, Noordhuis MG, Westra W, Koch W, Sidransky D. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget. 2016 Aug 9;7(32):51320-51334. [CrossRef] [PubMed] [PubMed Central]
- Tuominen H, Rautava S, Syrjänen S, Collado MC, Rautava J. HPV infection and bacterial microbiota in the placenta, uterine cervix and oral mucosa. Sci Rep. 2018 Jun 28;8(1):9787. [CrossRef] [PubMed] [PubMed Central]
- Dahlstrom KR, Sikora AG, Liu Y, Chang CC, Wei P, Sturgis EM, Li G. Characterization of the oral microbiota among middle-aged men with and without human papillomavirus infection. Oral Oncol. 2023 Jul;142:106401. Epub 2023 May 11. [CrossRef] [PubMed] [PubMed Central]
- Börnigen D, Ren B, Pickard R, Li J, Ozer E, Hartmann EM, Xiao W, Tickle T, Rider J, Gevers D, Franzosa EA, Davey ME, Gillison ML, Huttenhower C. Alterations in oral bacterial communities are associated with risk factors for oral and oropharyngeal cancer. Sci Rep. 2017 Dec 15;7(1):17686. [CrossRef] [PubMed] [PubMed Central]
- Mougeot JC, Beckman MF, Langdon HC, Lalla RV, Brennan MT, Bahrani Mougeot FK. Haemophilus pittmaniae and Leptotrichia spp. Constitute a Multi-Marker Signature in a Cohort of Human Papillomavirus-Positive Head and Neck Cancer Patients. Front Microbiol. 2022 Jan 18;12:794546. [CrossRef] [PubMed] [PubMed Central]
- Shigeishi H, Sugiyama M, Ohta K. Relationship between the prevalence of oral human papillomavirus DNA and periodontal disease (Review). Biomed Rep. 2021 May;14(5):40. Epub 2021 Feb 26. [CrossRef] [PubMed] [PubMed Central]
- Binder Gallimidi A, Fischman S, Revach B, Bulvik R, Maliutina A, Rubinstein AM, Nussbaum G, Elkin M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget. 2015 Sep 8;6(26):22613-23. [CrossRef] [PubMed] [PubMed Central]
- Ferraguti G, Terracina S, Petrella C, Greco A, Minni A, Lucarelli M, Agostinelli E, Ralli M, de Vincentiis M, Raponi G, Polimeni A, Ceccanti M, Caronti B, Di Certo MG, Barbato C, Mattia A, Tarani L, Fiore M. Alcohol and Head and Neck Cancer: Updates on the Role of Oxidative Stress, Genetic, Epigenetics, Oral Microbiota, Antioxidants, and Alkylating Agents. Antioxidants (Basel). 2022 Jan 11;11(1):145. [CrossRef] [PubMed] [PubMed Central]
- Zeng M, Li X, Jiao X, Cai X, Yao F, Xu S, Huang X, Zhang Q, Chen J. Roles of vaginal flora in human papillomavirus infection, virus persistence and clearance. Front Cell Infect Microbiol. 2023 Jan 4;12:1036869. [CrossRef] [PubMed] [PubMed Central]
- Verhoeven V, Renard N, Makar A, Van Royen P, Bogers JP, Lardon F, Peeters M, Baay M. Probiotics enhance the clearance of human papillomavirus-related cervical lesions: a prospective controlled pilot study. Eur J Cancer Prev. 2013 Jan;22(1):46-51. [CrossRef] [PubMed]
- Liu Y, Zhao X, Wu F, Chen J, Luo J, Wu C, Chen T. Effectiveness of vaginal probiotics Lactobacillus crispatus chen-01 in women with high-risk HPV infection: a prospective controlled pilot study. Aging (Albany NY). 2024 Jul 25;16(14):11446-11459. Epub 2024 Jul 25. [CrossRef] [PubMed] [PubMed Central]
- Ou YC, Fu HC, Tseng CW, Wu CH, Tsai CC, Lin H. The influence of probiotics on genital high-risk human papilloma virus clearance and quality of cervical smear: a randomized placebo-controlled trial. BMC Womens Health. 2019 Jul 24;19(1):103. [CrossRef] [PubMed] [PubMed Central]
- Zhai L, Yadav R, Kunda NK, Anderson D, Bruckner E, Miller EK, Basu R, Muttil P, Tumban E. Oral immunization with bacteriophage MS2-L2 VLPs protects against oral and genital infection with multiple HPV types associated with head & neck cancers and cervical cancer. Antiviral Res. 2019 Jun;166:56-65. Epub 2019 Mar 26. [CrossRef] [PubMed] [PubMed Central]
- Riaz Rajoka MS, Zhao H, Lu Y, Lian Z, Li N, Hussain N, Shao D, Jin M, Li Q, Shi J. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct. 2018 May 23;9(5):2705-2715. [CrossRef] [PubMed]
- Sungur T, Aslim B, Karaaslan C, Aktas B. Impact of Exopolysaccharides (EPSs) of Lactobacillus gasseri strains isolated from human vagina on cervical tumor cells (HeLa). Anaerobe. 2017 Oct;47:137-144. Epub 2017 May 26. [CrossRef] [PubMed]
- Nami Y, Abdullah N, Haghshenas B, Radiah D, Rosli R, Khosroushahi AY. Assessment of probiotic potential and anticancer activity of newly isolated vaginal bacterium Lactobacillus plantarum 5BL. Microbiol Immunol. 2014 Sep;58(9):492-502. [CrossRef] [PubMed]
- Nouri Z, Karami F, Neyazi N, Modarressi MH, Karimi R, Khorramizadeh MR, Motevaseli E (2016) Dual anti-metastatic and anti-proliferative activity assessment of two probiotics on HeLa and HT-29 cell lines.
- Motevaseli E, Azam R, Akrami SM, Mazlomy M, Saffari M, Modarressi MH, Daneshvar M, Ghafouri-Fard S. The Effect of Lactobacillus crispatus and Lactobacillus rhamnosusCulture Supernatants on Expression of Autophagy Genes and HPV E6 and E7 Oncogenes in The HeLa Cell Line. Cell J. 2016 Winter;17(4):601-7. Epub 2016 Jan 17. [CrossRef] [PubMed] [PubMed Central]
- Hu S, Hao Y, Zhang X, Yang Y, Liu M, Wang N, Zhang TC, He H. Lacticaseibacillus casei LH23 Suppressed HPV Gene Expression and Inhibited Cervical Cancer Cells. Probiotics Antimicrob Proteins. 2023 Jun;15(3):443-450. Epub 2021 Oct 2. [CrossRef] [PubMed]
- Cha MK, Lee DK, An HM, Lee SW, Shin SH, Kwon JH, Ha NJ (2012) Antiviral activity of Bifdobacterium adolescentis SPM1005-A on human papillomavirus type 16. BMC Med 10(1):1–6.
- Wang KD, Xu DJ, Wang BY, Yan DH, Lv Z, Su JR. Inhibitory Effect of Vaginal Lactobacillus Supernatants on Cervical Cancer Cells. Probiotics Antimicrob Proteins. 2018 Jun;10(2):236-242. [CrossRef] [PubMed]
- Pawar K, Aranha C. Lactobacilli metabolites restore E-cadherin and suppress MMP9 in cervical cancer cells. Curr Res Toxicol. 2022 Sep 21;3:100088. [CrossRef] [PubMed] [PubMed Central]
- Palma E, Recine N, Domenici L, Giorgini M, Pierangeli A, Panici PB. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: a promising solution against HPV-infection. BMC Infect Dis. 2018 Jan 5;18(1):13. [CrossRef] [PubMed] [PubMed Central]
- Dellino M, Cascardi E, Laganà AS, Di Vagno G, Malvasi A, Zaccaro R, Maggipinto K, Cazzato G, Scacco S, Tinelli R, De Luca A, Vinciguerra M, Loizzi V, Daniele A, Cicinelli E, Carriero C, Genco CA, Cormio G, Pinto V. Lactobacillus crispatus M247 oral administration: Is it really an effective strategy in the management of papillomavirus-infected women? Infect Agent Cancer. 2022 Oct 21;17(1):53. [CrossRef] [PubMed] [PubMed Central]
- DI Pierro F, Criscuolo AA, Dei Giudici A, Senatori R, Sesti F, Ciotti M, Piccione E. Oral administration of Lactobacillus crispatus M247 to papillomavirus-infected women: results of a preliminary, uncontrolled, open trial. Minerva Obstet Gynecol. 2021 Oct;73(5):621-631. Epub 2021 Apr 20. [CrossRef] [PubMed]
| Bacteria/probiotics | Cell line | Outcome | References |
| Milk-isolated L. casei and L. paracasei | Hela | upregulating the expression of apoptotic genes | [108] |
| Vagina-isolated L. gasseri | Hela | Inflammation and proliferation were reduced, and apoptosis was increased | [109] |
| Vagina-isolated L. plantarum | Hela | Suppression of proliferation and induction of apoptosis | [110] |
| Supernatants of L. rhamnosus and L. crispatus | Hela | Inhibited cell proliferation and metastasis | [111] |
| Supernatants of L. rhamnosus and L. crispatus | Hela | Decrease expression of HPV oncogenes | [112] |
| Lacticaseibacillus casei LH23 | Hela | suppress the proliferation and induced the apoptosis of cervical cancer cells | [113] |
| Bifdobacterium adolescentis SPM1005-A | SiHa | Inhibited E6 and E7 oncogenes | [114] |
| supernatants of L. crispatus, L. jensenii, and L. gasseri | CasKi | inhibitory effects on the viability of cervical cancer cells via regulation of HPV oncogenes and cell cycle-related genes | [115] |
| Twelve standard Lactobacillus | Hela and SiHa | reduce tumor invasion and metastasis | [116] |
| Sample size | Bacterial Strain | Condition | Intervention | Outcome | References |
| N=160 | L. crispatus M247 | ASCUS* or LSIL* HPV+ |
Oral administration (no fewer than 20 billion) for 12 months |
higher percentage of clearance of PAP-smear abnormalities in patients who took oral Lactobacillus crispatus M247 than in the control group |
[118] |
| N=35 | L. crispatus M247 | ASCUS or LSIL or NILM* HPV+ | Oral administration (no fewer than 20 billion) for 3 months |
reduction of approxi mately 70% in HPV positivity and a significant change in CST status with 94% of women | [119] |
| N-54 | L. casei Shirota | LSIL HPV+ | Oral administration (no fewer than 20 billion) for 6 months |
Probiotic users had a twice as high chance of clearance of cytological abnormalities | [104] |
| N-121 | L. rhamnosus and L. reuteri | High-risk HPV | Oral administration (no fewer than 5.4 billion) discontinued until negative HPV testing | No significant influence on HR-HPV clearance, but may have decreased the rates of mildly abnormal and unsatisfactory cervical smears | [106] |
| N=91 | L. crispatus chen01 | High-risk HPV | Intravaginal 1 × 109 CFU per capsule for 5 months | Significantly reduced viral load of HPV, ameliorated HPV clearance rate, and improved vaginal inflammation state | [105] |
| N=117 | L. rhamnosus BMX 54 | C1N1*, HPV+ | Intravaginal 1 × 104 CFU per tablet for 3 or 6 months | Long term user doubled the chance of resolving HPV-related cytological anomalies compared to short term user | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




