Submitted:
31 December 2024
Posted:
02 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
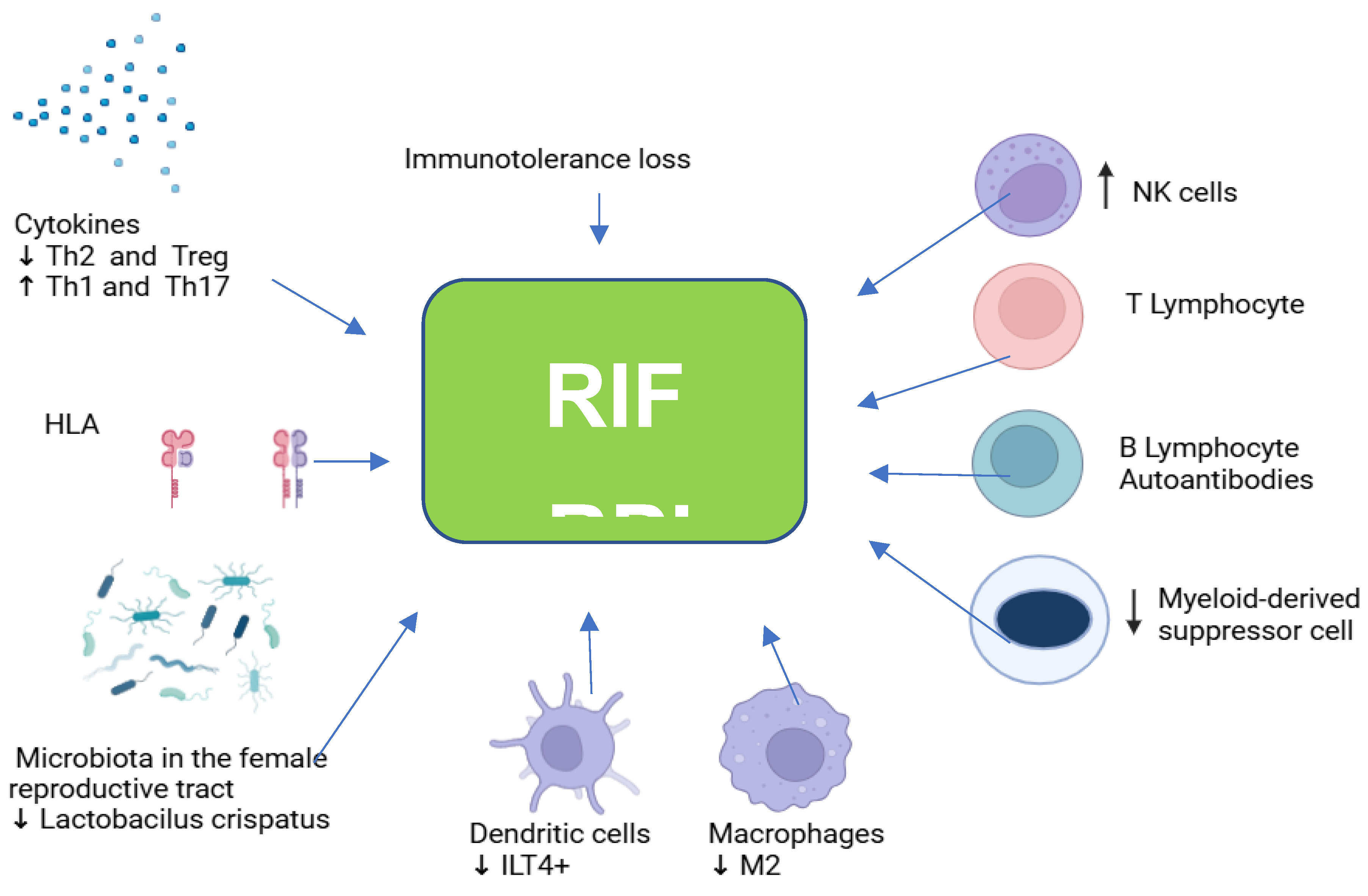
2. Innate Immune Response in RPL and RIF
2.1. Natural Killer (NK) Cells.
2.2. Macrophages and Dendritic Cells
2.3. Polymorphonuclear Cells.
2.4. T Cells
2.5. B Cells
2.6. Myeloid Suppressor Cells.
3. Cytokines
4. HLA in RPL and RIF
5. Immune Checkpoints in RPL and RIF
6. Autoimmunity
6.1. Antiphospholipid Antibodies (aPL) and Antiphospholipid Syndrome
6.2. Systemic Lupus Erythematosus and Other Autoimmune Diseases
6.3. Celiac Disease
6.4. Thyroid Autoimmunity
7. MicroRNAs (miRNAs) and RPL.
8. Microbiota in RPL and RIF
9. Immunological Treatment of RPL and RIF
9.1. Corticosteroids
9.2. Hydroxychloroquine
9.3. Calcineurin Inhibitors
9.4. Intravenous Immunoglobulins (IVIGs)
9.5. Granulocyte Colony-Stimulating Factor (G-CSF)
9.6. Tumor Necrosis Factor (TNF)-α Inhibitors
9.7. Allogenic Peripheral Blood Mononuclear Cell (PBMC) Immunotherapy
9.8. Intrauterine Peripheral Blood Mononuclear Cells
9.9. Intrauterine Autologous Platelet-Rich Plasma (PRP)
9.10. Lipid Emulsion (Intralipid) Intravenous Therapy
9.11. Omega 3 Fatty Acids Supplementation
9.11. Low Molecular Weight Heparin (LMWH)
9.12. Low Doses of Acetylsalicylic Acid
9.13. Vitamin D
9.14. Progesterone
9.15. Intrauterine Human Chorionic Gonadotropin (hCG) Infusion
9.16. Anti-Obesity Drugs to Increase Fertility.
10. Future Perspectives
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tomkiewicz, J.; Darmochwał-Kolarz, D The Diagnostics and Treatment of Recurrent Pregnancy Loss. J Clin Med. 2023;12(14):4768. [CrossRef]
- ESHRE Guideline Group on R.P.L, Bender Atik, R.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; et al. ESHRE guideline: recurrent pregnancy loss. Hum. Reprod. Open, 2018 (2018): 1-12. [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103-11. [CrossRef]
- WHO: Recommended Definitions, Terminology, and Format for Statistical Tables Related to The Perinatal Period And Use of A New Certificate For the Cause of Perinatal Deaths. Acta Obstet Gynecol Scand 1977, 56: 247-256. [CrossRef]
- Dimitriadis, E.; Menkhorst, E.; Saito, S.; Kutteh, W.H.; Brosens, J.J.. Recurrent pregnancy loss. Nat Rev Dis Primers. 2020; 6(1):98. [CrossRef]
- Dong, P.; Wen, X.Z.; Liu, J.; Yan, C.; Yuan, J.; Luo, L.; Hu, Q.F.; Li J. Simultaneous detection of decidual Th1/Th2 and NK1/NK2 immunophenotyping in unknown recurrent miscarriage using 8-color flow cytometry with FSC/Vt extended strategy. Biosci Rep 2017;37(3): BSR20170150. [CrossRef]
- Kohl Schwartz, A.S.; Wölfler, M.M.; Mitter, V.; Rauchfuss, M.; Haeberlin, F.; Eberhard, M.; von Orelli, S.; Imthurn, B.; Imesch, P.; Fink, D.; Leeners, B. Endometriosis, especially mild disease: a risk factor for miscarriages. Fertil Steril. 2017;108(5), pp.806-814.e2. [CrossRef]
- Harb, H.M.; Ghosh, J.; Al-Rshoud. F.; Karunakaran, B.; Gallos, I.D.; Coomarasamy, A. Hydrosalpinx and pregnancy loss: a systematic review and meta-analysis. Reprod Biomed Online. 2019;38(3):427–441. [CrossRef]
- Zhang, L.; Li, H.; Han, L.; Zhang, L.; Zu, Z,; Zhang, J. Association between semen parameters and recurrent pregnancy loss: An umbrella review of meta-analyses. J Obstet Gynaecol Res. 2024;50(4):545-556 . [CrossRef]
- Deshmukh, H.; Way, S.S. Immunological Basis for Recurrent Fetal Loss and Pregnancy Complications. Annu Rev Pathol. 2019;14(1):185–210. [CrossRef]
- Bagkou Dimakou, D.; Lissauer, D.; Tamblyn, J.; Coomarasamy, A.; Richter, A. Understanding human immunity in idiopathic recurrent pregnancy loss. Eur J Obstet Gynecol Reprod Biol. 2022; 270:17–29. [CrossRef]
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol and Endocrinol. 2018;16(1):121. [CrossRef]
- Comins Boo, A.; Segovia, A.G.; del Prado, N.N.; de la Fuente, L; Alonso, J.; Ramon, S.S. Evidence-based Update: Immunological Evaluation of Recurrent Implantation Failure. Reprod Immunol: Open Access. 2016; 01(04). [CrossRef]
- Wu, Y.; Li, L.; Liu, L.; Yang, X.; Yan, P.; Yang, K.; Zhang, X. Autologous peripheral blood mononuclear cells intrauterine instillation to improve pregnancy outcomes after recurrent implantation failure: a systematic review and meta-analysis. Arch Gynecol Obstet. 2019;300(5):1445–1459. [CrossRef]
- Wu, H.; You, Q.; Jiang, Y.; Mu, F. Tumor necrosis factor inhibitors as therapeutic agents for recurrent spontaneous abortion Mol Med Rep. 2021;24(6):847. [CrossRef]
- Saadaoui, M,; Singh, P.; Ortashi, O.; Al Khodor, S. Role of the vaginal microbiome in miscarriage: exploring the relationship. Front Cell Infect Microbiol. 2023;13:1232825. [CrossRef]
- Mrozikiewicz, A.E.; Ożarowski, M.; Jędrzejczak, P. Biomolecular Markers of Recurrent Implantation Failure—A Review. Int J Mol Sci. 2021;22(18):10082. [CrossRef]
- Wang, Q.; Sun, Y.; Fan, R.; Wang, M.; Ren, C.; Jiang, A.; Yang, T. Role of inflammatory factors in the etiology and treatment of recurrent implantation failure. Reprod Biol. 2022;22(4):100698. [CrossRef]
- Ma, J.; Gao, W.; Li, D. Recurrent implantation failure: A comprehensive summary from etiology to treatment. Frontiers Endocrinol, 2023; 13, 1061766. [CrossRef]
- Fathi, M.; Omrani, M. A.; Kadkhoda, S.; Ghahghaei-Nezamabadi, A.; Ghafouri-Fard, S. Impact of miRNAs in the pathoetiology of recurrent implantation failure. Mol cell probes, 2024; 74, 101955. DOI: 10.1016/j.mcp.2024.101955.
- Liu, L.; Liu, Y.; Tian, Y.; Cao, Y.; Wang, T.; Mi, S.; Yang, R.; Liu, S.; Ma, X.; Wang, J. Identification of Differentially Expressed mRNAs and lncRNAs Contributes to Elucidation of Underlying Pathogenesis and Therapeutic Strategy of Recurrent Implantation Failure. Reproductive sciences, 2024; 10.1007/s43032-024-01630-8.
- Zahir, M.; Tavakoli, B.; Zaki-Dizaji, M.; Hantoushzadeh, S.; Majidi Zolbin, M. Non-coding RNAs in Recurrent implantation failure. Clinica chimica acta; 2024, 553, 117731. [CrossRef]
- Colamatteo, A.; Fusco, C.; Micillo, T.; D'Hooghe, T.; de Candia, P.; Alviggi, C.; Longobardi, S.; Matarese, G. Immunobiology of pregnancy: from basic science to translational medicine. Trends Mol Med, 2023; 29(9), 711–725. [CrossRef]
- Zhao, F.; Hu, X.; Ying C. Advances in Research on the Relationship between Vaginal Microbiota and Adverse Pregnancy Outcomes and Gynecological Diseases. Microorganisms. 2023;11(4):991. /. [CrossRef]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 2016;215(6):684–703. [CrossRef]
- Garmendia, J.V.; De Sanctis, C.V.; Hajdúch, M.; De Sanctis, J.B. Microbiota and Recurrent Pregnancy Loss (RPL); More than a Simple Connection. Microorganisms. 2024 Aug 10;12(8):1641. [CrossRef]
- Jia, D.; Sun, F.; Han, S.; Lu, L.; Sun, Y.; Song, Q. Adverse outcomes in subsequent pregnancies in women with a history of recurrent spontaneous abortion: A meta-analysis. J Obstet Gynaecol Res. 2024; 50(3):281-297 . [CrossRef]
- Field, K.; Murphy, D.J. Perinatal outcomes in a subsequent pregnancy among women who have experienced recurrent miscarriage: a retrospective cohort study. Hum Reprod. 2015;30(5):1239–1245. [CrossRef]
- Fang, Y.; Jingjing, F.; Tiantain, C.; Huanhuan, X.; Qiaohua H. Impact of the number of previous embryo implantation failures on IVF/ICSI-ET pregnancy outcomes in patients younger than 40 years: a retrospective cohort study. Front Endocrinol. 2023;14: 1243402. [CrossRef]
- Cimadomo, D.; Rienzi, L.; Conforti, A.; Forman, E.; Canosa, S.; Innocenti, F.; et al. Opening the black box: why do euploid blastocysts fail to implant? A systematic review and meta-analysis. Hum Reprod Update. 2023;29(5):570–633. [CrossRef]
- Nitu, R.; Neamtu, R.; Lordache, O.; Stelea, L.; Dahma, G.; Sacarin, G.; et al. Cross-Sectional Analysis of Intimacy Problems, Stress Levels, and Couple Satisfaction among Women with Thrombophilia Affected by Recurrent Pregnancy Loss. Int J Environ Res Public Health. 2023;20(2);1208. [CrossRef]
- Chen, S.; Chang, S.; Kuo, P.; Chen, C. Stress, anxiety and depression perceived by couples with recurrent miscarriage. Int J Nurs Pract. 2020;26(2): e12796. [CrossRef]
- Quenby, S.; Gallos, I.D.; Dhillon-Smith, R.K.; Podesek, M.; Stephenson, M.D.; Fisher, J.; et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397(10285):1658–1667. [CrossRef]
- Voss, P.; Schick, M.; Langer, L.; Ainsworth, A.; Ditzen, B.; Strowitzki, T.; Wischmann, T.; Kuon, R.J. Recurrent pregnancy loss: a shared stressor---couple-orientated psychological research findings. Fertil Steril. 2020; 114(6):1288–1296. 10.1016/j.fertnstert.2020.08.1421.
- Mínguez-Alarcón, L.; Williams, P. L.; Souter, I.; Ford, J.B.; Hauser, R.; Chavarro, J.E. Women's preconception psychological stress and birth outcomes in a fertility clinic: the EARTH study. Front Glob Womens Health. 2024 ;5:1293255. [CrossRef]
- Garmendia, J.V.; De Sanctis, J.B. A Brief Analysis of Tissue-Resident NK Cells in Pregnancy and Endometrial Diseases: The Importance of Pharmacologic Modulation. Immuno. 2021, 1(3):174–193. [CrossRef]
- Lanier L. L. Five decades of natural killer cell discovery. J Exper Med 2024, 221(8), e20231222. [CrossRef]
- Rao, V.A.; Kurian, N.K.; Rao, K.A. Cytokines, NK cells, and regulatory T cell functions in normal pregnancy and reproductive failures. Am J Reprod Immunol. 2023;89(2): e13667. [CrossRef]
- Cavalcante, M.B.; da Silva, P.H.A.; Carvalho, T.R.; Sampaio, O.G.M.; Câmara, F.E.A.; et al. Peripheral blood natural killer cell cytotoxicity in recurrent miscarriage: a systematic review and meta-analysis. J Reprod Immunol. 2023;158:103956. [CrossRef]
- Sacks, G.; Yang, Y.; Gowen, E.; Smith, S.; Fay, L.; Chapman, M. Detailed Analysis of Peripheral Blood Natural Killer Cells in Women with Repeated IVF Failure. Am J Reprod Immunol. 2012;67(5):434–442. [CrossRef]
- Cai, J. Y.; Tang, Y. Y.; Deng, X. H.; Li, Y. J.; Liang, G.; Meng, Y. Q.; Zhou, H. Recurrent Implantation Failure May Be Identified by a Combination of Diagnostic Biomarkers: An Analysis of Peripheral Blood Lymphocyte Subsets. Front Endocrinol, 2024; 13, 865807. [CrossRef]
- Sacks, G. Enough! Stop the arguments and get on with the science of natural killer cell testing. Hum Reprod. 2015;30(7):1526–1531. [CrossRef]
- Dons’koi, B.V. Accentuated hypo- and hyper-NK lymphocyte CD8 expression is a marker of NK subsets’ misbalance and is predictive for reproductive failures. Immunobiol. 2015; 220(5):649–655. [CrossRef]
- Dons’koi, B.V.; Chernyshov, V.P.; Sirenko, V.Y.; Strelko, G.V.; Osypchuk, D.V. Peripheral blood natural killer cells activation status determined by CD69 upregulation predicts implantation outcome in IVF. Immunobiol 2014;219(3):167–171. [CrossRef]
- Gothe, J. P.; de Mattos, A. C.; Silveira, C. F.; Malavazi, K. C. Exploring Natural Killer Cell Testing in Embryo Implantation and Reproductive Failure: An Overview of Techniques and Controversies. Reproductive Sciences 2024; 31(3), 603–632. [CrossRef]
- Zhang, J.; Lye, S. J. The immune potential of decidua-resident CD16+CD56+ NK cells in human pregnancy. Human Immunol, 2021; 82(5), 332–339. [CrossRef]
- Salazar, M. D.; Wang, W. J.; Skariah, A.; He, Q.; Field, K.; Nixon, M.; et al. Post-hoc evaluation of peripheral blood natural killer cell cytotoxicity in predicting the risk of recurrent pregnancy losses and repeated implantation failures. J Reprod Immunol, 2022: 150, 103487. [CrossRef]
- Singh, N.; Dogra, Y.; Kumar, P.; Mathur, S.; Sharma, A.; Patel, G. Establishment of Cut-off Values for Uterine and Peripheral Blood Natural Killer Cells During the Peri-implantation Period in Fertile Controls and Women with Unexplained Recurrent Implantation Failure. J Reprod Infert, 2023; 24(4), 248–256. [CrossRef]
- Santillán, I.; Fernández Lozano, I.; Illán. J.; Verdú, V.; Coca, S.; Bajo-Arenas, J.; Martinez F. Where and when should natural killer cells be tested in women with repeated implantation failure? Journal Reprod Immunol. 2015 ;108:142–148. [CrossRef]
- Sfakianoudis, K.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Maziotis, E.; et al. The Role of Uterine Natural Killer Cells on Recurrent Miscarriage and Recurrent Implantation Failure: From Pathophysiology to Treatment. Biomed. 2021; 9(10):1425. [CrossRef]
- Bagkou Dimakou, D.; Tamblyn, J.; Justin, C.; Coomarasamy, A.; Richter, A. Diagnosis and management of idiopathic recurrent pregnancy loss (RPL): Current immune testing and immunomodulatory treatment practice in the United Kingdom. J Reprod Immunol, 2022; 153, 103662. [CrossRef]
- Seshadri, S.; Sunkara, S.K. Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis. Hum Reprod Update. 2013;20(3):429–438. [CrossRef]
- Lachapelle, M.; Miron, P.; Hemmings, R.; Roy, D. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J Immunol. 1996;156(10):4027–34.
- Ho, Y. K.; Chen, H. H.; Huang, C. C.; Lee, C. I.; Lin, P. Y.; Lee, M. S.; Lee, T. H. Peripheral CD56+CD16+ NK Cell Populations in the Early Follicular Phase Are Associated With Successful Clinical Outcomes of Intravenous Immunoglobulin Treatment in Women With Repeated Implantation Failure. Front Endocrinol, 2020; 10, 937. [CrossRef]
- Fukui, A.; Fujii, S.; Yamaguchi, E.; Kimura, H.; Sato, S.; Saito, Y. Natural Killer Cell Subpopulations and Cytotoxicity for Infertile Patients Undergoing In Vitro Fertilization. Am J Reprod Immunol. 1999;41(6): 413–422. [CrossRef]
- Strobel, L.; Vomstein, K.; Kyvelidou, C.; Hofer-Tollinger, S.; Feil, K.; Kuon, R. J.; Ebner, S.; Troppmair, J.; Toth, B. Different Background: Natural Killer Cell Profiles in Secondary versus Primary Recurrent Pregnancy Loss. J Clin Med, 2021; 10(2), 194. [CrossRef]
- Fukui, A.; Kwak-Kim, J.; Ntrivalas, E.; Gilman-Sachs, A.; Lee, S.K.; Beaman, K. Intracellular cytokine expression of peripheral blood natural killer cell subsets in women with recurrent spontaneous abortions and implantation failures. Fertil Steril. 2008;89(1):157–165. [CrossRef]
- Díaz-Peña, R.; de Los Santos, M. J.; Lucia, A.; Castro-Santos, P. Understanding the role of killer cell immunoglobulin-like receptors in pregnancy complications. J Assisted Reprod genetics, 2019, 36(5), 827–835. [CrossRef]
- Lin, Q.D.; Qiu, L.H. Pathogenesis, diagnosis, and treatment of recurrent spontaneous abortion with immune type. Front Med China. 2010;4(3):275–279. [CrossRef]
- Dambaeva, S. V.; Lee, D. H.; Sung, N.; Chen, C. Y.; Bao, S.; Gilman-Sachs, A.; Kwak-Kim, J.; Beaman, K. D. (2016). Recurrent Pregnancy Loss in Women with Killer Cell Immunoglobulin-Like Receptor KIR2DS1 is Associated with an Increased HLA-C2 Allelic Frequency. Am J Reprod Immunol. 2016; 75(2), 94–103. [CrossRef]
- Akbari, S.; Shahsavar, F.; Karami, R.; Yari, F.; Anbari, K.; Ahmadi, S.A.Y. Recurrent Spontaneous Abortion (RPL) and Maternal KIR Genes: A Comprehensive Meta-Analysis. JBRA Assist Reprod 2020;24(2), 197–213. [CrossRef]
- Yang, X.; Yang, E.; Wang, W.; He, Q.; Jubiz, G.; Dimantha Katukurundage, Dambaeva S.; Beaman, K.D.; Kwak-Kim, J. Decreased HLA-C1 alleles in couples of KIR2DL2 positive women with recurrent pregnancy loss. J Reprod Immunol. 2020 ;142:103186–103186. [CrossRef]
- Feyaerts, D.; Benner, M.; Comitini, G;, Shadmanfar, W.; van der Heijden, O. W. H.; Joosten, I.; van der Molen, R. G. NK cell receptor profiling of endometrial and decidual NK cells reveals pregnancy-induced adaptations. Front Immunol, 2024 15, 1353556. [CrossRef]
- Maftei, R.; Doroftei, B.; Popa, R.; Harabor, V.; Adam, A.M.; Popa, C.; et al. The Influence of Maternal KIR Haplotype on the Reproductive Outcomes after Single Embryo Transfer in IVF Cycles in Patients with Recurrent Pregnancy Loss and Implantation Failure—A Single Center Experience. J Clin Med. 2023;12(5):1905. [CrossRef]
- Nowak, I.; Wilczyńska, K.; Wilczyński, J.R.; Malinowski, A.; Radwan, P.; Radwan, M.; Kuśnierczyk, P. KIR, LILRB and their Ligands’ Genes as Potential Biomarkers in Recurrent Implantation Failure. Arch Immunol Ther Exp (Warsz). 2017;65(5):391-399 (2017). [CrossRef]
- Braun, A. S.; Vomstein, K.; Reiser, E.; Tollinger, S.; Kyvelidou, C.; Feil, K.; Toth, B. NK and T Cell Subtypes in the Endometrium of Patients with Recurrent Pregnancy Loss and Recurrent Implantation Failure: Implications for Pregnancy Success. J Clin Med, 2023; 12(17), 5585. [CrossRef]
- Morin, S. J.; Treff, N. R.; Tao, X.; Scott, R. T.; 3rd, Franasiak, J. M.; Juneau, C. R.; Maguire, M.; Scott, R. T. Combination of uterine natural killer cell immunoglobulin receptor haplotype and trophoblastic HLA-C ligand influences the risk of pregnancy loss: a retrospective cohort analysis of direct embryo genotyping data from euploid transfers. Fertility and sterility, 2017; 107(3), 677–683.e2. [CrossRef]
- Xu, Q. H.; Liu, H.; Wang, L. L.; Zhu, Q.; Zhang, Y. J.; Muyayalo, K. P.; Liao, A. H. Roles of γδT cells in pregnancy and pregnancy-related complications. Am J Reprod Immunol. 2021; 86(5), e13487. [CrossRef]
- Li, L.; Liu, Y.; Zhou, W.; Yang, C.; Feng, T.; Li, H. Human chorionic gonadotrophin indirectly activates peripheral γδT cells to produce interleukin-10 during early pregnancy. Immun Inflamm Dis, 2024 12(1), e1119. [CrossRef]
- Zhang, D.; Yu, Y.; Duan, T.; Zhou, Q. The role of macrophages in reproductive-related diseases. Heliyon. 2022;8(11):e11686–e11686. [CrossRef]
- Nagamatsu, T.; Schust, D.J. The Contribution of Macrophages to Normal and Pathological Pregnancies. Am J Reprod Immunol. 2010;63(6):460–471. [CrossRef]
- Tsao, F.Y.; Wu, M.Y.; Chang, Y.L.; Wu, C.T.; Ho H.N. M1 macrophages decrease in the deciduae from normal pregnancies but not from spontaneous abortions or unexplained recurrent spontaneous abortions. J Formo Med Assoc. 2018;117(3):204–211. [CrossRef]
- Robertson, S.A.; Moldenhauer, L.M.; Green, E.S.; Care, A.S.; Hull, M.L. Immune determinants of endometrial receptivity: a biological perspective. Fertil Steril. 2022;117(6):1107–1120. [CrossRef]
- Wang, W.J.; Hao, C.F.; Lin, Q.D. Dysregulation of macrophage activation by decidual regulatory T cells in unexplained recurrent miscarriage patients. J Reprod Immunol. 2011;92(1-2):97-102. [CrossRef]
- Quenby, S.; Bates, M.; Doig, T.; Brewster, J.; Lewis-Jones, D.I.; Johnson, P.M.; Vince, G. Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Hum Reprod 1999;14(9):2386–2391. [CrossRef]
- Krop, J.; Tian, X.; van der Hoorn, M. L.; Eikmans, M. The Mac Is Back: The Role of Macrophages in Human Healthy and Complicated Pregnancies. Int J Mol Sci, 2023; 24(6), 5300. [CrossRef]
- Tremellen, K.P.; Russell, P. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. II: adenomyosis and macrophages. J Reprod Immunol. 2012;93(1):58–63.
- Wei, R.; Lai, N.; Zhao, L.; Zhang, Z.; Zhu, X.; Guo, Q.; Chu, C.; Fu, X.; Li, X. Dendritic cells in pregnancy and pregnancy-associated diseases. Biomed Pharmacother 2021; 133, 110921. [CrossRef]
- Saito, S. Role of immune cells in the establishment of implantation and maintenance of pregnancy and immunomodulatory therapies for patients with repeated implantation failure and recurrent pregnancy loss. Reprod Med Biol. 2024;23(1):e12600. [CrossRef]
- Liu, S.; Wei, H., Li, Y.; Huang, C.; Lian, R.; Xu, J.; Chen, L.; Zeng, Y. Downregulation of ILT4+dendritic cells in recurrent miscarriage and recurrent implantation failure. Am J Reprod Immunol. 2018;80(4):e12998. [CrossRef]
- Zhu, X.X.; Yin, X.Q.; Hei, G.Z.; Wei, R.; Guo, Q.; Zhao, L.; Zhang, Z.; Chu, C.; Fu, X.X.; Xu, K.; Li, X. Increased miR-6875-5p inhibits plasmacytoid dendritic cell differentiation via the STAT3/E2-2 pathway in recurrent spontaneous abortion. Mol Hum Reprod. 2021;27(8):gaab044. [CrossRef]
- Huang, C.; Zhang, H.; Chen, X.; Diao, L.; Lian, R.; Zhang, X.; Hu, L.; Zeng, Y. Association of peripheral blood dendritic cells with recurrent pregnancy loss: a case-controlled study. Am J Reprod Immunol. 2016;76(4):326-32. [CrossRef]
- Kwiatek, M.; Gęca, T.; Krzyżanowski, A.; Malec, A.; Kwaśniewska, A. Peripheral Dendritic Cells and CD4+CD25+Foxp3+ Regulatory T Cells in the First Trimester of Normal Pregnancy and in Women with Recurrent Miscarriage. PLoS One. 2015;10(5):e0124747. [CrossRef]
- Sivridis, E.; Giatromanolaki, A.; Agnantis, N.; Anastasiadis, P. Mast cell distribution and density in the normal uterus--metachromatic staining using lectins. Europ J Obst Gynecol Reprod Biol, 2001 98(1), 109–113. [CrossRef]
- Norrby, K. On Connective Tissue Mast Cells as Protectors of Life, Reproduction, and Progeny. Int J Mol Sci, 2024; 25(8), 4499. [CrossRef]
- Lampiasi, N. Interactions between Macrophages and Mast Cells in the Female Reproductive System. Int J Mol Sci. 2022 May 12;23(10):5414. [CrossRef]
- Derbala, Y.; Elazzamy, H.; Bilal, M.; Reed R.; Salazar Garcia, M,D,; Skariah, A.; et al. Mast cell-induced immunopathology in recurrent pregnancy losses. Am J Reprod Immunol. 2019 Jul;82(1):e13128. [CrossRef]
- McCallion, A.; Nasirzadeh, Y.; Lingegowda, H.; Miller, J.E.; Khalaj, K.; Ahn, S.; et al. Estrogen mediates inflammatory role of mast cells in endometriosis pathophysiology. Front Immunol. 2022 Aug 9;13:961599. [CrossRef]
- Dunn, T.N.; Cope, D.I.; Tang, S.; Sirupangi, T.; Parks, S.E.; et al. . Inhibition of CSF1R and KIT With Pexidartinib Reduces Inflammatory Signaling and Cell Viability in Endometriosis. Endocrinology. 2024; 165(4):bqae003. [CrossRef]
- Blumenthal, R. D.; Samoszuk, M.; Taylor, A. P.; Brown, G.; Alisauskas, R.; Goldenberg, D. M. Degranulating eosinophils in human endometriosis. Am J Pathol, 2020; 156(5), 1581–1588. [CrossRef]
- Hornung, D.; Dohrn, K.; Sotlar, K.; Greb, R. R.; Wallwiener, D.; Kiesel, L.; Taylor, R. N. Localization in tissues and secretion of eotaxin by cells from normal endometrium and endometriosis. J Clin Endocrinol Met 2000; 85(7), 2604–2608. [CrossRef]
- Naseri, S.; Rosenberg-Hasson, Y.; Maecker, H. T.; Avrutsky, M. I.; Blumenthal, P. D. A cross-sectional study comparing the inflammatory profile of menstrual effluent vs. peripheral blood. Health Sci Rep, 2023; 6(1), e1038. [CrossRef]
- Wang, X.; Jia, Y.; Li, D.; Guo, X.; Zhou, Z.; Qi, M.; Wang, G.; Wang, F. The Abundance and Function of Neutrophils in the Endometriosis Systemic and Pelvic Microenvironment. Mediators Inflamm. 2023 Jan 31;2023:1481489.
- Hebeda, C. B.; Savioli, A. C.; Scharf, P.; de Paula-Silva, M.; Gil, C. D.; Farsky, S. H. P.; Sandri, S. Neutrophil depletion in the pre-implantation phase impairs pregnancy index, placenta and fetus development. Front Immunol, 2022; 13, 969336. [CrossRef]
- Ghafourian, M.; Abuhamidy, A.; Karami, N. Increase of peripheral blood TCD8+cells in women with recurrent miscarriage. J Obstet Gynaecol. 2013;34(1):36–39. [CrossRef]
- Morita, K.; Tsuda, S.; Kobayashi, E.; Hamana, H.; Tsuda, K.; Shima, T.; Nakashima, A.; Ushijima, A.; Kishi, H.; Saito, S. Analysis of TCR Repertoire and PD-1 Expression in Decidual and Peripheral CD8+ T Cells Reveals Distinct Immune Mechanisms in Miscarriage and Preeclampsia. Front Immunol. 2020; 11:1082. [CrossRef]
- Carbone, J.; Sarmiento, E.; Gallego, A.; Lanio, N.; Navarro, J.; Garcia, S.; Fernández-Cruz, E. Peripheral blood T- and B-cell immunophenotypic abnormalities in selected women with unexplained recurrent miscarriage. J Reprod Immunol. 2016; 113:50–53. [CrossRef]
- Huang, C.; Xiang, Z.; Zhang, Y.; Li, Y.; Xu, J.; Zhang, H.; Zeng, Y.; Tu, W. NKG2D as a Cell Surface Marker on γδ-T Cells for Predicting Pregnancy Outcomes in Patients With Unexplained Repeated Implantation Failure. Front Immunol, 2021; 12, 631077. [CrossRef]
- Yu, L.; Wang, L.; Wang, L.; Yan, S.; Chen, S.; Xu, Q.; Su, D.; Wang, X. Identification and validation of immune cells and hub genes alterations in recurrent implantation failure: A GEO data mining study. Front Genet. 2023 Jan 9;13:1094978. [CrossRef]
- Wang, X.; Ma, Z.; Hong, Y.; Zhao, A.; Qiu, L.; Lu, P.; Lin,, Q. The Skewed TCR-BV Repertoire Displayed at the Maternal-Fetal Interface of Women with Unexplained Pregnancy Loss. Am J Reprod Immunol. 2005;54(2):84–95.
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Invest. 2018 Oct 1;128(10):4224-4235. [CrossRef]
- Yang, H.; Qiu, L.; Chen, G.; Ye, Z.; Lü, C.; Lin, Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2008;89(3):656–661.
- Li, Q. H.; Zhao, Q. Y.; Yang, W. J.; Jiang, A. F.; Ren, C. E.; Meng, Y. H. Beyond Immune Balance: The Pivotal Role of Decidual Regulatory T Cells in Unexplained Recurrent Spontaneous Abortion. J Inflamm Res 2024, 17, 2697–2710. [CrossRef]
- Wang, W.J.; Hao, C.F.; Qu, Q.L.; Wang, X.; Qiu, L.H.; Lin, Q.D. The deregulation of regulatory T cells on interleukin-17-producing T helper cells in patients with unexplained early recurrent miscarriage. Hum Reprod 2010; 25(10):2591–2596. [CrossRef]
- Garmendia, J.V.; Blanca, I.; Peña, M.J.; De Sanctis, C.V.; De Sanctis, J.B. Unlocking the Puzzle: Investigating the Role of Interleukin 17 Genetic Polymorphisms, Circulating Lymphocytes, and Serum Levels in Venezuelan Women with Recurrent Pregnancy Loss. Immuno 2024; 4: 301–311. [CrossRef]
- Heitmann, R. J.; Weitzel, R. P.; Feng, Y.; Segars, J. H.; Tisdale, J. F.; Wolff, E. F. Maternal T Regulatory Cell Depletion Impairs Embryo Implantation Which Can Be Corrected With Adoptive T Regulatory Cell Transfer. Reproductive sciences 2017; 24(7), 1014–1024. [CrossRef]
- Granne, I.; Shen, M.; Rodriguez-Caro, H.; Chadha, G.; O'Donnell, E.; Brosens, J.J.; Quenby, S.; Child, T.; Southcombe, J.H. Characterisation of peri-implantation endometrial Treg and identification of an altered phenotype in recurrent pregnancy loss. Mucosal Immunol. 2022 Jan;15(1):120-129. [CrossRef]
- Moldenhauer, L.M.; Foyle, K.L.; Wilson, J.J.; Wong, Y.Y.; Sharkey. D.J.; Green, E.S.; Barry, S.C.; Hull, M.L.; Robertson, S.A. A disrupted FOXP3 transcriptional signature underpins systemic regulatory T cell insufficiency in early pregnancy failure. iScience. 2024;27(2):108994. [CrossRef]
- Winger, E.E.; Reed, J.L. Low Circulating CD4+ CD25+ Foxp3+ T Regulatory Cell Levels Predict Miscarriage Risk in Newly Pregnant Women with a History of Failure. Am J Reprod Immunol. 2011;66(4):320–328. [CrossRef]
- Jin, L.P.; Chen, Q.Y.; Zhang, T.; Guo, P.F.; Li, D.J. The CD4+CD25 bright regulatory T cells and CTLA-4 expression in peripheral and decidual lymphocytes are down-regulated in human miscarriage. Clin Immunol. 2009;133(3):402–410. [CrossRef]
- Tang, C.; Hu, W. The role of Th17 and Treg cells in normal pregnancy and unexplained recurrent spontaneous abortion (URSA): New insights into immune mechanisms. Placenta. 2023;142:18-26. [CrossRef]
- Farshchi, M.; Abdollahi, E.; Saghafi, N.; Hosseini, A.; Fallahi, S.; Rostami, S.; Rostami, P.; Rafatpanah, H.; Habibagahi, M. Evaluation of Th17 and Treg cytokines in patients with unexplained recurrent pregnancy loss. J Clin Transl Res. 2022 May 25;8(3):256-265.
- Ma, J.; Gao, W.; Li, D. Recurrent implantation failure: A comprehensive summary from etiology to treatment. Front Endocrinol, 2023; 13, 1061766. [CrossRef]
- Berdiaki, A.; Vergadi, E.; Makrygiannakis, F.; Vrekoussis, T.; Makrigiannakis, A. Title: Repeated implantation failure is associated with increased Th17/Treg cell ratio, during the secretory phase of the human endometrium. J Reprod Immunol. 2024 Feb;161:104170. [CrossRef]
- Niafar, M.; Samaie, V.; Soltani-Zangbar, M.S.; Motavalli, R.; Dolati, S.; Danaii, S.; Mehdizadeh, A.; Yousefi, M. The association of Treg and Th17 cells development factors and anti-TPO autoantibodies in patients with recurrent pregnancy loss. BMC Res Notes. 2023;16(1):302. [CrossRef]
- Wang, W.J.; Salazar Garcia, M.D.; Deutsch, G.; Sung, N.; Yang, X.; et al. PD-1 and PD-L1 expression on T-cell subsets in women with unexplained recurrent pregnancy losses. Am J Reprod Immunol. 2020 May;83(5):e13230. [CrossRef]
- Wang, W.; Sung, N;, Gilman-Sachs, A.; Kwak-Kim, J. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front Immunol, 2020; 11, 2025. [CrossRef]
- Weng, J.; Couture, C.; Girard, S. Innate and Adaptive Immune Systems in Physiological and Pathological Pregnancy. Biology (Basel). 2023;12(3):402. [CrossRef]
- Muzzio, D.; Zenclussen, A.C.; Jensen, F. The Role of B Cells in Pregnancy: the Good and the Bad. Am J Reprod Immunol. 2013;69(4):408–412. [CrossRef]
- Eblen, A.C.; Gercel-Taylor, C.; Shields, L.B.E.; Sanfilippo, J.S.; Nakajima, S.T.; Taylor, D.D. Alterations in humoral immune responses associated with recurrent pregnancy loss. Fertil Steril. 2000;73(2):305–313. [CrossRef]
- Marron, K.; Walsh, D.; Harrity, C. Detailed endometrial immune assessment of both normal and adverse reproductive outcome populations. J Assisted Reprod Genetics, 2019; 36(2), 199–210. [CrossRef]
- Vujisić, S.; Lepej, S.Ž.; Akšamija, A.; Jerković. L.; Sokolić, B.; Kupešić, S.; Vince, A. B- and T-cells in the Follicular Fluid and Peripheral Blood of Patients Undergoing IVF/ET Procedures. Am J Reprod Immunol. 2004; 52(6):379–385. [CrossRef]
- Liu, J. C.; Zeng, Q.; Duan, Y. G.; Yeung, W. S. B.; Li, R. H. W.; Ng, E. H. Y.; Cheung, K. W.; Zhang, Q.; Chiu, P. C. N. B cells: roles in physiology and pathology of pregnancy. Front Immunol, 2024 15, 1456171. [CrossRef]
- Danaii, S.; Ghorbani, F.; Ahmadi, M.; Abbaszadeh, H.; Koushaeian, L.; Soltani-Zangbar, M. S.; et al. IL-10-producing B cells play important role in the pathogenesis of recurrent pregnancy loss. Internat Immunopharmacol, 2020; 87, 106806. [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7(1):12150. [CrossRef]
- Ostrand-Rosenberg, S.; Sinha, P.; Figley, C.; Long, R.; Park, D.; Carter, D.; Clements, V.K. Frontline Science: Myeloid-derived suppressor cells (MDSCs) facilitate maternal–fetal tolerance in mice. J Leukoc Biol. 2016;101(5):1091–1101. [CrossRef]
- Köstlin, N.; Hofstädter, K.; Ostermeir, A.L.; Spring, B.; Leiber, A.; et al. Granulocytic Myeloid-Derived Suppressor Cells Accumulate in Human Placenta and Polarize toward a Th2 Phenotype. J Immunol. 2016;196(3):1132-45. [CrossRef]
- Bartmann, C.; Junker, M.; Segerer, S.E.; Häusler, S.F.; Krockenberger, M.; Kämmerer, U. CD33+/HLA-DRnegand CD33+/HLA-DR+/−Cells: Rare Populations in the Human Decidua with Characteristics of MDSC. Am J Reprod Immunol. 2016;75(5):539–556. [CrossRef]
- Pan, T.; Zhong, L.; Wu, S.; Cao, Y.; Yang, Q.; Cai, Z.; et al. 17β-Oestradiol enhances the expansion and activation of myeloid-derived suppressor cells via signal transducer and activator of transcription (STAT)-3 signalling in human pregnancy. Clin Exp Immunol. 2016;185(1):86-97. [CrossRef]
- Li, C.; Zhang, X.; Kang, X.; Chen, C.; Guo, F.; Wang, Q.; Zhao, A. Upregulated TRAIL and Reduced DcR2 Mediate Apoptosis of Decidual PMN-MDSC in Unexplained Recurrent Pregnancy Loss. Front Immunol. 2020;11: 1345. [CrossRef]
- Jiang, H.; Zhu, M.; Guo, P.; Bi, K.; Lu, Z.; Li, C.; Zhai, M.; Wang, K.; Cao, Y. Impaired myeloid-derived suppressor cells are associated with recurrent implantation failure: A case-control study. J Reprod Immunol. 2021 ;145:103316–103316. [CrossRef]
- Marin, N.S.; Fuente-Muñoz, E.; Gil-Laborda, R.; Villegas, Á.; Alonso-Arenilla, B.; et al. Myeloid-derived suppressor cells as a potential biomarker for recurrent pregnancy loss and recurrent implantation failure. Am J Reprod Immunol. 2023;90(5):e13783. [CrossRef]
- Pantos, K.; Grigoriadis, S.; Maziotis, E.; Pistola K.; Xystra, P.; Pantou, A.; et al. The Role of Interleukins in Recurrent Implantation Failure: A Comprehensive Review of the Literature. Int J of Mol Sci. 2022;23(4):2198. [CrossRef]
- Dong, X.; Zhou, M.; Li, X.; Huang, H.; Sun, Y. Gene profiling reveals the role of inflammation, abnormal uterine muscle contraction and vascularity in recurrent implantation failure. Front Genet 2023;14: 1108805 . [CrossRef]
- Kalu, E.; Bhaskaran, S.; Thum, M.Y.; Vishwanatha, R.; Croucher, C.; Sherriff, E.; Ford, B.; Bansal, A.S. Serial Estimation of Th1:Th2 Cytokines Profile in Women Undergoing In-Vitro Fertilization-Embryo Transfer. Am J Reprod Immunol. 2008;59(3):206–211. [CrossRef]
- Piekarska, K..; Dratwa, M.; Radwan, P.; Radwan, M.; Bogunia-Kubik, K.; Nowak, I. Pro- and anti-inflammatory cytokines and growth factors in patients undergoing in vitro fertilization procedure treated with prednisone. Front Immunol, 2023; 14, 1250488. [CrossRef]
- Mukherjee, N.; Sharma, R.; Modi, D. Immune alterations in recurrent implantation failure. Am J Reprod Immunol 2022;89(2). [CrossRef]
- Guo, L.; Guo, A.; Yang, F.; Li, L.; Yan, J.; Deng, X.; Dai, C.; Li, Y. Alterations of Cytokine Profiles in Patients With Recurrent Implantation Failure. Front Endocrinol. 2022 ;13:949123. [CrossRef]
- Yang, X,; Tian, Y.; Zheng, L.; Luu, T.; Kwak-Kim, J. The Update Immune-Regulatory Role of Pro- and Anti-Inflammatory Cytokines in Recurrent Pregnancy Losses. Int J Mol Sci. 2023;24(1):132. [CrossRef]
- Kwak-Kim, J.Y.H.; Chung-Bang, H.; Ng, S.; Ntrivalas, E.; Mangubat, C.; Beaman, K.; Beer, A.; Gilman-Sachs, A. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum Reprod. 2003;18(4):767–773. [CrossRef]
- Sereshki, N.; Gharagozloo, M.; Ostadi, V.; Ghahiri, A.; Roghaei, M.; Mehrabian, F.; Andalib, A.; Hassanzadeh, A.; Hosseini, H.; Rezaei, A.A. Variations in T-helper 17 and Regulatory T Cells during The Menstrual Cycle in Peripheral Blood of Women with Recurrent Spontaneous Abortion. Int J Fertil Steril. 2014;8(1):59–66.
- Inagaki, N.; Stern, C.; McBain, J.; Lopata, A.; Kornman, L.; Wilkinson, D. Analysis of intra-uterine cytokine concentration and matrix-metalloproteinase activity in women with recurrent failed embryo transfer. Hum Reprod. 2003;18(3):608–615. [CrossRef]
- Wang, W.J.; Zhang, H.; Chen, Z.Q.; Zhang, W.; Liu, X.M.; Fang, J.Y.; Liu, F.J.; Kwak-Kim, J. Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod Biol Endocrinol. 2019 Jan 3;17(1):2. [CrossRef]
- Sheikhansari, G.; Soltani-Zangbar, M.S.; Pourmoghadam, Z.; Kamrani, A.; Azizi, R.; Aghebati-Maleki, L.; et al. Oxidative stress, inflammatory settings, and microRNA regulation in the recurrent implantation failure patients with metabolic syndrome. Am J Reprod Immunol. 2019;82(4): e13170. DOI: 10.1111/aji.13170.
- O’Hern Perfetto, C.; Fan, X.; Dahl, S.; Krieg, S.A.; Westphal, L.M.; Lathi, R.B.; Nayak, N.R. Expression of interleukin-22 in decidua of patients with early pregnancy and unexplained recurrent pregnancy loss. J Assist Reprod Genet 2015;32(6):977–984. [CrossRef]
- Wang, W.J.; Liu, F.J.; Qu, H.M.; Hao, C.F.; Qu, Q.L.; Xiong-Wang, Bao H.C.; Wang, X.R. Regulation of the expression of Th17 cells and regulatory T cells by IL-27 in patients with unexplained early recurrent miscarriage. J Reprod Immunol. 2013;99(1-2):39–45. [CrossRef]
- Ma, Y.; Ma, M.; Ye, S.; Liu, Y.; Zhao, X.; Wang, Y. Association of IL-17 and IL-27 polymorphisms with susceptibility to recurrent pregnancy loss and pre-eclampsia: A systematic review and meta-analysis. Immun Inflamm Dis. 2023 Oct;11(10):e1057. [CrossRef]
- Zhao, L.; Fu, J.; Ding, F.; Liu, J.; Li, L.; Song, Q.; Fu, Y. IL-33 and Soluble ST2 Are Associated With Recurrent Spontaneous Abortion in Early Pregnancy. Front Physiol. 2021:12:789829. [CrossRef]
- Yue, C.; Zhang, B.; Ying, C. Elevated Serum Level of IL-35 Associated with the Maintenance of Maternal-Fetal Immune Tolerance in Normal Pregnancy. PLOS ONE. 2015;10(6):e0128219. [CrossRef]
- Karaer, A.; Cigremis, Y.; Celik, E.; Urhan Gonullu, R. Prokineticin 1 and leukemia inhibitory factor mRNA expression in the endometrium of women with idiopathic recurrent pregnancy loss. Fertil Steril 2014; 102(4):1091-1095.e1. [CrossRef]
- Raghupathy, R.; Al-Mutawa, E.; Al-Azemi, M.; Makhseed, M.; Azizieh, F.; Szekeres-Bartho, J. (2009). Progesterone-induced blocking factor (PIBF) modulates cytokine production by lymphocytes from women with recurrent miscarriage or preterm delivery. J Reproductive Immunology, 2009; 80(1-2), 91–99. [CrossRef]
- Kashyap, N.; Begum, A.; Ray Das, C.; Datta, R.; Verma, M. K.; Dongre, A.; Husain, S. A.; Ahmad Khan, L.; Deka Bose, P. Aberrations in the progesterone pathway and the Th1/Th2 cytokine dichotomy - An evaluation of RPL predisposition in the northeast Indian population. Am Reprod Immunol, 2023 90(2), e13745. [CrossRef]
- Amjadi, F.; Zandieh, Z.; Mehdizadeh, M.; Aghajanpour, S.; Raoufi, E.; Aghamajidi, A.; Aflatoonian, R. The uterine immunological changes may be responsible for repeated implantation failure. J Reprod Immunol. 2020;138:103080–103080. [CrossRef]
- Laitinen, T. A Set of MHC Haplotypes Found Among Finnish Couples Suffering From Recurrent Spontaneous Abortions. Am J Reprod Immunol. 1993;29(3):148–154. [CrossRef]
- Hsiao, T.W.; Chung, M.T.; Wen, J.Y.; Lin, Y.; Lin, L.Y.; Tsai Y. HLA sharing and maternal HLA expression in couples with recurrent pregnancy loss in Taiwan. Taiwan J Obstet Gynecol. 2022;61(5):854–857. [CrossRef]
- Gharesi-Fard, B.; Askarinejad-Behbahani, R.; Behdin, S. The effect of HLA-DRB1 sharing between the couples with recurrent pregnancy loss on the pregnancy outcome after leukocyte therapy. Iran J Immunol 2014;11(1):13–20.
- Wang, X.P.; Lin, Q.; Peng, L.; Ma, Z.; Zhao, A. Association of HLA-DQB1 coding region with unexplained recurrent spontaneous abortion. Chin Med J. 2004;117(4):492–497.
- Ho, H.N.; Yang, Y.S.; Hsieh, R.P.; Lin, H.R.; Chen, S.; Huang, S.; Lee, T.Y.; Gill, T.J. Sharing of human leukocyte antigens in couples with unexplained infertility affects the success of in vitro fertilization and tubal embryo transfer. Am J Obstet Gynecol 1994;170(1):63–71. [CrossRef]
- Weckstein, L.N.; Patrizio, P.; Balmaceda, J.P.; Asch, R.H.; Branch, D.W. Human leukocyte antigen compatibility and failure to achieve a viable pregnancy with assisted reproductive technology. Acta Eur fertile. 1991;22(2):103–107.
- Balasch, J.; Jové, I.; Martorell, J.; Gayà, A.; Vanrell, J.A. Histocompatibility in in vitro fertilization couples. Fertil Steril.1993;59(2):456–458. [CrossRef]
- Hiby, S. E.; Regan, L.; Lo, W.; Farrell, L.; Carrington, M.; Moffett, A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Human reproduction 2008, 23(4), 972–976. [CrossRef]
- Hiby, S. E.; Apps, R.; Sharkey, A. M.; Farrell, L. E.; Gardner, L.; Mulder, A.; et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest, 2010; 120(11), 4102–4110. [CrossRef]
- Yang, X.; Yang, E.; Wang, W. J.; He, Q.; Jubiz, G.; Katukurundage, D.; Dambaeva, S.; Beaman, K.; Kwak-Kim, J. Decreased HLA-C1 alleles in couples of KIR2DL2 positive women with recurrent pregnancy loss. J Reprod Immunol 2020, 142, 103186. [CrossRef]
- Gil Laborda, R.; de Frías, E. R.; Subhi-Issa, N.; de Albornoz, E. C.; Meliá, E.; Órdenes, M.; et al. Centromeric AA motif in KIR as an optimal surrogate marker for precision definition of alloimmune reproductive failure. Scientific reports, 2024; 14(1), 3354. [CrossRef]
- Dahl, M.; Djurisic, S.; Hviid, T.V. The many faces of human leukocyte antigen-G: relevance to the fate of pregnancy. J Immunol Res. 2014;2014:591489. [CrossRef]
- Fan, W.; Huang, Z.; Li, S.; Xiao, Z. The HLA-G 14-bp polymorphism and recurrent implantation failure: a meta-analysis. J Assist Reprod and Genet. 2017;34(11):1559–1565. [CrossRef]
- Hu, L.; He, D.; Zeng, H. Association of parental HLA-G polymorphisms with soluble HLA-G expressions and their roles on recurrent implantation failure: A systematic review and meta-analysis. Front Immunol 2022, 13, 988370. [CrossRef]
- Nowak, I.; Wilczyńska, K.; Radwan, P.; Wiśniewski, A.; Krasiński, R.; Radwan, M.; Wilczyński, J.R.; Malinowski, A.; Kuśnierczyk, P. Association of Soluble HLA-G Plasma Level and HLA-G Genetic Polymorphism With Pregnancy Outcome of Patients Undergoing in vitro Fertilization Embryo Transfer. Front Immunol 2020;10: 2982. [CrossRef]
- Zych, M.; Roszczyk, A.; Kniotek, M.; Dąbrowski, F.; Zagożdżon, R. Differences in Immune Checkpoints Expression (TIM-3 and PD-1) on T Cells in Women with Recurrent Miscarriages-Preliminary Studies. J Clin Med, 2021 10(18), 4182. [CrossRef]
- Zych, M.; Roszczyk, A.; Dąbrowski, F.; Kniotek, M.; Zagożdżon, R. Soluble Forms of Immune Checkpoints and Their Ligands as Potential Biomarkers in the Diagnosis of Recurrent Pregnancy Loss-A Preliminary Study. Int J Mol Sci, 2023 25(1), 499. [CrossRef]
- Esparvarinha, M.; Madadi, S.; Aslanian-Kalkhoran, L.; Nickho, H.; Dolati, S.; Pia, H.; Danaii, S.; Taghavi, S.; Yousefi, M. Dominant immune cells in pregnancy and pregnancy complications: T helper cells (TH1/TH2, TH17/Treg cells), NK cells, MDSCs, and the immune checkpoints. Cell Biology International, 2023 47(3), 507–519. [CrossRef]
- Qian, C.; Pan, C.; Liu, J.; Wu, L.; Pan, J.; Liu, C.; Zhang, H. Differential expression of immune checkpoints (OX40/OX40L and PD-1/PD-L1) in decidua of unexplained recurrent spontaneous abortion women. Human immunology, 2024 85(1), 110745. [CrossRef]
- Zych, M.; Kniotek, M.; Roszczyk, A.; Dąbrowski, F.; Jędra, R.; Zagożdżon, R. Surface Immune Checkpoints as Potential Biomarkers in Physiological Pregnancy and Recurrent Pregnancy Loss. Int J Mol Sci, 2024; 25(17), 9378. [CrossRef]
- Opatrny, L.; David, M.; Kahn, S.R.; Shrier, I.; Rey, E. Association between antiphospholipid antibodies and recurrent fetal loss in women without autoimmune disease: A metaanalysis. J Rheumatol. 2006; 33(11):2214–21.
- Thangaratinam, S.; Tan, A.; Knox, E.; Kilby, M.D.; Franklyn, J.; Coomarasamy, A. Association between thyroid autoantibodies and miscarriage and preterm birth: Metaanalysis of evidence. BMJ. 2011;342(7806):1–8. [CrossRef]
- Cavalcante, M.B.; Cavalgante, C.T.; Sarno, M.; Da Silva, A.; Barini, R. Antinuclear antibodies and recurrent miscarriage: Systematic review and meta-analysis. Am J Reprod Immunol. 2020;83(3):13215. [CrossRef]
- Chen, S.; Yang, G.; Wu, P.; Sun, Y.; Dai, F.; He, Y.; Qian, H.; Liu, Y.; Shi, G. Antinuclear antibodies positivity is a risk factor of recurrent pregnancy loss: A meta-analysis. Semin Arthritis Rheum. 2020 Aug;50(4):534-543. [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Ferrer-Oliveras, R.; Llurba, E.; Gris, J.M. Tumor Necrosis Factor-Alpha and Pregnancy: Focus on Biologics. An Updated and Comprehensive Review. Clin Rev Allergy Immunol 2017; 53(1):40–53. [CrossRef]
- Lockwood, C.J.; Romero, R.; Feinberg, R.F.; Clyne, L.P.; Coster, B.; Hobbins, J.C. The prevalence and biologic significance of lupus anticoagulant and antic ardiolipin antibodies in a general obstetric population. Am J Obstet Gynecol. 1989:161(2):369–373. [CrossRef]
- Bahar, A.M.; Kwak, J.Y.H.; Beer, A.E.; Kim, J.H.; Nelson, L.A.; Beaman, K.D.; Gilman-Sachs, A. Antibodies to phospholipids and nuclear antigens in non-pregnant women with unexplained spontaneous recurrent abortions. J Reprod Immunol. 1993;24(3):213–222. [CrossRef]
- Kwak, J.Y.H.; Beer, A.E.; Cubillos, J.; Muñoz Sandoval, P.; Mendoza, J.; Espinel, F. Biological Basis of Fetoplacental Antigenic Determinants in the Induction of the Antiphospholipid Antibody Syndrome and Recurrent Pregnancy Loss. Ann N Y Acad Sci. 1994;731(1):242–245. [CrossRef]
- Rai, R.S.; Regan, L.; Clifford, K.; Pickering, W.; Dave, M.; Mackie, I.; McNally, T.; Cohen, H. Immunology: Antiphospholipid antibodies and β2-glycoprotein-I in 500 women with recurrent miscarriage: results of a comprehensive screening approach. Hum Reprod. 1995;10(8):2001–2005. [CrossRef]
- Del Porto, F.; Ferrero, S.; Cifani, N.; Sesti, G.; Proietta, M. Antiphospholipid antibodies and idiopathic infertility. Lupus. 2022 Mar;31(3):347-353. [CrossRef]
- D’Ippolito, S.; Ticconi, C.; Tersigni, C.; Garofalo, S.; Martino, C.; Lanzone, A.; Scambia, G.; Di Simone, N. The pathogenic role of autoantibodies in recurrent pregnancy loss. Am J Reprod Immunol. 2019;83(1): e13200.
- Gibbins, K.J.; Mumford, S.L.; Sjaarda, L.A.; Branch, D.W.; Perkins, N.J.; Ye, A.; Schisterman, E.F.; Silver R.M. Preconception antiphospholipid antibodies and risk of subsequent early pregnancy loss. Lupus. 2018; 27(9):1437–1445. [CrossRef]
- Papadimitriou, E.; Boutzios, G.; Mathioudakis, A.G.; Vlahos, N.F.; Vlachoyiannopoulos, P.; Mastorakos, G. Presence of antiphospholipid antibodies is associated with increased implantation failure following in vitro fertilization technique and embryo transfer: A systematic review and meta-analysis. PloS One. 2022;17(7):e0260759. [CrossRef]
- Jarne-Borràs, M.; Miró-Mur, F.; Anunciación-Llunell, A.; Alijotas-Reig, J. Antiphospholipid antibodies in women with recurrent embryo implantation failure: A systematic review and meta-analysis. Autoimmun Rev 2022;21(6):103101. [CrossRef]
- Tan, X.F.; Xu, L.; Li, T.T.; Wu, Y.T.; Ma, W.W.; Ding, J.Y.; Dong, H.L. Serum antiphospholipid antibody status may not be associated with the pregnancy outcomes of patients undergoing in vitro fertilization. Medicine. 2022; 101(12):e29146. [CrossRef]
- Tan, X.; Ding, J.; Pu, D.; Wu, J. Anti-phospholipid antibody may reduce endometrial receptivity during the window of embryo implantation. J Gynecol Obstet Hum Reprod. 2021;50(6):101912–101912. [CrossRef]
- Matalon, S.T.; Blank, M.B.; Ornoy, A.; Shoenfeld, Y. The Association Between Anti-Thyroid Antibodies and Pregnancy Loss. Am J Reprod Immunol microbiol. 2001;45(2):72–77. [CrossRef]
- Valeff, N.J.; Ventimiglia, M.S.; Diao, L.; Jensen, F. Lupus and recurrent pregnancy loss: the role of female sex hormones and B cells. Front Endocrinol. 2023; 14:1233883. [CrossRef]
- Gao, R.; Zeng, X.; Qin, L. Systemic autoimmune diseases and recurrent pregnancy loss: research progress in diagnosis and treatment. Chin Med J 2021 134(17):2140–2. [CrossRef]
- Mankee, A.; Petri, M.; Magder, L.S. Lupus anticoagulant, disease activity and low complement in the first trimester are predictive of pregnancy loss. Lupus Sci Med. 2015;2(1):e000095. [CrossRef]
- Ticconi, C.; Inversetti, A.; Logruosso, E.; Ghio, M.; Casadei, L.; Selmi, C.; Di Simone, N. Antinuclear antibodies positivity in women in reproductive age: From infertility to adverse obstetrical outcomes - A meta-analysis. J Reprod Immunol, 2023 155, 103794. [CrossRef]
- Hardy, C.J.; Palmer, B.P.; Morton, S.J.; Muir, K.R.; Powell R.J. Pregnancy outcome and family size in systemic lupus erythematosus: a case-control study. Rheumatology 1999;38(6):559–563. [CrossRef]
- Singh, M.; Fayaz, F.F.A.; Wang, J.; Wambua, S.; Subramanian, A.; Reynolds, J.A.; Nirantharakumar, K.; Crowe, F. MuM-PreDiCT. Pregnancy complications and autoimmune diseases in women: systematic review and meta-analysis. BMC Med. 2024 Aug 26;22(1):339. [CrossRef]
- Motak-Pochrzest, H.; Malinowski, A. Does autoimmunity play a role in the risk of implantation failures? Neuro Endocrinol Lett. 2018 Feb;38(8):575-578.
- Salmeri, N.; Gennarelli, G.; Vanni, V.S.; Ferrari, S.; Ruffa, A.; Rovere-Querini, P.; Pagliardini, L.; Candiani, M.; Papaleo, E. Concomitant Autoimmunity in Endometriosis Impairs Endometrium-Embryo Crosstalk at the Implantation Site: A Multicenter Case-Control Study. J Clin Med. 2023 May 19;12(10):3557. [CrossRef]
- Ballester, C.; Grobost, V.; Roblot, P.; Pourrat, O.; Pierre, F.; Laurichesse-Delmas, H.; Gallot, D.; Aubard, Y.; Bezanahary, H.; Fauchais, A.L. Pregnancy and primary Sjögren's syndrome: management and outcomes in a multicentre retrospective study of 54 pregnancies. Scand J Rheumatol. 2017;46(1):56-63. [CrossRef]
- Gupta S, Gupta N. Sjögren Syndrome and Pregnancy: A Literature Review. Perm J. 2017 ;21:16-047. [CrossRef]
- Imbroane, M. R.; LeMoine, F.; Gibson, K. S. Autoimmune Condition Diagnosis Following Recurrent Pregnancy Loss. Am J Reprod Immunol, 2024; 92(4), e70006. [CrossRef]
- Masucci, L.; D’Ippolito, S.; De Maio, F.; Quaranta, G.; Mazzarella, R.; Bianco, D.M. et al. Celiac Disease Predisposition and Genital Tract Microbiota in Women Affected by Recurrent Pregnancy Loss. Nutrients. 2023;15(1):221–221. [CrossRef]
- Arvanitakis, K.; Siargkas, A.; Germanidis, G.; Dagklis, T.; Tsakiridis, I. Adverse pregnancy outcomes in women with celiac disease: a systematic review and meta-analysis. Ann Gastroenterol. 2023;36(1):12-24. [CrossRef]
- Tersigni, C.; Castellani, R.; de Waure, C.; Fattorossi, A.; De Spirito, M.; Gasbarrini, A.; Scambia, G.; Di Simone, N. Celiac disease and reproductive disorders: meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum Reprod Update. 2014;20(4):582-93. [CrossRef]
- Saccone, G.; Berghella, V.; Sarno, L.; Maruotti, G.M.; Cetin, I.; Greco, L.; et al. Celiac disease and obstetric complications: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;214(2):225-234. [CrossRef]
- Di Simone, N.; Silano, M.; Castellani, R.; Di Nicuolo, F.; D'Alessio, M.C.; Franceschi, F.; et al.. Anti-tissue transglutaminase antibodies from celiac patients are responsible for trophoblast damage via apoptosis in vitro. Am J Gastroenterol. 2010;105(10):2254-61. [CrossRef]
- Di Simone, N.; De Spirito, M.; Di Nicuolo, F.; Tersigni, C.; Castellani, R.; Silano, M.; et al. Potential new mechanisms of placental damage in celiac disease: anti-transglutaminase antibodies impair human endometrial angiogenesis. Biol Reprod. 2013;17;89(4):88. [CrossRef]
- D'Ippolito, S.; Gasbarrini, A.; Castellani, R.; Rocchetti, S.; Sisti, L.G.; Scambia, G.; Di Simone, N. Human leukocyte antigen (HLA) DQ2/DQ8 prevalence in recurrent pregnancy loss women. Autoimmun Rev. 2016 Jul;15(7):638-43. [CrossRef]
- Królik, M.; Wrześniak, M.; Jezela-Stanek, A. Possible effect of the HLA-DQ2/DQ8 polymorphism on autoimmune parameters and lymphocyte subpopulation in recurrent pregnancy losses. J Reprod Immunol. 2022;149:103467. [CrossRef]
- Huang, C.; Liang, P.; Diao, L.; Liu, C.; Chen, X.; Li, G.; Chen, C.; Zeng, Y. Thyroid Autoimmunity is Associated with Decreased Cytotoxicity T Cells in Women with Repeated Implantation Failure. Int J Environ Res Public Health. 2015;12(9):10352–10361. [CrossRef]
- Huisman, P.; Krogh, J.; Nielsen, C.H.; Nielsen, H.S.; Feldt-Rasmussen, U.; Bliddal, S. Thyroglobulin antibodies in women with recurrent pregnancy loss: A Systematic Review and Meta-Analysis. Thyroid. 2023;33(11):1287-1301. [CrossRef]
- Zhong, Y.; Ying, Y.; Wu, H.; Zhou, C.; Xu, Y.; Wang, Q.; Li, J.; Shen, X.; Jin, L. Relationship between Antithyroid Antibody and Pregnancy Outcome following in Vitro Fertilization and Embryo Transfer. Int J Med Sci. 2012;9(2):121–125. [CrossRef]
- Abdolmohammadi-Vahid, S.; Danaii, S., Hamdi, K.; Jadidi-Niaragh, F.; Ahmadi, M.; Yousefi, M. Novel immunotherapeutic approaches for treatment of infertility. Biomed Pharmacother, 2016; 84: 1449–1459. [CrossRef]
- Stewart-Akers, A.M.; Krasnow, J.S.; Brekosky, J.; Deloia, J.A. Endometrial Leukocytes Are Altered Numerically and Functionally in Women with Implantation Defects. Am J Reprod Immunol. 1998;39(1):1–11. [CrossRef]
- Dhillon-Smith, R.K.; Middleton, L.J.; Sunner, K.K.; Cheed, V.; Baker, K.; et al. Levothyroxine in Women with Thyroid Peroxidase Antibodies before Conception. N England J Med. 2019;380(14):1316–1325. [CrossRef]
- van Dijk, M.M;, Vissenberg, R.; Fliers, E.; van der Post, J.A.M.; van der Hoorn, M.P.; de Weerd, S.; et al. Levothyroxine in euthyroid thyroid peroxidase antibody positive women with recurrent pregnancy loss (T4LIFE trial): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10(5):322-329. [CrossRef]
- Leng, T.; Li, X.; Zhang, H. Levothyroxine treatment for subclinical hypothyroidism improves the rate of live births in pregnant women with recurrent pregnancy loss: a randomized clinical trial. Gynecol Endocrinol. 2022;38(6):488-494. [CrossRef]
- Gaál, Z. Role of microRNAs in Immune Regulation with Translational and Clinical Applications. Int. J. Mol. Sci. 2024, 25, 1942. [CrossRef]
- Dong, J.; Warner, L. M.; Lin, L. L.; Chen, M. C.; O'Connell, R. M.; Lu, L. F. miR-155 promotes T reg cell development by safeguarding medullary thymic epithelial cell maturation. J Exper Med, 2021 218(2), e20192423. [CrossRef]
- Zolfaghari, M.A.; Motavalli, R.; Soltani-Zangbar, M.S.; Parhizkar, F.; Danaii, S.; Aghebati-Maleki, L.; et al. A new approach to the preeclampsia puzzle; MicroRNA-326 in CD4+ lymphocytes might be as a potential suspect. J Reprod Immunol. 2021;145:103317. [CrossRef]
- Winger, E.E.; Reed, J.L.; Ji, X. First-trimester maternal cell microRNA is a superior pregnancy marker to immunological testing for predicting adverse pregnancy outcome. J Reprod Immunol. 2015;110:22–35. [CrossRef]
- Patronia, M.M.; Potiris, A.; Mavrogianni, D.; Drakaki, E.; Karampitsakos, T.; Machairoudias, P.; et al. The Expression of microRNAs and Their Involvement in Recurrent Pregnancy Loss. J Clin Med. 2024;13(12):3361. [CrossRef]
- Xu, N.; Zhou, X.; Shi, W.; Ye, M.; Cao, X.; Chen, S.; Xu, C. Integrative analysis of circulating microRNAs and the placental transcriptome in recurrent pregnancy loss. Front Physiol. 2022;13:893744. [CrossRef]
- Wang, X.; Li, B.; Wang, J.; Lei, J.; Liu, C.; Ma, Y.; Zhao, H. Evidence that miR-133a causes recurrent spontaneous abortion by reducing HLA-G expression. Reprod Biomed Online. 2012;25(4):415–424. [CrossRef]
- Li, L.; Feng, T.; Zhou, W.; Liu, Y.; Li, H. miRNAs in decidual NK cells: regulators worthy of attention during pregnancy. Reprod Biol Endocrinol. 2021;19(1):150. [CrossRef]
- Guo, C,; Yin, X.; Yao, S. The effect of MicroRNAs variants on idiopathic recurrent pregnancy loss. J Assist Reprod Genet. 2023;40(7):1589–1595. [CrossRef]
- Thapliyal, A.; Tomar, A.K.; Naglot, S.; Dhiman, S.; Datta, S.K.; Sharma, J.B.; Singh, N.; Yadav, S. Exploring Differentially Expressed Sperm miRNAs in Idiopathic Recurrent Pregnancy Loss and Their Association with Early Embryonic Development. Noncoding RNA. 2024;10(4):41. [CrossRef]
- Odendaal, J.; Black, N.; Bennett, P.R.; Brosens, J.; Quenby, S.; MacIntyre, D.A. The endometrial microbiota and early pregnancy loss. Hum Reprod. 2024;39(4):638–646. [CrossRef]
- Gao, X.; Louwers, Y.V.; Laven, E.; Schoenmakers, S. Clinical Relevance of Vaginal and Endometrial Microbiome Investigation in Women with Repeated Implantation Failure and Recurrent Pregnancy Loss. Inter J Mol Sci. 2024;25(1):622–622. [CrossRef]
- Soyer Caliskan, C.; Yurtcu, N.; Celik, S.; Sezer, O.; Kilic, S.S.; Cetin, A. Derangements of vaginal and cervical canal microbiota determined with real-time PCR in women with recurrent miscarriages. J Obstet Gynaecol. 2022 Aug;42(6):2105-2114. [CrossRef]
- Al-Memar, M.; Bobdiwala, S.; Fourie, H.; Mannino, R.; Lee, Y.S.; Smith, A.; et al. The association between vaginal bacterial composition and miscarriage: a nested case-control study. BJOG. 2020; 127(2):264–274. [CrossRef]
- Grewal, K.; Lee, Y.S.; Smith, A.; Brosens, J.J.; Bourne, T.; Al-Memar, M.; Kundu, S.; MacIntyre, D.A.; Bennett P.R. Chromosomally normal miscarriage is associated with vaginal dysbiosis and local inflammation. BMC Med. 2022;20(1):38. [CrossRef]
- Peuranpää, P.; Holster, T.; Saqib, S.; Kalliala, I.; Tiitinen, A.; Salonen, A.; Hautamäki, H. Female reproductive tract microbiota and recurrent pregnancy loss: a nested case-control study. Reprod BioMed Online. 2022;45(5):1021–1031. [CrossRef]
- Vomstein, K.; Reider, S.; Böttcher, B.; Watschinger, C.; Kyvelidou, C.; Tilg, H.; Moschen, A.R.; Toth, B. Uterine microbiota plasticity during the menstrual cycle: Differences between healthy controls and patients with recurrent miscarriage or implantation failure. J Reprod Immunol. 2022 Jun;151:103634. [CrossRef]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahçeci, M.; et al.. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022;10(1):1. [CrossRef]
- Shi, Y.; Yamada, H.; Sasagawa, Y.; Tanimura, K.; Deguchi, M. Uterine endometrium microbiota and pregnancy outcome in women with recurrent pregnancy loss. J Reprod Immunol. 2022;152:103653. [CrossRef]
- Wang, L.; Chen, J.; He, L.; Liu, H.; Liu, Y.; et al. Association between the vaginal and uterine microbiota and the risk of early embryonic arrest. Front Microbiol. 2023;14:1137869. [CrossRef]
- Quenby, S.; Kalumbi, C.; Bates, M.; Farquharson, R.; Vince, G. Prednisolone reduces preconceptual endometrial natural killer cells in women with recurrent miscarriage. Fertil Steril. 2005;84(4):980–984. [CrossRef]
- Gomaa, M.F.; Elkholy, A.G.; El-Said, M.M.; Abdel-Salam, N.E. Combined oral prednisolone and heparin versus heparin: the effect on peripheral NK cells and clinical outcome in patients with unexplained recurrent miscarriage. A double-blind placebo randomized controlled trial. Arch Gynecol Obstet 2014;290(4):757–762. [CrossRef]
- Rezayat, F.; Esmaeil, N.; Rezaei, A,; Sherkat, R. Contradictory Effect of Lymphocyte Therapy and Prednisolone Therapy on CD3+CD8+CD56+ Natural Killer T Population in Women with Recurrent Spontaneous Abortion. J Hum Reprod Sci. 2023;16(3):246–246. [CrossRef]
- Tang, A.W.; Alfirevic, Z.; Turner, M.A.; Drury, J.A.; Small, R.; Quenby, S. A feasibility trial of screening women with idiopathic recurrent miscarriage for high uterine natural killer cell density and randomizing to prednisolone or placebo when pregnant. Hum Reprod. 2013;28(7):1743–1752. [CrossRef]
- Boomsma, C.M.; Kamath, M.S.; Keay, S.D.; Macklon, N.S. Peri-implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst Rev. 2022 Jun 30;6(6):CD005996. [CrossRef]
- Cooper, S.; Laird, S.M.; Mariee, N.; Li, T.C.; Metwally, M. The effect of prednisolone on endometrial uterine NK cell concentrations and pregnancy outcome in women with reproductive failure. A retrospective cohort study. J Reprod Immunol. 2019;131:1–6. [CrossRef]
- Dan, S.; Wei, W.; Yichao, S.; Hongbo, C.; Shenmin, Y.; Jiaxiong, W.; Hong, L. Effect of Prednisolone Administration on Patients with Unexplained Recurrent Miscarriage and in Routine Intracytoplasmic Sperm Injection: A Meta-Analysis. Am J Reprod Immunol 2015;74(1):89–97. [CrossRef]
- He, Y.; Tang, R.; Yu, H.; Mu, H.; Jin, H.; Dong. J.; Wang, W.; Wang, L.; Chen, S.; Wang, X. Comparative effectiveness and safety of 36 therapies or interventions for pregnancy outcomes with recurrent implantation failure: a systematic review and network meta-analysis. J Assist Reprod Genet. 2023;40(10):2343–2356. [CrossRef]
- Huang, Q.; Wu. H.; Li, M.; Yang, Y.; Fu, X. Prednisone improves pregnancy outcome in repeated implantation failure by enhance regulatory T cells bias. J Reprod Immunol. 2021;143:103245. [CrossRef]
- Hasegawa, I.; Yamanoto, Y.; Suzuki, M.; Murakawa, H.; Kurabayashi, T.; Takakuwa, K.; Tanaka, K. Prednisolone plus low-dose aspirin improves the implantation rate in women with autoimmune conditions who are undergoing in vitro fertilization. Fertil Steril. 1998;70(6):pp.1044–1048. [CrossRef]
- Fan, J.; Zhong, Y.; Chen, C. Combined treatment of prednisone and aspirin, starting before ovulation induction, may improve reproductive outcomes in ANA-positive patients. Am J Reprod Immunol. 2016; 76(5):391–395. [CrossRef]
- Ando, T.; Suganuma, N.; Furuhashi, M.; Asada, Y.; Kondo, I.; Tsutsumi, Y. Successful glucocorticoid treatment for patients with abnormal autoimmunity on in vitro fertilization and embryo transfer. J Assist Reprod Genet. 1996; 13(10):776–781. [CrossRef]
- Sun, Y.; Cui, L.; Lu, Y.; Tan, J.; Dong, X.; et al. Prednisone vs Placebo and Live Birth in Patients With Recurrent Implantation Failure Undergoing In Vitro Fertilization. JAMA. 2023;329(17):1460–1460. [CrossRef]
- Bramham, K.; Thomas, M.; Nelson-Piercy, C.; Khamashta, M.; Hunt, B.J. First-trimester low-dose prednisolone in refractory antiphospholipid antibody-related pregnancy loss. Blood. 2011;117(25):6948–6951. [CrossRef]
- Riancho-Zarrabeitia, L.; Lopez-Marin, L.; Cacho, P.M.; López-Hoyos, M.; Barrio, R.D.; Haya, A.; Martínez-Taboada, V.M. Treatment with low-dose prednisone in refractory obstetric antiphospholipid syndrome: A retrospective cohort study and meta-analysis. Lupus. 2022;31(7):808–819. [CrossRef]
- Forges, T.; Monnier-Barbarino, P.; Guillet-May, F.; Faure, G.C.; Béné, M.C. Corticosteroids in patients with antiovarian antibodies undergoing in vitro fertilization: a prospective pilot study. Eur J Clin Pharmacol. 2006;62(9):699–705. [CrossRef]
- Bandoli, G.; Palmsten, K.; Forbess Smith, C.J.; Chambers, C.D. A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcomes. Rheum Dis Clin North Am. 2017; 43(3):489–502. [CrossRef]
- Hooper, A.; Bacal, V.; Bedaiwy, M.A. Does adding hydroxychloroquine to empiric treatment improve the live birth rate in refractory obstetrical antiphospholipid syndrome? A systematic review. Am J Reprod Immunol. 2023 Sep;90(3):e13761. [CrossRef]
- Mekinian, A.; Lazzaroni, M.G.; Kuzenko, A.; Alijotas-Reig, J.; Ruffatti, A.; Levy, P.; et al. The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: Data from a European multicenter retrospective study. Autoimmun Rev 2015;14(6):498–502. [CrossRef]
- Mekinian, A.; Alijotas-Reig, J.; Carrat, F.; Costedoat-Chalumeau, N.; Ruffatti, A.; Lazzaroni, M.G.; et al. Refractory obstetrical antiphospholipid syndrome: Features, treatment and outcome in a European multicenter retrospective study. Autoimmun Rev. 2017;16(7):730–734. [CrossRef]
- Ye, S.L.; Gu, X.K.; Tao, L.Y.; Cong, J.M.; Wang, Y.Q. Efficacy of Different Treatment Regimens for Antiphospholipid Syndrome-related Recurrent Spontaneous Abortion. Chin Med J 2017;130(12):1395–1399. [CrossRef]
- Gerde, M.; Ibarra, E.; Mac Kenzie, R.; Fernandez Suarez, C.; Heer, C.; Alvarez, R.; Iglesias, M.; Balparda, J.; Beruti, E.; Rubinstein, F. The impact of hydroxychloroquine on obstetric outcomes in refractory obstetric antiphospholipid syndrome. Thromb. Res. 2021;206:104–110. [CrossRef]
- Ruffatti, A.; Tonello, M.; Hoxha, A.; Sciascia, S.; Cuadrado, M.J.; Latino, J.O.; et al. Effect of Additional Treatments Combined with Conventional Therapies in Pregnant Patients with High-Risk Antiphospholipid Syndrome: A Multicentre Study. Thromb Haemost. 2018;47(04):639–646. [CrossRef]
- Sciascia, S.; Hunt, B.J.; Talavera-Garcia, E.; Lliso, G.; Khamashta, M.A.; Cuadrado, M.J. The impact of hydroxychloroquine treatment on pregnancy outcome in women with antiphospholipid antibodies. Am J Obstet Gynecol. 2016;214(2):273.e1–273.e8. [CrossRef]
- Sadeghpour, S.; Ghasemnejad Berenji, M.; Nazarian, H.; Ghasemnejad, T.; Nematollahi, M.H.; Abroon, S.; et al. Effects of treatment with hydroxychloroquine on the modulation of Th17/Treg ratio and pregnancy outcomes in women with recurrent implantation failure: clinical trial. Immunopharmacol Immunotoxicol. 2020;42(6):632-642. [CrossRef]
- Dernoncourt, A.; Hedhli, K.; Abisror, N.; Cheloufi, M.; Cohen, J. et al. Hydroxychloroquine in recurrent pregnancy loss: data from a French prospective multicenter registry. Hum Reprod. 2024 Jun 28:deae146. [CrossRef]
- Halloran, P.F. Molecular mechanisms of new immunosuppressants. Clin Transplant 1996;10:118-123.
- Saad, A.F.; Pacheco, L.D, Saade GR. Immunosuppressant Medications in Pregnancy. Obstet Gynecol. 2024;143(4):e94-e106. [CrossRef]
- Cavalcante, M.B.; Tavares, A.C.M.; Rocha, C.A.; de Souza, G.F.; Lima, E.M.; Simões, J.M.L.; et al. Calcineurin inhibitors in the management of recurrent miscarriage and recurrent implantation failure: Systematic review and meta-analysis. J Reprod Immunol. 2023 Dec;160:104157. [CrossRef]
- Nakagawa, K.; Sugiyama, R. Tacrolimus treatment in women with repeated implantation failures. Reproductive medicine and biology, 2024 23(1), e12558. [CrossRef]
- Ling, Y.; Huang, Y.; Chen, C.; Mao, J.; Zhang, H. Low dose Cyclosporin A treatment increases live birth rate of unexplained recurrent abortion - initial cohort study. Clin Exp Obst Gynecol. 2017;44(2):230–235. [CrossRef]
- Azizi, R.; Ahmadi, M.; Danaii, S.; Abdollahi-Fard, S.; Mosapour, P.; Eghbal-Fard, S.; et al. Cyclosporine A improves pregnancy outcomes in women with recurrent pregnancy loss and elevated Th1/Th2 ratio. J Cell Physiol.2019;234(10):19039–19047. [CrossRef]
- Fu, J.H. Analysis of the use of cyclosporin A to treat refractory immune recurrent spontaneous abortion. Clin Exp Obstet Gynecol. 2015;42(6):739–742. [CrossRef]
- Qu, D.; Tian, X.; Ding, L.; Li, Y.; Zhou, W. Impacts of Cyclosporin A on clinical pregnancy outcomes of patients with a history of unexplained transfer failure: a retrospective cohort study. Reprod Biol Endocrinol. 2021;19(1):44. [CrossRef]
- Liu, J.; Li, M.; Fu, J.; Yuan, G.; Li, N.; Fu, Y., Zhao, L. Tacrolimus improved the pregnancy outcomes of patients with refractory recurrent spontaneous abortion and immune bias disorders: a randomized controlled trial. Eur J Clin Pharmacol. 2023;79(5):627-634. [CrossRef]
- Kuroda, K.; Ikemoto, Y.; Horikawa, T.; Moriyama, A.; Ojiro, Y.; Takamizawa, S.; Uchida, T.; Nojiri, S.; Nakagawa, K.; Sugiyama, R. Novel approaches to the management of recurrent pregnancy loss: The OPTIMUM (OPtimization of Thyroid function, Thrombophilia, Immunity, and Uterine Milieu) treatment strategy. Reprod Med Biol. 2021;20(4):524-536. [CrossRef]
- Nakagawa, K.; Kuroda, K.; Sugiyama, R.; Yamaguchi, K. After 12 consecutive miscarriages, a patient received immunosuppressive treatment and delivered an intact baby. Reprod Med Biol. 2017;16(3):297-301. [CrossRef]
- Shen, P.; Zhang, T.; Han, R.; Xie, H.; Lv, Q. Co-administration of tacrolimus and low molecular weight heparin in patients with a history of implantation failure and elevated peripheral blood natural killer cell proportion. J Obstet Gynaecol Res. 2022;49(2):649–657. [CrossRef]
- Nakagawa, K.; Kwak-Kim, J.; Hisano, M.; Kasahara, Y.; Kuroda, K.; Sugiyama, R.; Yamaguchi, K. Obstetric and perinatal outcome of the women with repeated implantation failures or recurrent pregnancy losses who received pre- and post-conception tacrolimus treatment. Am J Reprod Immunol. 2019;82(2):e13142. [CrossRef]
- Nakamura, A.; Tanaka, Y.; Amano, T.; Takebayashi, A.; Takahashi, A.; Hanada, T.; Tsuji, S.; Murakami, T. mTOR inhibitors as potential therapeutics for endometriosis: a narrative review. Molecular human reproduction, 2024; 30(12), gaae041. [CrossRef]
- Li, M.Y.; Shen, H.H.; Cao, X.Y.; Gao, X.X.; Xu, F.Y.; Ha, S.Y.; Sun, J.S.; Liu, S.P.; Xie, F.; Li, M.Q. Targeting a mTOR/autophagy axis: a double-edged sword of rapamycin in spontaneous miscarriage. Biomed Pharmacother. 2024;177:116976. [CrossRef]
- Ahmadi, M.; Abdolmohamadi-Vahid, S.; Ghaebi, M.; Dolati, S.; Abbaspour-Aghdam, S.; Danaii, S.; et al. Sirolimus as a new drug to treat RIF patients with elevated Th17/Treg ratio: A double-blind, phase II randomized clinical trial. Int Immunopharmacol 2019;74:105730–105730. [CrossRef]
- Kwak, J.Y.H.; Kwak, F.M.Y.; Ainbinder, S.W.; Ruiz, A.M.; Beer A.E. Elevated Peripheral Blood Natural Killer Cells Are Effectively Downregulated by Immunoglobulin G Infusion in Women With Recurrent Spontaneous Abortions. Am J of Reprod Immunol. 1996;35(4):363–369. [CrossRef]
- Ahmadi, M.; Abdolmohammadi-Vahid, S.; Ghaebi, M.; Aghebati-Maleki, L.; Afkham, A.; Danaii, S.; et al. Effect of Intravenous immunoglobulin on Th1 and Th2 lymphocytes and improvement of pregnancy outcome in recurrent pregnancy loss (RPL). Biomed Pharmacother 2017a;92:1095–1102. [CrossRef]
- Ahmadi, M.; Abdolmohammadi-Vahid, S.; Ghaebi, M.; Aghebati-Maleki, L.; Dolati, S.; Farzadi, L.; et al. Regulatory T cells improve pregnancy rate in RIF patients after additional IVIG treatment. Syst Biol Reprod Med 2017b;63(6):350–359. [CrossRef]
- Yamada, H.; Deguchi, M.; Saito, S.; Takeshita, T.; Mitsui, M.; Saito, T.; et al. High doses of intravenous immunoglobulin stimulate regulatory T cell and suppress natural killer cell in women with recurrent pregnancy loss. J Reprod Immunol. 2023;158:103977. [CrossRef]
- Shi, Y.; Tan, D.; Hao, B.; Zhang, X.; Geng, W.; Wang, Y.; Sun, J.; Zhao Y. Efficacy of intravenous immunoglobulin in the treatment of recurrent spontaneous abortion: A systematic review and meta-analysis. Am J Reprod Immunol. 2022;88(5): e13615. [CrossRef]
- Christiansen, O.B.; Kolte, A.M.; Krog, M.C.; Nielsen, H.S.; Egerup, P. Treatment with intravenous immunoglobulin in patients with recurrent pregnancy loss: An update. J Reprod Immunol. 2019; 133:37–42. [CrossRef]
- Yamada, H.; Deguchi, M.; Saito, S.; Takeshita, T.; Mitsui, M.; Saito, T.; et Intravenous immunoglobulin treatment in women with four or more recurrent pregnancy losses: A double-blind, randomised, placebo-controlled trial. EClinicalMedicine, 2022; 50, 101527. [CrossRef]
- Ramos-Medina, R.; García-Segovia, A.; Gil, J.; Carbone, J.; Aguarón de la Cruz, A.; Seyfferth, A.; et al. Experience in IVIg Therapy for Selected Women with Recurrent Reproductive Failure and NK Cell Expansion. Am J Reprod Immunol. 2014;71(5):458–466. [CrossRef]
- Lee, S.K.; Kim, J.Y.; Han, A.R.; Hur, S.E.; Kim, C.J.; Kim, T.H.; Cho, B.R.; Han, J.W.; Han, S.G.; Na, B.J.; Kwak-Kim, J. Intravenous Immunoglobulin G Improves Pregnancy Outcome in Women with Recurrent Pregnancy Losses with Cellular Immune Abnormalities. Am J Reprod Immunol. 2016;75(1):59–68. [CrossRef]
- Banjar, S.; Kadour, E.; Khoudja, R.; Ton-Leclerc, S.; Beauchamp, C.; Beltempo, M.; et al. Intravenous immunoglobulin use in patients with unexplained recurrent pregnancy loss. Am J Reprod Immunol. 2023 Aug;90(2):e13737. [CrossRef]
- Kim, J.H.; Kim, S.H.; Yang, N.; Ko, Y.; Lee, S.R.; Chae, H.D. Outcomes of Empirical Treatment With Intravenous Immunoglobulin G Combined With Low-Dose Aspirin in Women With Unexplained Recurrent Pregnancy Loss. J Korean Med Sci. 2022 Jun 27;37(25):e200. [CrossRef]
- Habets, D.H.J.; Pelzner, K.; Wieten, L.; Spaanderman, M.E.A.; Villamor, E.; Al-Nasiry, S. Intravenous immunoglobulins improve live birth rate among women with underlying immune conditions and recurrent pregnancy loss: a systematic review and meta-analysis. Allergy Asthma Clin Immunol. 2022; 18(1):23. [CrossRef]
- Clark, D.A.; Coulam, C.B.; Stricker, R.B. Is intravenous immunoglobulins (IVIG) efficacious in early pregnancy failure? A critical review and meta-analysis for patients who fail in vitro fertilization and embryo transfer (IVF). J Assist Reprod Genet 2006;23(1):1–13. [CrossRef]
- Kumar, P.; Philip, C. E.; Eskandar, K.; Marron, K.; Harrity, C. Effect of intravenous immunoglobulin therapy in recurrent implantation failure: A Systematic review and meta-analysis. J Reprod Immunol, 2024 166, 104323. [CrossRef]
- Park, J. S.; Song, A. Y.; Bae, J. Y.; Han, J. W.; Kim, T. H.; Kim, C. J.; Lee, S. K. IL-17 Producing T to Foxp3+CD4+ Regulatory T Cell Ratio as a Diagnostic and Prognostic Marker in Women With Recurrent Pregnancy Loss and Its Implications for Intravenous Immunoglobulin Therapy. Am J Reprod Immunol, 2024 92(5), e70020. [CrossRef]
- Velikova, T.; Sekulovski, M.; Bogdanova, S.; Vasilev, G.; Peshevska-Sekulovska, M.; Miteva, D.; Georgiev, T. Intravenous Immunoglobulins as Immunomodulators in Autoimmune Diseases and Reproductive Medicine. Antibodies (Basel). 2023 Mar 2;12(1):20. [CrossRef]
- Perricone, R.; De Carolis, K.B.; Greco, E.; Giacomelli, R.; Cipriani, P.; Fontana, L.; Perricone, C. Intravenous immunoglobulin therapy in pregnant patients affected with systemic lupus erythematosus and recurrent spontaneous abortion. Rheumatology (Oxford). 2008;47(5):646–651. [CrossRef]
- Wang, S.W.; Zhong, S.Y.; Lou, L.J.; Hu, Z.F.; Sun, H.Y.; Zhu, H.Y. The effect of intravenous immunoglobulin passive immunotherapy on unexplained recurrent spontaneous abortion: a meta-analysis. Reprod BioMed Online. 2016;33(6):720–736. [CrossRef]
- Winger, E.E.; Reed, J.L.; Ashoush, S.; El-Toukhy, T.; Ahuja, S.; Taranissi, M. Elevated Preconception CD56+16+ and/or Th1:Th2 Levels Predict Benefit from IVIG Therapy in Subfertile Women Undergoing IVF. Am J Reprod Immunol. 2011;66(5):394–403. [CrossRef]
- Sung, N.; Han, A.R.; Park, C.W.; Park, D.W.; Park, J.C.; Kim, N.Y.; Lim, K.S., Shin, J.E.; Joo, C.W.; Lee, S.E.; Kim, J.W.; Lee, S.K.; IVIG Task Force, Korean Society for Reproductive Immunology. Intravenous immunoglobulin G in women with reproductive failure: The Korean Society for Reproductive Immunology practice guidelines. Clin Exp Reprod Med. 2017 Mar;44(1):1-7. [CrossRef]
- Woon, E.V.; Day, A.; Bracewell-Milnes, T.; Male, V.; Johnson, M. Immunotherapy to improve pregnancy outcome in women with abnormal natural killer cell levels/activity and recurrent miscarriage or implantation failure: A systematic review and meta-analysis. J Reprod Immunol. 2020;142:103189. [CrossRef]
- Porter, T.A.; Lacoursiere, Y.; Scott, J. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2006;(2):CD000112. [CrossRef]
- Urban, M.L.; Bettiol, A.; Serena, C.; Comito, C.; Turrini, I.; Fruttuoso, S.; et al. Intravenous immunoglobulin for the secondary prevention of stillbirth in obstetric antiphospholipid syndrome: A case series and systematic review of literature. Autoimmun Rev. 2020;19(9):102620. [CrossRef]
- Perricone, R.; Di Muzio, G.; Perricone, C.; Giacomelli, R.; De Nardo, D.; Fontana, L.; De Carolis, C. High Levels of Peripheral Blood NK Cells in Women Suffering from Recurrent Spontaneous Abortion are Reverted from High-Dose Intravenous Immunoglobulins. Am J Reprod Immunol. 2006;55(3):232–239. [CrossRef]
- Elram, T.; Simon, A.; Israel, S.; Revel, A.; Shveiky, D.; Laufer, N. Treatment of recurrent IVF failure and human leukocyte antigen similarity by intravenous immunoglobulin. Reproduc BioMed Online. 2005;11(6):745–749. [CrossRef]
- Rutella, S. Granulocyte Colony-Stimulating Factor for the Induction of T-Cell Tolerance. Transplantation. 2007;84(Supplement):S26–S30. [CrossRef]
- Perobelli, S. M.; Mercadante, A. C.; Galvani, R. G.; Gonçalves-Silva, T.; Alves, A. P.; Pereira-Neves, A.; Benchimol, M.; Nóbrega, A.; Bonomo, A. G-CSF-Induced Suppressor IL-10+ Neutrophils Promote Regulatory T Cells That Inhibit Graft-Versus-Host Disease in a Long-Lasting and Specific Way. J Immunol 2016; 197(9), 3725–3734. [CrossRef]
- Scarpellini, F.; Sbracia, M. Use of granulocyte colony-stimulating factor for the treatment of unexplained recurrent miscarriage: a randomised controlled trial. Hum Reprod. 2009;24(11):2703–2708. [CrossRef]
- Eapen, A.; Joing, M.; Kwon, P.; Tong, J.; Maneta, E.; Santo, C.D.; Mussai, F.; Lissauer, D.; Carter, D.; De Santo, C.; RESPONSE study group. Recombinant human granulocyte- colony stimulating factor in women with unexplained recurrent pregnancy losses: a randomized clinical trial. Hum Reprod 2019;34(3):424–432. [CrossRef]
- Busnelli, A.; Somigliana, E.; Cirillo, F.; Baggiani, A.; Levi-Setti, P.E. Efficacy of therapies and interventions for repeated embryo implantation failure: a systematic review and meta-analysis. Sci Rep. 2021;11(1):1747. [CrossRef]
- Kamath, M.S.; Chittawar, P.B.; Kirubakaran, R.; Mascarenhas, M. Use of granulocyte-colony stimulating factor in assisted reproductive technology: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2017;214:16–24. [CrossRef]
- Arefi, S.; Fazeli, E.; Esfahani, M.; Borhani, N.; Yamini, N.; Hosseini, A.; Farifteh, F. Granulocyte-colony stimulating factor may improve pregnancy outcome in patients with history of unexplained recurrent implantation failure: An RCT. Int J Reprod Biomed. 2018, 16(5):299–304. [CrossRef]
- Liu, M.; Yuan, Y.; Qiao, Y.; Tang, Y.; Sui, X.; Yin, P.; Yang, D. The effectiveness of immunomodulatory therapies for patients with repeated implantation failure: a systematic review and network meta-analysis. Sci Rep. 2022;12(1):18434. [CrossRef]
- Li, J.; Mo, S.; Chen, Y. The effect of G-CSF on infertile women undergoing IVF treatment: A meta-analysis. Syst Biol Reprod Med. 2017:63(4):239–247. [CrossRef]
- Fu, J.; Li, L.; Qi, L.; Zhao, L. A randomized controlled trial of etanercept in the treatment of refractory recurrent spontaneous abortion with innate immune disorders. Taiwan J Obstet Gynecol. 2019;58(5):621–625. [CrossRef]
- Santiago, K.Y.; Porchia, L.M.; López-Bayghen, E. Endometrial preparation with etanercept increased embryo implantation and live birth rates in women suffering from recurrent implantation failure during IVF. Reprod Biol. 2021;21(1):100480. [CrossRef]
- Winger, E,E,; Reed, J.L. Treatment with Tumor Necrosis Factor Inhibitors and Intravenous Immunoglobulin Improves Live Birth Rates in Women with Recurrent Spontaneous Abortion. Am J Reprod Immunol. 2008;60(1):8–16. [CrossRef]
- Winger, E.E.; Reed, J.L.; Ashoush, S.; Ahuja, S.; El-Toukhy, T.; Taranissi, M. Treatment with Adalimumab (Humira®) and Intravenous Immunoglobulin Improves Pregnancy Rates in Women Undergoing IVF. Am J Reprod Immunol. 2008;61(2):113–120. [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Anunciación-Llunell, A.; Marques-Soares, J.; Pardos-Gea, J.; Miró-Mur, F. Pathogenesis, Diagnosis and Management of Obstetric Antiphospholipid Syndrome: A Comprehensive Review. J Clin Med. 2022 Jan 28;11(3):675. [CrossRef]
- Hajipour, H.; Nejabati, H.R.; Latifi, Z.; Hamdi, K.; Bahrami-Asl, Z.; Fattahi, A.; Nouri, M. Lymphocytes immunotherapy for preserving pregnancy: Mechanisms and Challenges. Am J Reprod Immunol 2018., 80(3):12853–e12853. [CrossRef]
- Yang, H.; Qiu, L.; Di, W.; Zhao, A.; Chen, G.; Hu, K.; Lin, Q. Proportional change of CD4+CD25+ regulatory T cells after lymphocyte therapy in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2009; 92(1):301–305. [CrossRef]
- Sarkesh, A.; Sorkhabi, A.D.; Parhizkar, F.; Soltani-Zangbar, M.S.; Yousefi, M.; Aghebati-Maleki, L. The immunomodulatory effect of intradermal allogeneic PBMC therapy in patients with recurrent spontaneous abortion. J Reprod Immunol. 2023;156:103818–103818. [CrossRef]
- Liu, S.; Gu, X.; Weng, R. Clinical effect of lymphocyte immunotherapy on patients with unexplained recurrent spontaneous abortion. Immun Inflamm Dis. 2021 Dec;9(4):1272-1278. [CrossRef]
- Fainboim, L.; Belén, S.; González, V.; Fernández, P. Evaluation of paternal lymphocyte immunotherapy and potential biomarker mixed lymphocyte reaction-blocking factor in an Argentinian cohort of women with unexplained recurrent spontaneous abortion and unexplained infertility. Am J Reprod Immunol. 2021 Aug;86(2):e13422. [CrossRef]
- Sarno, M.; Cavalcante, M.B.; Niag, M.; Pimentel, K.; Luzm I.; Figueiredom B.; et al. Gestational and perinatal outcomes in recurrent miscarriages couples treated with lymphocyte immunotherapy. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100036. [CrossRef]
- Chen, J.L.; Yang, J.M.; Huang, Y.Z.; Li, Y. Clinical observation of lymphocyte active immunotherapy in 380 patients with unexplained recurrent spontaneous abortion. Int Immunopharmacol. 2016;40:347–350. [CrossRef]
- Gharesi-Fard, B.; Zolghadri, J.; Foroughinia, L.; Tavazoo, F.; Samsami Dehaghani, A. Effectiveness of leukocyte immunotherapy in primary recurrent spontaneous abortion (RPL). Iran J Immunol. 2007;4(3):173–8.
- Pandey, M.K.; Agrawal, S. Induction of MLR-Bf and protection of fetal loss: a current double blind randomized trial of paternal lymphocyte immunization for women with recurrent spontaneous abortion. Int Immunopharmacol. 2004;4(2):289–298. [CrossRef]
- Ober, C.; Karrison, T.; Odem, R.R.; Barnes, R.B.; Branch, DW, Stephenson MD, Baron B, Walker MA, Scott JR, Schreiber JR. Mononuclear-cell immunisation in prevention of recurrent miscarriages: a randomised trial. Lancet. 1999;354(9176):365-9. [CrossRef]
- Wong, L.F.; Porter, T.F.; Scott, J.R. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2014;2014(10):CD000112. [CrossRef]
- Günther, V.; Alkatout, I.; Meyerholz, L.; Maass, N.; Görg, S.; von Otte, S.; Ziemann, M. Live Birth Rates after Active Immunization with Partner Lymphocytes. Biomedicines. 2021 Sep 29;9(10):1350. [CrossRef]
- Liu, Z.; Xu, H.; Kang, X.; Wang, T.; He, L.; Zhao, A. Allogenic Lymphocyte Immunotherapy for Unexplained Recurrent Spontaneous Abortion: A Meta-Analysis. Am J Reprod Immunol. 2016; 76(6):443–453. [CrossRef]
- Rasmark Roepke, E.; Hellgren, M.; Hjertberg, R.; Blomqvist, L.; Matthiesen, L.; Henic, E.; Lalitkumar, S.; Strandell, A. Treatment efficacy for idiopathic recurrent pregnancy loss - a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2018;97(8):921–941. [CrossRef]
- Ma, J.; Gao, W,; Li, D. Recurrent implantation failure: A comprehensive summary from etiology to treatment. Front Endocrinol (Lausanne). 2023;13:1061766. [CrossRef]
- Yu, N.; Zhang, B.; Xu, M.; Wang, S.; Liu, R.; Wu, J.; Yang, J.; Feng, L Intrauterine administration of autologous peripheral blood mononuclear cells (PBMCs) activated by HCG improves the implantation and pregnancy rates in patients with repeated implantation failure: a prospective randomized study. Am J Reprod Immunol. 2016;76(3):212–216. [CrossRef]
- Li, S.; Wang, J.; Cheng, Y.; Zhou, D.; Yin, T.; Xu, W.; Yu, N.; Yang, J. Intrauterine administration of hCG-activated autologous human peripheral blood mononuclear cells (PBMC) promotes live birth rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. J Reprod Immunol 2017;119:15–22. [CrossRef]
- Maleki-Hajiagha, A.; Razavi, M.; Rezaeinejad, M.; Rouholamin, S.; Almasi-Hashiani, A.; Pirjani, R.; Sepidarkish, M. Intrauterine administration of autologous peripheral blood mononuclear cells in patients with recurrent implantation failure: A systematic review and meta-analysis. J Reprod Immunol. 2019;131:50–56. [CrossRef]
- Pourmoghadam, Z.; Abdolmohammadi-Vahid, S.; Pashazadeh, F.; Aghebati-Maleki, L.; Ansari, F.; Yousefi, M. Efficacy of intrauterine administration of autologous peripheral blood mononuclear cells on the pregnancy outcomes in patients with recurrent implantation failure: A systematic review and meta-analysis. J Reprod Immunol. 2020;137:103077. [CrossRef]
- Yakin, K.; Oktem, O. Urman B. Intrauterine administration of peripheral mononuclear cells in recurrent implantation failure: a systematic review and meta-analysis. Sci Rep. 2019;9(1):3897. [CrossRef]
- Cai, S.; Dai, S.; Lin, R.; Huang, C.; Zeng, Y.; Diao, L.; Lian, R.; Tu, W. The effectiveness and safety of intrauterine infusion of autologous regulatory T cells (Tregs) in patients with recurrent pregnancy loss and low levels of endometrial FoxP3+ cells: A retrospective cohort study. Am J Reprod Immunol. 2023 Aug;90(2):e13735. [CrossRef]
- Ban, Y.; Yang, X.; Xing, Y.; Que, W.; Yu, Z.; Gui, W.; Chen, Y.; Liu, X. Intrauterine Infusion of Leukocyte-Poor Platelet-Rich Plasma Is an Effective Therapeutic Protocol for Patients with Recurrent Implantation Failure: A Retrospective Cohort Study. J Clin Med. 2023;12(8):2823–2823. [CrossRef]
- Kong, X.; Tang, G.; Liu, Y.; Zheng, Z.; Li, Y.; Yan, F. Efficacy of intrauterine infusion therapy before embryo transfer in recurrent implantation failure: A systematic review and network meta-analysis. J Reprod Immunol. 2023;156:103819. [CrossRef]
- Deng, H.; Wang, S.; Li, Z.; Xiao, L.; Mao, Y. Effect of intrauterine infusion of platelet-rich plasma for women with recurrent implantation failure: a systematic review and meta-analysis. J Obstet Gynaecol. 2023;43(1):2144177. [CrossRef]
- Mehrafza, M.; Pourseify, G.; Zare Yousefi, T.; Azadeh, R.; Saghati Jalali, S.; Hosseinzadeh, E.; Samadnia, S.; Habibdoost, M.; Tamimi, A.; Hosseini, A. The Efficiency of Introducing Intrauterine Infusion of Autologous Platelet-Rich Plasma versus Granulocyte Colony-Stimulating Factor in Repeated Implantation Failure Patients: An Unblinded Randomised Clinical Trial. Int J Fertil Steril. 2024;18(Suppl 1):30-34. [CrossRef]
- Kumar, P.; Marron, K.; Harrity, C. Intralipid therapy and adverse reproductive outcome: is there any evidence? Reprod Fertil. 2021;2(3):173–186. [CrossRef]
- Roussev, R.G.; Acacio, B.; Ng, S.C.; Coulam, C.B. Duration of Intralipid’s Suppressive Effect on NK Cell’s Functional Activity. Am J Reprod Immunol. 2008;60(3):258–263. [CrossRef]
- Singh, N.; Davis, A.A.; Kumar, S.; Kriplani, A. The effect of administration of intravenous intralipid on pregnancy outcomes in women with implantation failure after IVF/ICSI with non-donor oocytes: A randomised controlled trial. Eur J Obstet Gynecol Reprod Biol. 2019;240:45–51. [CrossRef]
- Dakhly, D.M.R.; Bayoumi, Y.A.; Sharkawy, M.; Gad Allah, S.H.; Hassan, M.A.; Gouda, H.M.; Hashem, A.T.; Hatem, D.L.; Ahmed, M.F.; El-Khayat, W. Intralipid supplementation in women with recurrent spontaneous abortion and elevated levels of natural killer cells. Inter J Gynecol Obstet 2016;135(3):324–327. [CrossRef]
- Han, E.J.; Lee, H.N.; Kim, M.K.; Lyu, S.W.; Lee, W.S. Efficacy of intralipid administration to improve in vitro fertilization outcomes: A systematic review and meta-analysis. Clin Exp Reprod Med. 2021;48(3): 203–210. [CrossRef]
- Rimmer, M.P.; Black, N.; Keay, S.; Quenby, S.; Al Wattar, B.H. Intralipid infusion at time of embryo transfer in women with history of recurrent implantation failure: A systematic review and meta-analysis. J Obstet and Gynaecol Res. 2021;47(6):2149–2156. [CrossRef]
- Marchand, G.J.; Masoud, A.T.; Ulibarri, H.; Arroyo, A.; Coriell, C.; Goetz, S.; Moir, C.; Moberly, A.; Gonzalez, D.; Blanco, M.; Craig, H.R. Effect of a 20% intravenous fat emulsion therapy on pregnancy outcomes in women with RPL or RIF undergoing IVF/ICSI: a systematic review and meta-analysis. J Clin Transl Res. 2023 Jul 12;9(4):236-245.
- Ndukwe, G. Recurrent embryo implantation failure after in vitro fertilisation: improved outcome following intralipid infusion in women with elevated T Helper 1 response. Hum. Fertil. (Camb.). 2011; 14(2):1–8.
- Coulam, C,B. Intralipid treatment for women with reproductive failures. Am J Reprod Immunol. 2021;85(4):e13290. [CrossRef]
- Martini, A.; Jasulaitis, S.; Fogg, L.; Uhler, M.; Hirshfeld-Cytron, J. Evaluating the utility of intralipid infusion to improve live birth rates in patients with recurrent pregnancy loss or recurrent implantation failure. J Hum Reproductive Sciences. 2018;11(3):261. [CrossRef]
- Carta, G.; Iovenitti, P.; Falciglia, K. Recurrent miscarriage associated with antiphospholipid antibodies: prophylactic treatment with low-dose aspirin and fish oil derivates. Clin Exper Obstetrics Gynecol, 2005: 32(1), 49–51.
- Mu, F.; Huo, H.; Wang, M.; Wang, F. Omega-3 fatty acid supplements and recurrent miscarriage: A perspective on potential mechanisms and clinical evidence. Food Sci Nutr. 2023 Jun 1;11(8):4460-4471. [CrossRef]
- Canella, P. R. B. C.; Vinces, S. S.; Silva, Á. A. R.; Sanches, P. H. G.; Barini, R.; Porcari, A. M.; Razolli, D. S.; Carvalho, P. O. Altered profile of plasma phospholipids in woman with recurrent pregnancy loss and recurrent implantation failure treated with lipid emulsion therapy. Am J Reprod Immunol, 2023; 89(3), e13673. [CrossRef]
- Bender Atik, R.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; et al. ESHRE guideline: recurrent pregnancy loss: an update in 2022. Hum Reprod Open, 2023(1):hoad002. [CrossRef]
- Royal College of Obstetricians and Gynaecologists. The Investigation and Treatment of Couples with Recurrent First- trimester and Second-trimester Miscarriage Green-top Guideline No. 17, 2022. Available at: https://www.rcog.org.uk/media/3cbgonl0/gtg_17.pdf. Assessed December 8, 2024.
- Hamulyák, E.N.; Scheres, L.J.; Marijnen, M.C.; Goddijn, M.; Middeldorp, S. Aspirin or heparin or both for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss. Cochrane Database Syst Rev. 2020;5(5):CD012852. [CrossRef]
- Liu, X.; Qiu, Y.; Yu, E.D.; Xiang, S.; Meng, R.; Niu, K.F.; Zhu, H. Comparison of therapeutic interventions for recurrent pregnancy loss in association with antiphospholipid syndrome: A systematic review and network meta-analysis. Am J Reprod Immunol. 2020;83(4):e13219. [CrossRef]
- Grandone, E.; Tiscia, G.L.; Mastroianno, M.; Larciprete, G.; Kovač, M.; Tamborini Permunian, E.; et al. Findings from a multicentre, observational study on reproductive outcomes in women with unexplained recurrent pregnancy loss: the OTTILIA registry. Human Reproduction. 2021;36(8):2083–2090. [CrossRef]
- Aynıoglu, O.; Isik. H.; Sahbaz, A.; Alptekın, H.; Bayar, U. Does anticoagulant therapy improve adverse pregnancy outcomes in patients with history of recurrent pregnancy loss? Ginekol Pol. 2016; 87(8):585–591. [CrossRef]
- Shaaban, O.M.; Abbas, A.M.; Zahran, K.M.; Fathalla, M.M.; Anan, M.A.; Salman, S.A. Low-Molecular-Weight Heparin for the Treatment of Unexplained Recurrent Miscarriage With Negative Antiphospholipid Antibodies: A Randomized Controlled Trial. Clin Appl Thromb Hemost. 2016;23(6):567–572. [CrossRef]
- Jiang, F.; Hu, X.; Jiang, K.; Pi, H.; He, Q.; Chen, X. The role of low molecular weight heparin on recurrent pregnancy loss: A systematic review and meta-analysis. Taiwan J Obstet Gynecol. 2021;60(1):1–8. [CrossRef]
- Li, J.; Gao, Y.H.; Xu, L.; Li, Z.Y. Meta-analysis of heparin combined with aspirin versus aspirin alone for unexplained recurrent spontaneous abortion. Int J Gynaecol Obstet. 2020;151(1):23-32. [CrossRef]
- Skeith, L.; Carrier, M.; Kaaja, R.; Martinelli, I.; Petroff, D.; Schleußner, E.; Laskin, C.A.; Rodger, M.A. A meta-analysis of low-molecular-weight heparin to prevent pregnancy loss in women with inherited thrombophilia. Blood. 2016;127(13):1650–1655. [CrossRef]
- de Jong, P.; Kaandorp, S.; Di Nisio, M.; Goddijn, M.; Middeldorp, S. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev. 2014;(7):CD004734.
- Schleussner, E.; Kamin, G.; Seliger, G.; Rogenhofer, N.; Ebner, S.; Toth, B.; et al. Low-Molecular-Weight Heparin for Women With Unexplained Recurrent Pregnancy Loss. Ann Intern Med. 2015;162(9):601–609. [CrossRef]
- Karadağ, C.; Akar, B.; Gönenç, G.; Aslancan, R.; Yılmaz, N.; Çalışkan, E. Aspirin, low molecular weight heparin, or both in preventing pregnancy complications in women with recurrent pregnancy loss and factor V Leiden mutation. J Matern Fetal Neonatal Med. 2020, 33(11):1934–1939. [CrossRef]
- Lin, T.; Chen, Y.; Cheng, X.; Li, N.; Sheng, X. Enoxaparin (or plus aspirin) for the prevention of recurrent miscarriage: A meta-analysis of randomized controlled studies. Eur J Obstet Gynecol Reprod Biol. 2019;234:53–57. [CrossRef]
- Wang, G.; Zhang, R.; Li, C.; Chen, A. Evaluation of the effect of low molecular weight heparin in unexplained recurrent pregnancy loss: a meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med. 2021;35(25):7601–7608. [CrossRef]
- Scarrone, M.; Salmeri, N.; Buzzaccarini, G.; Canti, V.; Pasi, F.; Papaleo, E.; Rovere-Querini, P.; Candiani, M.; Alteri, A.; Busnelli, A.; Vanni, V.S. Low-molecular-weight heparin in the prevention of unexplained recurrent miscarriage: a systematic review and meta-analysis. Sci Rep. 2024 Jun 19;14(1):14168. [CrossRef]
- Scarrone, M.; Canti, V.; Vanni, V.S.; Bordoli, S.; Pasi, F.; Quaranta, L; et al. Treating unexplained recurrent pregnancy loss based on lessons learned from obstetric antiphospholipid syndrome and inherited thrombophilia: A propensity-score adjusted retrospective study. J Reprod Immunol. 2022;154:103760. [CrossRef]
- Quenby, S.; Booth K, Hiller, L.; Coomarasamy, A.; de Jong, P.G.; Hamulyák, E.N.; et al. Heparin for women with recurrent miscarriage and inherited thrombophilia (ALIFE2): an international open-label, randomised controlled trial. Lancet 2023;402(10395):54–61. [CrossRef]
- Giouleka, S.: Tsakiridis, I.; Arsenaki, E.; Kalogiannidis, I.; Mamopoulos, A.; Papanikolaou, E.; Athanasiadis, A.; Dagklis, T. Investigation and Management of Recurrent Pregnancy Loss: A Comprehensive Review of Guidelines. Obstet Gynecol Surv. 2023 May;78(5):287-301. [CrossRef]
- Kuroda K, Matsumura Y, Ikemoto Y, Segawa T, Hashimoto T, Fukuda J, Nakagawa K, Uchida T, Ochiai A, Horimoto Y, Arakawa A, Nojiri S, Itakura A, Sugiyama R. Analysis of the risk factors and treatment for repeated implantation failure: OPtimization of Thyroid function, IMmunity, and Uterine Milieu (OPTIMUM) treatment strategy. Am J Reprod Immunol. 2021;85(5):e13376. [CrossRef]
- Kuroda, K.; Horikawa, T.; Moriyama, A.; Ojiro, Y.; Takamizawa, S.; Watanabe, H.; Maruyama, T.; Nojiri, S.; Nakagawa, K.; Sugiyama, R. Therapeutic efficacy of the optimization of thyroid function, thrombophilia, immunity and uterine milieu (OPTIMUM) treatment strategy on pregnancy outcomes after single euploid blastocyst transfer in advanced age women with recurrent reproductive failure. Reprod Med Biol. 2023;22(1):e12554. [CrossRef]
- Mohammad-Akbari, A.; Mohazzab, A.; Tavakoli, M.; Karimi, A.; Zafardoust, S.; Zolghadri, Z.; Shahali, S.; Tokhmechi, R.; Ansaripour, S. The effect of low-molecular-weight heparin on live birth rate of patients with unexplained early recurrent pregnancy loss: A two-arm randomized clinical trial. J Res Med Sci. 2022;27:78. [CrossRef] [PubMed]
- Dolitzky, M.; Inbal, A.; Segal, Y.; Weiss, A.; Brenner, B.; Carp, H. A randomized study of thromboprophylaxis in women with unexplained consecutive recurrent miscarriages. Fertil Steril. 2006 Aug;86(2):362-6. [CrossRef]
- Naimi, A.I.; Perkins, N.J.; Sjaarda, L.A.; Mumford, S.L.; Platt, R.W,; Silver, R.M.; Schisterman, E.F. The Effect of Preconception-Initiated Low-Dose Aspirin on Human Chorionic Gonadotropin-Detected Pregnancy, Pregnancy Loss, and Live Birth: Per Protocol Analysis of a Randomized Trial. Ann Intern Med. 2021;174(5):595-601. [CrossRef]
- Mumford, S.L.; Silver, R.M.; Sjaarda, L.A.; Wactawski-Wende, J.; Townsend, J.M.; Lynch, A.M.; et al. Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Hum Reprod. 2016;31(3):657-65. [CrossRef]
- Blomqvist, L.; Hellgren, M.; Strandell, A. Acetylsalicylic acid does not prevent first-trimester unexplained recurrent pregnancy loss: A randomized controlled trial. Acta Obstet Gynecol Scand. 2018 Nov;97(11):1365-1372. [CrossRef]
- Ikemoto, Y.; Kuroda, K.; Nakagawa, K.; Ochiai, A.: Ozaki, R.; Murakami, K.; Jinushi, M.: Matsumoto, A.; Sugiyama, R.; Takeda, S. Vitamin D Regulates Maternal T-Helper Cytokine Production in Infertile Women. Nutrients. 2018 Jul 13;10(7):902. [CrossRef]
- Ota, K.; Dambaeva, S.; Kim, M.W.; Han, A.R.; Fukui, A.; Gilman-Sachs, A.; Beaman, K.; Kwak-Kim, J. 1,25-Dihydroxy-vitamin D3 regulates NK-cell cytotoxicity, cytokine secretion, and degranulation in women with recurrent pregnancy losses. Eur J Immunol. 2015;45(11):3188-99. [CrossRef]
- Ichikawa, T.; Toyoshima, M.; Watanabe, T.; Negishi, Y.; Kuwabara, Y.; Takeshita, T.; Suzuki, S. Associations of Nutrients and Dietary Preferences with Recurrent Pregnancy Loss and Infertility. J Nippon Med Sch. 2024;91(3):254-260. [CrossRef]
- Chen, X.; Yin, B.; Lian, R.C.; Zhang, T.; Zhang, H.Z.; Diao, L.H.; Li, Y.Y.; Huang, C.Y.; Liang, D.S.; Zeng, Y. Modulatory effects of vitamin D on peripheral cellular immunity in patients with recurrent miscarriage. Am J Reprod Immunol. 2016 Dec;76(6):432-438. [CrossRef]
- Tamblyn, J.A.; Pilarski, N.S.P.; Markland, A.D.; Marson, E.J.; Devall, A.; Hewison, M.; Morris, R.K.; Coomarasamy, A. Vitamin D and miscarriage: a systematic review and meta-analysis. Fertil Steril. 2022;118(1), pp.111–122. [CrossRef]
- Raghupathy, R.; Szekeres-Bartho, J. Progesterone: A Unique Hormone with Immunomodulatory Roles in Pregnancy. Int J Mol Sci, 2022; 23(3), 1333. [CrossRef]
- Lee, J.H.; Lydon, J.P.; Kim, C.H. Progesterone suppresses the mTOR pathway and promotes generation of induced regulatory T cells with increased stability. Eur J of Immunol. 2012;42(10):2683–2696. [CrossRef]
- Green, E. S.; Moldenhauer, L. M.; Groome, H. M.; Sharkey, D. J., Chin, P. Y.; Care, A. S.; Robker, R. L.; McColl, S. R.; Robertson, S. A. Regulatory T cells are paramount effectors in progesterone regulation of embryo implantation and fetal growth. JCI insight, 2023; 8(11), e162995. [CrossRef]
- Haas, D. M.; Hathaway, T. J.; Ramsey, P. S. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. The Cochrane database of systematic reviews, 2019(11), CD003511. [CrossRef]
- Devall, A.J.; Papadopoulou, A.; Haas, D.M.; Price, M.J.; Coomarasamy, A.; Gallos, I.D. Progestogens for preventing miscarriage: a network meta-analysis. Cochrane Database of Syst Rev. 2021;4(4):CD013792. [CrossRef]
- Zhao, Y.; D'Souza, R.; Gao, Y.; Hao, Q.; Kallas-Silva, L.; Steen, J.P.; Guyatt, G. Progestogens in women with threatened miscarriage or recurrent miscarriage: A meta-analysis. Acta Obstet Gynecol Scand. 2024;103(9):1689-1701. [CrossRef]
- Xie, H.; Zeng, H.; He, D.; Liu, N. Effect of intrauterine perfusion of human chorionic gonadotropin before embryo transfer after two or more implantation failures: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2019;243:133–138. [CrossRef]
- Bakry, M.S.; Eldesouky, E.; Alghazaly, M.M.; Farag, E.; Sultan, E.E.K.; Elazzazy, H.; Mohamed, A.; Ali, S.M.S.; Anwar, A.; Elrashedy, A.A.; Abdelmonem, M.; Abd-ElGawad, M.A.H. Granulocyte colony stimulating factor versus human chorionic gonadotropin for recurrent implantation failure in intra cytoplasmic sperm injection: a randomized clinical trial. BMC Pregnancy and Childbirth. 2022; 22(1):881. [CrossRef]
- Torky, H.; El-Desouky, E.S.; El-Baz, A.; Aly, R.; El-Taher, O.; Shata, A.; Hussein, A.; Marie, H.; Deif, O.; Eldemery, A.; Abo-Louz, A. Effect of Intra Uterine Granulocyte Colony Stimulating Factor vs. Human Chorionic Gonadotropin at Ovum Pick Up Day on Pregnancy Rate in IVF/ICSI Cases With Recurrent Implantation Failure. JBRA Assist Reprod. 2021;26(2):274–279. [CrossRef]
- Bellver, J.; Marín, C.; Lathi, R. B.; Murugappan, G.; Labarta, E.; Vidal, C.; et al. Obesity Affects Endometrial Receptivity by Displacing the Window of Implantation. Reproductive Sci 2021, 28(11), 3171–3180. [CrossRef]
- Gonnella, F., Konstantinidou, F.; Donato, M.; Gatta, D. M. P.; Peserico, A.; Barboni, B.; Stuppia, L.; Nothnick, W. B.; Gatta, V. The Molecular Link between Obesity and the Endometrial Environment: A Starting Point for Female Infertility. Int J Mol Sci, 2024 25(13), 6855. [CrossRef]
- Gonçalves, C. C. R. A.; Feitosa, B. M.; Cavalcante, B. V.; Lima, A. L. G. S. B.; de Souza, C. M.; Joventino, L. B.; Cavalcante, M. B. Obesity and recurrent miscarriage: The interconnections between adipose tissue and the immune system. Am J Reprod Immunol, 2023 90(3), e13757. [CrossRef]
- Ramidi, G.; Khan, N.; Glueck, C. J.; Wang, P.; Goldenberg, N. Enoxaparin-metformin and enoxaparin alone may safely reduce pregnancy loss. Trans Res J Lab Clin Med, 2009 153(1), 33–43. [CrossRef]
- Silverii, G. A. Optimizing metformin therapy in practice: Tailoring therapy in specific patient groups to improve tolerability, efficacy and outcomes. Diabetes, obesity & metabolism, 2024; 26 Suppl 3, 42–54. [CrossRef]
- Rajeev, D.; MacIver, N. J. Metformin as a Therapeutic Agent for Obesity-Associated Immune Dysfunction. The Journal of Nutrition, 2024; 154(8), 2534–2542. [CrossRef]
- Sola-Leyva, A.; Pathare, A.D.S.; Apostolov, A,; Aleksejeva, E.; Kask, K.; Tammiste, T.; Ruiz-Durán, S.; Risal, S.; Acharya, G.; Salumets, A. The hidden impact of GLP-1 receptor agonists on endometrial receptivity and implantation. Acta Obstet Gynecol Scand. 2024 Dec 18. [CrossRef]
- Maslin, K.; Alkutbe, R.; Gilbert, J.; Pinkney, J.; Shawe, J. What is known about the use of weight loss medication in women with overweight/obesity on fertility and reproductive health outcomes? A scoping review. Clinical obesity, 2024; 14(6), e12690. [CrossRef]
| Treatment | Rationale | Effect | References |
| Corticosteroids | Decrease peripheral NK cells and increase tolerogenic activity. Combined with aspirin in patients with autoimmune antibodies. Combined with aspirin and heparin in antiphospholipid syndrome. |
Decrease cytotoxic function. No suppressive effect. Increase implantation rate in IVF. Increase implantation rate and pregnancy success. No increase in live births. Increase implantation and pregnancy success. |
[238,239,241,243] [240] [244,245,246] [247,248] [249] [250,251,252,253,254] |
| Hydroxy- chloroquine |
Anti-thrombotic and immunomodulatory properties Combined with conventional treatment in antiphospholipid syndrome |
Decrease pregnancy loss. Effect dependent on dose. Enhanced Treg diminished Th17. Does not prevent further miscarriage. |
[255,256,257,258,259] [259,260,261] [262] [263] |
| Calcineurin inhibitors | Cyclosporine and Tacrolimus. Immunosuppressive agents with risk of birth defects [264,265]. Tacrolimus low dose in women with immune disorders alone or combined with heparin. Low side effects Sirolimus (rapamycin) inhibits the mTOR pathway altered in some RIF and RPL patients [279,280] |
Increase implantation and pregnancy rate. Hypertensive disorders with treatment No increase in implantation rate Increase implantation success and pregnancy outcome. Decrease Th1/Th2 ratio. Phase II clinical in altered Th17/Treg patients. Increase implantation and pregnancy success. |
[266–269 [270] [271] [272,273,274] [275,276] [277,278] [279] |
| Intravenous immunoglobulins | Inhibition of HLA antibodies decreases Fc receptor expression and modulates NK cells. | Increase pregnancy success. Better efficiency at high doses. Effective in women with immunological |
[280,281,282,283,284,285,286,287,288,289,290] [291,292,293,294,295,296,297,298,299,300,301,302,303,304] |
| Granulocyte colony-stimulating factor (G-CSF) | Tolerogenic response. Increase in Treg/IL-10 [305,306]. | Increase in pregnancy success. There is no difference compared to placebo. Subcutaneous G-CSF increases implantation success in RIF patients. Subcutaneous injections have a better effect on women's ongoing procedures. |
[307] [308] [309] [310,311,312,313] |
| Anti-TNFα | Inhibition of TNFα decreases local inflammatory milieu. | Benefit for RPL and RIF patients with autoimmune spectrum. Combined with IVIG, it increases pregnancy success. |
[314,315] [318 |
| Allogenic peripheral blood mononuclear cell (PBMC) immunotherapy | Generation of tolerogenic response to HLA antigens from the father and fetus. [319,320,321] | Increased in successful pregnancies in some trials. Benefit in primary RPL only. No beneficial effect Therapy may have complications. |
[322,323,324,326,327,330,331] [325] [328–329 [332,333] |
| Autologous Intrauterine (PBMC) | PBMC is activated by human chorionic gonadotropin to generate a local tolerogenic response. | Increase in successful pregnancy in RPL patients. Increase in Treg in patients with low endometrial Treg. |
[334,335,336,337,338] [339] |
| Intrauterine autologous platelet-rich plasma (PRP) | Decrease local inflammatory response. | No significant effects. Improved live pregnancy in RIF patients. PRP therapy was superior to G-CSF infusion. |
[340] [341,342] [343] |
| Intralipid/Intravenous lipid emulsions | Suppression of NK cytotoxic function [344,345] and probably T CD8 cells. | Increased pregnancy rate in previously failed IVF. No effect on pregnancy rate. Effective in patients with high Th1 in endometrial biopsy. No effect in patients with high endometrial NK cells |
[344,346,349] [347,348,350] [351] [352] |
| Omega-3 fatty acid oral supplementation | Decreases peroxide formation—generation of resolvins to decrease the inflammatory response. | Positive effect in antiphospholipid syndrome RPL patients with conventional treatment. | [354] |
| Low molecular weight heparin (LMWH). | Decreases thrombotic risk in patients with antiphospholipid syndrome. Used as a guideline [357,358]. |
Increase in live birth rate in RPL patients with persistent antiphospholipid antibodies. Increase in live births in patients with thrombophilia and RPL. There are no significant differences in the patients with inherited thrombophilia and heterogeneous pregnancy morbidity. No beneficial effects |
[359,360,363,364,365] [361,362,373,374] [366,370,371,372] |
| Low dose acetylsalicylic acid | A co-treatment in antiphospholipid syndrome. | Combination treatment with LMWH enhances birth rates as compared to aspirin monotherapy. | [375–380 [381,382] |
| Vitamin D | Deficiency in vitamin D is related to impaired immune response. Decreases Th17 cell population | Vitamin D deficiency is observed in RPL patients. Decrease of vitamin D in antiphospholipid syndrome |
[385] [318,387] |
| Progesterone | Decreases inflammatory response—decreases macrophage, NK, and T cell activation [388,389,390]. Suppresses mTOR pathway. | Increases pregnancy rate. No effect |
[391,393] [392] |
| Intrauterine human chorionic gonadotropin (hCG) | Induces tolerogenic milieu | Increases fertility rate, but not live birth. Lower effect than GM-CSF |
[394,396,397] [395] |
| Anti-obesity drugs | Obesity decreases fertility rates. Subclinical inflammation may be responsible for decreased implantation rate and pregnancy success [397,398,399]. | Metformin increases pregnancy success in polycystic ovary syndrome patients. |
[400,401,402] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





