1. Introduction
Hydrogen energy is a highly efficient and clean source of green energy, widely utilized for energy storage and conversion, which aids in reducing reliance on fossil fuels[
1]. The slow kinetics of the alkaline oxygen evolution reaction (OER) limit hydrogen production efficiency from water electrolysis and hinder the industrial application of related technologies, necessitating additional energy for effective reaction maintenance[
2,
3]. It is known that transition metal oxides, hydroxides (e.g., NiFeOH), and nickel-iron alloys exhibit good OER electrocatalytic performance, but their limited stability still restricts their use[
4]. Therefore, developing new catalysts that combine high performance with durability is essential. Perovskite oxides, represented by the general formula ABO
3 (where A denotes alkali or rare-earth metals and B denotes transition metals), are employed for electrocatalysis with the advantages of the low cost, versatile structures, and adjustable physicochemical properties[
5,
6]. Recently, more and more attention has been paid to highly active perovskite materials such as PrCoO
3, BaCoO
3, and SrCoO
3. Zhao et al.[
7] successfully synthesized the Pr
0.7Sr
0.3Co
0.95Ru
0.05O
3 perovskite electrocatalyst. In 1 M KOH, Pr
0.7Sr
0.3Co
0.95Ru
0.05O
3 achieved 10 mA cm
-2 with only 321 mV, and its OER overpotential was lower than that of commercial RuO
2. The Ba
0.5Sr
0.5Co
0.8Fe
0.2O
3-δ oxides were optimized using molecular orbital theory. And their intrinsic OER catalytic activity in alkaline solution was significantly higher than that of iridium oxide catalysts[
8]. In addition, perovskites are comparatively simple to synthesize, as ideal candidates for OER electrocatalysts in alkaline media.
To date, the preparing methods for perovskite materials include electrostatic spinning method, solution combustion method, and the high-temperature method[16,17,18]. Niu et al.[12] prepared the La0.6Sr0.4Co0.8Ni0.2O3-δ (LSCN-0.8) electrocatalyst using the electrostatic spinning method. LSCN-0.8 exhibited an overpotential of 327 mV in 1 M KOH at a current density of 10 mA cm-2. Saraswati et al.[13] synthesized La0.6Sr0.4Co0.8Ni0.2O3 using the solution combustion method, achieving a low overpotential of 250 mV at a current density of 10 mA cm-2. However, the perovskite materials prepared by the methods described above often suffer from grain agglomerations and inhomogeneity during the sintered process. To address this issue, some scholars have successfully synthesized perovskite materials with minimal agglomeration in a closed system at high temperature and pressure by the hydrothermal method. After hydrothermal synthesis and sintering, the average particle size of La0.8Sr0.2CrO3 is less than 5 μm, resulting in more uniform grain size and shape[14]. Thanh et al.[15] successfully synthesized LaNiO3 electrocatalysts with a porous and hollow structure via the hydrothermal method. The durability of these electrocatalysts far exceeds that of Pt/C, with the current density decreasing by 5 % after a continuous stability test lasting 10,000 seconds. In addition, perovskite materials can also be prepared by the sol-gel method. For example, Omari et al.[16] prepared LaNi1-xCoxO3 nanoparticles with a porous structure using the sol-gel method. Their optical band gap exceeds 3 eV, indicating that these perovskite materials are good semiconductors as good catalysts. Liang et al.[17] synthesized impurity-free La0.4Sr0.6Co0.8Ni0.2O3 using the sol-gel method, exhibiting fewer agglomerates and a higher density of small particles and interstitials on the La0.4Sr0.6Co0.8Ni0.2O3 surface. This result indicates that high-purity, less agglomerated perovskite materials with controllable particle sizes can be successfully prepared via the sol-gel method. However, there are limited studies regarding the preparation of the LaSrCoO3-δ system by the sol-gel method.
In general, for perovskite materials the abundance of substitutable elements at the A and B sites is crucial for perovskite oxides to achieve oxygen evolution reaction (OER) during water electrolysis. The catalytic performance of electrocatalysts can be improved by modulating the elemental composition and altering the crystal and electronic structures[18]. For example, Zhu et al.[19] synthesized a novel tetragonal structured SrCo0.95P0.05O3-δby doping phosphorus into SrCoO3-δ. The prepared SrCo0.95P0.05O3-δ materials can provide a current density of 10 mA cm-2 at a relatively small overpotential of 0.48 V. Luo et al.[20] synthesized a hexagonal structure of Ba0.9Sr0.1Co0.8Fe0.1Ir0.1O3-δ, which demonstrated good OER activity under alkaline conditions. It was due to the substitution of iridium for iron, resulting in an overpotential of 300 mV at a current density of 10 mA cm-2 and stable performance for 10 h in a 1.0 M KOH electrolyte. The SrCoO3-δ based perovskite materials exhibit some catalytic activity for OER. However, their overpotential remains high. Currently, the oxide La1-xCoxO3, a useful type of perovskite, has been applied to catalyze the alkaline oxygen evolution reaction (OER). Zheng et al[21] reported that the LaCoO3 perovskite undergoes lattice distortion in an oxygen-deficient environment, accompanied by the reconfiguration of A/B-O bonds. This reconfiguration generates more oxygen vacancies. The researchers further optimized the A-site defects by introducing Sr defects into the La-based perovskite, which enhanced oxygen mobility and improved catalytic performance of the material[22]. The results states that the perovskite material changes from a rhombic structure (LaCoO3) to a cubic structure (La0.2Sr0.8CoO3) with the gradual substitution of La by Sr. This change is accompanied by the alignment of Co-O-Co bonds and an increase in the Co oxidation state, which enhances the overlap of the energy bands of Co 3d and O 2p. Consequently, the conductivity and catalytic performance of La0.2Sr0.8CoO3 is improved. It indicates that La1-xSrxCoO3 has superior basic OER performance. In addition, Co-doping at the B-site of perovskite oxides with two transition metals is also promising. For example, Hua et al.[23] synthesized fluorine-doped La0.5Ba0.25Sr0.25CoO2.9-δF0.1. The results showed that fluorine doping activated the lattice oxygen and promoted proton and electron transfer, thereby enhancing the catalytic properties of the perovskite material. Wei et al.[24] doped La0.3Sr0.7CoO3-δ with aluminum, and they found that the surface remodeling and structure of La0.3Sr0.7CoO3-δ material and were enhanced. Notably, among the various dopants, nickel is the most effective candidate for enhancing the OER reaction[25]. For example, Han et al[26]. synthesized nickel-doped perovskite nanorods (La5Ni3Co2) by optimizing the molar ratios of La, Ni, and Co. La5Ni3Co2 achieved a current density of 10 mA cm-2 at only 0.360 V in 1.0 M KOH. Nickel doping is beneficial for catalyzing the OER reaction, and precise regulation of the catalyst properties can be achieved by modulating the Ni doping ratio. However, reports on Ni-doped LaSrCoO3-δ for alkaline OER are relatively scarce, and further studies on the catalytic performance of Ni-doped LaSrCoO3-δ under different conditions are still needed.
In this work, nickel-doped perovskites La0.5Sr0.5Co1-xNixO3-δ (x = 0.2, 0.5, 0.8) were successfully synthesized by the sol-gel method for the alkaline oxygen evolution reaction. the electrochemical measurements show that La0.5Sr0.5Co1-xNixO3-δ exhibits superior performance of OER. As a comparison, a series of La0.5Sr0.5Co0.8Ni0.2O3-δ (x = 0.2, 0.5, 0.8) was prepared using the hydrothermal method. The crystal structure of prepared perovskites La0.5Sr0.5Co1-xNixO3-δ was confirmed by powder X-ray diffraction (XRD) analysis. Additionally, the morphology of La0.5Sr0.5Co1-xNixO3-δ was characterized using field emission scanning electron microscopy (FESEM). The amount of Ni doping is optimized. XRD reveals that La0.5Sr0.5Co1-xNixO3-δ still is the perovskite structure. SEM results show that La0.5Sr0.5Co0.8Ni0.2O3-δ prepared by the sol-gel method presents a layered structure with pores dispersing on the surface and La0.5Sr0.5Co0.8Ni0.2O3-δ prepared by the hydrothermal method is spherical particles. The formed pore structures of La0.5Sr0.5Co0.8Ni0.2O3-δ increase the specific surface area and consequently enhance active sites of La0.5Sr0.5Co0.8Ni0.2O3-δ. As an OER electrocatalyst, La0.5Sr0.5Co0.8Ni0.2O3-δ prepared by the sol-gel method achieved a current density of 10 mA cm-2 at a low overpotential of 213 mV and exhibited good stability for 30 h in a 1.0 M KOH electrolyte. Furthermore, X-ray photoelectron spectroscopy (XPS) results show that high electrocatalytic performance of La0.5Sr0.5Co0.8Ni0.2O3-δ is related with changes in the electronic configuration of Co during the sol-gel preparation process.
2. Experimental Section
La0.5Sr0.5Co1-xNixO3-δ perovskite materials were prepared by the sol-gel method and the hydrothermal method, respectively. The raw materials, equipment, and calcination processes were same except using the preparation methods. In addition, the ratios of nickel and cobalt were also consistent. The primary difference is the preparation process by the precursors.
2.1. Preparation of La0.5Sr0.5Co1-xNixO3-δ Using the Sol-Gel Method
The molar ratio of cobalt and nickel was 10:1. Lanthanum nitrate (5 mmoL), strontium nitrate (5 mmoL), nickel nitrate (8 mmoL), and cobalt nitrate (2 mmoL) were dissolved in 80 mL of deionized water. Citric acid (5 mmoL) was added, and the solution was ultrasonically dispersed for 10 min. After complete dispersion, a magnetic stir bar was placed in the beaker, which was then positioned on a magnetic heating stirrer rotating at 400 rpm, maintaining a heating temperature of 95 ℃. The solution evaporated until it became viscous. Heating and stirring were stopped, and the stir bars were removed once the beaker cooled to room temperature. Subsequently, the beaker underwent further drying in a blast drying oven at 180 ℃ for 6 h. The dried material was then ground in mortar and transferred to an alumina crucible, followed by placement in a muffle furnace. The furnace temperature was ramped from room temperature to 750 ℃ at 3 ℃/min, held at 750 ℃ for 4 h, and naturally cooled to room temperature. This synthesis process was repeated for perovskite materials with x values of 0.5 and 0.8, maintaining consistent conditions, named as La0.5Sr0.5Co0.5Ni0.5O3-δ-S, La0.5Sr0.5Co0.5Ni0.8O3-δ-S, La0.5Sr0.5CoO3-δ-S, and La0.5Sr0.5Co0.2Ni0.8O3-δ-S.
2.2. Preparation of La0.5Sr0.5Co1-xNixO3-δ by the Hydrothermal Method
In a beaker containing 60 mL of deionized water, 5 mmol each of lanthanum nitrate and strontium nitrate, 8 mmoL of nickel nitrate, and 2 mmoL of cobalt nitrate were dissolved. Then, 5 mmoL of citric acid was added, and the solution was stirred continuously at 400 rpm for 2 h at room temperature. The resulting solution was transferred to a 100 mL Teflon hydrothermal reactor vessel and heated in an oven at 180 °C for 24 h. After cooling naturally, the product in the reactor liner was removed and washed with deionized water and ethanol using centrifugation. Once washing was complete, the product was placed in a blast drying oven at 110 °C for 12 h. The dried powder was then transferred to an alumina crucible, which was placed in a muffle furnace and heated at a rate of 3 °C/min until 750 °C. The powder was held at 750 °C for 4 h before cooling naturally to room temperature. The resulting material was La0.5Sr0.5Co0.8Ni0.2O3-δ. In the same preparation process, the cobalt-nickel ratio was adjusted to produce La0.5Sr0.5CoO3-δ-H, La0.5Sr0.5Co0.5Ni0.5O3-δ-H, La0.5Sr0.5Co0.8Ni0.2O3-δ-H and La0.5Sr0.5Co0.2Ni0.8O3-δ-H.
3. Results and Discussions
3.1. Materials Characterization
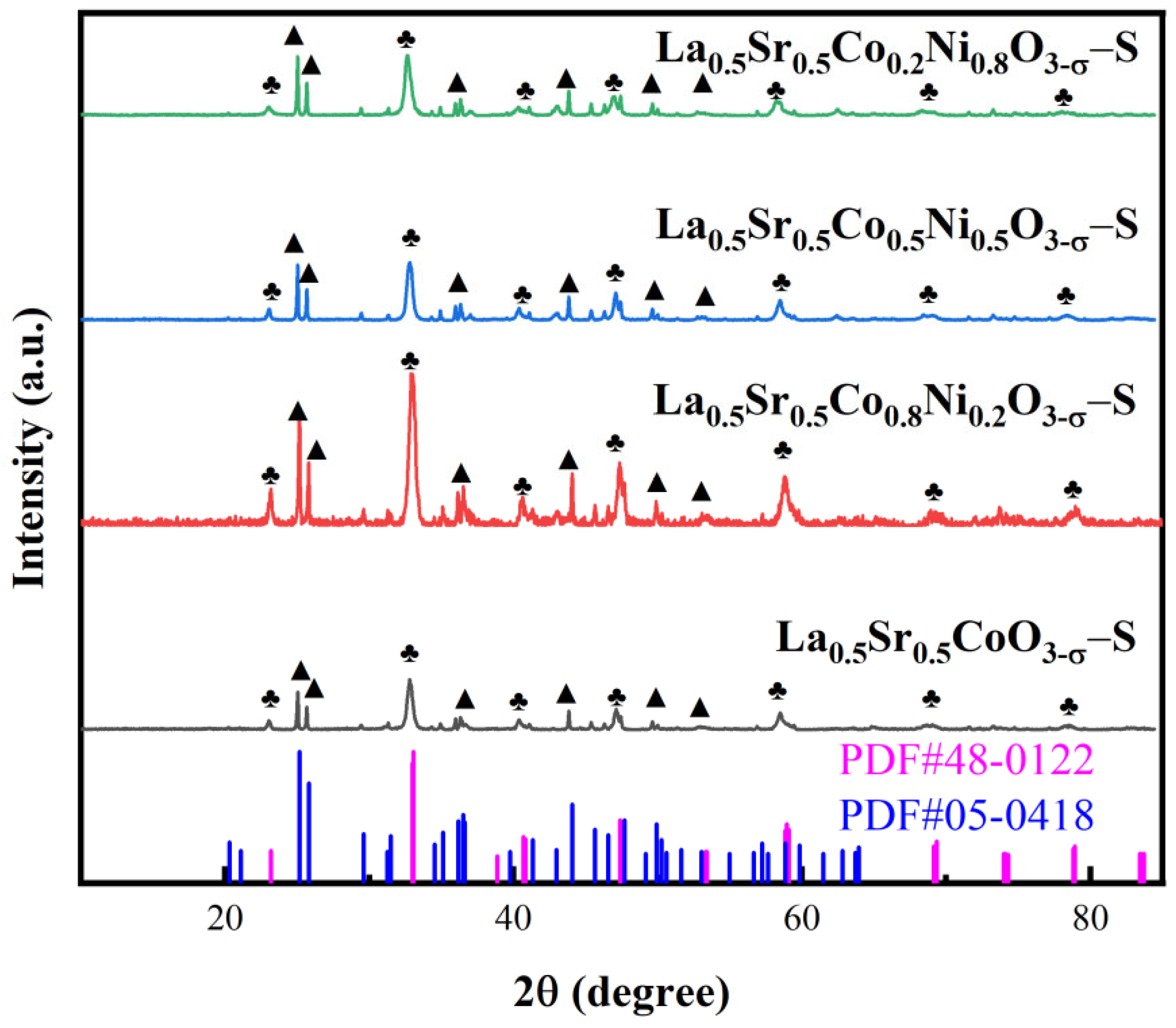
The crystal structures of La
0.5Sr
0.5Co
1-xNi
xO
3-δ prepared by sol-gel method and hydrothermal method were analyzed using XRD. As depicted in
Figure 1, the diffraction peaks observed at 23.2°, 33.1°, 40.7°, 47.4°, 58.9°, 69.3°, and 78.9° in the four samples correspond to crystallographic planes of (012), (104), (202), (024), (300), (208), and (128) of La
0.5Sr
0.5CoO
2.91 (PDF#48-0122). The diffraction peaks in the nickel-doped samples exhibit a leftward shift compared to the standard card, indicating that nickel ion doping has increased the lattice constant and expanded the crystal lattice spacing. Additionally, peaks at 25.2°, 25.8°, 36.5°, 44.1°, and 49.9° in the four samples correspond to SrCO
3 (PDF#05-0418), with crystallographic planes identified as (111), (021), (130), (221), and (113). These peaks also demonstrate a leftward shift, suggesting the coexistence of SrCO
3 alongside La
0.5Sr
0.5CoO
2.91 during the preparation process. Moreover, nickel-doped samples also exhibit small diffraction peaks at 43.0° and 62.7°. However, the La
0.5Sr
0.5CoO
2.91 crystalline structure remains predominant in all samples, indicating that the perovskite structure formed after nickel doping resembles that of the pre-doped materials. Notably, the diffraction peak of La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ is more prominent in the prepared samples.
To obtain the morphologies of La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ, Field emission scanning electron microscopy was used to analyze the morphology and surface element distribution of La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ prepared by the sol-gel method and hydrothermal method.
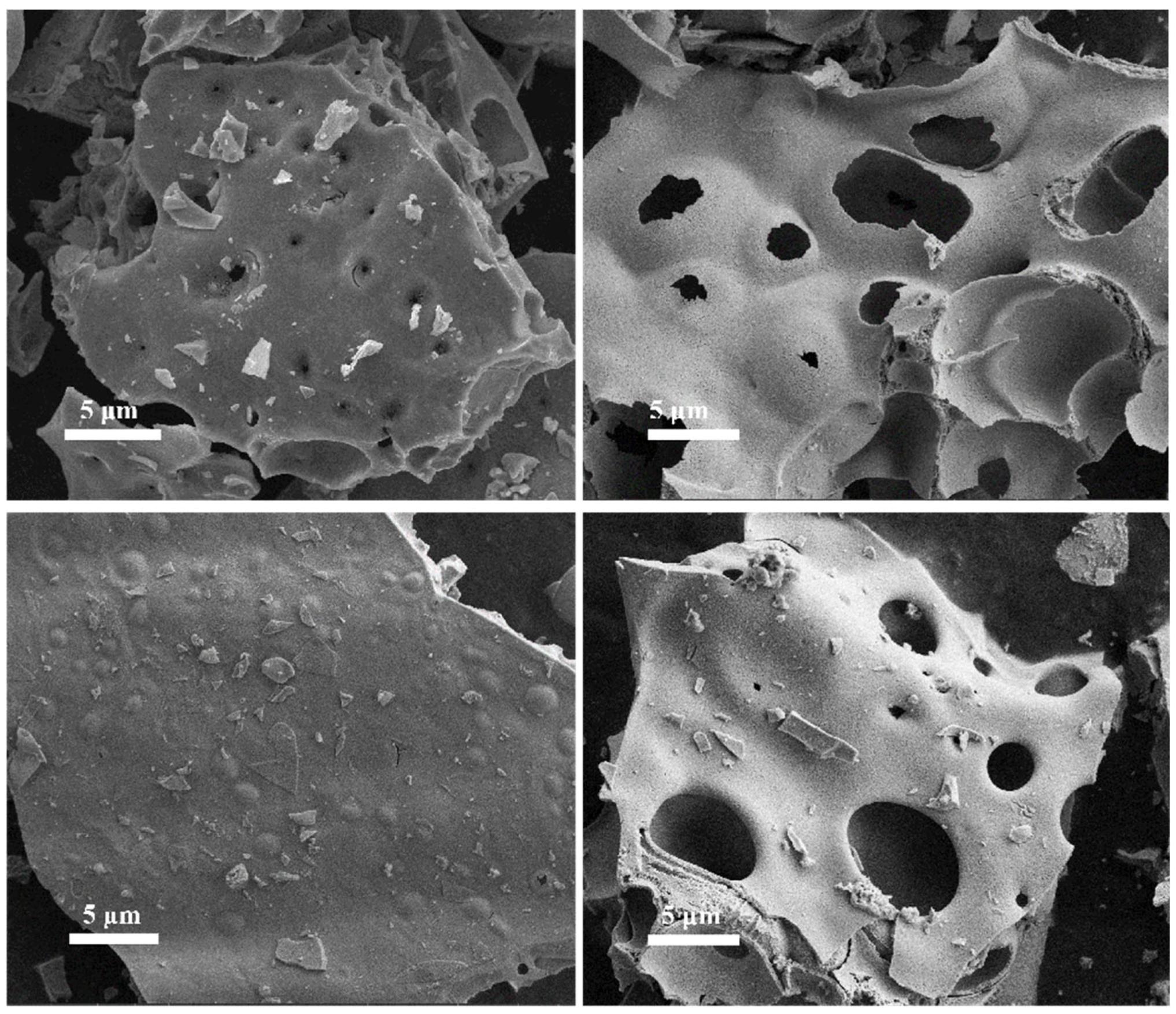
Figure 2(a-d) displays the morphology of La
0.5Sr
0.5Co
1-xNi
xO
3-δ-S series materials synthesized using the sol-gel method at low magnification, showing a lumpy and lamellar structure across all materials. During oven drying, citric acid in the gel structure swells and combusts, producing carbon dioxide that escapes. This results in the formation of porous voids in the material. The structure of La
0.5Sr
0.5Co
1-xNi
xO
3-δ consists of multiple laminated layers stacked on top of each other, with small debris on the surface without part of the main stack. Comparing materials with different nickel doping ratios, it is observed that La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-S and La
0.5Sr
0.5Co
0.5Ni
0.5O
3-δ-S have similar surfaces at low magnification. However, most blocks have several irregularly distributed, more pronounced pores on their surfaces. The La
0.5Sr
0.5Co
0.2Ni
0.8O
3-δ-S appears as broken blocks with few obvious holes, indicating gas escape during expansion and resulting in fragmentation.
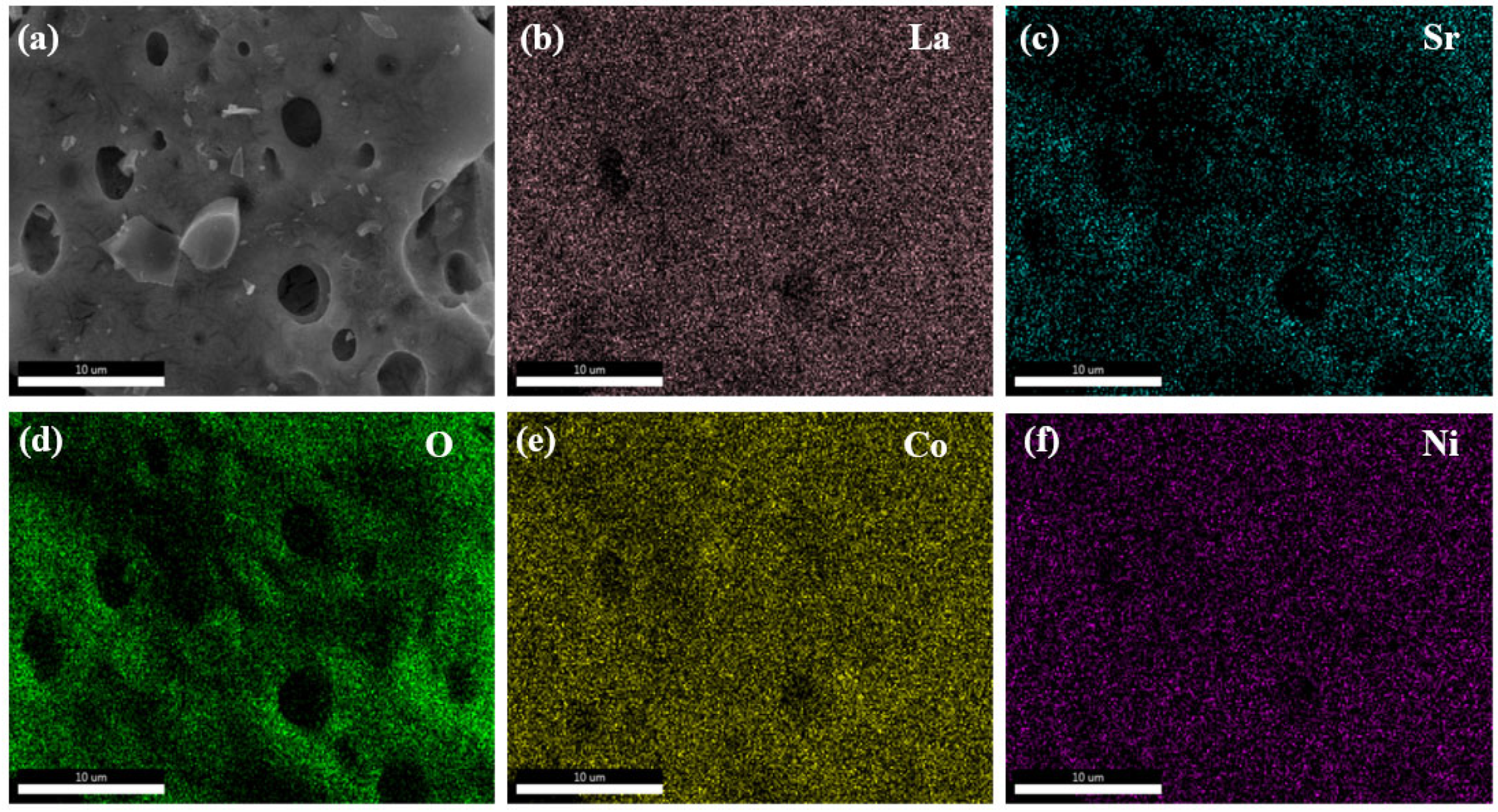
Figure 3 illustrates the elemental distribution on the surface of La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-S. The elements La, Sr, Co, Ni, and O are evenly distributed on the surface. Some elements appear shaded or in darker colors, which mostly correspond to holes in the actual sample.
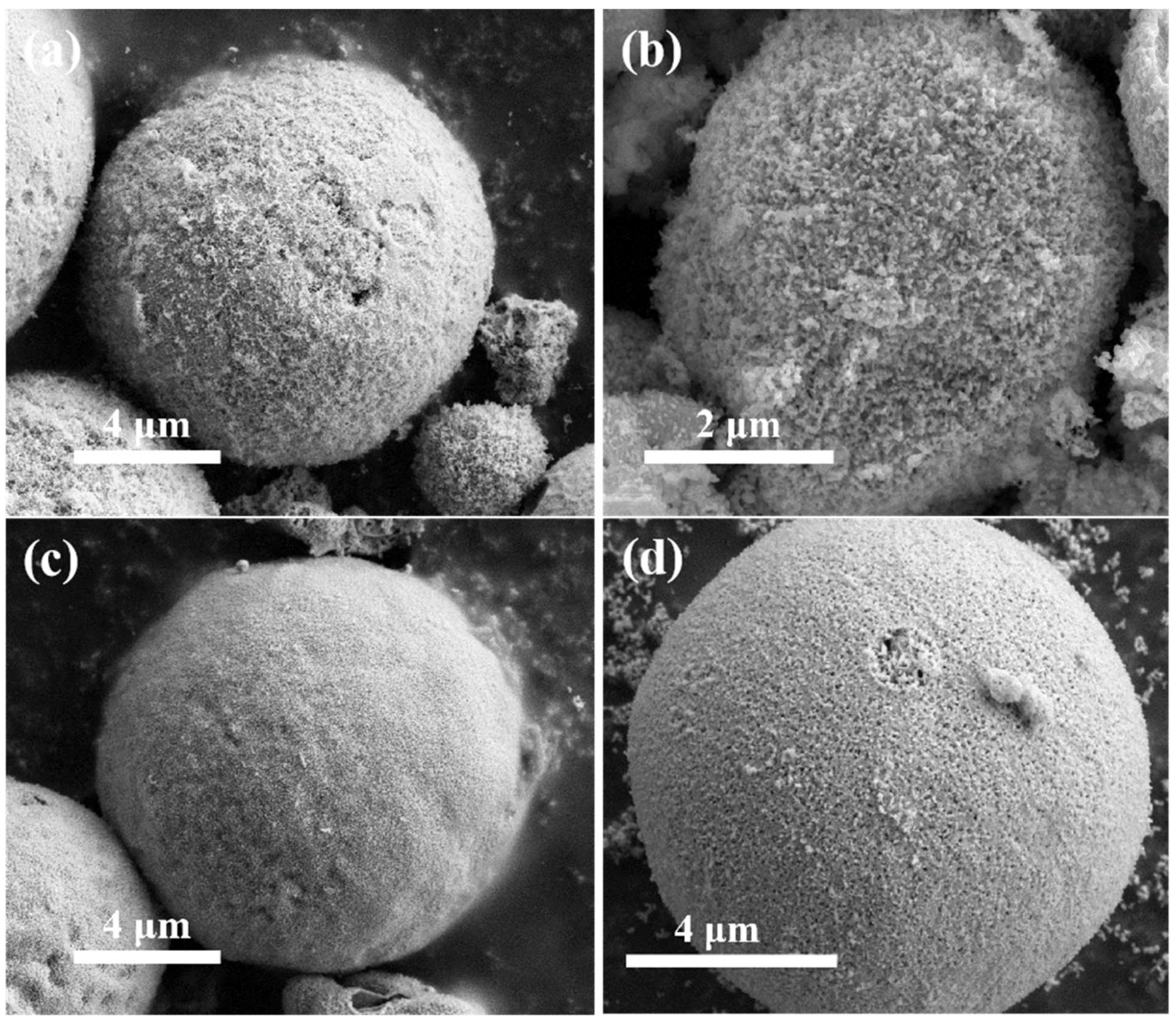
Figure 4 depicts the morphology of La
0.5Sr
0.5Co
1-xNi
xO
3-δ-H series materials synthesized using the hydrothermal method. These materials take on the shape of spherical particles. The overall volume is smaller than that of the blocks prepared by the sol-gel method, and some of the particles are broken, and there is no obvious pore structure on their surfaces. Some particles are fragmented, and their surfaces lack obvious pore structures. Comparison of the materials with different cobalt-nickel doping ratios revealed that the particles of La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-H were significantly smaller than those with other ratios. The particle structures of all four samples are indistinguishable, with particles ranging in size from micrometers and covered with dendrites. La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-H is notable for its smaller particle size and dendrites measuring 50-60 nm upon closer examination.
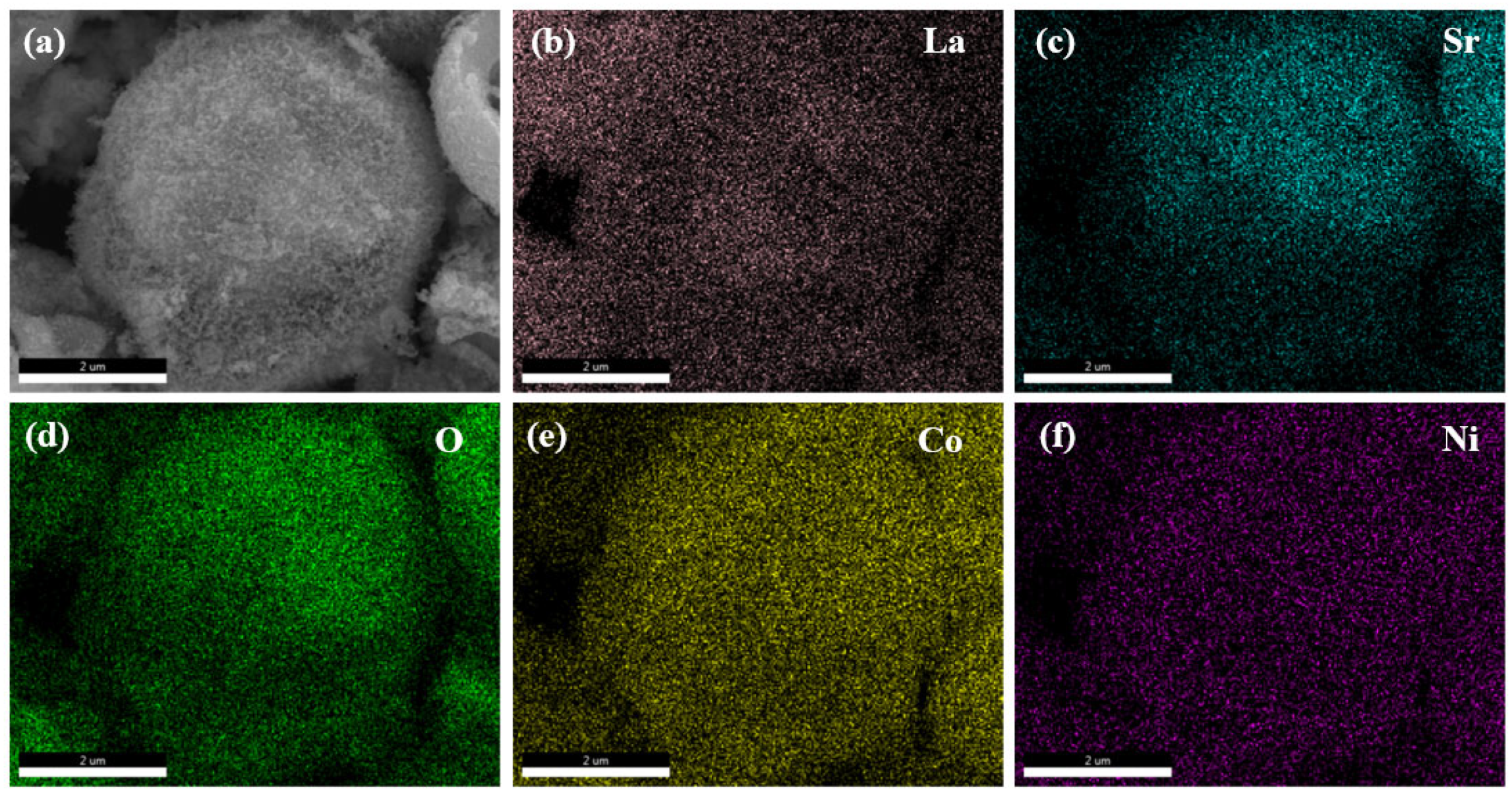
The energy dispersive spectroscopy (EDS) of hydrothermally synthesized La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-H is shown in
Figure 5. The sample surface exhibits a uniform distribution of five elements, with strontium being the least uniformly distributed among them. The prominent distribution of lanthanides suggests that a substantial fraction of these elements is concentrated on the surface of the sample.
3.2. Electrochemical Performance
The OER catalytic performance of La
0.5Sr
0.5Co
1-xNi
xO
3-δ-S was evaluated as shown in
Figure 6.
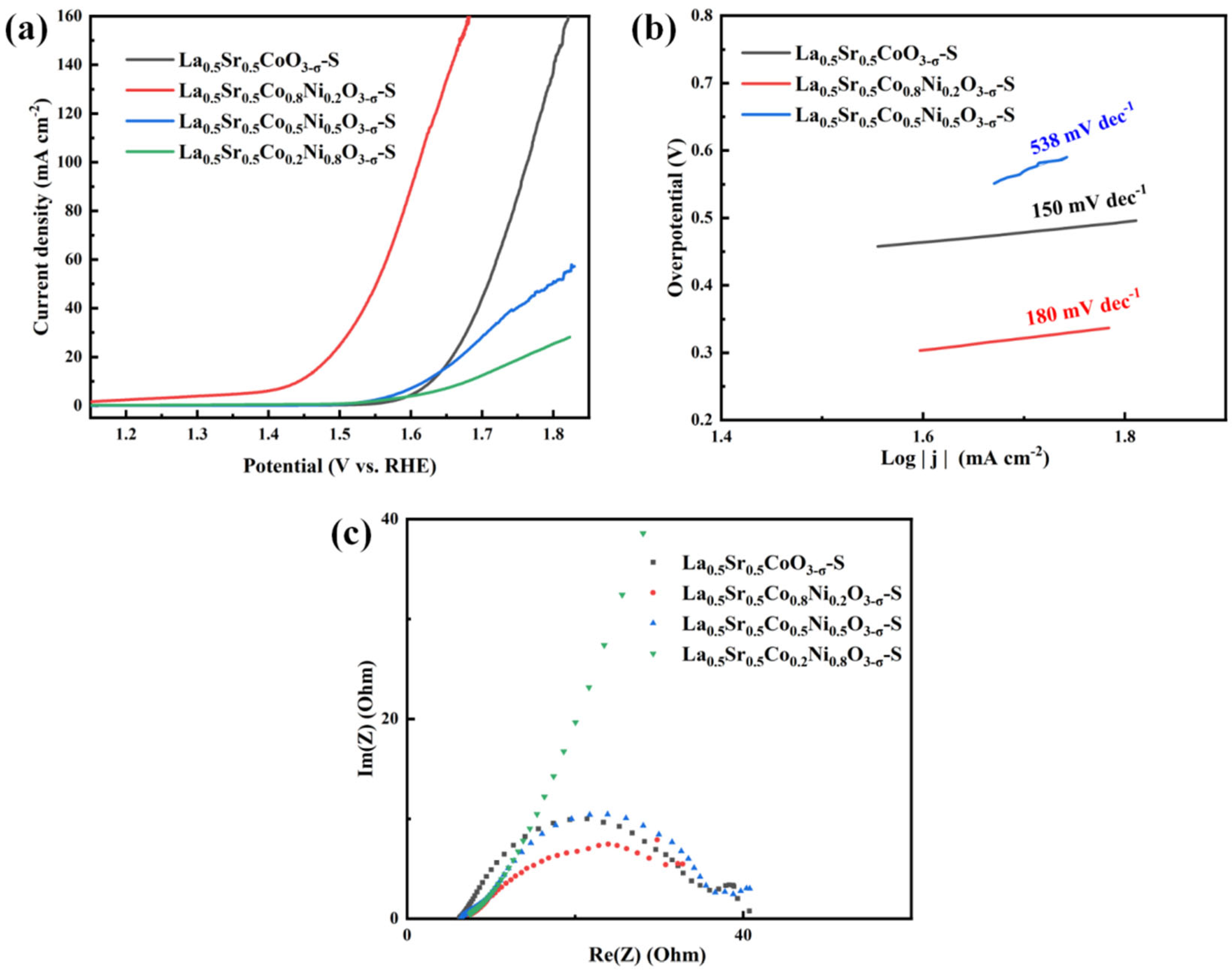
Figure 6a shows that the LSV curves indicate that La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-S requires only 213 mV and 320 mV overpotentials to achieve current densities of 10 mA cm
-2 and 50 mA cm
-2, respectively. La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-S demonstrated excellent OER performance. Subsequently, La
0.5Sr
0.5CoO
3-δ-S shows the next best performance, with current densities of 10 mA cm
-2 at 397 mV overpotential and 50 mA cm
-2 at 492 mV overpotential. La
0.5Sr
0.5Co
0.5Ni
0.5O
3-δ-S and La
0.5Sr
0.5Co
0.2Ni
0.8O
3-δ-S exhibited comparatively poor performance. The former exhibited an initial overpotential of 390 mV at 10 mA cm
-2, but the current density did not increase proportionately thereafter. The latter displayed weak trends in both initial overpotential and the subsequent increase in current density, suggesting a potential deficiency in OER catalytic performance. Tafel plots were also used to analyze reaction kinetics. La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-S showed a Tafel slope of 180 mV dec
-1, while La
0.5Sr
0.5CoO
3-δ-S exhibited a lower slope of 150 mV dec
-1. La
0.5Sr
0.5Co
0.5Ni
0.5O
3-δ-S had a Tafel slope of 538 mV dec
-1, indicating severely restricted reaction kinetics. In the impedance test, La
0.5Sr
0.5CoO
3-δ-S, La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-S, and La
0.5Sr
0.5Co
0.5Ni
0.5O
3-δ-S displayed similar electrical impedance values of 23.17 Ω,17.6 Ω, and 23.19 Ω, respectively. In contrast, La
0.5Sr
0.5Co
0.2Ni
0.8O
3-δ-S had a much higher impedance. This result mirrors the pattern observed in samples prepared via the hydrothermal method, suggesting that difficult electron transfer during catalysis significantly impacts the performance of material.
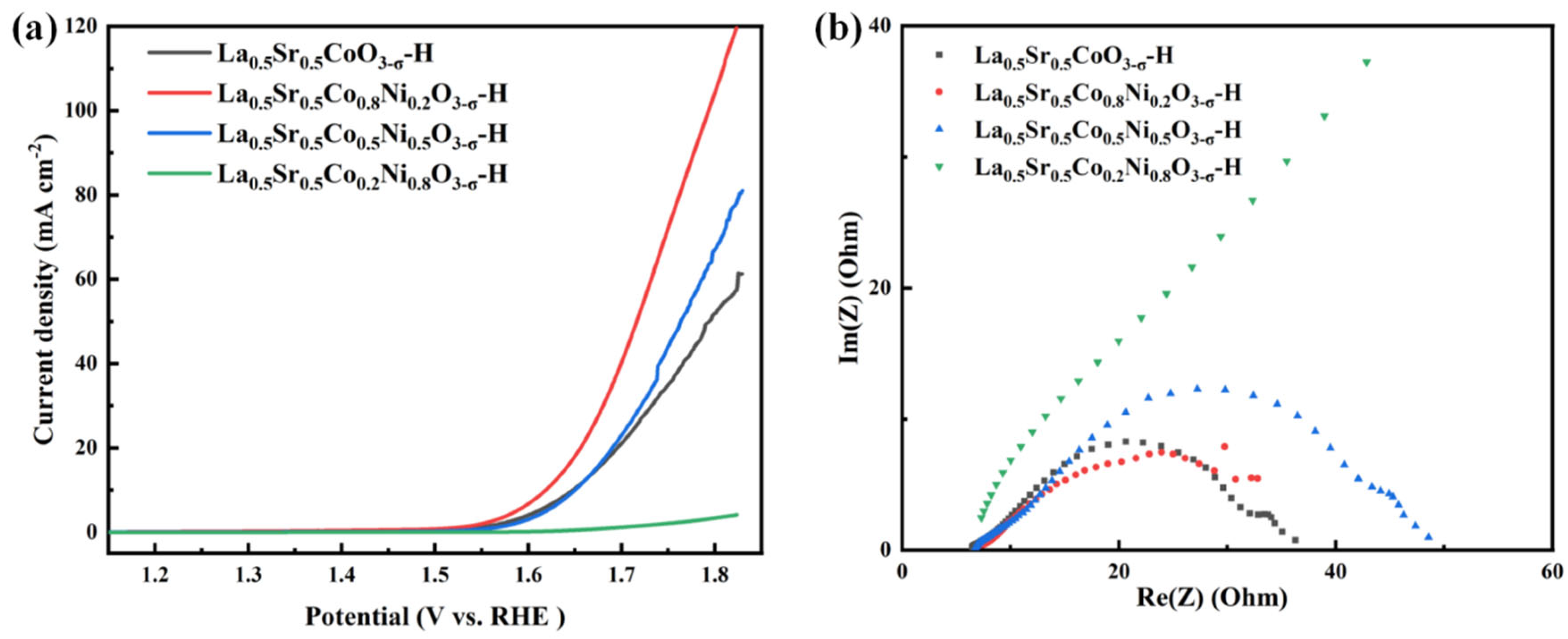
The OER catalytic performance of the La
0.5Sr
0.5Co
1-xNi
xO
3-δ-H was tested. The results are shown in
Figure 7. The LSV performance curves reveal that La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-H exhibits good performance, with current densities of 10 mA cm
-2 and 50 mA cm
-2 and overpotentials of 389 mV and 490 mV, respectively. The Tafel value of La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-H is 179 mV dec
-1. La
0.5Sr
0.5Co
0.2Ni
0.8O
3-δ-H has the worst negligible OER performance. Overall, the catalytic performance through La
0.5Sr
0.5Co
1-xNi
xO
3-δ-H is much worse than that of La
0.5Sr
0.5Co
1-xNi
xO
3-δ-S, with a lower current density. The EIS results show that the catalytic performances of La
0.5Sr
0.5Co
0.5CoO
3-δ-H, La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-H, and La
0.5Sr
0.5Co
0.5Ni
0.5O
3-δ-H have electrical impedance values of 32.22 Ω, 33.31 Ω, and 31.29 Ω, respectively, and the differences between them are small. However, La
0.5sr
0.5Co
0.2Ni
0.8O
3-δ-H also has a higher resistance, and the poor performance may be due to its extremely high electrical impedance, which hinders charge transfer during electrolysis.
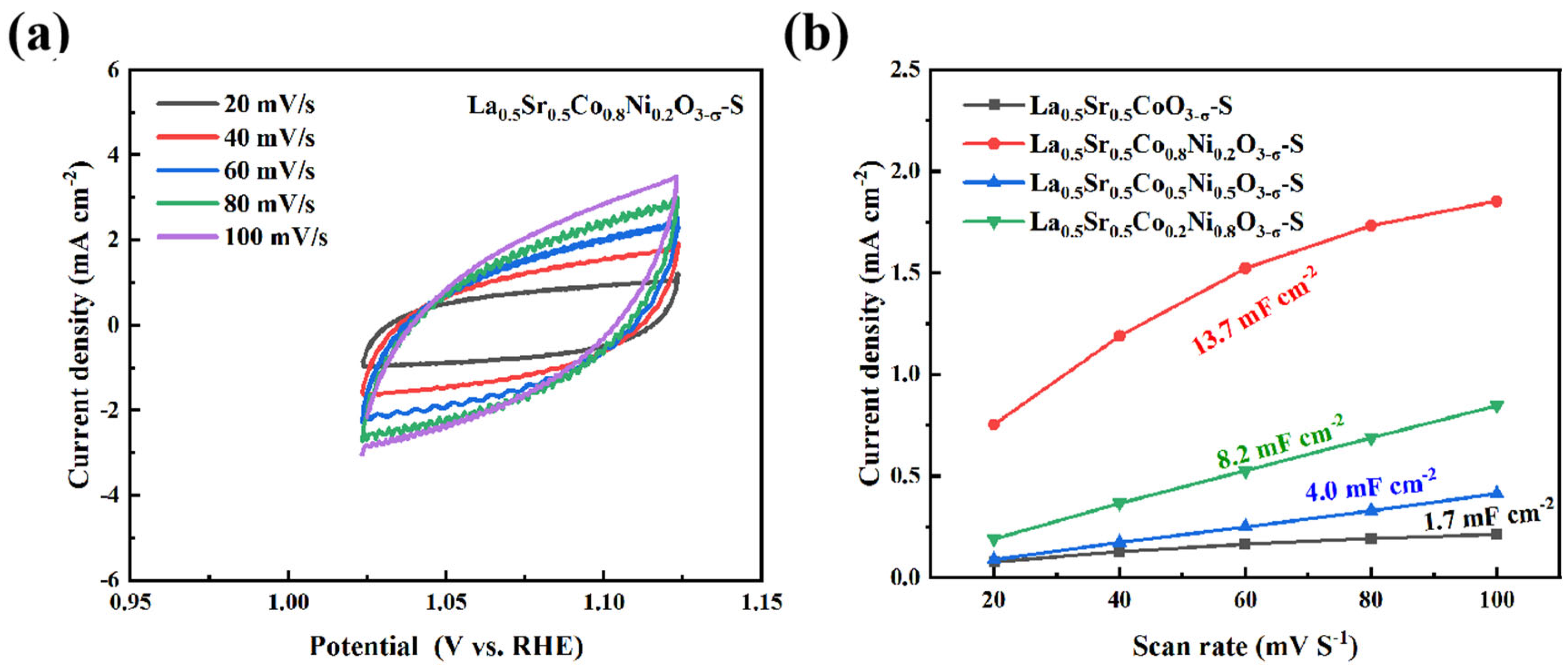
La
0.5Sr
0.5Co
1-xNi
xO
3-δ-S was scanned at various speeds to generate cyclic voltammetry curves for each series and to plot the double-layer capacitance slope. According to
Figure 8, La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-S exhibits the highest capacitance among the catalysts in this series, measuring 13.7 mF cm
-2. La
0.5Sr
0.5Co
0.2Ni
0.8O
3-δ-S follows closely with 8.2 mF cm
-2. La
0.5Sr
0.5Co
0.5Ni
0.5O
3-δ-S and La
0.5Sr
0.5CoO
3-δ-S exhibit lower capacitances of 4.0 mF cm
-2 and 1.7 mF cm
-2, respectively. Combined SEM and double-layer capacitance analyses revealed that the morphology of the catalyst significantly affects the electrochemically active surface area, thereby influencing the OER.
During the CV test, the cyclic voltammetry curves of the La0.5Sr0.5Co1-xNixO3-δ-H show remarkably low current densities. Despite its superior oxygen evolution performance, La0.5Sr0.5Co0.8Ni0.2O3-δ-H exhibits current densities less than one-tenth of those La0.5Sr0.5Co0.8Ni0.2O3-δ-S. The double-layer capacitance for La0.5Sr0.5Co0.8Ni0.2O3-δ-H are negligible according to CV calculations.
The comparison of SEM results from the two methods, using the double-layer capacitance test, revealed that the La0.5Sr0.5Co0.8Ni0.2O3-δ-S forms a porous structure during preparation. This improvement enhances the catalyst's specific surface area, thereby increasing the number of active sites. In contrast, La0.5Sr0.5Co0.8Ni0.2O3-δ-H prepared by the hydrothermal method show either complete or broken particle morphologies, with larger particle sizes and no change in specific surface area. Comprehensive analyses of bilayer capacitance tests indicate that the controllable preparation of the material morphology can have an impact on the catalytic activity of the material.
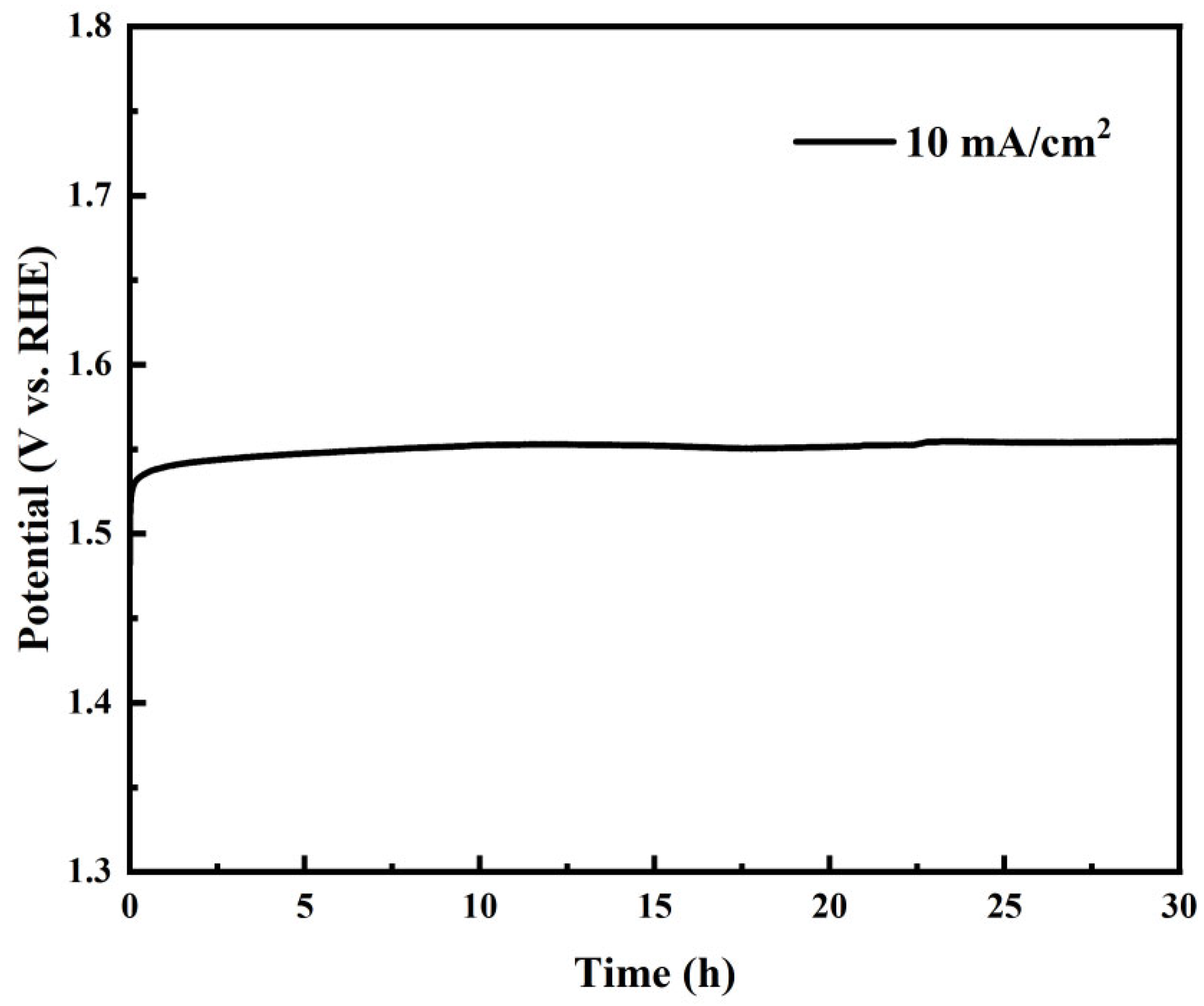
Stability testing of La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-S demonstrated the best OER catalytic performance. Nickel foam was selected as the substrate to prevent catalyst detachment and oxidation of the glassy carbon electrode surface during stability testing for oxygen evolution reactions. The catalyst was drop-coated onto the nickel foam, ensuring a consistent loading per unit area equivalent to that on the glassy carbon electrode during testing.
Figure 9 shows that La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-S exhibits excellent catalytic stability even without pre-activation. Its potential remained stable, varying by approximately 10 mV over 30 h, demonstrating its robustness in alkaline conditions for OER.
3.3. XPS Analysis of La0.5Sr0.5Co0.8Ni0.2O3-δ by the Sol-Gel Method
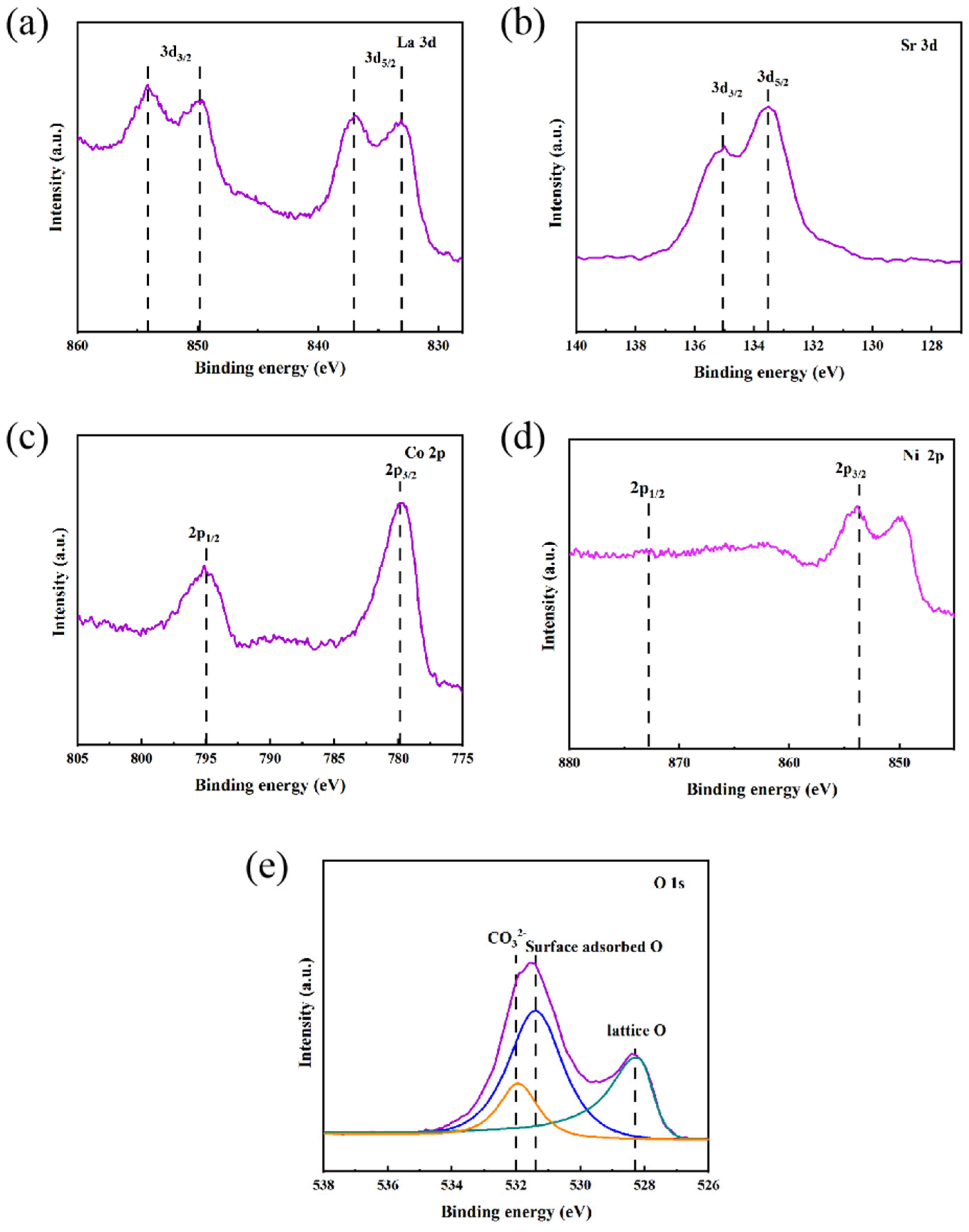
Valence analysis of La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-S prepared by the sol-gel method using XPS was conducted.
Figure 10 illustrates the binding energy peaks for La 3d, Sr 3d, Co 2p, Ni 2p, and O 1s. The La 3d peaks at 833.5 eV and 837.1 eV correspond to the 3d
5/2 orbitals, while the 3d
3/2 orbitals appear at 849.7 eV and 854.2 eV, all indicating the presence of La
3+. The Sr 3d peaks at 133.5 eV and 135.1 eV relate to the 3d
5/2 and 3d
3/2 orbitals of Sr
2+, respectively. In the Co 2p region, peaks at 779.6 eV and 794.9 eV correspond to the 2p
3/2 and 2p
1/2 orbitals of Co
3+. Although the overlapping La peaks obscure the Ni 2p features, the positions for Ni
2+ can be approximately identified. The O 1s peaks reveal distinct components: lattice oxygen (O
2-) at 528.4 eV, surface-adsorbed oxygen at 531.6 eV, and CO
32- at 532 eV, with the oxygen p-band center positioned near the Fermi level. Nickel doping is proposed to increase the B-O distance, affecting the electronic structure of Co and enhancing the binding energy of Co
3+, which may improve electronic conductivity and catalytic activity[27]. This doping also leads to a left shift in diffraction peaks, indicating an increase in the lattice constant and spacing, which collectively enhances the ionic and electronic conductivity. Overall, the synergistic effects of lattice expansion, modifications in electronic structure, and improved oxygen mobility contribute to the superior performance of La
0.5Sr
0.5Co
0.8Ni
0.2O
3-δ-S, reflecting its potential for applications requiring high catalytic efficiency and stability.
4. Conclusions
In summary, nickel-doped perovskite oxides La0.5Sr0.5Co1-xNixO3-δ-S (x = 0.2, 0.5, 0.8) were synthesized using sol-gel methods. SEM and EDS results indicate that the La0.5Sr0.5Co1-xNixO3-δ-S has numerous surface pores, a multilayered laminar structure, and a homogeneous distribution of surface elements, in contrast to the spherical granular samples La0.5Sr0.5Co1-xNixO3-δ-H prepared by the hydrothermal method. Furthermore, La0.5Sr0.5Co0.8Ni0.2O3-δ-S requires only 213 mV and 320 mV overpotentials to achieve current densities of 10 mA cm-2 and 50 mA cm-2, respectively. La0.5Sr0.5Co0.8Ni0.2O3-δ-S shows excellent stability in alkaline solutions, and the potential change remains within about 10 mV after 30 h of stabilization tests. Comprehensive XRD and XPS analyses showed that nickel doping extended the lattice and increased the B-O spacing, which changed the electronic configuration of cobalt and increased the oxygen mobility during sol-gel process. As a result, the catalytic performance of the La0.5Sr0.5Co0.8Ni0.2O3-δ-S material for OER is improved. La0.5Sr0.5Co0.8Ni0.2O3-δ-S is a highly efficient, durable, and cost-effective electrocatalyst for water splitting in alkaline solutions. It offers valuable insights for improving the OER catalytic performance of non-precious metal perovskite materials in the future.
Funding
This research was funded by the National Natural Science Foundation of China (No. 22278125) and the Central Guidance on Local Science and Technology Development Fund of Hebei Province (Grant No. 246Z4412G), and the key scientific research project of Suzhou university (No: 2022xhx251, 2024yzd18).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- S. Han, J. Park, J. Yoon. Surface reconstruction of Co-based catalysts for enhanced oxygen evolution activity in anion exchange membrane water electrolysis. Advanced Functional Materials 34 (2024) 2314573. [CrossRef]
- H. Wang, H. Wang, Q. Hu, B. Wang, X. Lei, J. You, R. Guo, The latest advances in the deep reconstruction of pre-catalysts for the oxygen evolution reaction, Journal of Alloys and Compounds 1010 (2025) 177225. [CrossRef]
- S. Zhu, L. Song, Z. Xu, F. Chen, H. Tao, X. Tang, Y. Wang, Theoretical and experimental aspects of electrocatalysts for oxygen evolution reaction, Chemistry - A European Journal 30 (2024) e202303672. [CrossRef]
- X. Xie, L. Du, L. Yan, S. Park, Y. Qiu, J. Sokolowski, W. Wang, Y. Shao, Oxygen evolution reaction in alkaline environment: Material challenges and solutions, Advanced Functional Materials 32 (2022) 2110036. [CrossRef]
- X. Liang, K.-X. Zhang, Y.-C. Shen, K. Sun, L. Shi, H. Chen, K.-Y. Zheng, X.-X. Zou, Perovskite-type water oxidation electrocatalysts, Journal of Electrochemistry 28 (2022) 2214004.
- J. Hwang, R.R. Rao, L. Giordano, Y. Katayama, Y. Yu, Y. Shao-Horn, Perovskites in catalysis and electrocatalysis, Science 358 (2017) 751-756. [CrossRef]
- Y.-N. Zhao, C. Liu, S. Xu, S. Min, W. Wang, N. Mitsuzaki, Z. Chen, A/B-site management strategy to boost electrocatalytic overall water splitting on perovskite oxides in an alkaline medium, inorg. Chem. 62 (2023) 12590-2599.
- J. Suntivich, K.J. May, H.A. Gasteiger, J.B. Goodenough, Y. Shao-Horn, A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles, Science 334 (2011) 1383-1385. [CrossRef]
- M.-C. Sung, C.H. Kim, B. Hwang, D.-W. Kim, Rationalizing the catalytic surface area of oxygen vacancy-enriched layered perovskite LaSrCrO4 nanowires on oxygen electrocatalyst for enhanced performance of Li-O2 batteries, Carbon Energy 6 (2024) e550. [CrossRef]
- M. Christy, H. Rajan, H. Yang, Y.-B. Kim, Optimizing the surface characteristics of La0.6Sr0.4CoO3-δ perovskite oxide by rapid flash sintering technology for easy fabrication and fast reaction kinetics in alkaline medium, Energy Fuels 34 (2020) 16838-16846.
- C. Cao, C. Shang, X. Li, Y. Wang, C. Liu, X. Wang, S. Zhou, J. Zeng, Dimensionality control of electrocatalytic activity in perovskite nickelates, Nano Letter 20 (2020) 2837-2842. [CrossRef]
- Y. Niu, X. Chang, M. Zhang, J. Mu, Surface reconstruction of La0.6Sr0.4Co0.8Ni0.2O3-δ perovskite nanofibers for oxygen evolution reaction, Ceramics International 50 (2024) 13014-13021.
- S. Roy, T. Yoshida, A. Kumar, S.M. Yusuf, C. Chakraborty, S. Roy, Tailoring Co site reactivity via Sr and Ni doping in LaCoO3 for enhanced water splitting performance, Catalysis Today 441 (2024) 114885. [CrossRef]
- J.C. Rendón-Angeles, K. Yanagisawa, Z. Matamoros-Veloza, M.I. Pech-Canul, J. Mendez-Nonell, S.D. la Torre, Hydrothermal synthesis of perovskite strontium doped lanthanum chromite fine powders and its sintering, Journal of Alloys and Compounds 504 (2010) 251-256. [CrossRef]
- T.D. Thanh, N.D. Chuong, J. Balamurugan, H. Van Hien, N.H. Kim, J.H. Lee, Porous hollow-structured LaNiO3 stabilized N,S-Codoped graphene as an active electrocatalyst for oxygen reduction reaction, Small 13 (2017).
- E. Omari, S. Makhloufi, M. Omari, Preparation by sol-gel method and characterization of Co-doped LaNiO3 perovskite, J Inorg Organomet Polym 27 (2017) 1466-1472. [CrossRef]
- D. Liang, H. Huang, J. Liu, H. Wang, Preparation and modification of La0.4Sr0.6Co1-xNixO3 (x = 0-0.8) perovskite oxide for bi-functional catalysis in alkaline medium, Inorganic Chemistry Communications 127 (2021) 108533. [CrossRef]
- Y. Liu, H. Huang, L. Xue, J. Sun, X. Wang, P. Xiong, J. Zhu, Recent advances in the heteroatom doping of perovskite oxides for efficient electrocatalytic reactions, Nanoscale 13 (2021) 19840-19856. [CrossRef]
- Y. Zhu, W. Zhou, J. Sunarso, Y. Zhong, Z. Shao, Phosphorus-doped perovskite oxide as highly efficient water oxidation electrocatalyst in alkaline solution, Advanced Functional Materials 26 (2016) 5862-5872. [CrossRef]
- Q. Luo, D. Lin, W. Zhan, W. Zhang, L. Tang, J. Luo, Z. Gao, P. Jiang, M. Wang, L. Hao, K. Tang, Hexagonal perovskite Ba0.9Sr0.1Co0.8Fe0.1Ir0.1O3-δ as an efficient electrocatalyst towards the oxygen evolution reaction, ACS Appl. Energy Mater. 3 (2020) 7149-7158.
- H. Zheng, Y. Zhang, Y. Wang, Z. Wu, F. Lai, G. Chao, N. Zhang, L. Zhang, T. Liu, Perovskites with enriched oxygen vacancies as a family of electrocatalysts for efficient nitrate reduction to ammonia, Small 19 (2023) 2205625. [CrossRef]
- J.T. Mefford, X. Rong, A.M. Abakumov, W.G. Hardin, S. Dai, A.M. Kolpak, K.P. Johnston, K.J. Stevenson, Water electrolysis on La1-xSrxCoO3-δ perovskite electrocatalysts, Nat Commun 7 (2016) 11053.
- B. Hua, M. Li, W. Pang, W. Tang, S. Zhao, Z. Jin, Y. Zeng, B. Shalchi Amirkhiz, J.-L. Luo, Activating p-blocking centers in perovskite for efficient water splitting, Chem 4 (2018) 2902-2916. [CrossRef]
- Y. Wei, Y. Hu, P. Da, Z. Weng, P. Xi, C.-H. Yan, Triggered lattice-oxygen oxidation with active-site generation and self-termination of surface reconstruction during water oxidation, Proc Natl Acad Sci U S A 120 (2023) e2312224120. [CrossRef]
- [25] K. Bera, A. Karmakar, K. Karthick, S. S. Sankar, S. Kumaravel, R.Madhu, S. Kindu, Enhancement of the OER Kinetics of the Less-Explored α-MnO2 via Nickel Doping Approaches in Alkaline Medium, Inorganic Chemistry 60 (2021) 19429-19439.
- [26] Y. Han, Z. Zhu, L. Huang, Y. Guo, Y. Zhai, S. Dong, Hydrothermal Synthesis of Polydopamine functionalized Cobalt doped Lanthanum Nickelate Perovskite Nanorod for Efficient Water Oxidation in Alkaline Solution, Nanoscale 41 (2019) 19579-19585. [CrossRef]
- F. Dong, L. Li, Z. Kong, X. Xu, Y. Zhang, Z. Gao, B. Dong, M. Ni, Q. Liu, Z. Lin, Materials engineering in perovskite for optimized oxygen evolution electrocatalysis alkaline condition, Small 17 (2020) 2006638. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).