Submitted:
31 December 2024
Posted:
03 January 2025
You are already at the latest version
Abstract
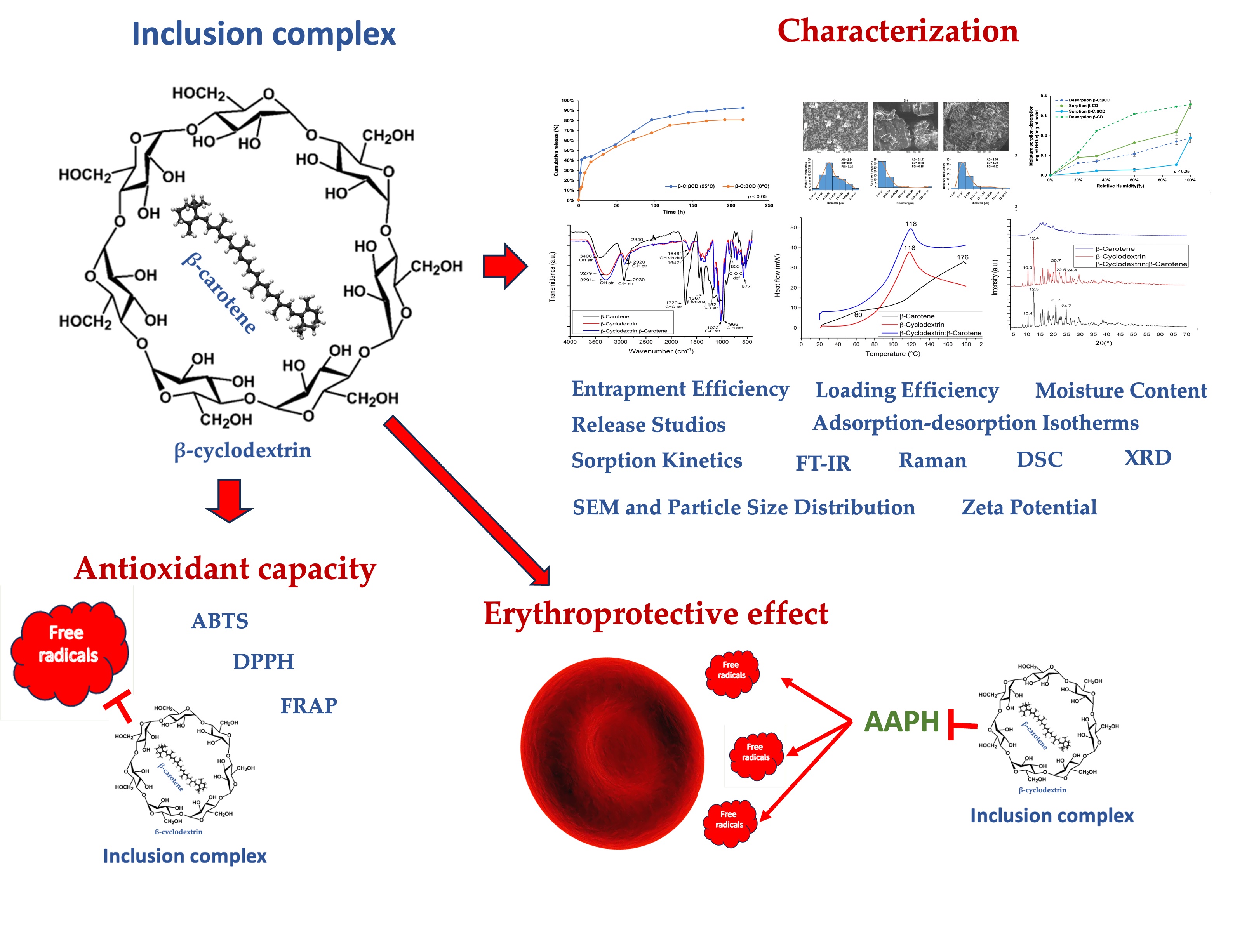
Keywords:
1. Introduction
2. Results
2.1. Yield, Entrapment Efficiency and Loading Determination
2.2. Moisture Content
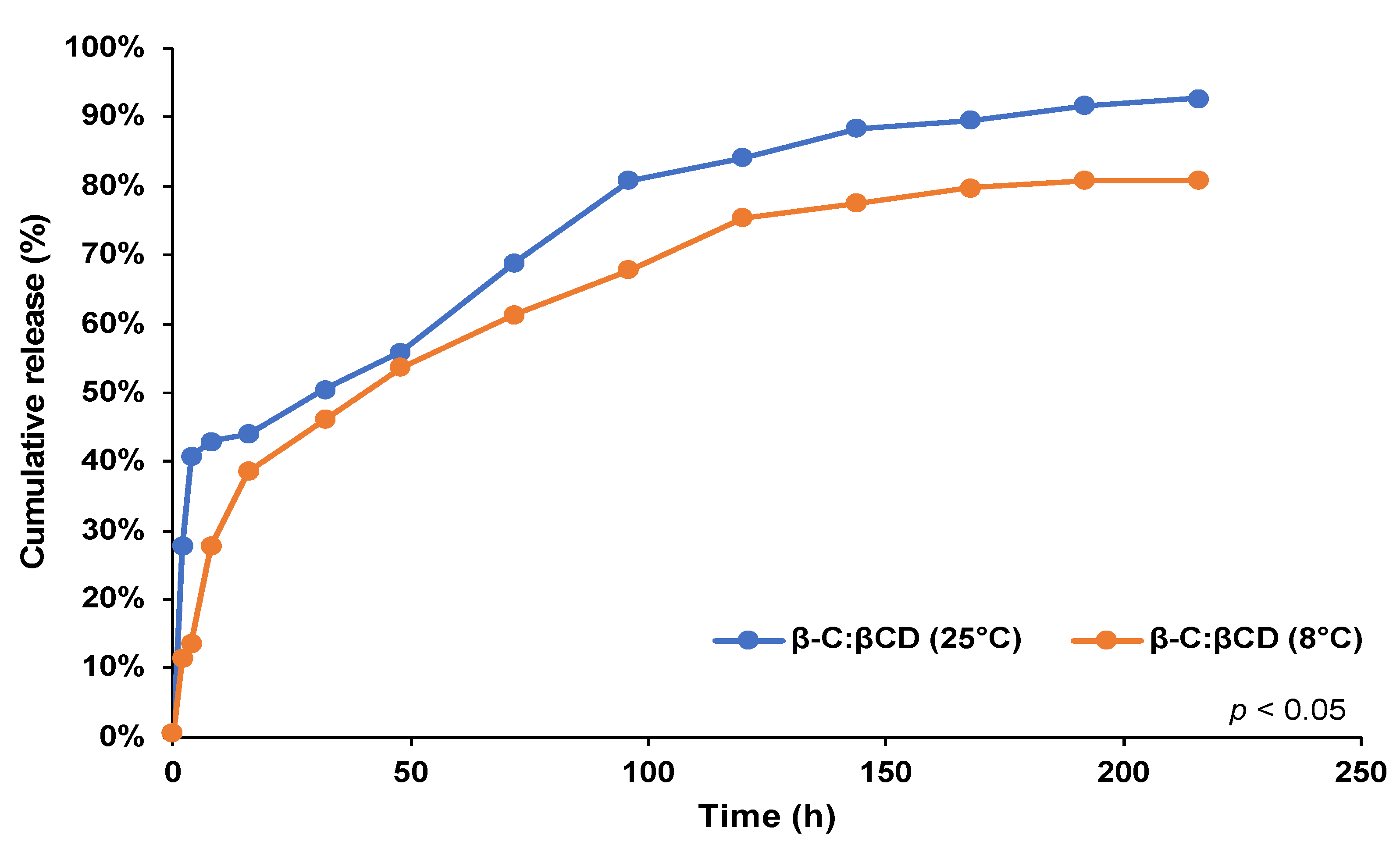
2.3. Determination of Release Profiles
2.4. Sorption Studies
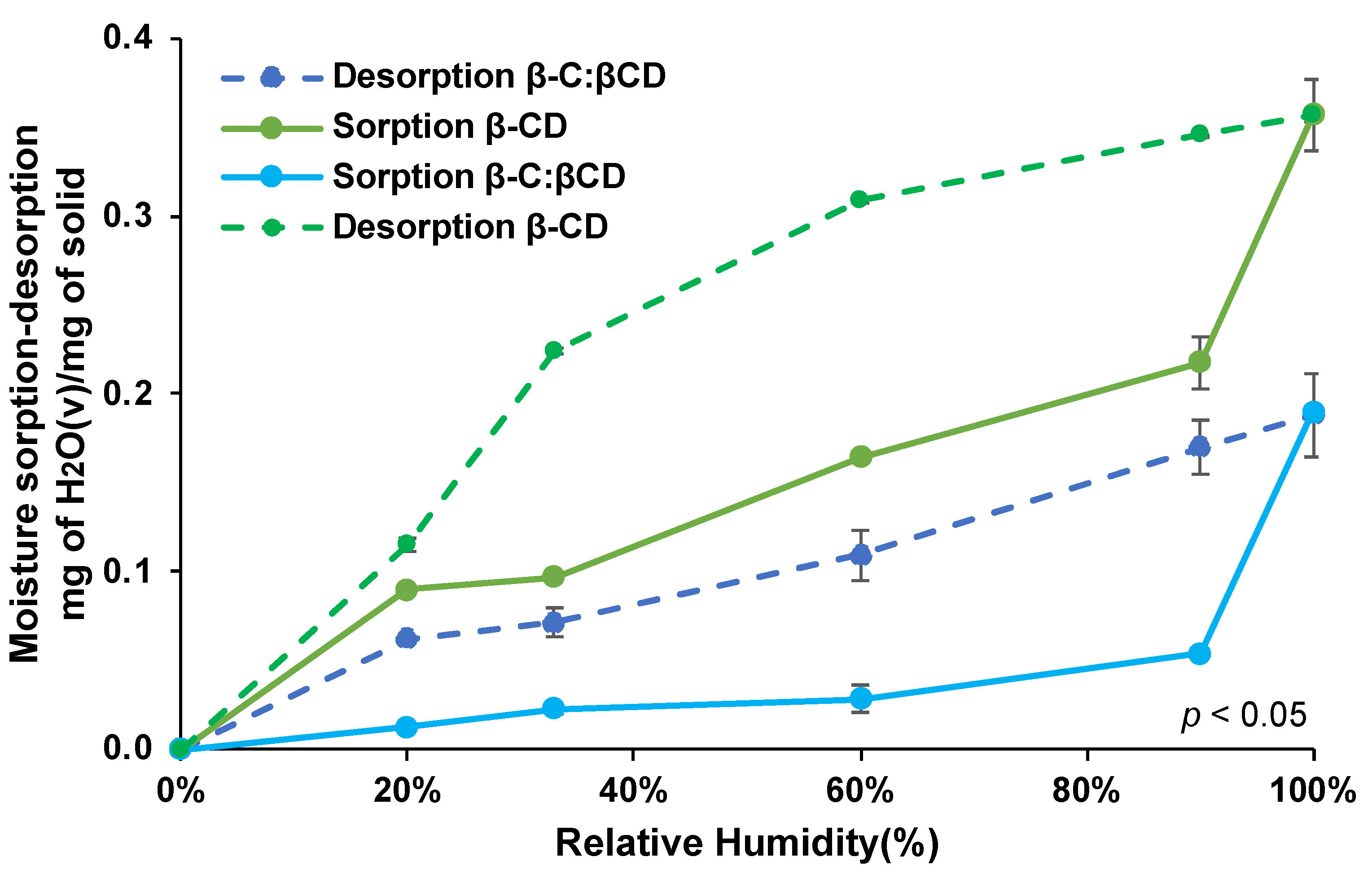
2.4.1. Adsorption-Desorption Isotherms
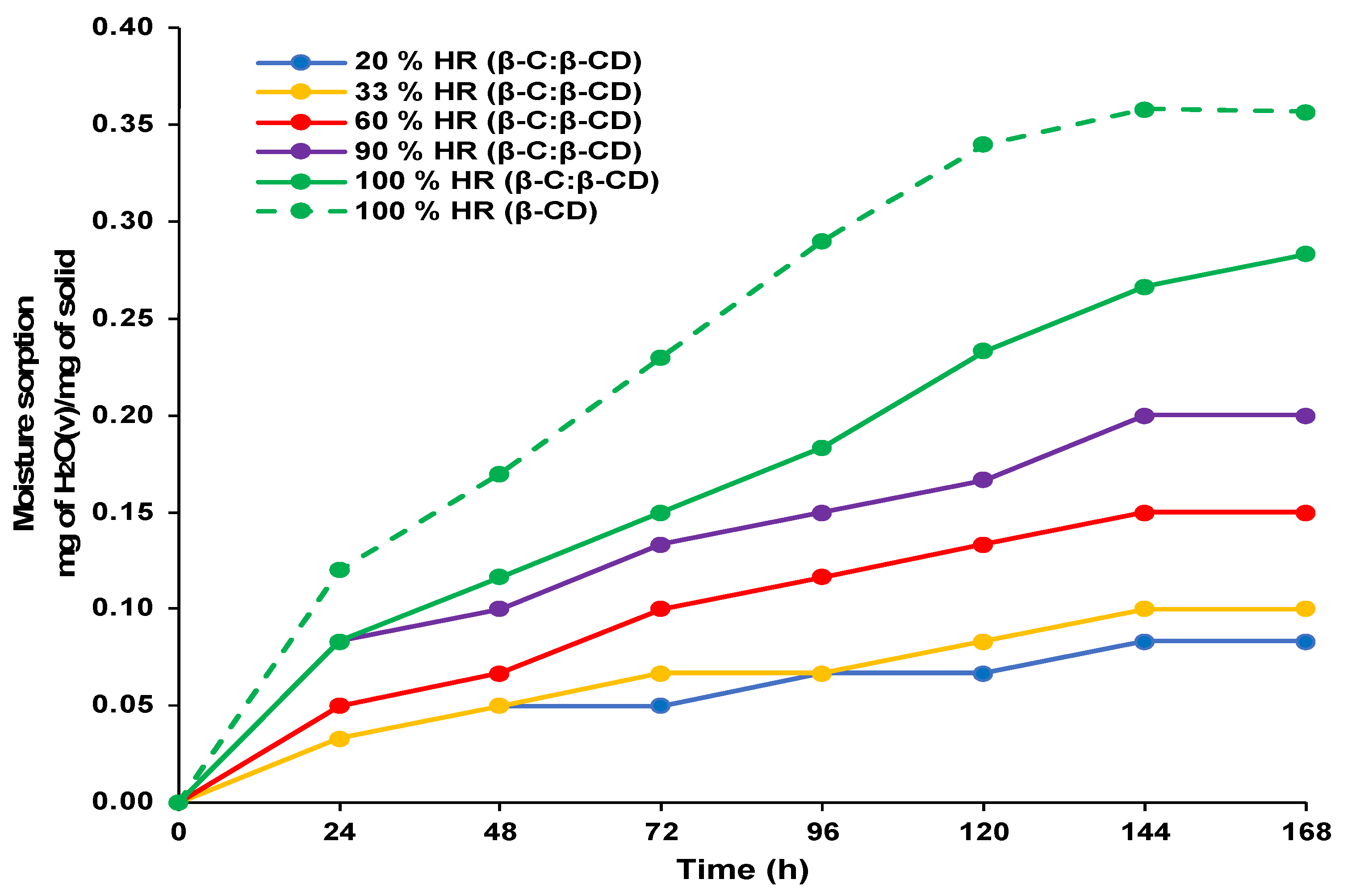
2.4.2. Sorption Kinetics
2.5. Fourier Transform Infrared Spectroscopy (FT-IR)
2.6. Raman
2.7. Differential Scanning Calorimetry (DSC)
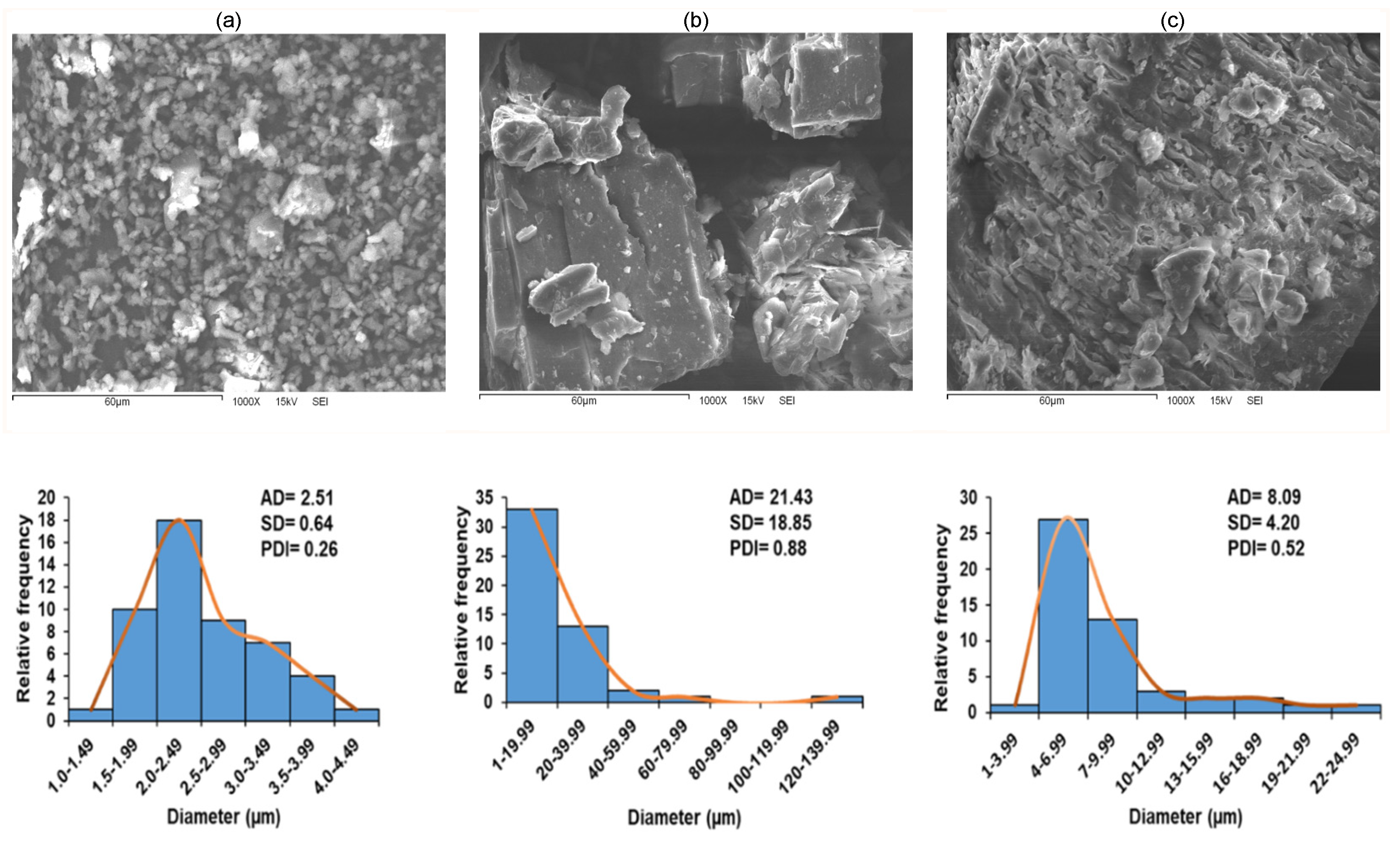
2.8. Scanning Electron Microscopy (SEM)
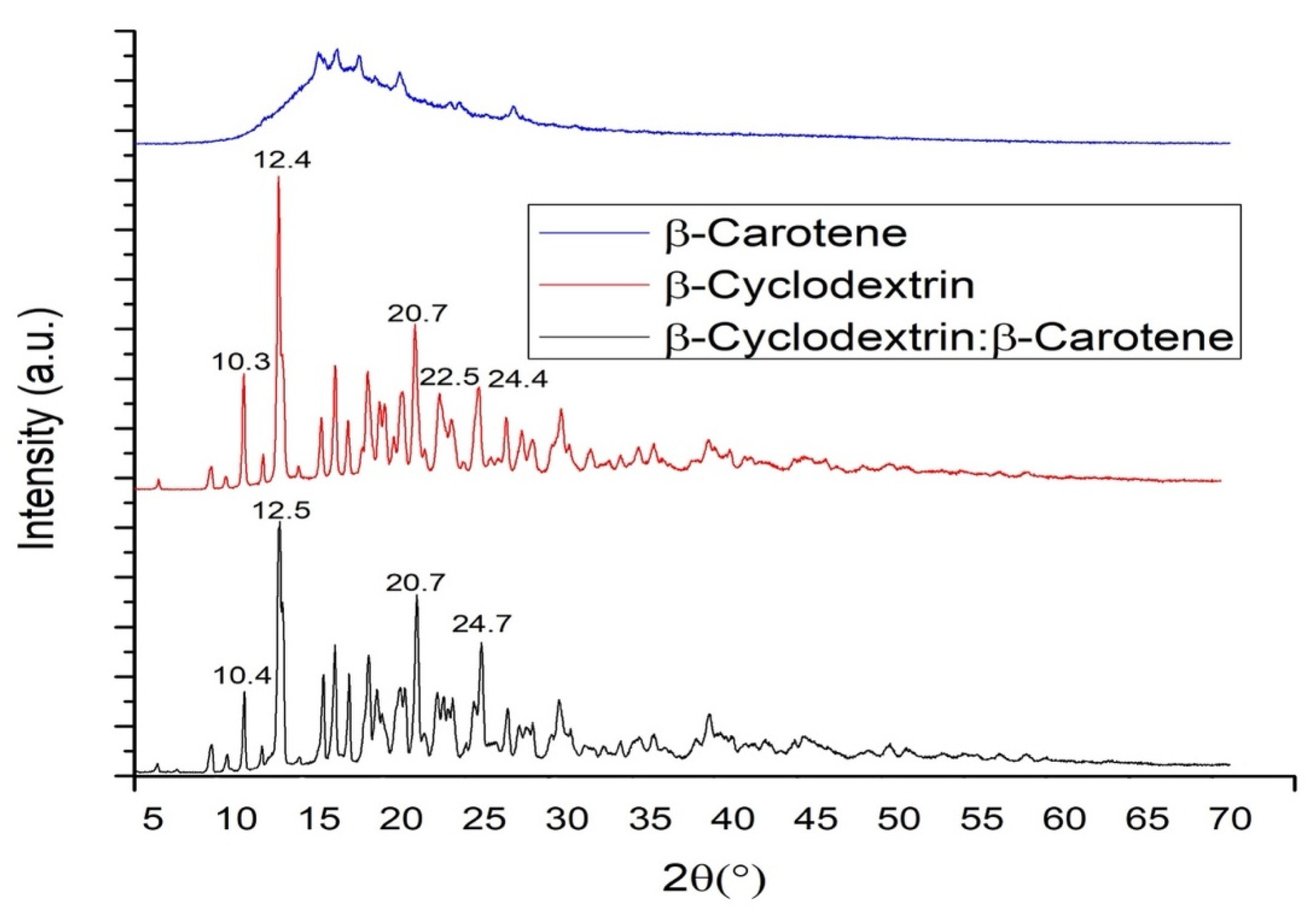
2.9. X-ray Diffraction (XRD)
2.10. Zeta Potential (ζ)
2.11. Antioxidant Activity
2.12. Protective Effect on Human Erythrocytes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of β-C/β-CD Inclusion Complex
4.3. Entrapment Efficiency (EE %) and Loading Efficiency (LE %)
4.4. Moisture Content
4.5. Release Studios
4.6. Adsorption-Desorption Isotherms
4.7. Sorption Kinetics
4.8. Fourier Transform Infrared Spectroscopy (FT-IR)
4.9. Raman
4.10. Differential Scanning Calorimetry (DSC)
4.11. Scanning Electron Microscopy (SEM) and Particle Size Distribution
4.12. X-Ray Diffraction (XRD)
4.13. Zeta Potential (ζ)
4.14. Antioxidant Activity
4.14.1. ABTS•+ Assay
4.14.2. DPPH Assay
4.14.3. FRAP Assay
4.15. Protective Effect on Human Erythrocytes
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgment
Conflicts of Interest
Abbreviations
| β-C | β-Carotene |
| β-CD | β-cyclodextrin |
| β-C/β-CD | β-Carotene in β-cyclodextrin inclusion complexes |
| FT-IR | Fourier transform infrared spectroscopy |
| DCS | Differential scanning calorimetry |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| ZP | Zeta potential |
| ABTS | 2,2′-azinobis(3-ethylbenzothiazoline |
| DPPH | 2,2-diphenyl-1-picrilhydrazil |
| FRAP | Ferric reducing antioxidant power |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
| AAPH | 2,2′-Azobis(2-amidinopropane) dihydrochloride |
| AD | Average diameter |
| SD | Standard deviation |
| PDI | Polydispersity index |
| RH | Relative humidity |
| TE | Trolox Equivalents |
References
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; Chen, H.; Qin, W.; Wu, H.; Chen, S. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Trusova, M.E.; Postnikov, P.S.; Sedlarik, V. Enhancement of the antioxidant activity and stability of β-carotene using amphiphilic chitosan/nucleic acid polyplexes. Int. J. Biol. Macromol. 2018, 117, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Rohmah, M.; Rahmadi, A.; Raharjo, S. Bioaccessibility and antioxidant activity of β-carotene loaded nanostructured lipid carrier (NLC) from binary mixtures of palm stearin and palm olein. Heliyon 2022, 8, e08913. [Google Scholar] [CrossRef]
- Shin, K.-C.; Seo, M.-J.; Kim, Y.-S.; Yeom, S.-J. Molecular properties of β-carotene oxygenases and their potential in industrial production of vitamin A and its derivatives. Antioxidants 2022, 11, 1180. [Google Scholar] [CrossRef]
- Lin, Q.; Wu, D.; Singh, H.; Ye, A. Improving solubility and stability of β-carotene by microencapsulation in soluble complexes formed with whey protein and OSA-modified starch. Food Chem. 2021, 352, 129267. [Google Scholar] [CrossRef]
- Păduraru, D.N.; Niculescu, A.-G.; Bolocan, A.; Andronic, O.; Grumezescu, A.M.; Bîrlă, R. An updated overview of cyclodextrin-based drug delivery systems for cancer therapy. Pharmaceutics 2022, 14, 1748. [Google Scholar] [CrossRef]
- Gonzalez Pereira, A.; Carpena, M.; García Oliveira, P.; Mejuto, J.C.; Prieto, M.A.; Simal Gandara, J. Main applications of cyclodextrins in the food industry as the compounds of choice to form host–guest complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular view into the cyclodextrin cavity: Structure and hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef]
- Alvira, E. Theoretical study of the β-cyclodextrin inclusion complex formation of eugenol in water. Molecules 2018, 23, 928. [Google Scholar] [CrossRef]
- Braithwaite, M.C.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Tomar, L.K.; Tyagi, C.; Pillay, V. A novel multi-tiered experimental approach unfolding the mechanisms behind cyclodextrin-vitamin inclusion complexes for enhanced vitamin solubility and stability. Int. J. Pharm. 2017, 532, 90–104. [Google Scholar] [CrossRef]
- Szente, L.; Fenyvesi, É. Cyclodextrin-enabled polymer composites for packaging. Molecules 2018, 23, 1556. [Google Scholar] [CrossRef] [PubMed]
- Sarabia-Vallejo, Á.; Caja, M.d.M.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Cyclodextrin inclusion complexes for improved drug bioavailability and activity: synthetic and analytical aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opinion on Drug Delivery 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Puebla-Duarte, A.L.; Santos-Sauceda, I.; Rodríguez-Félix, F.; Iturralde-García, R.D.; Fernández-Quiroz, D.; Pérez-Cabral, I.D.; Del-Toro-Sánchez, C.L. Active and intelligent packaging: A review of the possible application of cyclodextrins in food storage and safety indicators. Polymers 2023, 15, 4317. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Nilofar; Yildiztugay, E.; Bouyahya, A.; Cavusoglu, H.; Gevrenova, R. Zheleva-Dimitrova, D. A comparative study on UHPLC-HRMS profiles and biological activities of inula sarana different extracts and its beta-cyclodextrin complex: Effective insights for novel applications. Antioxidants 2023, 12, 1842.
- Stelling-Férez, J.; López-Miranda, S.; Gabaldón, J.A.; Nicolás, F.J. Oleanolic acid complexation with cyclodextrins improves its cell bio-availability and biological activities for cell migration. Int. J. Mol. Sci. 2023, 24, 14860. [Google Scholar] [CrossRef]
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: A review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- Hosseini, H.; Jafari, S.M. Introducing nano/microencapsulated bioactive ingredients for extending the shelf-life of food products. Adv. Colloid Interface Sci. 2020, 282, 102210. [Google Scholar] [CrossRef]
- Núñez-Delicado, E.; Sánchez-Ferrer, A.; García-Carmona, F. Cyclodextrins as secondary antioxidants: Synergism with ascorbic acid. J. Agric. Food Chem. 1997, 45, 2830–2835. [Google Scholar] [CrossRef]
- Messias, M.A.; Ferreira, S.M.; Tavares, L.; Santos, L. A Comparative study between onion peel extracts, free and complexed with β-cyclodextrin, as a natural uv filter to cosmetic formulations. Int. J. Mol. Sci. 2023, 24, 15854. [Google Scholar] [CrossRef]
- Borel, P.; Troadec, R.; Damiani, M.; Halimi, C.; Nowicki, M.; Guichard, P.; Margier, M.; Astier, J.; Grino, M.; Reboul, E. β-Carotene bioavailability and conversion efficiency are significantly affected by sex in rats: First Observation suggesting a possible hormetic regulation of vitamin a metabolism in female rats. Mol. Nutr. Food Res. 2021, 65, 2100650. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Kobayashi, S.; Warang, P.; Madkaikar, M.; Mukherjee, M.B. Erythrocytes as a preferential target of oxidative stress in blood. Free Radical Res. 2021, 55, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.; Havránek, O. Redox status of erythrocytes as an important factor in eryptosis and erythronecroptosis. Folia Biologica 2023, 69. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.M.; Mortada, S.M.; Sallam, M.A. Carboxylate cross-linked cyclodextrin: A nanoporous scaffold for enhancement of rosuvastatin oral bioavailability. Eur. J. Pharm. Sci. 2018, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.; El-Meligy, M.A.; Sharaf, M.; Soliman, A.T.; AbuKhadra, M.R. Insight into chitosan/zeolite-A nanocomposite as an advanced carrier for levofloxacin and its anti-inflammatory properties; loading, release, and anti-inflammatory studies. Int. J. Biol. Macromol. 2021, 179, 206–216. [Google Scholar] [CrossRef]
- Tan, D.; Yuan, P.; Dong, F.; He, H.; Sun, S.; Liu, Z. Selective loading of 5-fluorouracil in the interlayer space of methoxy-modified kaolinite for controlled release. Applied Clay Science 2018, 159, 102–106. [Google Scholar] [CrossRef]
- Sujja-areevath, J.; Munday, D.L.; Cox, P.J.; Khan, K.A. Relationship between swelling, erosion and drug release in hydrophillic natural gum mini-matrix formulations. Eur. J. Pharm. Sci. 1998, 6, 207–217. [Google Scholar] [CrossRef]
- Jacques, C.H.M.; Hopfenberg, H.B.; Stannett, V. , Super case II transport of organic vapors in glassy polymers. In Permeability of Plastic Films and Coatings, Springer US: 1974; pp 73-86.
- Ahmed, L.; Atif, R.; Eldeen, T.S.; Yahya, I.; Omara, A.; Eltayeb, M. Study the using of nanoparticles as drug delivery system based on mathematical models for controlled release. IJLTEMAS 2019, 8, 52–56. [Google Scholar]
- Borandeh, S.; van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric drug delivery systems by additive manufacturing. Adv. Drug Del. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Sobczak, M.; Kędra, K. Biomedical polyurethanes for anti-cancer drug delivery systems: A brief, comprehensive review. Int. J. Mol. Sci. 2022, 23, 8181. [Google Scholar] [CrossRef]
- Duan, G.; Zhong, Q.; Bi, L.; Yang, L.; Liu, T.; Shi, X.; Wu, W. The Poly(acrylonitrule-co-acrylic acid)-graft-β-cyclodextrin hydrogel for thorium(IV) adsorption. Polymers 2017, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Taulier, N.; Chalikian, T.V. Hydrophobic hydration in cyclodextrin complexation. J. Phys. Chem. B. 2006, 110, 12222–12224. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Zavala, J.F.; Soto-Valdez, H.; González-León, A.; Álvarez-Parrilla, E.; Martín-Belloso, O.; González-Aguilar, G.A. Microencapsulation of cinnamon leaf (Cinnamomum zeylanicum) and garlic (Allium sativum) oils in β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2007, 60, 359–368. [Google Scholar] [CrossRef]
- Del Toro-Sánchez, C.; Ayala-Zavala, J.; Machi, L.; Santacruz, H.; Villegas-Ochoa, M.; Alvarez-Parrilla, E.; González-Aguilar, G. Controlled release of antifungal volatiles of thyme essential oil from β-cyclodextrin capsules. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 431–441. [Google Scholar] [CrossRef]
- Crini, G.; Peindy, H.; Gimbert, F.; Robert, C. Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007; 53, 97–110. [Google Scholar]
- Filho, C.M.C.; Bueno, P.V.A.; Matsushita, A.F.Y.; Vilsinski, B.H.; Rubira, A.F.; Muniz, E.C.; Murtinho, D.M.B.; Valente, A.J.M. Uncommon sorption mechanism of aromatic compounds onto poly(vinyl alcohol)/chitosan/maleic anhydride-β-cyclodextrin hydrogels. Polymers 2020, 12, 877. [Google Scholar] [CrossRef]
- da Costa, J.P.; Avellan, A.; Tubić, A.; Duarte, A.C.; Rocha-Santos, T. Understanding Interface Exchanges for Assessing Environmental Sorption of Additives from Microplastics: Current Knowledge and Perspectives. Molecules 2024, 29, 333. [Google Scholar] [CrossRef]
- Kaleta, A.; Górnicki, K.; Obranović, M.; Kosiorek, K. Some aspects of the modelling of dried red beets rehydration process. Appl. Sci. 2024, 14, 1016. [Google Scholar] [CrossRef]
- Rafiq, A.; Chowdhary, J.; Hazarika, M.K.; Makroo, H.A. Temperature dependence on hydration kinetic model parameters during rehydration of parboiled rice. J. Food Sci. Technol. 2015, 52, 6090–6094. [Google Scholar] [CrossRef]
- Genovese, J.; Tappi, S.; Tylewicz, U.; D'Elia, F.; De Aguiar Saldanha Pinheiro, A.C.; Rocculi, P. Dry-salted cod (Gadus morhua) rehydration assisted by pulsed electric fields: modelling of mass transfer kinetics. J. Sci. Food Agric. 2022, 102, 4961–4965. [Google Scholar] [CrossRef]
- Peleg, M. An empirical model for the description of moisture sorption curves. J. Food Sci. 1988, 53, 1216–1217. [Google Scholar] [CrossRef]
- Guo, X.; Pang, J.; Chen, S.; Jia, H. Sorption properties of tylosin on four different microplastics. Chemosphere 2018, 209, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Abukhadra, M.R.; Refay, N.M.; Sharaf, M.F.; El-Meligy, M.A.; Awwad, E.M. Synthesis of chitosan/MCM-48 and β-cyclodextrin/MCM-48 composites as bio-adsorbents for environmental removal of Cd2+ ions; kinetic and equilibrium studies. React. Funct. Polym. 2020, 154, 104675. [Google Scholar] [CrossRef]
- Li, H.; Chang, S.-L.; Chang, T.-R.; You, Y.; Wang, X.-D.; Wang, L.-W.; Yuan, X.-F.; Tan, M.-H.; Wang, P.-D.; Xu, P.-W.; Gao, W.-B.; Zhao, Q.-S.; Zhao, B. Inclusion complexes of cannabidiol with β-cyclodextrin and its derivative: Physicochemical properties, water solubility, and antioxidant activity. J. Mol. Liq. 2021, 334, 116070. [Google Scholar] [CrossRef]
- Wang, H.; Hu, L.; Peng, L.; Du, J.; Lan, M.; Cheng, Y.; Ma, L.; Zhang, Y. Dual encapsulation of β-carotene by β-cyclodextrin and chitosan for 3D printing application. Food Chem. 2022, 378, 132088. [Google Scholar] [CrossRef] [PubMed]
- Rostamabadi, H.; Sadeghi Mahoonak, A.; Allafchian, A.; Ghorbani, M. Fabrication of β-carotene loaded glucuronoxylan-based nanostructures through electrohydrodynamic processing. Int. J. Biol. Macromol. 2019, 139, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Low, Z.X.; Teo, M.Y.M.; Nordin, F.J.; Dewi, F.R.P.; Palanirajan, V.K.; In, L.L.A. Biophysical evaluation of water-soluble curcumin encapsulated in β-cyclodextrins on colorectal cancer cells. Int. J. Mol. Sci. 2022, 23, 12866. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Szakal, R.N.; Chirilă, C.A.; Lukinich-Gruia, A.T.; Păunescu, V.; Muntean, C.; Rusu, G.; Bujancă, G.; Hădărugă, D.I. Complexation of Danube common nase (Chondrostoma nasus L.) oil by β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin. Food Chem. 2020; 303, 125419. [Google Scholar]
- Kapoor, M.P.; Moriwaki, M.; Minoura, K.; Timm, D.; Abe, A.; Kito, K. Structural investigation of hesperetin-7-O-glucoside Inclusion complex with β-cyclodextrin: A spectroscopic assessment. Molecules 2022, 27, 5395. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, X.; Wang, C.; Fang, Q.; Zhong, L.; Wei, Q. Preparation, optimization, and characterization of inclusion complexes of Cinnamomum longepaniculatum essential oil in β-cyclodextrin. Sustainability 2022, 14, 9513. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Formation of rutin–β-cyclodextrin inclusion complexes by supercritical antisolvent precipitation. Polymers 2021, 13, 246. [Google Scholar] [CrossRef]
- de Oliveira, V.E.; Almeida, E.W.C.; Castro, H.V.; Edwards, H.G.M.; Dos Santos, H.F.; de Oliveira, L.F.C. Carotenoids and β-cyclodextrin inclusion complexes: Raman spectroscopy and theoretical investigation. J. Phys. Chem. A 2011, 115, 8511–8519. [Google Scholar] [CrossRef]
- Celitan, E.; Gruskiene, R.; Sereikaite, J. An optimization procedure for preparing aqueous CAR/HP-CD aggregate dispersions. Molecules 2021, 26, 7562. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Latosiński, J.; Gumułka, P.; Dąbrowska, M.; Kępczyński, M.; Sulka, G.D.; Starek, M. Controlled delivery of celecoxib-β-cyclodextrin complexes from the nanostructured titanium dioxide layers. Pharmaceutics 2023, 15, 1861. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yu, T.; Yuan, L.; Rao, G.; Li, D.; Mu, C. Preparation, physicochemical characterization and release behavior of the inclusion complex of trans -anethole and β-cyclodextrin. Food Res. Int. 2015, 74, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Dai, S.; Lian, Z.; Tong, X.; Yang, S.; Chen, Y.; Qi, W.; Peng, X.; Wang, H.; Jiang, L. The layered encapsulation of vitamin B2 and β-carotene in multilayer alginate/chitosan gel microspheres: improving the bioaccessibility of vitamin B2 and β-carotene. Foods 2021, 11, 20. [Google Scholar] [CrossRef]
- Christaki, S.; Spanidi, E.; Panagiotidou, E.; Athanasopoulou, S.; Kyriakoudi, A.; Mourtzinos, I.; Gardikis, K. Cyclodextrins for the Delivery of Bioactive Compounds from Natural Sources: Medicinal, Food and Cosmetics Applications. Pharmaceuticals 2023, 16, 1274. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Cyclodextrin encapsulated catechin: Effect of pH, relative humidity and various food models on antioxidant stability. LWT - Food Sci. Tech. 2017; 85, 232–239. [Google Scholar]
- Dai, Y.; Lu, X.; Li, R.; Cao, Y.; Zhou, W.; Li, J.; Zheng, B. Fabrication and characterization of W/O/W emulgels by sipunculus nudus salt-soluble proteins: co-encapsulation of vitamin C and β-carotene. Foods 2022, 11, 2720. [Google Scholar] [CrossRef]
- Ma, X.; Yan, T.; Miao, S.; Mao, L.; Liu, D. In vitro digestion and storage stability of β-carotene-loaded nanoemulsion stabilized by soy protein isolate (SPI)-citrus pectin (CP) complex/conjugate prepared with ultrasound. Foods 2022, 11, 2410. [Google Scholar] [CrossRef]
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar] [CrossRef]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragrance J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, B.; Jia, S.; Ma, M.; Hao, J. Novel supramolecular organogel based on β-cyclodextrin as a green drug carrier for enhancing anticancer effects. J. Mol. Liq. 2018, 250, 19–25. [Google Scholar] [CrossRef]
- Chulurks, S.; Jitapunkul, K.; Katanyutanon, S.; Toochinda, P.; Lawtrakul, L. Stability enhancement and skin permeation application of nicotine by forming inclusion complex with β-cyclodextrin and methyl-β-cyclodextrin. Sci. Pharm. 2021, 89, 43. [Google Scholar] [CrossRef]
- Fuenmayor, C.A.; Baron-Cangrejo, O.G.; Salgado-Rivera, P.A. Encapsulation of carotenoids as food colorants via formation of cyclodextrin inclusion complexes: A review. Polysaccharides 2021, 2, 454–476. [Google Scholar] [CrossRef]
- Ćujić Nikolić, N.; Žilić, S.; Simić, M.; Nikolić, V.; Živković, J.; Marković, S.; Šavikin, K. Microencapsulates of blue maize polyphenolics as a promising ingredient in the food and pharmaceutical industry: characterization, antioxidant properties, and in vitro-simulated digestion. Foods 2023, 12, 1870. [Google Scholar] [CrossRef]
- Čulina, P.; Zorić, Z.; Garofulić, I.E.; Repajić, M.; Dragović-Uzelac, V.; Pedisić, S. Optimization of the spray-drying encapsulation of sea buckthorn berry oil. Foods 2023, 12, 2448. [Google Scholar] [CrossRef]
- Li, Q.; Ghadiani, H.; Jalilvand, V.; Alam, T.; Farhat, Z.; Islam, M.A. Hydrogen impact: A review on diffusibility, embrittlement mechanisms, and characterization. Materials 2024, 17, 965. [Google Scholar] [CrossRef]
- Rashed, A.S.; Nasr, E.H.; Mabrouk, S.M. Influence of gyrotactic microorganisms on bioconvection in electromagnetohydrodynamic hybrid nanofluid through a permeable sheet. Computation 2024, 12, 17. [Google Scholar] [CrossRef]
- Horablaga, A.; Şibu Ciobanu, A.; Megyesi, C.I.; Gligor Pane, D.; Bujancă, G.S.; Velciov, A.B.; Morariu, F.E.; Hădărugă, D.I.; Mişcă, C.D.; Hădărugă, N.G. Estimation of the controlled release of antioxidants from β-cyclodextrin/chamomile (Matricaria chamomilla L.) or milk thistle (Silybum marianum L.), asteraceae, hydrophilic extract complexes through the fast and cheap spectrophotometric technique. Plants, 2023; 12, 2352. [Google Scholar]
- González-Manzano, S.; Dueñas, M. Applications of natural products in food. Foods 2021, 10, 300. [Google Scholar] [CrossRef]
- Wu, C.; Ouyang, X.; Zhou, X.; Li, X.; Li, H.; Li, W.; Wan, C.; Yu, B.; El-Sohaimy, S.; Wu, Z. Dry nutrition delivery system based on defatted soybean particles and its application with β-carotene. Molecules 2023, 28, 3429. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Liu, B.; Zhao, J.; Thomas, D.S.; Hook, J.M. An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem. 2013, 136, 186–192. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Perez-Perez, L.M.; Armenta-Villegas, L.; Santacruz-Ortega, H.; Gutiérrez-Lomelí, M.; Aguilar, J.A.; Reynoso-Marin, F.J.; Robles-García, M.A.; Robles-Zepeda, R.E.; Ruiz-Cruz, S.; Del-Toro-Sánchez, C.L. Characterization of Anemopsis californica essential oil–β-cyclodextrin inclusion complex as antioxidant prolonged-release system. Chemical Papers 2017, 71, 1331–1342. [Google Scholar] [CrossRef]
- González-Vega, R.I.; Robles-García, M.Á.; Mendoza-Urizabel, L.Y.; Cárdenas-Enríquez, K.N.; Ruiz-Cruz, S.; Gutiérrez-Lomelí, M.; Iturralde-García, R.D.; Avila-Novoa, M.G.; Villalpando-Vargas, F.V.; Del-Toro-Sánchez, C.L. Impact of the ABO and RhD blood groups on the evaluation of the erythroprotective potential of fucoxanthin, β-carotene, gallic acid, quercetin and ascorbic acid as therapeutic agents against oxidative stress. Antioxidants 2023, 12, 2092. [Google Scholar] [CrossRef]
- Huang, M.; Wang, J.; Tan, C. Tunable high internal phase emulsions stabilized by cross-linking/ electrostatic deposition of polysaccharides for delivery of hydrophobic bioactives. Food Hydrocoll. 2021, 118, 106742. [Google Scholar] [CrossRef]
- AOAC Official Method 950.02Animal Feed. In Official Methods of Analysis of AOAC INTERNATIONAL, Oxford University Press: 2023.
- Mohammadi, M.; Hamishehkar, H.; McClements, D.J.; Shahvalizadeh, R.; Barri, A. Encapsulation of Spirulina protein hydrolysates in liposomes: Impact on antioxidant activity and gastrointestinal behavior. Food Chem. 2023, 400, 133973. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.D.; Bin Jumah, M.N.; AlZahrani, S.A.; Allam, A.A.; Abukhadra, M.R.; Bellucci, S. Insights into the effect of chitosan and β-cyclodextrin hybridization of zeolite-a on its physicochemical and cytotoxic properties as a bio-carrier for 5-fluorouracil: Equilibrium and release kinetics studies. Molecules 2023, 28, 5427. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Hernández-Ruiz, K.L.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Gassos-Ortega, L.E.; Ornelas-Paz, J.d.J.; Del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; López-Mata, M.A.; Rodríguez-Félix, F. Evaluation of antioxidant capacity, protective effect on human erythrocytes and phenolic compound identification in two varieties of plum fruit (Spondias spp.) by UPLC-MS. Molecules, 2018; 23, 3200. [Google Scholar]
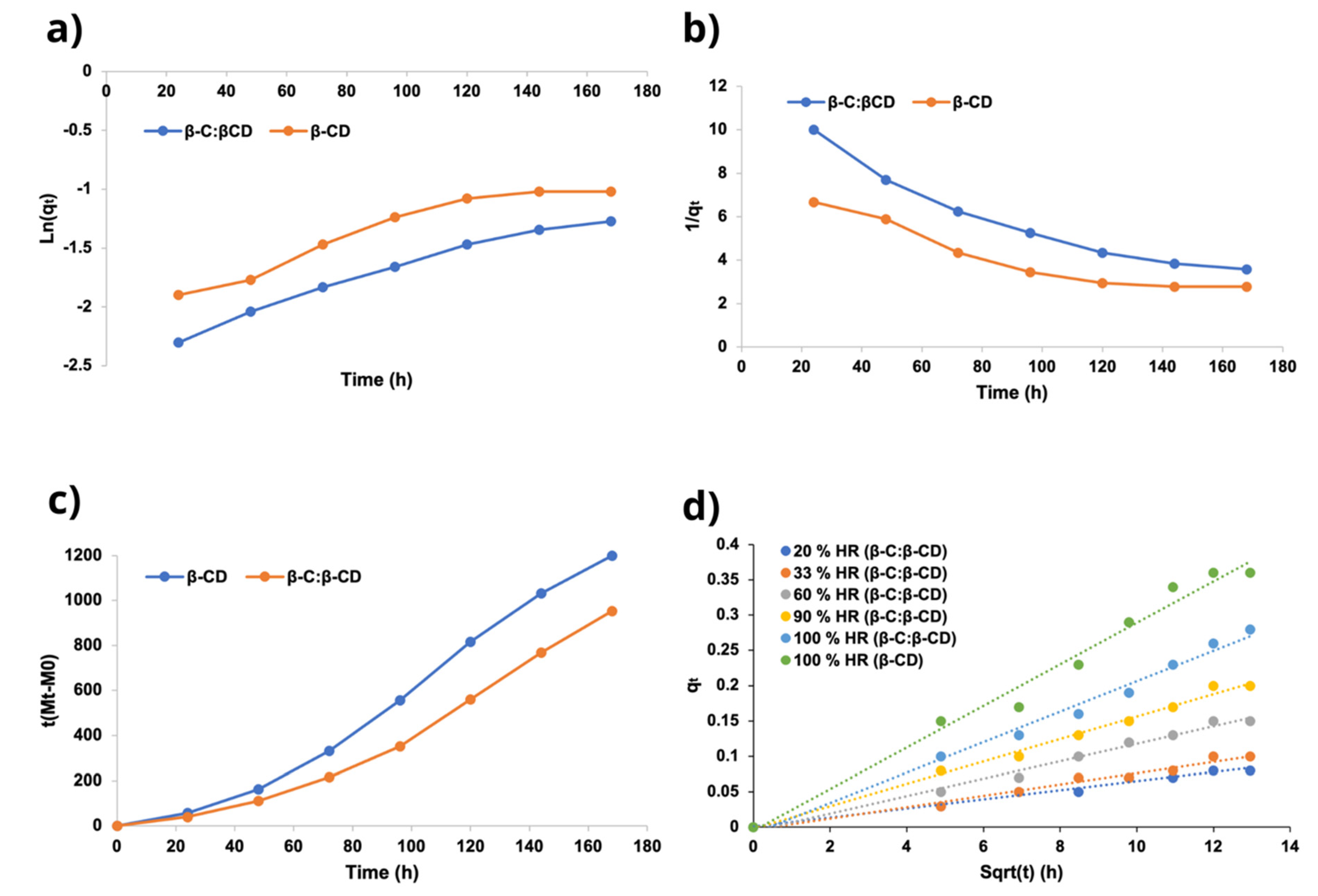
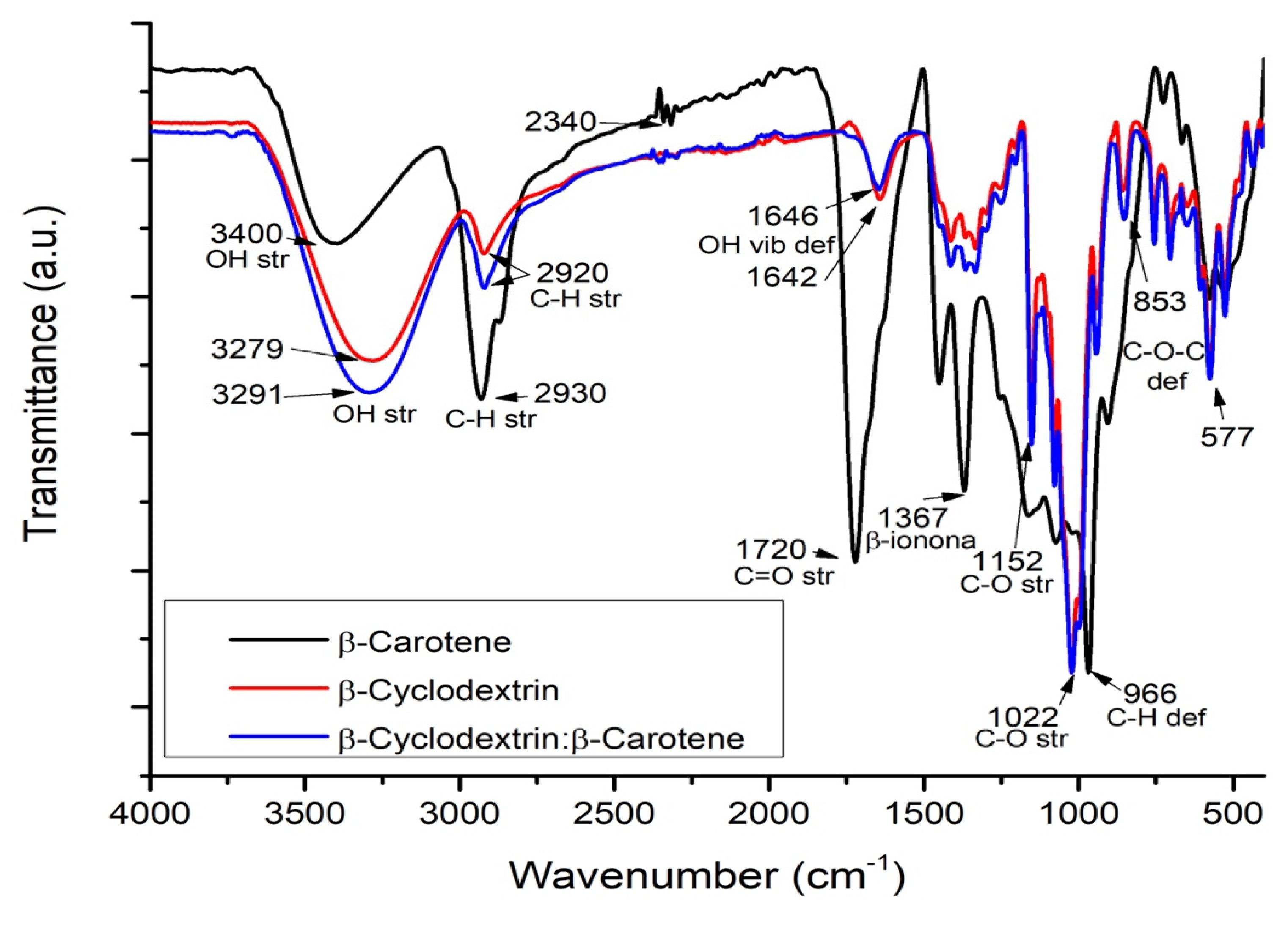
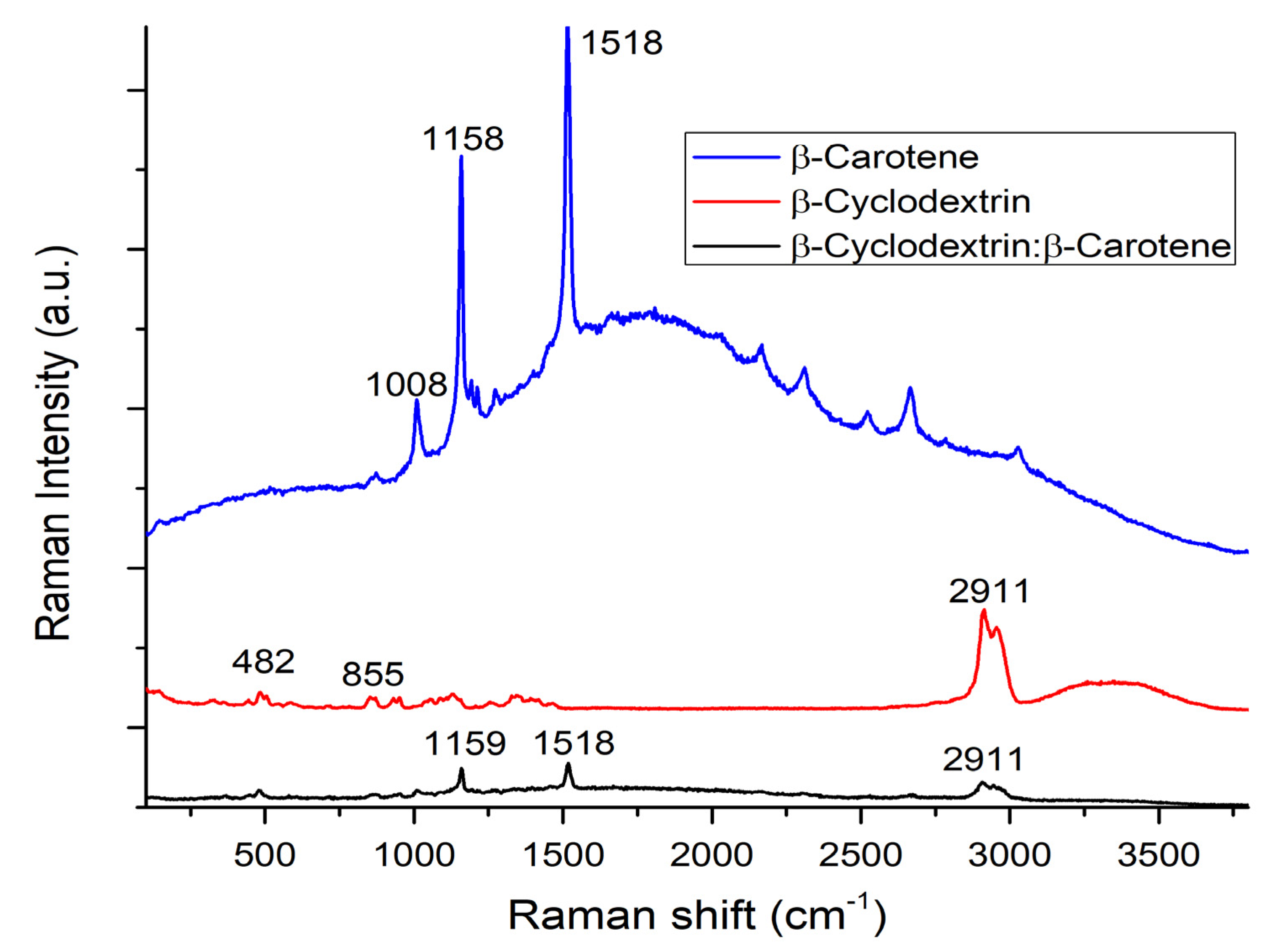
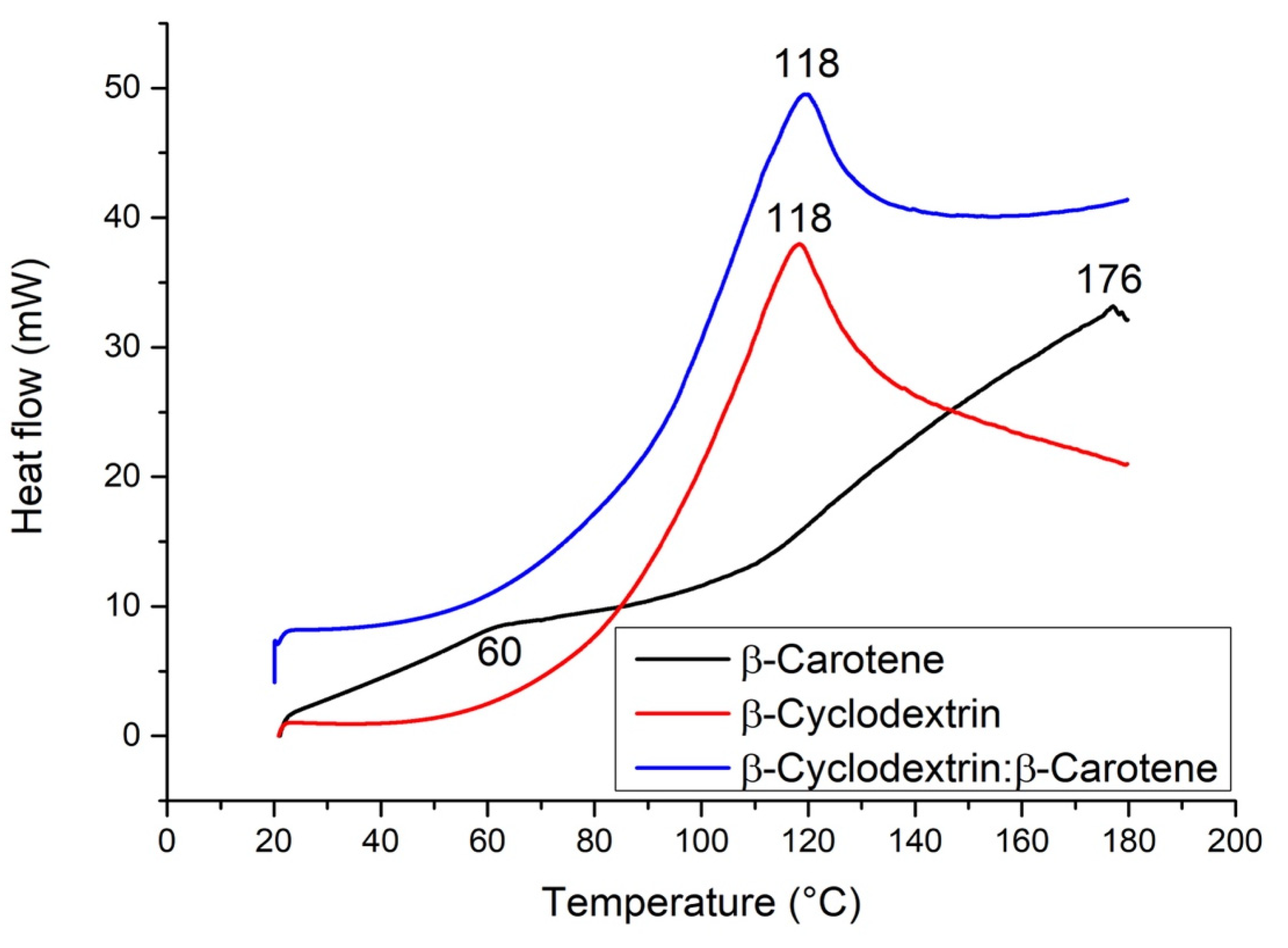
| β-C/β-CD ratio (%w/w) | Initial weight β-C (g) | Initial weight β-CD (g) | Initial weight β-C/β-CD (g) | Recovery β-C/β-CD (g) | Yield (%)A | Weight of encapsulated β-C (g) A | EE (%)A | LE (%)A |
|---|---|---|---|---|---|---|---|---|
| 40:60 | 0.4 | 0.6 | 1.0 | 0.941 ± 0.012 | 94.10 ± 1.21 | 0.3298 ± 0.03 | 82.47 ± 0.40 | 11.92 ± 0.24% |
| Temperature | Zero order | First order | Higuchi | Korsmeyer-Peppas | |
|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | n | |
| 8 °C | 0.8079 | 0.4052 | 0.9544 | 0.9909 | 0.349 |
| 25 °C | 0.8070 | 0.2972 | 0.9332 | 0.9887 | 0.264 |
| Molecule | Pseudo-first-order | Pseudo-second-order | Peleg's model | |||||
| R2 | R2 | R2 | K1 | K2 | R0 | M∞ | R2 | |
| β-CD | 0.9191 | 0.8802 | 0.9507 | 17.412 | 0.079 | 13.825 | 33.825 | 0.9958 |
| β-C:β-CD | 0.9745 | 0.9142 | 0.9778 | 9.245 | 0.072 | 12.690 | 32.690 | 0.9999 |
| Sample | ABTS (% inhibition) |
DPPH (% inhibition) |
FRAP (mM TE/g) |
|---|---|---|---|
| β-C | 8.21 ± 0.3a | 5.99 ± 0.5a | 1.26 ± 0.1a |
| β-C/βCD | 8.63 ± 0.42a | 6.35 ± 0.3a | 1.57 ± 0.5a |
| % inhibition of hemolysis | ||
|---|---|---|
| β-C | β-C/β-CD | AAPH |
| 25.6 ± 1.23b | 32.57 ± 1.55a | 0c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





