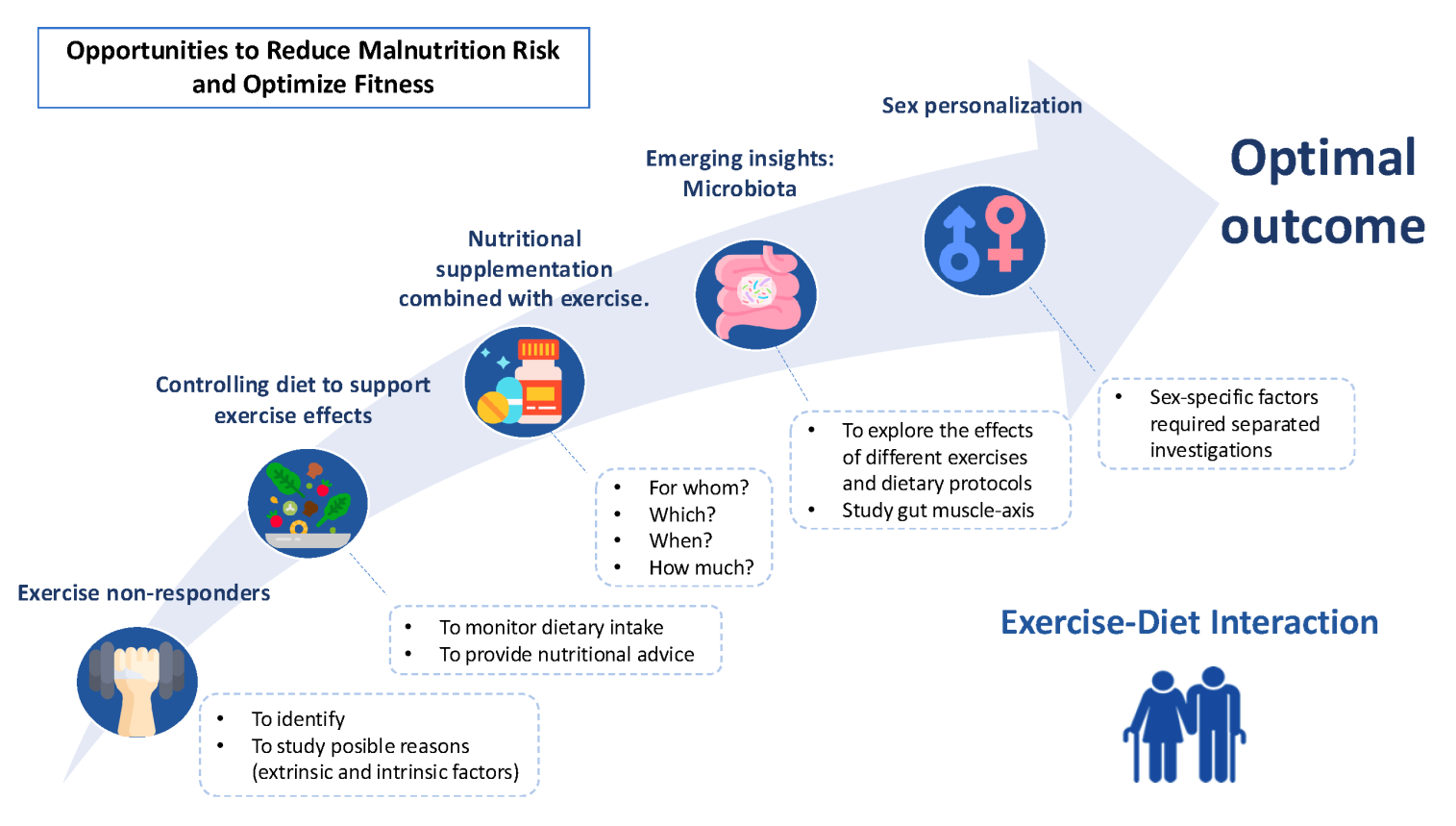
1. Introduction
Population aging is a global phenomenon that poses significant challenges to health systems and public policies. However, instead of focusing solely on longevity, the concept of healthy aging aims to ensure that older adults maintain a good quality of life, independence, and functionality for as long as possible. The World Health Organization (WHO) defines healthy aging as developing and maintaining the functional capacity that enables well-being in older age [
1,
2]. Among the multiple factors influencing healthy aging, diet and exercise are key elements. Proper nutrition can prevent chronic diseases, improve cognitive function, and maintain muscle mass, while physical activity and exercise contribute to preserving muscle mass, strength and mobility, and both are associated with autonomy and quality of life [
3,
4].
In this context, a critical challenge is malnutrition, which affects individuals with undernutrition and those with obesity and specific micronutrient deficiencies [
5]. Prevalence rates are typically under 10% in older adults living at home, but rise to as much as two-thirds among older patients who are hospitalized [
5]. On the other hand, in the case of Europe, it is estimated that 63.4% of the older population between 65-75 ages are at least overweight and close to has 20% obesity. Thus, addressing malnutrition in older adults is crucial, as its impact on health and quality of life, requiring urgent and sustained intervention [
6]. Malnutrition can exacerbate the negative effects of aging, including sarcopenia, frailty [
7,
8,
9], and an increased risk of chronic diseases. Moreover, malnutrition can also lead to a decline in physical fitness, a critical marker of mortality [
10,
11], highlighting the importance of addressing nutritional and fitness-related needs through diet and physical exercise in older adults.
For all these reasons, the main aim of this perspective article is to explore the interactions between diet and exercise, focusing on their role in maintaining muscle mass, cognitive function, and quality of life. It highlights existing knowledge gaps and proposes integrated approaches to personalize nutritional and exercise interventions to maximize their benefits for healthy ageing.
2. Exercise and Future Perspectives Related to Non-Responders
Exercise has been demonstrated to be a powerful non-pharmacological treatment to delay and reverse physiological detriments and diseases associated with ageing. Numerous studies highlight the potential benefits of multicomponent exercise programs in improving function and preventing frailty [
12,
13]. Other exercise modalities have been recommended to prevent cognitive impairment or reduce falls, such as dual-task or balance training, respectively [
14]. However, there are significant gaps in defining the best methodologies for evaluating dual-task activities [
15] and addressing these gaps is essential, as dual-task training may play a vital role in enhancing both physical and cognitive functions, which are crucial for maintaining autonomy and reducing fall risk in older adults.
Moreover, several challenges remain in exercise for older adults, including identifying non-responders—individuals who do not show the expected improvements in fitness or health outcomes after following an exercise regimen[
14]. This variability may be due to a range of factors, including intrinsic elements such as genetic predispositions, low-grade inflammation, or chronic diseases, as well as extrinsic factors like medication, baseline fitness levels, and adherence to exercise protocols [
16]. However, as discussed in subsequent sections, nutritional interaction factors, such as dietary inadequacies or gut microbiota composition, could also contribute to these non-responses.
3. Controlling Dietary Strategies to Support Exercise Effects
As the population ages, understanding dietary patterns becomes increasingly important, particularly in the context of malnutrition, which poses significant health risks for this special age group. Meeting daily energy and nutrient requirements can be challenging for this demographic population due to several factors, including reduced appetite, changes in taste and smell, dental issues, and difficulties in meal preparation [
17]. Additionally, age-related physiological changes, such as a decline in digestive efficiency and alterations in basal metabolic rate, may further complicate nutrient absorption and utilization [
5]. Social factors, such as isolation and limited access to support systems, further exacerbate these challenges, underscoring the need for a comprehensive approach to nutrition in older populations [
18].
Protein intake has been highlighted as a priority due to its crucial role in preserving muscle mass. ESPEN guidelines recommend at least an intake of 1.0-1.2 g/kg of body mass of protein, higher for those with chronic diseases and up to 2 g/kg of body mass in those with malnutrition [
19]. Moreover, it is thought that consuming approximately 25-30 g of protein per meal may enhance muscle protein synthesis more effectively than lower amounts spread across meals [
20], but this issue needs more confirmation. Various observational studies indicate that protein intake in this population is insufficient to meet their needs [
21]. A study analyzing data from the National Health and Nutrition Examination Survey (NHANES) found that low dietary protein intake is associated with functional limitations in older adults, highlighting the need for increased protein consumption in this age group [
22]. Similarly, a meta-analysis indicated that a significant proportion of community-dwelling older adults have protein intakes below the recommended levels, with many studies advocating for an increase to 1.0–1.2 g/kg of body weight per day to support health and prevent functional decline [
23,
24]. Furthermore, a longitudinal study revealed that older adults with protein intakes below 1.0 g/kg/day had a greater risk of developing mobility limitations over time [
25]. This underlines the importance of adequate protein to maintain physical function and independence in old age and, more importantly, to ensure the effect of exercise on muscle mass.
Other essential nutrients, such as vitamin D, calcium, magnesium, and B vitamins, have been observed to have diminished status [
26], potentially influencing bone and muscle adaptations to exercise. Adopting a healthy diet, such as the Mediterranean diet, could facilitate meeting these nutritional requirements. This type of diet has also been beneficial when combined with exercise in this population, as it is associated with anti-inflammatory effects [
27], reduced cardiovascular risk, and improvements in body composition [
28,
29]. Furthermore, adherence to this type of diet does not appear to be a significant challenge, as more than 80% of older adults individuals in European countries reported follow it [
30]. Consequently, some studies in older adults have begun focusing on nutritional counselling, often combined with exercise, to meet recommended dietary requirements and promote long-term adherence. Providing tailored nutritional advice may be more practical and effective than supplementation alone, serving as a first-line approach to prevent pathological conditions [
31,
32]. Future research needs to explore whether improving dietary habits in older adults is both a viable and appealing approach, offering a sustainable alternative to the seemingly easier supplementation route.
Monitoring dietary intake through 24-hour recalls before and after interventions can help evaluate the impact of exercise on appetite. This may benefit participants by addressing frailty and malnutrition. Additionally, further research is needed to explore whether meeting recommended dietary intakes enhances exercise’s effects on health outcomes. In this regard, the vast majority of exercise interventions found in the literature were conducted without considering the dietary deficiencies of older adult participants, even though this should be one of the first variables considered when analyzing intervention.
4. Nutritional Supplementation Combined with Exercise
The evidence found in the literature regarding low protein consumption has led new studies to focus on supplementation with these products [
33]. The combined effect of exercise with nutritional supplements has been studied to improve the main outcomes of body composition and physical function [
34,
35] but, to a lesser extent, quality of life [
36]. Although existing studies on protein supplementation combined with exercise are promising, there remains a significant gap in our understanding, especially concerning conditions such as malnutrition or sarcopenia [
37]. This recent meta-analysis did not find significant differences between exercise alone and various supplements in people with sarcopenic obesity. However, it did not differentiate between the types of supplements or the exercise modalities [
38]. Similarly, Shlisky et al. reported comparable findings when attempting to draw conclusions about supplementation in the context of sarcopenia, frailty, and malnutrition. They treated exercise as a rehabilitation therapy with a very low load, which likely did not provide a sufficient stimulus to induce meaningful changes in muscle strength, fat-free mass or physical function [
39]. However, when it comes to malnutrition little is known, as studies focusing on it as a primary outcome have only recently begun to emerge [
40,
41]. Exercise has already demonstrated its potential to improve nutritional status [
42], so combining exercise with nutritional supplementation could be particularly beneficial for this population, as the increase in appetite and enhanced muscle protein synthesis may lead to improved functionality and quality of life. This combination shows greater results in individuals with poorer conditions of frailty and nutritional status compared to other healthy populations [
36]. However, further research is needed to determine the optimal types and doses of exercise and supplementation to maximize the effects and improve quality of life. Different types of supplementations commonly used and worth mentioning include whey protein [
43,
44], β-hidroxi-β-metilbutirate (HMB) [
44,
45], essential amino acids (EAAs) [
46], leucine [
47], and multi-ingredient shakes [
48]. However, it is not yet clear which of these most effectively enhances exercise outcomes. Nevertheless, the International Society of Sports Nutrition has recently supported the intake of EAAs in clinical conditions and aging, emphasizing the need for further research on the EAA profile, as those richer in leucine appear to elicit a greater anabolic response [
49]. Nevertheless, a key limitation in not consuming adequate amounts of protein and micronutrients is insufficient intake of food and energy. Protein consumption without adequate energy intake is ineffective. While supplements may provide a short-term solution, this highlights the importance of addressing overall energy consumption as a critical factor for optimizing nutrition and exercise outcomes.
In addition, other types of supplements have been investigated in this population, such as omega-3 fatty acids [
50,
51], creatine, vitamin D, antioxidants or foods that are easier to consume and more user-friendly, like milk [
52]. Omega-3 fatty acids are often included due to their anti-inflammatory properties, which can help mitigate exercise-induced inflammation and promote muscle recovery [
53,
54]. Creatine is widely used for its role in enhancing strength and muscle mass by supporting energy production during high-intensity exercise, and moreover, it has been started to be studied concerning bone health and cognitive function [
55]. Vitamin D supplementation is particularly relevant for maintaining bone health, improving muscle function, and compensating for potential deficiencies common in older populations. Antioxidants are studied for their ability to counteract oxidative stress caused by exercise, potentially reducing muscle damage and fatigue [
53]. Lastly, milk and other easy-to-consume foods are considered practical options because they combine essential nutrients like protein, calcium, and other vitamins in a palatable and accessible form, making them ideal for individuals with reduced appetite or difficulty consuming complex meals [
52]. These diverse supplementation strategies underline the importance of tailored nutritional approaches, integrated with specific exercise interventions, to address both the physiological demands of exercise and the unique needs of different populations, thereby ensuring optimal health and performance outcomes. However, further research and definition are still needed to determine which supplement, which type of exercise, and which dosage to apply in each individual case in order to maximize the benefits and improve, at the very least, quality of life.
Due to the extended and different methodologies used, current evidence is inconclusive regarding which supplements, at what doses and in combination with which types of exercise are most effective for specific outcomes, such as preserving muscle mass, enhancing bone health, or reducing oxidative stress. Furthermore, the timing of supplementation and its interaction with exercise regimens are yet to be clearly defined, and these factors may play a critical role in maximizing benefits. Additional research is also needed to understand how individual factors, such as health status, comorbidities, and baseline nutritional levels, influence the effectiveness of these interventions. Ultimately, developing personalized strategies that optimize supplementation and exercise based on individual characteristics is essential to improve quality of life in this population.
5. The Need for Sex-Personalization in Nutritional and Exercise Interventions
The adaptation of exercise interventions to sex in older adults is a topic of increasing relevance in geriatric health. Research indicates notable physiological and psychological differences between older men and women, suggesting that tailored exercise programs and supplement prescriptions may be beneficial for optimizing health outcomes in this demographic. For instance, women demonstrate smaller skeletal muscle metabolic perturbations during exercise, which may influence their recovery and adaptation to training [
56]. Additionally, women have a more favorable skeletal muscle metabolism, as evidenced by a lesser decrease in adenosine triphosphate (ATP) concentrations during resistance exercise [
57]. Furthermore, cardiovascular adaptations to exercise also differ by sex, with older men showing more pronounced endothelial function improvements following endurance training than women [
58]. All of this research suggests that women might require different training intensities or modalities to achieve similar gains in muscle strength and endurance as men [
59] and probably different supplementation.
Moreover, psychological factors significantly influence exercise adherence and motivation among older adults. They often prefer exercise programs segmented according to their physical capabilities and personal preferences, which can vary significantly between sexes [
60]. Programs that foster social connections and provide clear guidance on the benefits of exercise are particularly effective in promoting adherence among older women [
61]. Thus, tailoring exercise programs to account for these sex related factors can enhance efficacy, participation rates and satisfaction. Future research should continue to explore these differences to refine exercise recommendations and improve health outcomes in older populations.
Similarly, in terms of supplementation, the effectiveness of protein supplementation may vary by sex, as men typically have higher muscle mass and may require different amounts of protein to achieve similar benefits compared to women. This is supported by findings from Kenkmann et al., which highlight the importance of considering sex when designing nutritional interventions, as older men may have different baseline nutritional needs compared to older women [
62]. Moreover, the impact of nutritional supplementation on health outcomes such as malnutrition and mortality also appear to differ by sex. Söderström et al. found that dietary advice and oral nutritional supplements did not significantly increase survival rates among older malnourished adults, but subgroup analyses indicated that malnourished individuals, particularly men, showed different responses to supplementation compared to women [
63]. Likewise, the role of specific nutrients, such as omega-3 fatty acids, has been shown to interact with exercise adaptations differently in older men and women [
64].
Sex-specific factors may influence the effectiveness of nutritional and exercise interventions, particularly in populations at risk of malnutrition. Given that older adults may respond differently to exercise and supplementation based on sex and have distinct health goals, research that acknowledges these differences and advocates for separate investigations is highly recommended.
6. Emerging Insights into the Role of Gut Microbiota
The relationship between gut microbiota, exercise, and nutrition is a complex and dynamic interplay that significantly influences human health. Research has increasingly focused on how dietary habits and physical activity or exercise can shape the gut microbiome, which in turn affects various physiological processes, including metabolism, immune function, and even mental health [
65]. Specific dietary patterns, such as the Mediterranean diet, have been shown to promote a healthy microbiome by providing a rich source of fiber, polyphenols, and healthy fats, which are beneficial for microbial growth and activity [
66,
67]. At the same time, some studies suggest that exercise can lead to a more favorable gut microbiome, which may contribute to improved metabolic outcomes, such as better glucose regulation and reduced risk of obesity [
68]. The interaction between diet and exercise can synergistically affect gut health, but it still needs more study. This interplay is particularly important in chronic diseases, where diet and exercise can be leveraged to improve gut health and overall well-being [
69]. The concept of personalized nutrition is gaining traction, emphasizing the need to tailor dietary recommendations based on individual microbiota profiles and responses to exercise [
70]. However, it is not convinced that nutrition should be personalized based on microbiota composition as it seems that plant-based dietary patterns, rich in foods with “prebiotic” functions, are the most beneficial [
71]. If this consumption is not maintained, the changes are quickly reversible. It is essential to sustain this over time. In any case, there is still much to learn in this area.
Future research should aim to address current limitations by ensuring data homogeneity in terms of age, BMI, and sex and investigating the specific effects of exercise modalities, intensities, and participant characteristics on gut microbiota. Controlling dietary intake during interventions and employing advanced sequencing technologies with detailed taxonomic resolution are essential for a deeper understanding of microbial changes and their functional implications. Finally, mechanistic studies focusing on the gut-muscle axis may elucidate the pathways linking exercise to microbiota adaptations, paving the way for more targeted and effective interventions [
72].
7. Conclusions
Combining exercise and tailored nutritional strategies offers significant potential to enhance health and well-being in older adults, yet important gaps remain. Individual variability in response to exercise, influenced by factors such as fitness level, genetics, inflammation, and nutritional deficiencies, highlights the need for personalized interventions. Integrating dietary approaches or supplementation with exercise could address age-related conditions like frailty and malnutrition, but further research is needed to define optimal protocols, including the type, dosage, and timing of interventions. Future studies should prioritize controlling dietary intake, exploring the gut-muscle axis, and addressing sex-specific responses to develop effective, targeted strategies that maximize benefits and improve quality of life in this population.
Author Contributions
Conceptualization, A.M. and G.V.-R.; investigation, A.M., L.A.M., J.A.C. and G.V.-R.; writing—original draft preparation, A.M.; writing—review and editing, L.A.M., J.A.C. and G.V.-R.; supervision, L.A.M., J.A.C. and G.V.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. World Report on Ageing and Health, /: PP - Geneva: World Health Organization; 2015. https, 2015.
- World Health Organization. Decade of Healthy Ageing: Baseline Report, 9: 2020. ISBN, 2020.
- Govindaraju T, Sahle BW, McCaffrey TA, McNeil JJ, Owen A. Dietary Patterns and Quality of Life in Older Adults: A Systematic Review. Nutrients. 2018, 10, 971. [Google Scholar] [CrossRef]
- Campbell E, Petermann-Rocha F, Welsh P, et al. The effect of exercise on quality of life and activities of daily life in frail older adults: A systematic review of randomised control trials. Exp Gerontol, 1112. [CrossRef]
- Dent E, Wright ORL, Woo J, Hoogendijk EO. Malnutrition in older adults. The Lancet. 2023, 401, 951–966. [Google Scholar] [CrossRef]
- Eurostat. Overweight and Obesity-BMI Statistics. Statistics Explained.; 2024. https://ec.europa.eu/eurostat/statisticsexplained/.
- Sieber, CC. Malnutrition and sarcopenia. Aging Clin Exp Res. 2019, 31, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Verlaan S, Ligthart-Melis GC, Wijers SLJ, Cederholm T, Maier AB, de van der Schueren MAE. High prevalence of physical frailty among community-dwelling malnourished older adults–a systematic review and meta-analysis. J Am Med Dir Assoc. 2017, 18, 374–382. [Google Scholar] [CrossRef]
- Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017, 29, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009, 301, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Erikssen, G. Physical fitness and changes in mortality: the survival of the fittest. Sports Med. 2001, 31, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Casas-Herrero Á, Sáez de Asteasu ML, Antón-Rodrigo I, et al. Effects of Vivifrail multicomponent intervention on functional capacity: a multicentre, randomized controlled trial. J Cachexia Sarcopenia Muscle. 2022, 13, 884–893. [Google Scholar] [CrossRef]
- Fernández-García ÁIván, Gómez-Cabello Alba, Moradell Ana, et al. How to Improve the Functional Capacity of Frail and Pre-Frail Elderly People? Health, Nutritional Status and Exercise Intervention. The EXERNET-Elder 3.0 Project. Sustainability. 2020, 12, 6246. [Google Scholar] [CrossRef]
- Izquierdo M, Merchant RA, Morley JE, et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J Nutr Health Aging. 2021, 25, 824–853. [Google Scholar] [CrossRef]
- Verhaeghen P, Steitz D, Sliwinski M, Cerella J. Aging and Dual-Task Performance: A Meta-Analysis. Psychol Aging. [CrossRef]
- Pickering C, Kiely J. Do Non-Responders to Exercise Exist-and If So, What Should We Do About Them? Sports Med. 2019, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clinical nutrition. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Katsas K, Mamalaki E, Kontogianni MD, et al. Malnutrition in older adults: Correlations with social, diet-related, and neuropsychological factors. Nutrition, 1106. [CrossRef]
- Volkert D, Beck AM, Cederholm T, et al. ESPEN practical guideline: Clinical nutrition and hydration in geriatrics. Clinical Nutrition. 2022, 41, 958–989. [Google Scholar] [CrossRef]
- Paddon-Jones D, Campbell WW, Jacques PF, et al. Protein and healthy aging. Am J Clin Nutr. 2015, 101, 1339S–1345S. [Google Scholar] [CrossRef] [PubMed]
- Gaytán-González A, Ocampo-Alfaro M de J, Torres-Naranjo F, et al. The Consumption of Two or Three Meals Per Day With Adequate Protein Content Is Associated With Lower Risk of Physical Disability in Mexican Adults Aged 60 Years and Older. Geriatrics. 2020, 5, 1. [Google Scholar] [CrossRef]
- Krok-Schoen JL, Price A, Luo M, Kelly O, Taylor CA. Low Dietary Protein Intakes and Associated Dietary Patterns and Functional Limitations in an Aging Population: A NHANES Analysis. J Nutr Health Aging. 2019, 23, 338–347. [Google Scholar] [CrossRef]
- Hengeveld LM, Boer JMA, Gaudreau P, et al. Prevalence of Protein Intake Below Recommended in Community-dwelling Older Adults: A Meta-analysis Across Cohorts From the PROMISS Consortium. J Cachexia Sarcopenia Muscle. 2020, 11, 1212–1222. [Google Scholar] [CrossRef]
- Mendonça N, Hengeveld LM, Visser M, et al. Low Protein Intake, Physical Activity, and Physical Function in European and North American Community-Dwelling Older Adults: A Pooled Analysis of Four Longitudinal Aging Cohorts. American Journal of Clinical Nutrition. 2021, 114, 29–41. [Google Scholar] [CrossRef]
- Mendonça N, Granic A, Hill TR, et al. Protein Intake and Disability Trajectories in Very Old Adults: The Newcastle 85+ Study. J Am Geriatr Soc. 2018, 67, 50–56. [Google Scholar] [CrossRef]
- Kehoe L, Walton J, Flynn A. Nutritional challenges for older adults in Europe: current status and future directions. Proceedings of the Nutrition Society. 2019, 78, 221–233. [Google Scholar] [CrossRef]
- Esposito K, Marfella R, Ciotola M, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporosis International. 2013, 24, 1587–1598. [Google Scholar] [CrossRef]
- Ruiz-Canela M, Zazpe I, Shivappa N, et al. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvención con DIeta MEDiterránea) trial. British Journal of Nutrition. 2015, 113, 984–995. [Google Scholar] [CrossRef]
- Cacciatore S, Calvani R, Marzetti E, et al. Low Adherence to Mediterranean Diet Is Associated With Probable Sarcopenia in Community-Dwelling Older Adults: Results From the Longevity Check-Up (Lookup) 7+ Project. Nutrients. 2023, 15, 1026. [Google Scholar] [CrossRef]
- Maderuelo-Fernandez JA, Recio-Rodriguez JI, Patino-Alonso MC, et al. Effectiveness of interventions applicable to primary health care settings to promote Mediterranean diet or healthy eating adherence in adults: A systematic review. Prev Med (Baltim), S: Suppl. [CrossRef]
- Ji W, Lee D, Kim M, et al. Efficacy of a combined exercise and nutrition intervention study for outpatients with possible sarcopenia in community-based primary care clinics (ENdSarC): study protocol for a multicenter single-blinded randomized controlled trial. BMC Geriatr. 2024, 24, 861. [Google Scholar] [CrossRef]
- Campbell WW, Deutz NEP, Volpi E, Apovian CM. Nutritional Interventions: Dietary Protein Needs and Influences on Skeletal Muscle of Older Adults. The Journals of Gerontology: Series A. [CrossRef]
- Hernández-Lepe MA, Miranda-Gil MI, Valbuena-Gregorio E, Olivas-Aguirre FJ. Exercise Programs Combined with Diet Supplementation Improve Body Composition and Physical Function in Older Adults with Sarcopenia: A Systematic Review. Nutrients. [CrossRef]
- Sirikul W, Buawangpong N, Pinyopornpanish K, Siviroj P. Impact of multicomponent exercise and nutritional supplement interventions for improving physical frailty in community-dwelling older adults: a systematic review and meta-analysis. BMC Geriatr. [CrossRef]
- Abizanda P, López MD, García VP, et al. Effects of an Oral Nutritional Supplementation Plus Physical Exercise Intervention on the Physical Function, Nutritional Status, and Quality of Life in Frail Institutionalized Older Adults: The ACTIVNES Study. J Am Med Dir Assoc. 2015, 16, e9–e439. [Google Scholar] [CrossRef]
- Wu P-Y, Huang K-S, Chen K-M, Chou C-P, Tu Y-K. Exercise, Nutrition, and Combined Exercise and Nutrition in Older Adults with Sarcopenia: A Systematic Review and Network Meta-analysis. Maturitas. [CrossRef]
- Contillo AT, Rodriguez NR, Pescatello LS. Exercise and Protein Supplementation Recommendations for Older Adults With Sarcopenic Obesity: A Meta-Review. J Aging Phys Act. 2023, 31, 878–886. [Google Scholar] [CrossRef]
- Shlisky J, Bloom DE, Beaudreault AR, et al. Nutritional Considerations for Healthy Aging and Reduction in Age-Related Chronic Disease. Adv Nutr. 2017, 8, 17–26. [Google Scholar] [CrossRef]
- Jayawardena R, Weerasinghe K, Gamage M, Hills AP. Enhancing physical function and activity level in malnourished older adults through oral nutrition supplements: a randomized controlled trial. BMC Geriatr. 2024, 24, 566. [Google Scholar] [CrossRef]
- Chen B, Zhao H, Li M, et al. Effect of multicomponent intervention on malnutrition in older adults: A multicenter randomized clinical trial. Clin Nutr ESPEN. [CrossRef]
- Moradell A, Fernández-García ÁI, Navarrete-Villanueva D, et al. Does nutritional status influence the effects of a multicomponent exercise programme on body composition and physical fitness in older adults with limited physical function? Eur J Sport Sci. 2023, 23, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Camargo L da R, Doneda D, Oliveira VR. Whey protein ingestion in elderly diet and the association with physical, performance and clinical outcomes. Exp Gerontol, 1109. [CrossRef]
- Courel-Ibáñez J, Vetrovsky T, Dadova K, Pallarés JG, Steffl M. Health Benefits of β-Hydroxy-β-Methylbutyrate (HMB) Supplementation in Addition to Physical Exercise in Older Adults: A Systematic Review with Meta-Analysis. Nutrients. [CrossRef]
- Gutiérrez-Reguero H, Buendía-Romero Á, Franco-López F, et al. Effects of multicomponent training and HMB supplementation on disability, cognitive and physical function in institutionalized older adults aged over 70 years: a cluster-randomized controlled trial. J Nutr Health Aging. 2024, 28, 100208. [Google Scholar] [CrossRef]
- Cheng H, Kong J, Underwood C, et al. Systematic review and meta-analysis of the effect of protein and amino acid supplements in older adults with acute or chronic conditions. British Journal of Nutrition. 2018, 119, 527–542. [Google Scholar] [CrossRef]
- Komar B, Schwingshackl L, Hoffmann G. Effects of leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: a systematic review and meta-analysis. J Nutr Health Aging. 2015, 19, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Moran C, Scotto di Palumbo A, Bramham J, et al. Effects of a Six-Month Multi-Ingredient Nutrition Supplement Intervention of Omega-3 Polyunsaturated Fatty Acids, vitamin D, Resveratrol, and Whey Protein on Cognitive Function in Older Adults: A Randomised, Double-Blind, Controlled Trial. J Prev Alzheimers Dis. 2018, 5, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Ferrando AA, Wolfe RR, Hirsch KR, et al. International society of sports nutrition position stand: essential amino acid supplementation on skeletal muscle and Performance. J Int Soc Sports Nutr. [CrossRef]
- Pastor R, Tur JA. Response to exercise in older adults who take supplements of antioxidants and/or omega-3 polyunsaturated fatty acids: A systematic review. Biochem Pharmacol, 1136. [CrossRef]
- Stocks J, Valdes AM. Effect of dietary omega-3 fatty acid supplementation on frailty-related phenotypes in older adults: a systematic review and meta-analysis protocol. BMJ Open. 2018, 8, e021344–e021344. [Google Scholar] [CrossRef]
- Hidayat K, Chen G-C, Wang Y, et al. Effects of milk proteins supplementation in older adults undergoing resistance training: A meta-analysis of randomized control trials. J Nutr Health Aging. 2018, 22, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Custodero C, Mankowski RT, Lee SA, et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res Rev. [CrossRef]
- Pastor R, Tur JA. Response to exercise in older adults who take supplements of antioxidants and/or omega-3 polyunsaturated fatty acids: A systematic review. Biochem Pharmacol, 1136. [CrossRef]
- Candow DG, Forbes SC, Kirk B, Duque G. Current Evidence and Possible Future Applications of Creatine Supplementation for Older Adults. Nutrients. [CrossRef]
- D’Souza AW, Takeda R, Manabe K, et al. The Interactive Effects of Age and Sex on the Neuro-cardiovascular Responses During Fatiguing Rhythmic Handgrip Exercise. J Physiol. 2023, 601, 2877–2898. [Google Scholar] [CrossRef]
- Noh, K. Effects of Resistance Exercise on Older Individuals With Sarcopenia: Sex Differences in Humans. Exercise Science. 2023, 32, 255–265. [Google Scholar] [CrossRef]
- Moreau KL, Ozemek C. Vascular Adaptations to Habitual Exercise in Older Adults: Time for the Sex Talk. Exerc Sport Sci Rev. 2017, 45, 116–123. [Google Scholar] [CrossRef]
- Boit MD, Sibson R, Meakin JR, et al. Sex Differences in the Response to Resistance Exercise Training in Older People. Physiol Rep. 2016, 4, e12834. [Google Scholar] [CrossRef]
- Jung Y-H, Park J-B, Kang A, Cho K-C. The Elderly’s Satisfaction With Physical Activity Programs in Senior Welfare Centers. Front Public Health. [CrossRef]
- Mehra S, Dadema T, Kröse BJA, et al. Attitudes of Older Adults in a Group-Based Exercise Program Toward a Blended Intervention; A Focus-Group Study. Front Psychol. [CrossRef]
- Kenkmann A, Price GM, Bolton J, Hooper L. Health, Wellbeing and Nutritional Status of Older People Living in UK Care Homes: An Exploratory Evaluation of Changes in Food and Drink Provision. BMC Geriatr. [CrossRef]
- Söderström L, Rosenblad A, Bergkvist L, Frid H, Adolfsson ET. Dietary Advice and Oral Nutritional Supplements Do Not Increase Survival in Older Malnourished Adults: A Multicentre Randomised Controlled Trial. Ups J Med Sci. [CrossRef]
- Boit MD, Sibson R, Selvaraj S, et al. Sex Differences in the Effect of Fish-Oil Supplementation on the Adaptive Response to Resistance Exercise Training in Older People: A Randomized Controlled Trial. American Journal of Clinical Nutrition. 2017, 105, 151–158. [Google Scholar] [CrossRef]
- Cryan JF, O KJ, M Cowan CS, et al. The Microbiota-Gut-Brain Axis. Physiol Rev, 1877. [CrossRef]
- Gibiino G, Siena MD, Sbrancia M, et al. Dietary Habits and Gut Microbiota in Healthy Adults: Focusing on the Right Diet. A Systematic Review. Int J Mol Sci. 2021, 22, 6728. [Google Scholar] [CrossRef]
- Gentile CL, Weir TL. The Gut Microbiota at the Intersection of Diet and Human Health. Science (1979). 2018, 362, 776–780. [Google Scholar] [CrossRef]
- Sánchez-Rodriguez E, Egea-Zorrilla A, Plaza-Díaz J, et al. The Gut Microbiota and Its Implication in the Development of Atherosclerosis and Related Cardiovascular Diseases. Nutrients. 2020, 12, 605. [Google Scholar] [CrossRef]
- Zyoud SH, Shakhshir M, Abushanab AS, et al. Mapping the Global Research Landscape on Nutrition and the Gut Microbiota: Visualization and Bibliometric Analysis. World J Gastroenterol. 2022, 28, 2981–2993. [Google Scholar] [CrossRef]
- Kolodziejczyk AA, Zheng D, Elinav E. Diet–microbiota Interactions and Personalized Nutrition. Nat Rev Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Sidhu SRK, Kok CW, Kunasegaran T, Ramadas A. Effect of Plant-Based Diets on Gut Microbiota: A Systematic Review of Interventional Studies. Nutrients. [CrossRef]
- Ortiz-Alvarez L, Xu H, Martinez-Tellez B. Influence of Exercise on the Human Gut Microbiota of Healthy Adults: A Systematic Review. Clin Transl Gastroenterol. 2020, 11, e00126. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).