1. Introduction
Mycoplasma pneumoniae (
M pneumoniae) is a common etiology of community acquired pneumonia in school-aged children, with periodic epidemic surges occurring every few years [
1].
Prior to the COVID-19 pandemic,
M pneumoniae was a prevalent cause of respiratory tract infections, with a global incidence of 8.61% between 2017 and 2020, as determined by direct diagnostic methods. A reduced incidence to 1.69% was observed between 2020-2021 due to the implementation of non-pharmaceutical interventions (NPI) to combat COVID-19. Furthermore, even after the relaxation or discontinuation of NPIs,
M pneumoniae transmission continued to show long-term reduction, with an incidence of 0.70% between 2021-2022 and 0.82% between 2022-2023. Conversely, infections with other pathogens like respiratory syncytial virus (RSV) experienced a resurgence, indicating an increase in community transmission [
2].
The dynamic of the
M pneumoniae infections showed an increased pneumonia outbreak across multiple geographic locations in late 2023 according to global prospective surveillance group [the
European Society of Clinical Microbiology and Infectious Diseases Study Group for Mycoplasma and Chlamydia Infections (ESGMAC), ESGMAC Mycoplasma pneumoniae Surveillance (MAPS) Study]. This late re-emergence is hypothesized to be the consequence of
M pneumoniae characteristics like slow growth, prolonged incubation period (2-3 weeks) and low transmission rates [
3].
The Centre for Diseases Control and Prevention (CDC) reported in October 2024 that infections caused by
M pneumoniae have been on the rise since spring 2024, remaining elevated over the subsequent six months, with a peak incidence observed in August 2024. Notably, the infection rates were higher among children, increasing from 3.5% to 7.4% in those aged 5–17 years. The most remarkable increase, however, was seen in children aged 2–4 years, where rates rose from 1.0% to 7.2%. This finding is particularly significant, as
M pneumoniae has not historically been recognized as a major cause of pneumonia in this younger age group [
4].
M pneumoniae typically causes self-limiting respiratory infections and commonly referred to as “walking pneumonia” [
5]. However, in certain cases, the condition may progress to refractory
M pneumoniae pneumonia (RMPP), a more severe form of the disease. While a consensus definition for RMPP is lacking, it is generally characterized by persistence or exacerbation of clinical symptoms, prolonged fever, deterioration of radiological findings and development of extrapulmonary complications. These manifestations occur despite appropriate macrolide antibiotic therapy for at least 7 days and markers like C-reactive protein (CRP), lactate-dehydrogenase (LDH), erythrocytes sedimentation rate (ESR), neutrophils (%) and lymphocytes (%) may have predictive value [
6].
Mild
M pneumoniae infections are common among children above 5 years of age. Respiratory infections with
M pneumoniae frequently manifest with an array of systemic and respiratory symptoms. The systemic manifestations typically include headache, fever and muscle pain. Respiratory symptoms often encompass pharyngitis, non-productive cough, and mucopurulent expectoration. Young children and infants may exhibit wheezing and dyspnea in a small proportion. The extrapulmonary manifestations can involve the musculoskeletal, neurological, dermatological, cardiovascular, genitourinary, and hematological systems. Disease duration vary between 7-10 days, for most patients the evolution being favorable [
7,
8].
Radiological features of
M pneumoniae are non-specific and consist of patchy areas of consolidation, interstitial infiltrates or both [
9]. Pleural effusion (PE) is a recognized complication of
M pneumoniae pneumonia, occurring with variable frequency and often associated with heightened inflammatory responses and prolonged febrile states. Recent research has proposed a dichotomous classification of PE based on pathogen genome isolation and cytokine profiles. This categorization distinguishes between PE that is genome-positive with elevated cytokine levels, and PE that is genome-negative with lower cytokine concentrations [
10]. Atelectasis is described recently in 40 out of 572 patients with
M pneumoniae and also in a cohort of 122 children hospitalized in Children’s Hospital of Xiamen between December 2015 and December 2018 [
11].
Diagnosis of
M pneumoniae is achieved either by PCR testing which represents the “gold-standard”, with high specificity, sensitivity (detection of <100 colony-forming units (CFU) /mL) and rapidity of result acquisition, although the presence in upper respiratory tract may not necessarily indicate respiratory disease as the pathogen may be isolated in 3% to 56% of asymptomatic children. Given the long incubation period and special media, culture-based identification is not routinely used in practice. Rapid antigen testing identifies the pathogen with a cutoff limit of 1x10
3 CFU /mL, with a lower sensitivity than PCR, but faster [
12]. Serological diagnosis consists of specific antibody detection either immunoglobulin (Ig) A, IgG or IgM. The “gold-standard” consists in collection of a serum sample after ≥ 2 weeks, to observe the seroconversion or the rise in antibodies titer ≥ 4 times. IgM antibodies are usually detected as early as one week after the clinical onset with a maximum increase in the third week; IgA antibodies increase, reach the maximum and decline faster than IgM ones and IgG antibodies commonly appear 2 weeks after the infection and are detectable for a long period [
7,
13,
14].
The aim of this study is to comprehensively evaluate the clinical, radiological, and laboratory characteristics of M pneumoniae infection in pediatric patients, in the context of its re-surgency and elevated global incidence rates observed in the post-pandemic era. This research seeks to delineate regionally specific patterns of M pneumoniae infection in Romania, contributing nationally representative data to the global epidemiological landscape. Furthermore, it will provide critical insights into the Romanian epidemiological context, enhancing international understanding of M pneumoniae dynamics in the context of evolving post-COVID-19 respiratory pathogens transmission patterns.
2. Materials and Methods
This is a retrospective single center study. Pediatric patients diagnosed with laboratory-confirmed M pneumoniae respiratory infection between March and December 2024 admitted in our pulmonology department. All clinical, laboratory, and radiological data were extracted from the institutional electronic medical records system. Inclusion criteria comprised the confirmation of M pneumoniae infection through either molecular diagnostic techniques (polymerase chain reaction, PCR assay of nasopharyngeal specimens from a multiplex panel including influenza virus, parainfluenzae virus 1,2,3 and 4, rhinovirus, respiratory syncytial virus, metapneumovirus, coronavirus, bocavirus, Bordetella pertussis, Bordetella parapertussis, Chlamydia pneumoniae and M pneumoniae through Biofire ® method) or serological evidence (detection of pathogen-specific IgM antibodies through Chemiluminescence Immunoassay) defined as an IgM concentration ≥10 UA/mL in a patient with compatible clinical and paraclinical (biochemical and/or radiological) signs of respiratory infection. Subjects with inadequate or incomplete documentation were excluded from the final analysis.
Demographic parameters were recorded for all participants. Clinical and laboratory variables collected included: interval from symptom onset to definitive diagnosis (measured in days), maximum recorded body temperature (expressed in degrees Celsius), peripheral blood leukocyte count (cells/mm³), serum CRP concentration (mg/dL), and serum procalcitonin level (ng/mL, available for 23 patients) at presentation.
Radiographic evaluation of pulmonary involvement was systematically categorized according to anatomical distribution (bilateral, unilateral left, or unilateral right lung involvement) and predominant pathological pattern (presence or absence of alveolar or interstitial infiltrates). Additional variables documented included concurrent infection with other respiratory pathogens (viral, bacterial) detected through PCR panel testing. Identification of bacteria from the upper respiratory tract was based on cultures from the nasopharyngeal swabs. Supplementary, prior antimicrobial therapy before hospital admission, associated pulmonary complications including atelectasis, pleural effusion, and clinical manifestation of respiratory distress characterized by dyspnea were noted.
In-hospital etiologic treatment was standardized in three categories: azithromycin-group, clarithromycin-group and newer quinolones (levofloxacin)- group. Hospitalization length was also noted.
The study was approved by the Ethics Committee of our Institution (reference number #87/17.12.2024).
Statistics
Data were analyzed using SPSS v.26 (Chicago, IL, USA). Descriptive statistics were computed for both continuous and categorical variables. Continuous variables were evaluated for normality using the Kolmogorov–Smirnov test given the sample size. For continuous variables, the mean/ median with standard deviations (SD) or interquartile range (IQR) as appropriate were used, regarding their distribution. To analyze the association between categorical variables, we used chi-square test. A Chi-square goodness of fit test was performed to evaluate the distribution of categorical outcomes in our sample. Pearson correlation was used to evaluate the associations between continuous variables that demonstrated normal distribution. To compare means and medians of continuous variables between more than two groups, one-way Anova was used for the normally distributed data and the Kruskal–Wallis one-way for the non-normally distributed data. Independent-T-test was used to compare means of normally distributed variables between two groups. Post hoc analysis was performed for ANOVA and Kruskal–Wallis, when significant differences were found between groups. A p value < 0.05 was considered as the threshold for statistical significance.
3. Results
Sixty-six patients were recruited initially. Three were excluded due to insufficient data. The final analysis comprised 63 patients, 30 (47.6%) boys. Median age at inclusion [IQR] was 12.6 years [8-15]. Three patients (4.8%) were diagnosed through serological testing. Patients’ characteristics are shown in
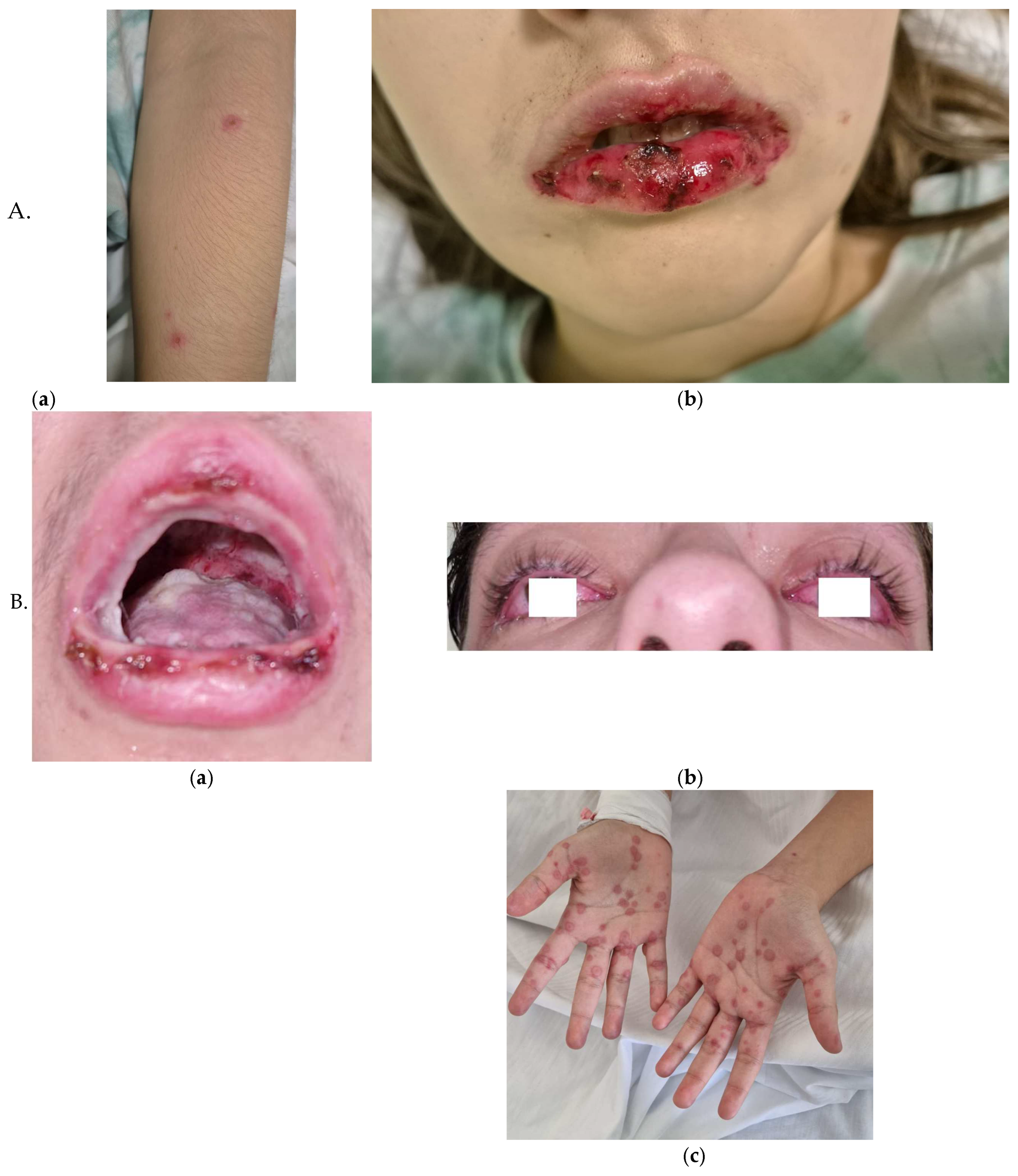
Table 1. Complications were pleural effusion (PE) in almost a quarter of patients (n=17, 27%), meningoencephalitis in 1 patient (1.6%) and 2 cases (3.2%) of reactive infectious mucocutaneous eruption (RIME) syndrome (
Figure 1). Monthly distribution of cases indicates a two-peaks prevalence, one in the summer (July-August) and one in the winter (December) (
Figure 2) with a progressive reduction in mean hospitalization duration from 11±1.52 days in March 2024 to 6.2±2.1 days in December (
Figure 3). Identification of different pathogens detected on the PCR panel (viral/bacterial) or culture-based methods were observed in 19 (30.2%) of patients out of which 8 (12.7%) had co-infections with ≥ 1 virus and/or
Bordetella pertussis (as identified on the multiplex PCR panel) (
Table 2).
Duration of hospitalization was positively correlated with fever value on admission (Pearson r coefficient = -0.247, p=0.05) but not with the number of leukocytes (p=0.181), CRP level (p=0.178) or procalcitonin level (p=0.698) or age (p=0.107).
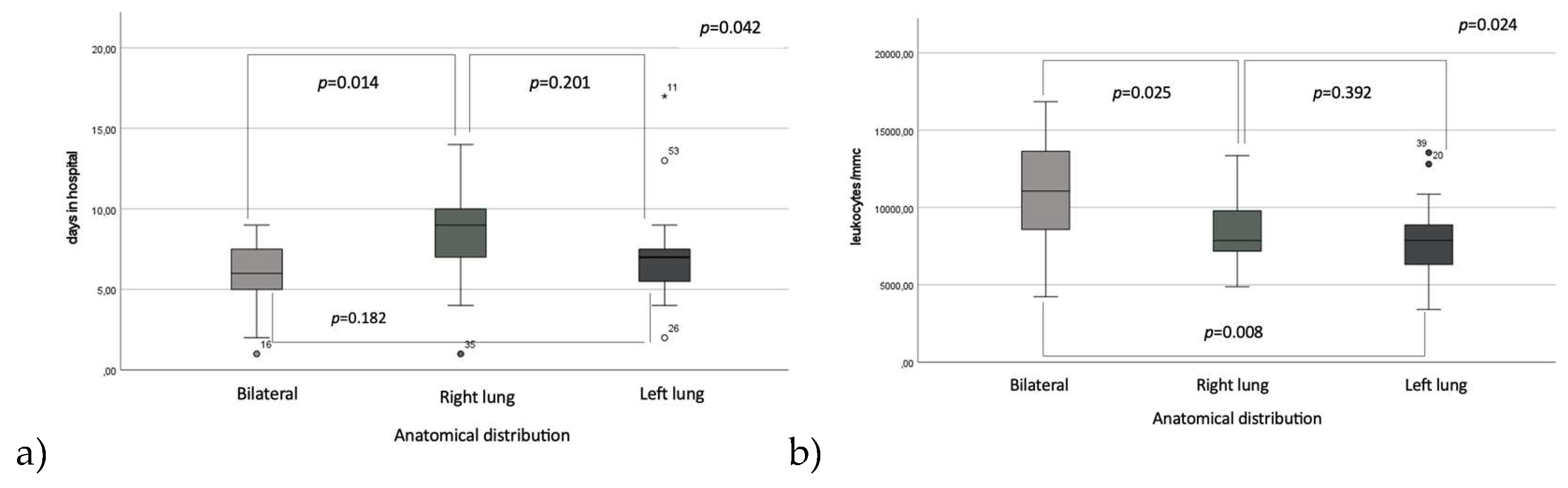
Regarding the duration of hospitalization in relation to the anatomical distribution of pulmonary involvement, right lung localization was associated with the longest hospitalization period compared to bilateral involvement, with means of 8.2 ± 2.7 vs. 5.7 ± 2.5 days (p=0.014), but not significantly different when compared to left lung involvement, 7.2±3.3 days, p=0.201; there were no significantly statistic differences between the length of hospitalization between the left lung pneumonia group and bilateral involvement group (p=0.182). Hospitalization length varied across seasons, with the longest mean stay observed during the initial trimester, March-June, (8.7 ± 4.1 days). This reached the shortest duration in the middle trimester to 6.8 ± 2.1 days. The third trimester (October-December) had a mean hospitalization time of 7.4±2.5 days. While ANOVA revealed no statistically significant differences across all trimesters (p = 0.091), post-hoc analysis identified a significant reduction in hospitalization time between the first and second trimesters (p = 0.029)
Conversely, leukocyte counts were significantly elevated in patients with bilateral pulmonary involvement (10721 ± 3954.2 cells/mm³) compared to those observed in patients with right pulmonary involvement (8582.4 ± 2203 cells/mm³), p=0.025 and those with left pulmonary involvement (7917.9 ± 2555 cells/mm³), p=0.008, while we did not observe any significantly differences between left and right pneumonia group, p=0.392 (
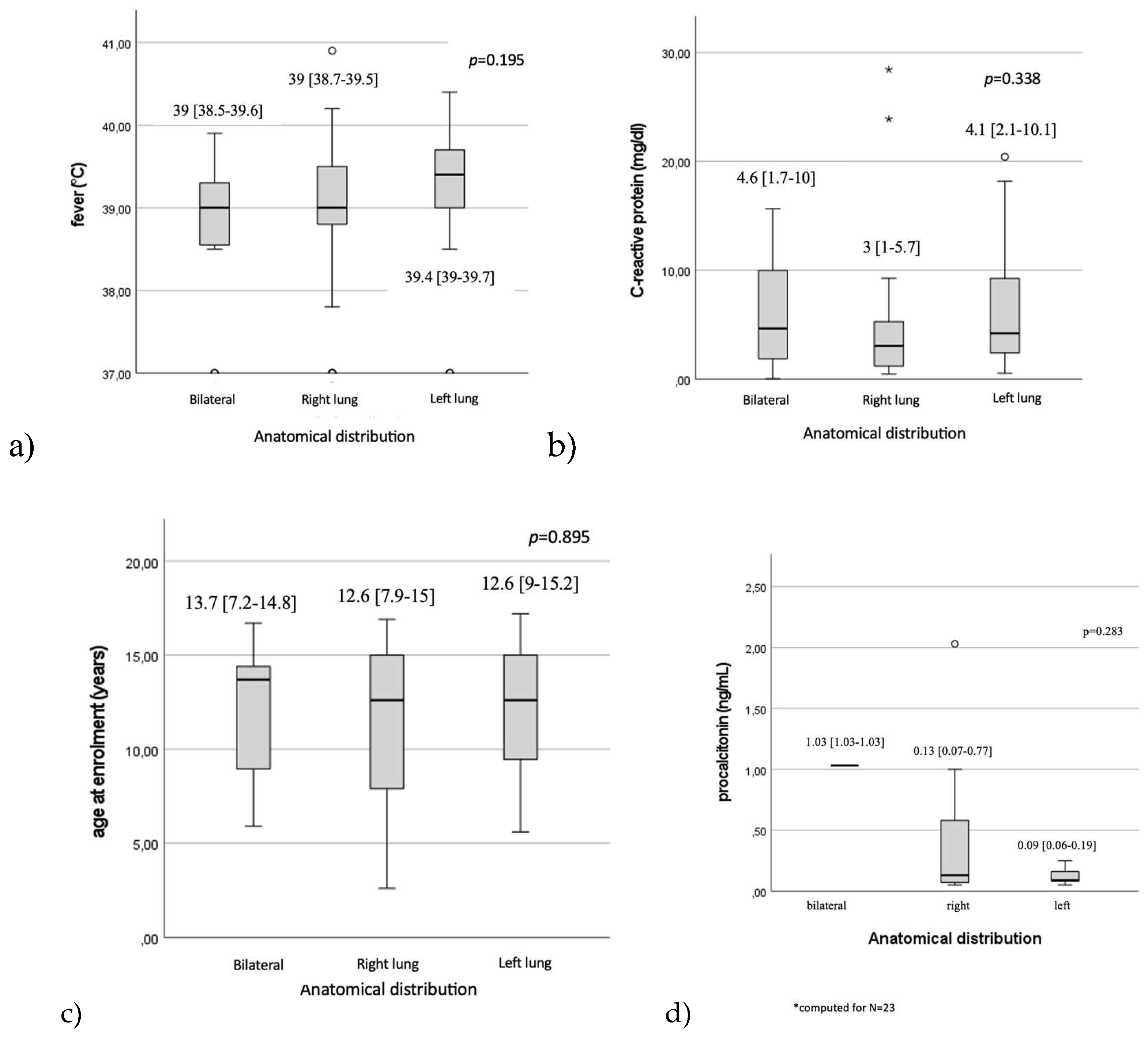
Figure 2). No statistically significant differences were observed regarding fever values, 39 [38.5-39.6], p=0.195), CRP levels (p=0.338), procalcitonin levels (p=0.283) or patients’ age (p=0.895) in relation to the anatomical distribution of lesions. (
Figure 3)
When assessed between Mycoplasma infection alone and co-infection with viral and/or bacterial pathogens identified on the PCR multiplex panel, no significantly different distributions were observed regarding mean hospitalization time (7.3±2.7 vs. 8.4±4.1, p=0.349), fever (39 [38.9-39.6] vs. 39.2 [37.2-39.6], p=0.494) or biochemical parameters (leukocytes count 8727.3±2854.1 vs. 8950±2557.4, p=0.835, CRP (3.7 [1.3-8.5] vs.4.2 [1-5.8], p=0.536, procalcitonin (0.13 [0.07-0.4] vs. 0.31 (only two patients had procalcitonin level available) p=0.791.
Etiologic treatment with either azithromycin (N=16), clarithromycin (N=37) or newer quinolones (N=8) did not influence the mean hospitalization length (7.1±2.4 vs.7.4±2.9 respectively 8.1±2.7, p=0.734). Two patients were excluded from this analysis because they received specific treatment after discharge.
Seven (11.1%) patients were ≤ 6 years of age. Leucocyte count was significantly higher among the children aged 6 years and younger when compared to the older children group (9790 [8680-12930] vs. 7945 [6952.5-10497.5], p= 0.046). There were no differences between the younger than 6 years of age group and >6 years group in terms of days of hospitalization (5.7±2.1 vs. 7.6±2.9, p=0.09), fever (39 [37.8-39.7] vs. 39 [38.8-39.5], p=0.645), CRP 1.1 [1.3-4.6] vs. 3.9 [1.2-8.9], p=0.420), procalcitonin level (0.07 [0.07-0.07] vs. 0.13 [0.07-0.52], p=0.522).
The majority of patients (n=48, 76.2%) was hospitalized > 5 days. Hospitalization length (≤5 days or >5 days) could not be predicted by a cutoff value for leukocytes (AUC=0.457), CRP (AUC=0.567), procalcitonin (AUC=0.580) or fever (AUC= 0.589) on admission as observed after ROC analysis.
Two patients (3.2%) were transferred into the Intensive Care Unit (ICU), one with respiratory failure and one with meningoencephalitis.
The occurrence of pleural effusion was associated with higher leukocytes count on admission (9322.8±2846.6 vs. 7220.5±2038.6, p=0.007) but not with the grade of fever (39.5 [38.9 -39.8] vs. 38.9 [38.5-39.5], p=0.152), CRP (3.1 [1.6-7.2] vs. 3.9 [1.2-8.6], p=0.938), procalcitonin levels (0.1 [ 0.07-0.1] vs. 0.1 [0.07-0.85], p=0.769) or age at inclusion (13.1 [8.3-15.4] vs.11.8 [7.9-14.8], p= 0.481). We did not find significant associations between localization of pneumonia and PE occurrence, p=0.337.
4. Discussion
Due to the advent of highly sensitive diagnostic techniques enabling real-time and precise detection of
M pneumoniae, coupled with increased recognition of this pathogen as a potential etiologic agent in pediatric respiratory infections, the reported prevalence of
M pneumoniae infections has demonstrated a progressive increase over the past decade. Our findings indicate that
M pneumoniae infection is prevalent among school-aged children, aligning with previous studies that report its higher incidence in children older than 5 years. For instance, Kutty et al. demonstrated a significant prevalence of
M pneumoniae among children hospitalized with community-acquired pneumonia, particularly in those aged 5–17 years [
15] similar to the reports of Shah et al. [
16]. Conversely, Zhang et al. reported a higher incidence (41.3%) in children aged 5 years and younger, which differs from the observations in our study [
1] This discrepancy may be attributed to the elevated co-circulation of influenza, RSV and
M pneumoniae infections during the peak of the epidemic in China in the fall of 2023. Such regional differences suggest the importance of considering local epidemiological factors when interpreting variations in
M pneumoniae incidence across age groups.
Our study revealed a lower rate of viral co-infection compared to previous reports in the literature. For instance, Chen et al. observed co-infection in 14.5% of hospitalized children with
M pneumoniae respiratory tract infections, RSV, bocavirus and parainfluenzae being the most prevalent [
17]. Notably, our findings corroborate the seasonal pattern reported by Chen et al., with peak incidence occurring during the summer months, although different etiologies were observed. More recent studies have reported varying co-infection rates, with some indicating rates as high as 38.75% [
18]. The lower co-infection rate observed in our study may be attributed to several factors, including regional variations in pathogen prevalence or temporal changes in infection dynamics.
The mean time of hospitalization observed in our study aligns with the repors of Wang et al. They found different periods, based on disease severity, between 6.54±2.58 days for mild cases and 9.25± 2.53 days for severe
M pneumoniae pneumonia [
19]. A progressive reduction in mean hospitalization time was observed during the third trimester (October–December), likely reflecting increasing clinician familiarity with disease progression patterns. However, a notable exception occurred in November, marked by a temporary increase in hospitalization length attributable to two cases of RIME syndrome. This isolated deviation highlights the impact of rare, severe extrapulmonary manifestations on variations in hospitalization measure and underscore the importance of accounting for atypical presentations when analyzing trends in infectious disease management.
Consistent with previous findings, the mean leukocyte count at enrollment was within the normal range. However, our results are different from those reported by Youn et al., as we observed that children over six years of age presented with higher leukocyte counts compared to younger children. This discrepancy may be attributed to the fact that a substantial proportion of our study cohort had received antibiotic treatment prior to enrollment, potentially influencing the observed leukocyte dynamics. The higher leukocyte counts in older children may reflect age-specific variations in immune responses or differences in disease severity. However, the confounding effect of pre-enrollment antibiotic use must be considered when interpreting these results [
20].
Median CRP levels at baseline were moderately elevated, approximately seven times the normal value, consistent with previous studies linking increased CRP levels to pneumonia severity [
21]. However, contrary to earlier findings, our analysis did not reveal a significant relationship between the severity of pneumonia, as assessed by its anatomical distribution, and the degree of inflammation. This observation suggests that while CRP is a reliable marker of systemic inflammation, its utility in predicting localized pulmonary involvement may be limited.
Contrary to the findings reported by Chen et al. [
17], our study did not reveal statistically significant differences in inflammatory markers (CRP levels and leukocyte counts) or length of hospitalization between patients with isolated
M pneumoniae infections and those with co-infections involving other pathogens. We speculate that this can be explained by the fact that association of additional pathogens did not substantially alter the inflammatory response or clinical course compared to only
M pneumoniae infections.
Kutty et al., observed no statistically significant differences in length of hospitalization between children who received antibiotics active against
M pneumoniae and those who did not [
15]. Our study demonstrated that the choice of specific antimicrobial therapy did not significantly impact the duration of hospitalization. These observations across studies suggests that factors beyond antibiotic selection may play a more crucial role in determining the length of hospital stay for pediatric patients with
M pneumoniae pneumonia. Several potential explanations can be proposed from host immune response, disease severity, timing of antibiotic introduction but also factors like on-demand discharge or the occurrence of complications. Thus, the variability in hospitalization length may be influenced by factors beyond the direct pathophysiological impact of the infection, potentially confounding its utility as a standalone measure for disease course and severity assessment. We have noticed an isolated increase in mean duration of hospitalization in November (11±2 days) most probable this observation is due to the fact that in this specific time-frame 2 cases of RIME syndrome were identified which were associated with prolonged hospitalization time.
The radiological manifestations of
M pneumoniae pneumonia are predominantly categorized as bronchopneumonia or segmental/lobar pneumonia/atelectasis, reflecting distinct pathological processes. As demonstrated by
Huang et al., bronchopneumonia represents the most frequent imaging pattern (59.6% of cases), while consolidation/atelectasis correlates with severe clinical outcomes, including prolonged hospitalization, elevated inflammatory markers (e.g., leukocytosis, CRP), and complications such as necrotizing pneumonia or bronchiolitis obliterans [
22]. Contrary to the reported bilateral involvement being the most prevalent [
23], right lung predominance observed in our cohort, is more likely due to anatomical factors (shorter right bronchus) facilitating pathogen deposition. Bilateral involvement indicates diffuse disease progression, correlating with higher leukocyte counts as observed in our research but not prolonged hospitalization, as reported by Huang
et al. [
22]. Variability in radiological classification may account for interstudy discrepancies. However, it is suggested that the severity of
M pneumoniae pneumonia is intrinsically tied to its radiological phenotype, with consolidation/atelectasis and bilateral involvement serving as markers of systemic inflammation and clinical severity. A unified imaging-based classification system, as proposed by Huang et al. could improve prognostic stratification and management strategies [
22].
Pleural effusion represents a significant complication of
M pneumoniae pneumonia serving as a marker of disease severity and prolonged clinical course. Its reported prevalence varies across studies, ranging from 4-28% in adults [
24]and 20.3-20.7 % in children from small sample size studies [
25]. Shen et al. reported a proportion of 19% children with
M pneumoniae parapneumonic pleural effusion in 59 children with bacterial pneumonia complicated with PE and empyema [
26]. Lee et al. observed that 65% of ICU-admitted patients presented with pleural effusion, compared to only 10% of non-ICU patients [
27]. Lin et al. report that 6.7% of patients with pleural effusion or empyema were
M pneumoniae PCR-positive [
28]. In our research, PE did not require ICU admission. Contrary to previous findings [
25] we did not observe differences in CRP markers or in the hospitalization time between groups with and without PE, although leukocyte count was higher among the PE group. Differences in observations may be explained by diagnostic modalities [X-ray or computed tomography (CT)], antibiotic regimens and time of their initiation, age at enrolment or regional pathogen dynamics. While pleural effusion remains a critical indicator of
M pneumoniae pneumonia severity, its clinical significance must be interpreted within context of regional epidemiology, diagnostic practices, and treatment protocols.
Central nervous system (CNS) complications associated with
M pneumoniae are more prevalent in pediatric populations and linked to elevated morbidity and mortality. The pathogenesis primarily involves immune-mediated mechanisms, particularly molecular mimicry, where antibodies targeting
M pneumoniae cross-react with host neuronal components such as myelin glycolipids, leading to autoimmune-driven neurological damage [
29]. although direct invasion of the parenchyma, vascular injury and hypercoagulable state are noted [
30,
31]. Neurological involvement has been reported in 1-10% of hospitalized patients.
M pneumoniae is a common cause of encephalitis in children and is reported in 5-10% of cases. Younger children particularly those under 10-years of age are most often affected. Encephalitis presents in two distinct patterns: early-onset, characterized by acute neurological symptoms concurrent with respiratory infection, and late-onset, which manifests days to weeks after initial symptoms and is often immune-mediated [
30]
Similar to its respiratory manifestations,
M pneumoniae encephalitis often occurs in an epidemic pattern [
32].
Our patient developed meningoencephalitis on the fifth day of hospitalization (day 11 of illness), manifesting with fever, diplopia, photophobia, strabismus, nuchal rigidity, and vomiting. CT of the brain revealed no structural abnormalities. Cerebrospinal fluid (CSF) analysis via lumbar puncture demonstrated a positive Pandy reaction. The patient was transferred to the ICU for five days, where she received broad-spectrum antibiotics and intravenous corticosteroids, resulting in favorable clinical improvement.
RIME syndrome was first defined as a distinct clinical entity in 2015; historically, it was described as Stevens-Johnson syndrome and toxic epidermal necrolysis. Pathogenesis involves molecular mimicry between
M pneumoniae P1 adhesin and keratinocyte antigens, immune complex deposition, B-cell activation, genetic susceptibility, and triggers from infections (e.g.
, M pneumoniae,
Chlamydia pneumoniae, viruses) or medications. Clinically, RIME preferentially affects mucous membranes, with oral (94–100%), ocular (82–92%), and urogenital (63–78%) involvement, while cutaneous manifestations are variable—absent in 34% of cases or polymorphic (e.g., targetoid, vesiculobullous) in 47% [
33]. In a meta-analysis by Canavan et al. mean age at diagnosis was 11.9 ±8.8 years with a male predominance [
34]. In our study median age was similar, 12 [9.0-15] years, with equal distribution between genders, most probably due to reduced incidence in our study group. One patient presented with mucositis, ocular involvement and also uro-genital lessions. RIME syndrome exhibits a seasonal pattern, with a predominance of cases reported during winter months. This seasonal distribution is associated with extended hospitalization periods [
35]. Our observations align with this trend, as we documented cases in late November and were characterized by prolonged hospital stays, with a mean of 7±2.4 days.
Despite its significant contribution to the current literature, the present study is subject to certain limitations. Primarily, its retrospective design may introduce selection biases in data collection including incomplete data and inconsistences in treatment strategies. The absence of a control group limits the possibility to generalize results beyond study group population. These methodological limitations should be considered when interpreting the findings and their broader applicability to clinical practice. Additionally, hospitalization duration may be influenced, in few cases, by patient-requested discharge was on demand or, on the contrary, hospitalization was prolonged by extrapulmonary complications. A significant limitation was the complete unavailability of testing kits for M pneumoniae during May 2024. This methodological constraint may have impacted the ability to accurately diagnose and include cases from this period, potentially leading to an underestimation of the true incidence of M pneumoniae infections in the study population. These limitations underscore the need for cautious interpretation of the findings and highlight areas for improvement in future prospective studies on M pneumoniae infections.