1. Introduction
Mycoplasma pneumoniae is a well-recognized respiratory pathogen affecting children, adolescents, and adults that can cause community-acquired pneumonia severe enough to require hospitalization [
1,
2]. The organism has a slow incubation period ranging from one to four weeks and is associated with cyclic epidemics occurring every three to five years [
3,
4]. This extended incubation period may contribute to prolonged outbreaks, allowing the infection to spread undetected over time. The cyclical nature of these regular
M. pneumoniae outbreaks is likely influenced by changes in population immunity. Following an outbreak, a significant proportion of those infected typically develops immunity, temporarily reducing the number of susceptible individuals and decreasing the potential for widespread transmission until immunity in the population wanes.
Several studies have observed a decrease in
M. pneumoniae infections during the COVID-19 pandemic, likely due to public health measures such as masking, physical distancing, and reduced social interactions. Globally, a subsequent rebound in number of infections to the pre-pandemic levels beginning in 2023 was observed [
5,
6,
7]. Reemergence of
M. pneumoniae infections, producing local outbreaks, have been reported in France and Switzerland [
8,
9,
10]. In the United States, the Centers for Disease Control and Prevention (CDC) reported an increase in
M. pneumoniae infections among children and adolescents starting in the fall of 2023, although the number of cases remained below the pre-COVID-19 pandemic levels [
11]. Additionally, in October 2024, the CDC issued an alert regarding an increase in the percentage of
M. pneumoniae test positivity across all age groups nationwide, with the most significant increase observed among children [
12].
In the city of Louisville, Kentucky, we identified an increase in hospitalized adult patients with M. pneumoniae as part of an ongoing local respiratory infection surveillance study. Because of this local finding, we decided to analyze national data with the goal to define if the local increase reflected a broader outbreak of M. pneumoniae infections within the United States.
2. Materials and Methods
This was a retrospective analysis of the United States Epic Cosmos database [
13,
14]. Epic Cosmos is a de-identified research database created in collaboration with a community of Epic health systems representing over 287 million patient records from over 1,600 hospitals and 36,000 clinics from all 50 states in the United States and Washington D.C. as of the study period. More than 11 billion patient interactions with an Epic-using medical facility (encounters such as a clinic visit, emergency department visit, or hospitalization) from January 1, 2017 to September 30, 2024 were queried for analysis.
A patient was defined as having a
M. pneumoniae infection if they met the following criteria: 1) they had a patient encounter at an Epic-using facility in the United States and 2) they had a positive test for
M. pneumoniae during that encounter. A positive test for
M. pneumoniae was identified using specified Logical Observation Identifiers Names and Codes (LOINC) [
14] for nucleic acid amplification tests (see
Table S1 in the supplementary materials).
Patients with encounters during the timeframe of January 1, 2017, to September 30, 2024, were included in this analysis. Infections documented before the emergence of the COVID-19 pandemic were defined as baseline infections from the timeframe of January 1, 2017, to March 31, 2020. Infections documented after the COVID-19 pandemic were defined as current infections from the timeframe of July 1, 2022, to September 30, 2024.
To identify the presence of an outbreak, all patients with
M. pneumoniae were aggregated in annual quarters and depicted in count charts (c-charts) [
15]. Additionally, c-charts were created for pediatric (age <18) and adult (age ≥18) patients. Expected control limits were derived from the baseline infection period. The mean number of monthly cases (µ) and standard error (SE) were calculated for the baseline infections, with the upper control limit (UCL) set at 3 SE above the mean. The presence of an outbreak was defined as any quarter above the baseline UCL.
The number of excess cases for each annual quarter, if any, was calculated by taking the number of cases in excess of the baseline UCL. Cumulative excess cases for all patients, pediatric patients, and adult patients were calculated and depicted in line graphs. Additionally, excess cases for patients hospitalized with M. pneumoniae pneumonia were calculated. Cumulative excess cases of all patients, pediatric patients, and adult patients hospitalized with M. pneumoniae pneumonia were also depicted in line graphs. The magnitude of excess cases was defined as the fold increase in excess cases above the baseline UCL for the quarter with the highest excess.
Patient demographics of age, sex, race, and ethnicity were compared between current infections and baseline infections. Severity of disease for baseline and current infections was measured by 1) the number of patients hospitalized with pneumonia, as defined by an admission using tenth International Classification of Disease (ICD-10) codes to define pneumonia, 2) the number of patients requiring mechanical ventilation, defined by Current Procedural Terminology (CPT) codes, and 3) the number of patients who died, defined by the Epic-reported disposition (see
Tables S2 and S3 in the supplementary materials for ICD-10 and CPT codes).
C-chart epidemic curves and line graphs were produced to visualize data. Data was aggregated by quarter. Baseline and current patient characteristics and severity of disease were compared using Chi-Squared tests of independence. Groups with less than or equal to 10 observations were censored as ≤10 to prevent identification of data. P-values of less than 0.05 were considered statistically significant. All data analysis was performed using R version 4.4.2.
3. Results
A total of 17,454 patients with
M. pneumoniae infection were identified, with 3,062 during the baseline period, and 14,007 during the current period (see
Figure S1 Study Flow Diagram). Of these patients, 3,946 were hospitalized with
M. pneumoniae pneumonia, with 1,000 patients hospitalized during the baseline period and 2,830 hospitalized during the current period.
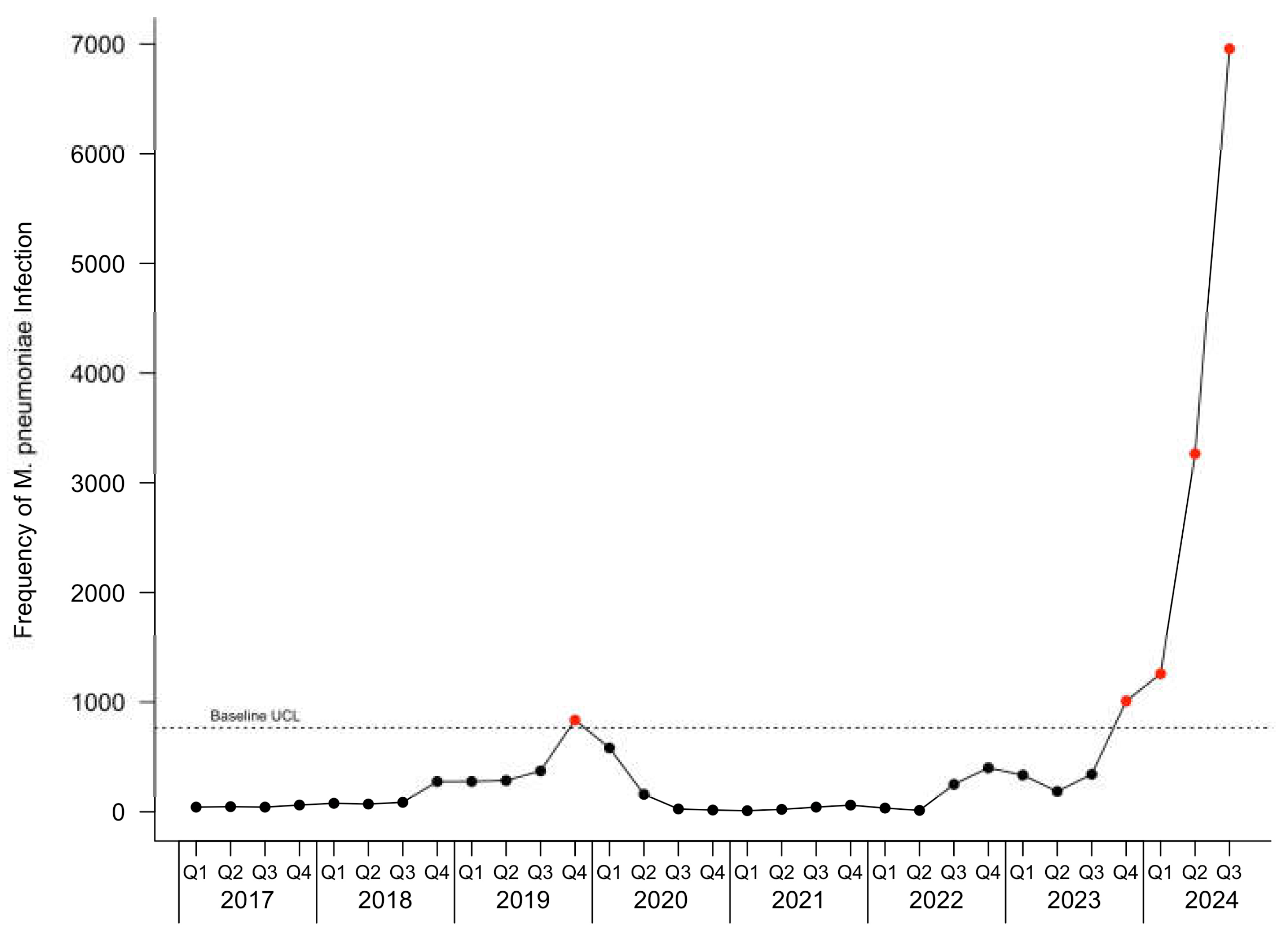
The c-chart for patients with
M. pneumoniae infection is depicted in
Figure 1.
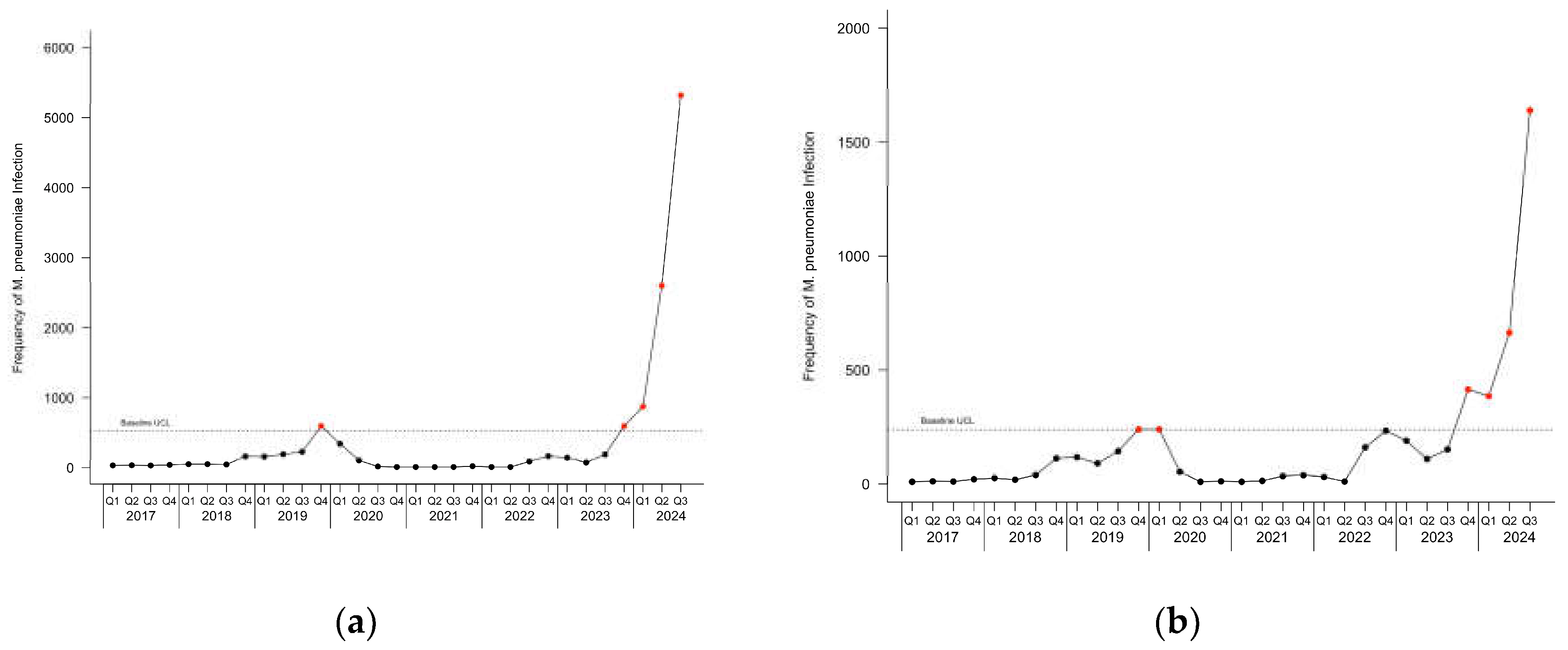
Figure 2 depicts the c-charts for pediatric and adult patients with
M. pneumoniae infection. During the baseline period, one point was observed above the UCL in the fourth quarter of 2019 for all patients and pediatric patients, and two points were observed above the UCL in the fourth quarter of 2019 and the first quarter of 2020 in adult patients. During the current infection period, an outbreak started in the fourth quarter of 2023 and continued through the last observed quarter.
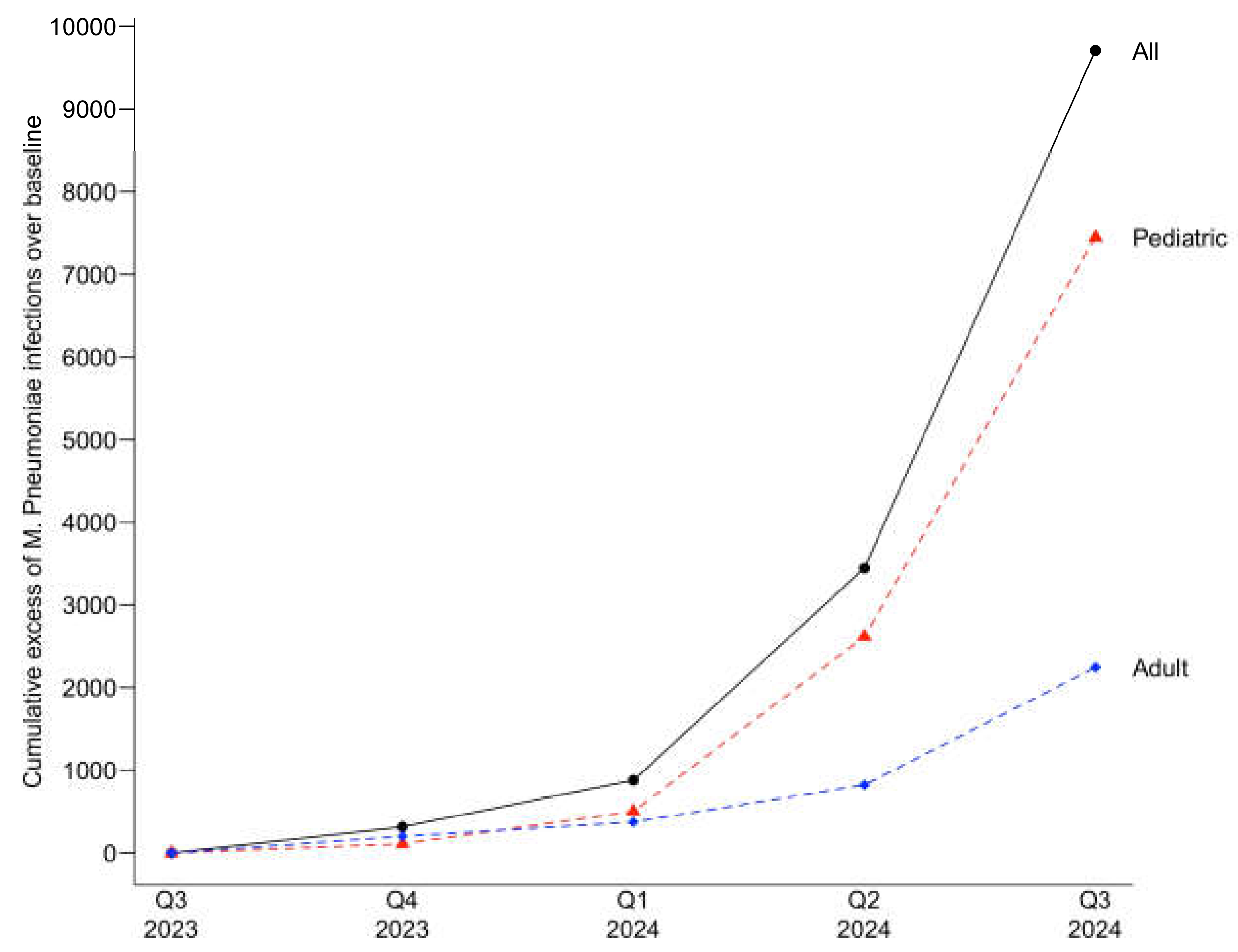
The cumulative excess case count for the current infection period was 9,708 for all patients. The highest increase above the UCL, representing a 9.0-fold increase, was observed in the third quarter 2024, the last quarter evaluated in the current infection period in this study. For pediatric patients, the cumulative excess was 7,450, with a 10.0-fold increase over baseline UCL observed in the third quarter of 2024. For adults, the cumulative excess was 2,264, with a 6.6-fold increase over the baseline UCL also in the third quarter of 2024.
Figure 3 depicts the cumulative excess cases for the current infection period.
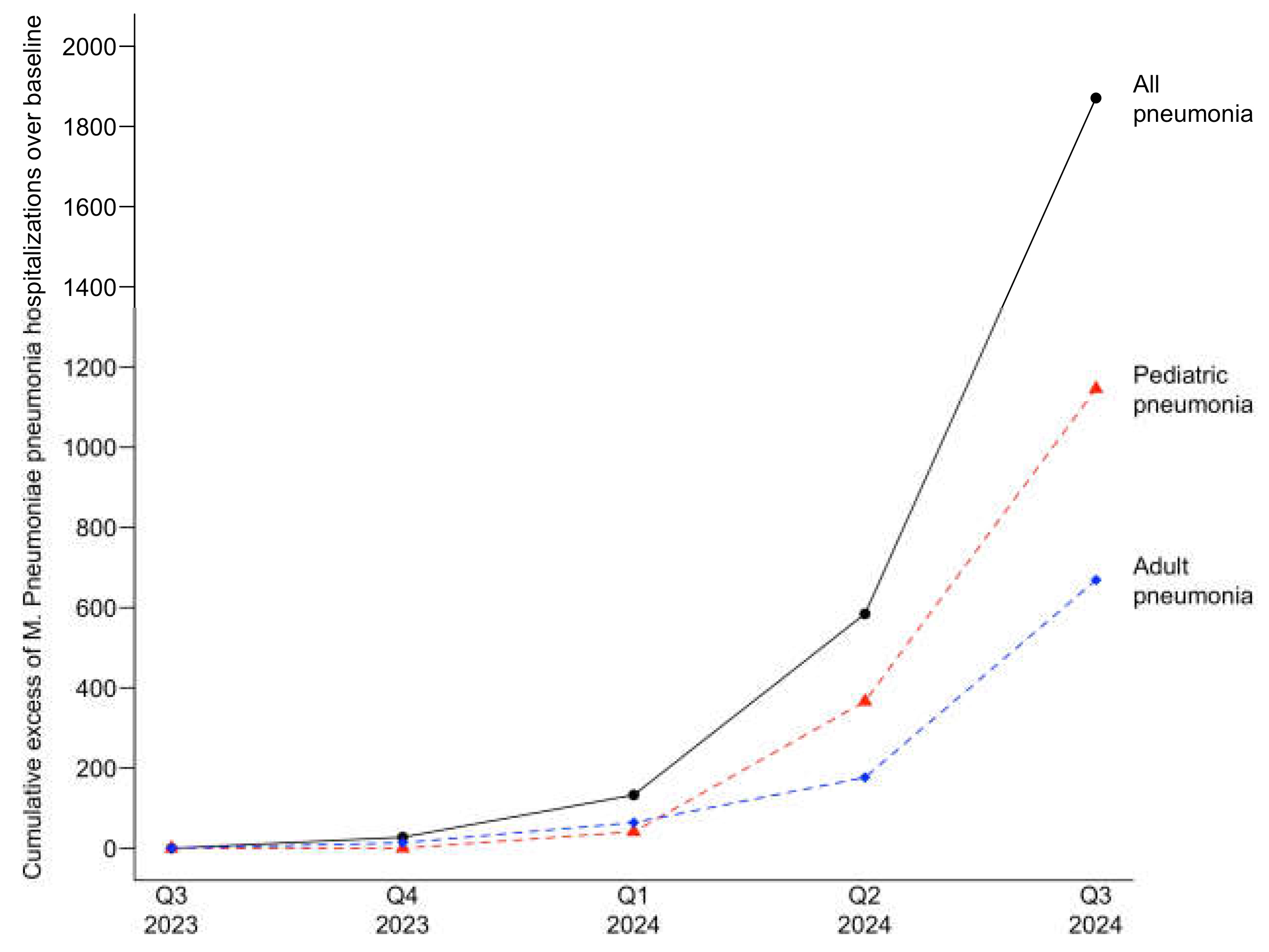
Excess cases of patients hospitalized with
M. pneumoniae pneumonia were also observed for the current infection period. Cumulative excess cases of were 1,870 for all patients, 1,147 for pediatric patients, and 670 for adult patients, with a 7.7-fold, 8.6-fold, and 5.4-fold increase over baseline UCL, respectively, observed in the third quarter of 2024.
Figure 4 depicts the cumulative excess cases of patients hospitalized with
M. pneumoniae pneumonia for the current infection period.
Pediatric and adult patient demographics comparing baseline and current infection periods are depicted in
Table 1. Overall, patient characteristics for both populations were similar between baseline and current infection periods.
Severity of disease for all patients is depicted in
Table 2. A smaller proportion of patients with
M. pneumoniae were hospitalized during the current infection period. Need for mechanical ventilation and death were similar among baseline and current infection periods.
4. Discussion
This study provides evidence of an ongoing outbreak of M. pneumoniae infections in the United States that began in late 2023 and is affecting both pediatric and adult populations. To our knowledge, this is the first report of the current outbreak in the United States. Given the absence of a plateau in the number of new cases, it is likely that this outbreak will extend into the 2024-2025 winter pneumonia season. Analysis of the outbreak, including the number and magnitude of excess cases, indicates that the pediatric population is disproportionally more affected compared to the adult population.
Similar demographic characteristics were observed in patients with M. pneumoniae infection in the current and baseline periods, suggesting that the current outbreak is affecting a similar patient population. We did not document an increase in the severity of disease for M. pneumoniae infections in the current outbreak considering the lack of increase in the number of patients with pneumonia requiring hospitalization, the number of patients with need for mechanical ventilation and the number of deaths compared to the pre-COVID-19 baseline period.
Data from our study suggest that a regular cyclic outbreak of
M. pneumoniae was developing in the United Sates by late 2019 but was abruptly interrupted by the occurrence of the COVID-19 pandemic. This interruption was likely due to the introduction of non-pharmacological interventions against COVID-19. At the end of 2019, outbreaks of
M. pneumoniae infections were also reported in several countries in Europe and Asia [
16]. Detection of
M. pneumoniae in the United Sates decreased significantly during the period of the COVID-19 pandemic. A decrease of other bacterial respiratory pathogens such as
Streptococcus pneumoniae and
Haemophilus influenzae was also reported during the COVID-19 pandemic period [
17]. This decline in infections likely resulted in decreased global population immunity, making the global community more susceptible to
M. pneumoniae infections as COVID-19 related restrictions were lifted. Although our data documents an ongoing
M. pneumoniae outbreak in the United States, it is possible that similar outbreaks are occurring in other regions with limited surveillance capabilities, leading to underdiagnosis on a global scale.
The current outbreak is associated with a significant increase in cases of
M. pneumoniae community-acquired pneumonia, with patients of all ages requiring hospitalization for care. This resurgence has several implications for clinical practice: 1) Physicians should maintain a high index of suspicion for
M. pneumoniae as a cause of respiratory infections, particularly in patients presenting with community-acquired pneumonia; 2) The diagnosis of
M. pneumoniae infections is challenging since the organism is not detectable on Gram stain, due to the lack of cell wall, and does not grow from respiratory or blood samples using standard culture methods. The primary method of diagnosis is polymerase chain reaction to detect the genetic material of the organism from respiratory specimens; 3)
M. pneumoniae is intrinsically resistant to all beta-lactam antibiotics because it lacks a cell wall, the target for beta-lactam bactericidal activity. Consequently, empiric monotherapy with a beta-lactams is ineffective against this pathogen; 4) Physicians should consider the possibility of macrolide-resistant
M. pneumoniae in hospitalized patients with pneumonia who fail to respond to macrolide therapy. Macrolide resistance in the U.S. varies regionally, with reported rates between 2% to 22% [
12]. Alternatives for treating macrolide resistant
M. pneumoniae include tetracyclines and fluoroquinolones, as clinical isolates with acquired resistance to these antibiotics have not been reported [
18]; 5) Physicians should remain vigilant about the range of possible extrapulmonary manifestations of
M. pneumoniae infections such as dermatological, cardiovascular or neurological complications. These manifestations may be present in patients with minimal or absent respiratory symptoms [
19].
This study has several strengths including the capability to evaluate more than 280 million patients from across the United States, the ability to obtain data on M. pneumoniae infections before the COVID-19 pandemic to define a pre-pandemic baseline level of infections for comparison, the inclusion of patients with diagnosis based solely on polymerase chain reaction of respiratory samples, and the capability to analyze subpopulations such as children and adults, with and without M. pneumoniae community-acquired pneumonia requiring hospitalization. Since the Epic Cosmos database is standardized, reflects real-world clinical practice, and is representative of a large proportion of the United States population, our findings are likely highly generalizable across the country.
The primary limitations of this study include the lack of data on the total number of diagnostic tests ordered for M. pneumoniae infections which did not allow us to calculate the percentage of positive tests. Additionally, in this analysis we considered every positive test as a case of M. pneumoniae infection. If a patient had a positive test in a clinic, and later required hospitalization for the same infection and was tested an additional time, while rare, this case would have been counted as two infections. Lastly, we were unable to assess the incidence of M. pneumoniae coinfections with other viral or bacterial respiratory pathogens.
5. Conclusions
In conclusion this study documents the presence of an ongoing outbreak of M. pneumoniae infections across the United States affecting both pediatric and adult populations. The current outbreak has significant implications for the management of patients with respiratory infections during the 2024-2025 winter pneumonia season. Continued surveillance at a national and global level is necessary to define the extent and clinical impact of this current M. pneumoniae outbreak.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1: Study flowchart of patients with
M. pneumoniae infections in the Cosmos database; Table S1: Logical Observation Identifiers Names and Codes (LOINC) queried to identify the presence of
M. pneumoniae infection; Table S2: International Classification of Diseases, Tenth Revision (ICD-10) codes queried to identify encounters pneumonia; Table S3: Study flowchart of patients with
M. pneumoniae infections in the Cosmos database.
Author Contributions
Conceptualization, A.R., S.F., T.C., S.R., W.M., and J.R.; methodology, A.R. and J.R.; validation, A.R., S.F., T.C., and J.R.; formal analysis, S.F. and T.C.; data curation, S.F., T.C., S.R., and W.M.; writing—original draft preparation, A.R., S.F., T.C., and J.R.; writing—review and editing, A.R., S.F., T.C., S.R., W.M., and J.R.; visualization, S.F., and T.C.; supervision, A.R. and J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. It was reviewed by the Western-Copernicus Group (WCG) Institutional Re-view Board (IRB# 1332986) and the Norton Research Institute and considered exempt with waiver of consent.
Informed Consent Statement
Informed consent was not obtained as this study was exempt with waiver of consent and utilized a de-identified dataset.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| COVID-19 |
Coronavirus Disease of 2019 |
| SE |
Standard error |
| UCL |
Upper confidence limit |
| LOINC |
Logical Observation Identifiers Names and Codes |
| ICD-10 |
Tenth International Classification of Disease |
| CPT |
Current Procedural Terminology |
References
- Jain, S. , Williams D.J., Arnold S.R., Ampofo K. et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. [CrossRef]
- Jain, S. , Self W.H., Wunderink R.G., Fakhran S. et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med. [CrossRef]
- Lind, K. , Benzon M.W., Jensen J.S., Clyde W.A. Jr. A seroepidemiological study of Mycoplasma pneumoniae infections in Denmark over the 50-year period 1946-1995. Eur J Epidemiol. [CrossRef]
- Atkinson, T.P. , Balish M.F., Waites K.B. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. [CrossRef]
- You, J. , Zhang L., Chen W., Wu Q. et al. Epidemiological characteristics of Mycoplasma pneumoniae in hospitalized children before, during, and after COVID-19 pandemic restrictions in Chongqing, China. Front Cell Infect Microbiol, 2024. [Google Scholar] [CrossRef]
- Boyanton, B.L. Jr, Frenner R.A., Ingold A., Ambroggio L. et al. SARS-CoV-2 pandemic non-pharmacologic interventions temporally associated with reduced pediatric infections due to Mycoplasma pneumoniae and co-infecting respiratory viruses in Arkansas. Microbiol Spectr, 2024. [Google Scholar] [CrossRef]
- Lan, S. , Gu C., Lu S., Zhou N., et al. Post-Pandemic Epidemiology of Respiratory Infections among Pediatric Inpatients in a Tertiary Hospital in Shanghai, China. Children (Basel). [CrossRef]
- Edouard, S. , Boughammoura H., Colson P., La Scola B. et al. Large-Scale Outbreak of Mycoplasma pneumoniae Infection, Marseille, France, 2023-2024. Emerg Infect Dis, 2024. [Google Scholar] [CrossRef]
- Zayet, S. , Poloni S., Plantin J., Hamani A. et al. Outbreak of Mycoplasma pneumoniae pneumonia in hospitalized patients: Who is concerned? Nord Franche-Comté Hospital, France, 2023-2024. Epidemiol Infect. [CrossRef]
- Garzoni, C. , Bernasconi E., Zehnder C., Malossa S.F. et al. Unexpected increase of severe Mycoplasma pneumoniae pneumonia in adults in Southern Switzerland. Clin Microbiol Infect. [CrossRef]
- Edens, C. , Clopper B.R., DeVies J., Benitez A. et al. Notes from the Field: Reemergence of Mycoplasma pneumoniae Infections in Children and Adolescents After the COVID-19 Pandemic, United States, 2018-2024. MMWR Morb Mortal Wkly Rep. [CrossRef]
- Centers for Disease Control and Prevention. Mycoplasma pneumoniae infection surveillance and Trends. Available online: https://www.cdc.gov/mycoplasma/php/surveillance/index.html (accessed on 1 December, 2024).
- Epic Systems. Epic Cosmos. Available online: https://cosmos.epic.com/ (accessed on 12 December, 2024).
- Tarabichi, Y. , Frees A., Honeywell S., Huang C. et al. The Cosmos Collaborative: A Vendor-Facilitated Electronic Health Record Data Aggregation Platform. ACI open. [CrossRef]
- National Institute of Standards and Technology. NIST/SEMATECH e-Handbook of Statistical Methods. Available online: http://www.itl.nist.gov/div898/handbook/ (accessed on 1 December, 2024).
- Sauteur, P.M. , Beeton M.L., Uldum S.A., Bossuyt N. et al. Mycoplasma pneumoniae detections before and during the COVID-19 pandemic: results of a global survey, 2017 to 2021. Eurosurveillance 2022, 27, 2100746. [Google Scholar]
- Brueggemann, A.B. , van Rensburg M.J., Shaw D., McCarthy N.D. et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. The Lancet Digital Health.
- Pereyre, S. , Goret, J. and Bébéar, C. Mycoplasma pneumoniae: current knowledge on macrolide resistance and treatment. Frontiers in microbiology.
- Narita, M. Classification of extrapulmonary manifestations due to Mycoplasma pneumoniae infection on the basis of possible pathogenesis. Frontiers in microbiology.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).