1. Introduction
Familial hypercholesterolemia (FH) is an autosomal dominant disease that leads to early cardiovascular events. FH manifests in two forms, heterozygous and homozygous. Diagnosis is usually confirmed by identifying pathogenic mutations; however, the disease can also be diagnosed using various scoring systems without genetic testing. The Dutch Lipid Clinical Network (DLCN) score is currently the most widely used scoring system for diagnosing FH. Prompt diagnosis, early detection of atherosclerosis, and initiation of lipid-lowering therapy are pivotal to save the lives of patients with FH [
1,
2,
3].
Subclinical atherosclerosis refers to the formation of atheromatous plaques in arterial territories, such as the coronary, carotid, or iliofemoral arterial segments, before symptomatic events related to the atherosclerotic process. Subclinical atherosclerosis is an early indicator of atherosclerotic burden and can be identified using noninvasive imaging techniques. The involvement of multiple vascular territories provides insight into the extent of subclinical atherosclerosis, and assessing various territories collectively enables the accurate measurement of the overall atherosclerotic burden [
4,
5,
6,
7].
In our study, we investigated the presence, extent and independent predictors of subclinical atherosclerosis among patients diagnosed with FH based on the DLCN score.
2. Materials and Methods
This was a single-center, prospective, cross-sectional study. Patients managed at outpatient clinic of our hospital underwent screening based on the DLCN criteria. Among these patients, those aged 18 years or older who were clinically diagnosed with FH according to the DLCN criteria were included in the study. The exclusion criteria were individuals under 18 years of age, patients with secondary diagnoses potentially leading to hypercholesterolemia (such as hypothyroidism, nephrotic syndrome, pregnancy, Cushing's syndrome and those receiving corticosteroids or immunosuppressive therapy), individuals with a history of secondary prevention (history of atherosclerotic cardiovascular disease [ASCVD]), and patients with advanced organ failure or malignancy. Demographic information and clinical characteristics of the patients were recorded. Laboratory parameters were analyzed using blood samples collected within the preceding month, followed by a fasting period of at least 12 h. The pre- and post-treatment lipid levels of patients receiving lipid-lowering therapy were recorded separately. A flowchart of this study is shown in
Figure 1.
2.1. Carotid Ultrasonography (USG)
Ultrasonographic examination of the carotid arteries was conducted using a Samsung RS85 Prestige system (Samsung Electronics Health & Medical Equipment and Samsung Medison, South Korea), along with a linear probe (model LA2-14A). The patients were positioned supine with their heads rotated at a 45-degree angle towards the contralateral side. The left and right common carotid arteries (within the first 10 mm before the bifurcation), the carotid bifurcation region (bulbus carotid), and the proximal segment (first 10 mm) of the internal and external carotid arteries were imaged. All anatomical regions were meticulously examined from diverse viewing angles, encompassing both cross-sectional and longitudinal perspectives. Measurements were conducted independently by two expert radiologists who were blinded to the patients’ clinical characteristics. Carotid plaques were assessed according to the criteria outlined in the European Mannheim Consensus [
8]. Focal thickening characterized by a 0.5 mm indentation into the lumen, thickening representing 50% of the surrounding intima-medial thickness (IMT), and thickening resulting in an IMT of >1.5 mm were identified as carotid plaques. The maximum plaque thickness and plaque structure were also evaluated using carotid USG. To determine the maximum plaque thickness, all plaque lesions visualized in the right and left carotid arteries were evaluated, and the thickest part of the lesion was recorded as the maximum plaque thickness. Plaque characteristics were classified according to the Gray-Weale classification, which is based on the echogenicity of the adjacent tissues. The plaques were categorized into four subgroups: type 1 (totally hypoechoic), type 2 (predominantly hypoechoic), type 3 (predominantly hyperechoic), and type 4 (totally hyperechoic). The number of patients with type 2 and type 3 plaques was limited; therefore, these groups were combined into the mixed plaques group [
9].
2.2. Femoral Ultrasonography
The femoral arteries were examined ultrasonographically using a Samsung RS85 Prestige system (Samsung Electronics Health & Medical Equipment and Samsung Medison, South Korea) with a linear probe (model LA2-14A). Plaques were investigated within the 20 mm segment of the bifurcation region located at the confluence of the common femoral artery with the superficial and deep femoral arteries. All anatomical regions were comprehensively examined from both cross-sectional and longitudinal perspectives. The measurement methodologies for plaque assessment via femoral ultrasonography were adapted from the established criteria for carotid plaque measurement. Two expert radiologists blinded to the clinical profiles of the patients performed all measurements. The plaque presence and characterization and maximum thickness measurements were conducted in a manner consistent with the carotid plaque assessment protocols.
2.3. Coronary Artery Calcium (CAC) Score
CAC scores were measured using a 128-slice computed tomography scanner (Philips Ingenuity 128 Circular Edition; Philips Medical Systems, Best, Netherlands). Contrast material was not administered during the examination. The imaging protocol involved a slice thickness of 3 mm, scenogram parameters set at 80–200 mAs and 120 kV, and a gantry rotation time of 0.3 seconds. After acquiring a topogram image with the patient in the supine position, single breath-hold imaging lasting approximately 8-10 seconds was performed, covering the entire heart from 1 cm below the carina. Cardiac CT images were simultaneously reviewed by two expert radiologists blinded to the clinical characteristics of the patients. The Philips Intellispace Portal Workstation software was used for the review. CAC measurements were conducted according to the Agatston scoring criteria. Lesions demonstrating a CT density >130 Hounsfield Units (HU) within an area >1 mm2 and spanning 2–3 adjacent pixels were classified as calcification. The area, density, and calcium score of each lesion identified on axial sections were automatically computed using dedicated software. The total CAC was determined by summing the individual calcium scores of the left main coronary artery (LMCA), left anterior descending coronary artery (LAD), circumflex artery (Cx), and right coronary artery (RCA). Coronary atherosclerosis was defined as a CAC score of ≥1.
2.4. Measurement of Laboratory Parameters
To measure the levels of Apo A-I, Apo B, and lipoprotein (Lp) (a), blood samples were obtained from patients after a 12-h fasting period. The samples were then centrifuged at 4,000 rpm for 15 min. After centrifugation, the separated plasma and sera were frozen at -80°C and stored for later analysis. A nephelometric method was used to measure the levels of Apo A-I, Apo B, and Lp(a). Serum samples, which had been previously frozen at -80°C, were thawed before analysis. Following the completion of appropriate controls and calibrations of the Siemens BNProspec® analyzer, the thawed sera were analyzed.
The study received ethical approval from the Istanbul University-Cerrahpasa Ethics Committee, with approval granted under decision dated 19.09.2023, bearing reference number E-83045809-604.01.01-789808. The research adhered to the ethical principles outlined in the Declaration of Helsinki, and informed consent was obtained from all participants.
2.5. Statistical Analysis
The Statistical Package for Social Sciences (SPSS) version 23 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. The Kolmogorov-Smirnov and Shapiro-Wilk tests were performed to determine the distribution patterns of numerical variables. Normally distributed variables were analyzed using the Student’s t-test or one-way analysis of variance (ANOVA) and are presented as means and standard deviations. Variables that did not show a normal distribution were analyzed using the Mann-Whitney U or Kruskal-Wallis test and expressed as median and interquartile range (between the 25th and 75th percentiles). Categorical variables were analyzed using the chi-square or Fisher’s exact test and presented as frequency and percentage distributions. The relationships among subclinical atherosclerosis, coronary atherosclerosis, carotid and femoral atherosclerosis, and other variables were examined using multivariable logistic regression. The Hosmer–Lemeshow test was used to assess the model’s goodness of fit. The association between the extent of subclinical atherosclerosis and numerical variables was analyzed using the Pearson and Spearman correlation tests. The Receiver Operating Characteristic (ROC) curve analysis was conducted to assess the predictive value of significant numerical variables identified in the correlation analysis for the dependent variable and to determine the corresponding cut-off levels. The significance threshold was set at p < 0.05 for all analyses.
3. Results
After screening, 1,145 patients with suspected FH were identified. The patients were analyzed to determine their eligibility based on the study's inclusion and exclusion criteria. Finally, 215 patients with no history of ASCVD were included in the study (
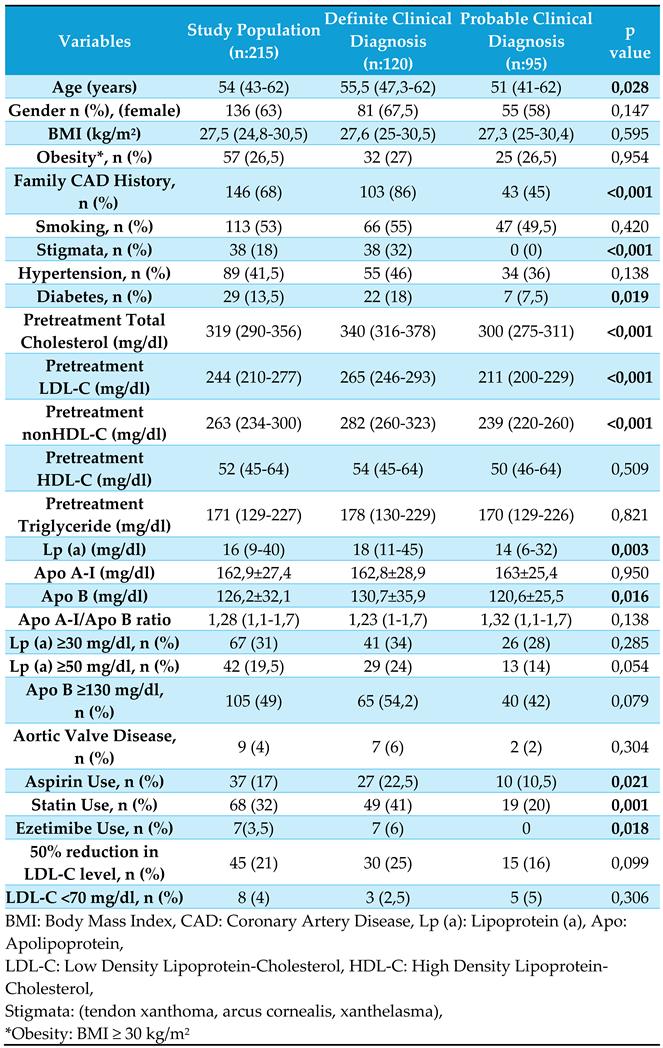
Figure 1). Among them, 120 and 95 patients had definite and probable FH, respectively, according to the DLCN scoring. The study cohort comprised 136 females (63%) and 79 males (37%), with a mean age of 54 (43–62) years. The most prevalent comorbidities were hypertension (41%) and diabetes (13.5%). Physical examinations revealed stigmata in 38 patients (18%). Genetic test results were available for 46 patients (42 patients had LDL-R gene mutations and 4 patients had Apo B gene mutations). Among the study population, 17% reported using aspirin, 32% reported using statins, and 19.5% reported using potent statins (10%, rosuvastatin; 9.5% atorvastatin). Additionally, 3.5% reported using ezetimibe, and 1% reported using proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors. The clinical characteristics of the study population and respective study groups are presented in
Table 1.
When evaluating the mean pretreatment lipid levels of patients, we observed that Low Density Lipoprotein-Cholesterol (LDL-C) was 252 mg/dL, Total Cholesterol (TC) was 329 mg/dL, Triglyceride (TG) was 190 mg/dL, High Density Lipoprotein-Cholesterol (HDL-C) was 55 mg/dL, and non-HDL-C was 273 mg/dL. The rate of statin use during the subclinical atherosclerosis screening was 32%, with only 45 patients (21%) achieving a 50% reduction in LDL-C levels. Furthermore, only eight patients (4%) attained LDL-C values below 70 mg/dL. These findings highlight that, within this high-risk patient group, both the rate of statin use and the attainment of treatment goals are notably low.
Patients with definite FH were older, on average, and all patients with stigmata were included in this group. The rates of aspirin, statin, potent statin, and ezetimibe use were significantly higher in patients with definite FH. While treatment differences were significant, the achievement of target LDL-C levels did not differ between the groups. Pretreatment lipid levels and levels of certain biomarkers, such as Lp (a) and Apo B, were higher in the definite FH group. Females generally had higher lipid and biomarker levels than males. Other laboratory parameters showed no significant differences between the groups.
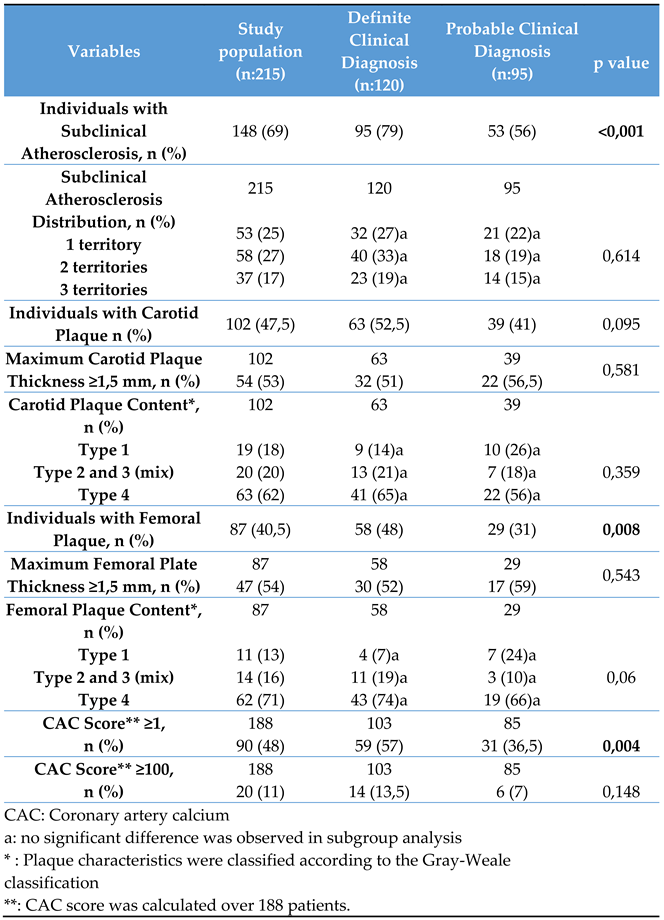
Subclinical atherosclerosis was observed in 148 patients (69%), with rates of 48%, 47.5%, and 40.5% in the coronary arteries, carotid bifurcation, and femoral bifurcation, respectively. Atherosclerosis occurred in one, two, or all three regions in 25%, 27%, and 17% of the cases, respectively. Type 4 plaques were most prevalent in the carotid and femoral arteries (62% and 71%, respectively), followed by mixed type (20% and 16%, respectively), and type 1 plaques (18% and 13%, respectively). Over half of the patients had a maximum plaque thickness ≥1.5 mm in both territories (53% and 54%). In 188 patients who underwent CAC score assessment, 48% had a score of ≥1, with 11% having a score of ≥100. Significant differences were observed between the groups in terms of femoral and coronary atherosclerosis but not in terms of carotid atherosclerosis (
Table 2).
The group with subclinical atherosclerosis was older and had higher pretreatment levels of LDL-C, TC, non-HDL-C, and Apo B. However, Lp(a) and Apo A-I levels and the Apo A-I/Apo B ratio were similar between groups. Patients with subclinical atherosclerosis had higher rates of hypertension and diabetes; however, no significant differences were found in statin use between the two groups.
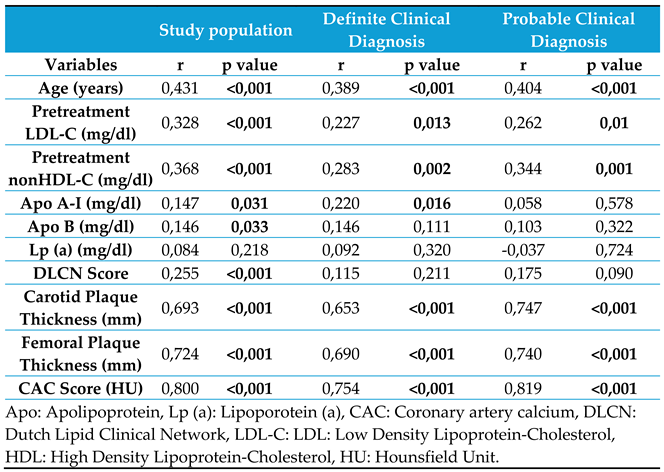
Variables correlated with the extent of subclinical atherosclerosis were assessed based on the affected vascular regions. While the age, femoral and carotid plaque thicknesses, and CAC score showed strong correlations, the pretreatment LDL-C and non-HDL-C levels and DLCN scoring had weak correlations (
Table 3).
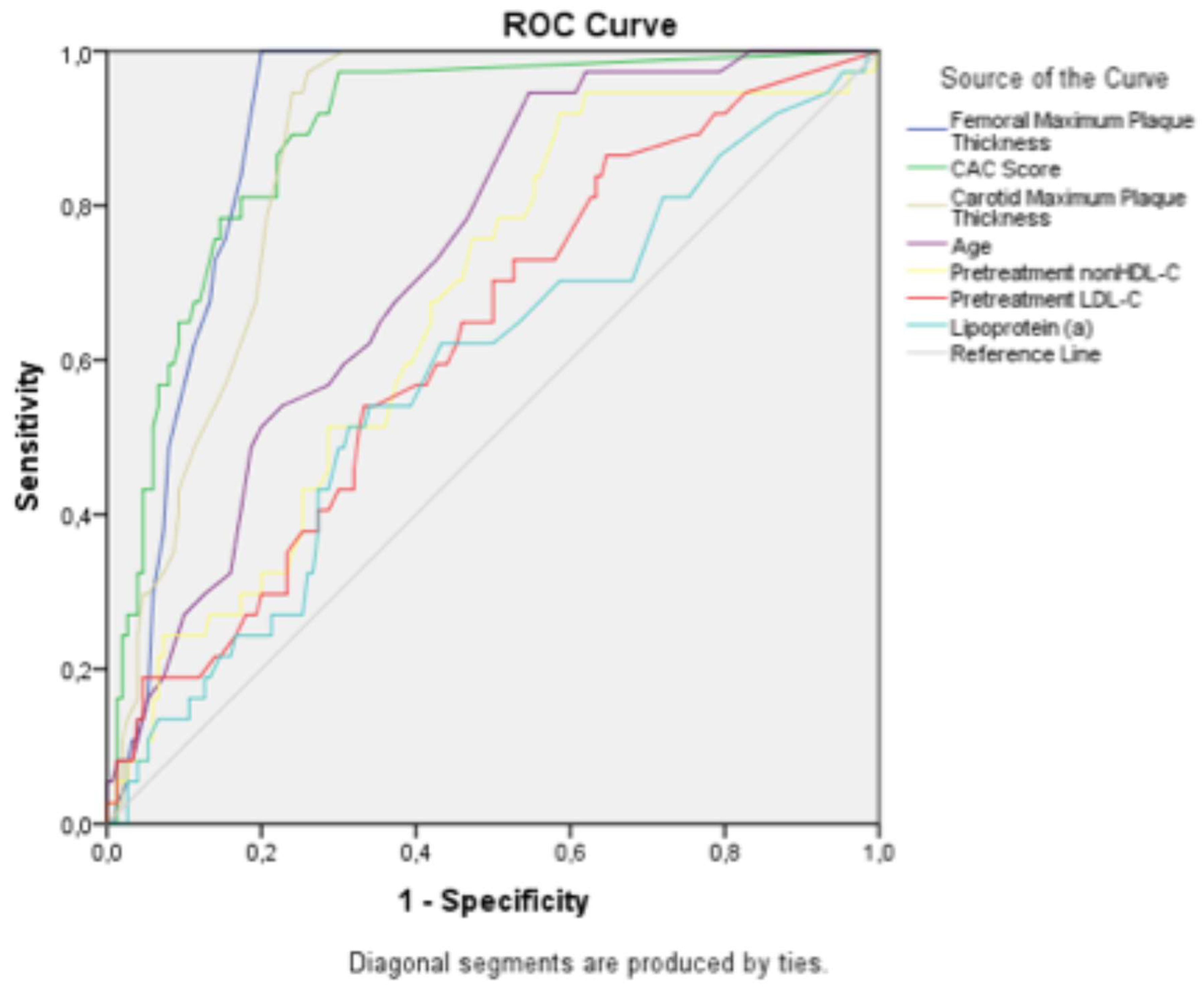
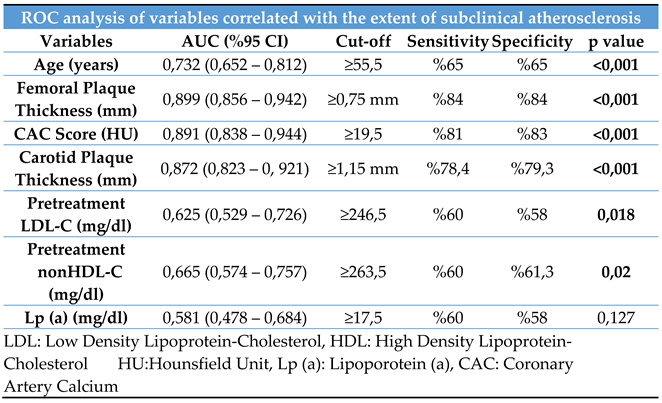
The study population was stratified into the definite and probable diagnosis groups. Correlation analyses for subclinical atherosclerosis were conducted separately for each group. Both groups showed strong correlations between age, femoral and carotid plaque thicknesses, and CAC score and the extent of subclinical atherosclerosis. Receiver operating characteristic (ROC) analysis was used to determine the cutoff values, sensitivity, and specificity of these correlated variables. The ROC curve is shown in
Figure 2, and the corresponding cutoff values, along with the sensitivity and specificity ratios, are presented in
Table 4.
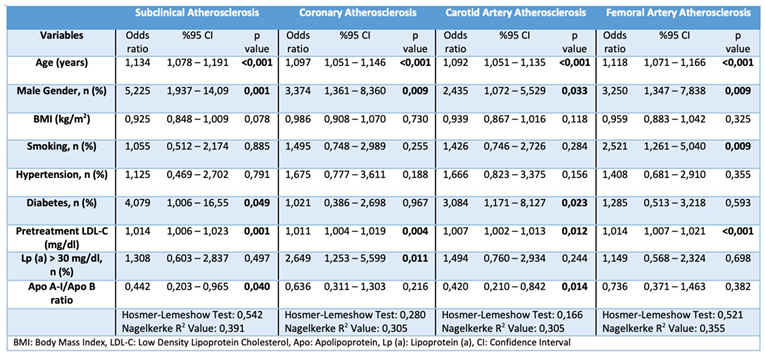
Multiple logistic regression analysis identified several predictors of subclinical atherosclerosis, including advanced age, male sex, high pretreatment LDL-C level, low Apo A-I/Apo B ratio, and diabetes. Interestingly, sex and the Apo A-I/Apo B ratio, which were initially insignificant in the univariable analysis, became significant in the multivariable analysis. Conversely, hypertension and diabetes were initially significant but lost significance. When examining atherosclerosis-prone regions separately, advanced age, male sex, and high pretreatment LDL-C levels were consistent predictors. Additionally, Lp(a) levels >30 mg/dL predicted coronary atherosclerosis, diabetes and low Apo A-I/Apo B ratios predicted carotid atherosclerosis, and smoking predicted femoral atherosclerosis (
Table 5).
4. Discussion
In our study of patients with FH, a high rate of subclinical atherosclerosis (69%) was observed, with similar rates across the coronary (48%), carotid (48%), and femoral (41%) regions. Independent predictors of subclinical atherosclerosis in patients with FH included advanced age, male sex, high pretreatment LDL-C level, presence of diabetes, and low Apo A-I/Apo B ratio. Advanced age, male sex, and high pretreatment LDL-C levels were consistent predictors across all vascular regions. Lp(a) levels ≥30 mg/dL predicted coronary atherosclerosis, while diabetes and low Apo A-I/Apo B ratios predicted carotid atherosclerosis, and smoking predicted femoral atherosclerosis. Correlation analysis showed strong associations between advanced age, femoral and carotid plaque thicknesses, CAC scores, and extent of subclinical atherosclerosis.
In patients with FH, the risk of ASCVD is notably elevated, as demonstrated by studies such as the CASCADE FH Registry and SAFEHEART trials [
10,
11]. In the CASCADE FH Registry, 3.6% of patients aged >20 months experienced ASCVD events, and an older age, male sex, low HDL-C level, diabetes, and hypertension were significant factors [
10]. In the SAFEHEART study, which followed patients with FH for 5.5 years, ASCVD events occurred in 5.6% of the cases. A predictive model in the SAFEHEART study highlighted an advanced age, male sex, history of ASCVD, high blood pressure, high body mass index (BMI), smoking, high LDL-C levels, and high Lp(a) levels as independent predictors of future major adverse cardiovascular event (MACE) risk (11). Familial hypercholesterolemia increases the risk of ASCVD, which is compounded by traditional risk factors. Guidelines underscore screening for subclinical atherosclerosis and achieving target LDL-C levels in managing the ASCVD risk in patients [
2,
3].
Patients with FH are expected to have a high prevalence of subclinical atherosclerosis because of their elevated ASCVD risk. Mattina et al. assessed 154 patients with FH for subclinical atherosclerosis and identified its presence in 83% of patients. The rates of CAC, carotid plaques, and femoral plaques were 62%, 55%, and 56%, respectively, with no significant differences in atherosclerosis in the coronary, carotid, and femoral regions; however, coronary atherosclerosis was higher in males, while carotid atherosclerosis was higher in females [
12]. In our study, subclinical atherosclerosis was found in 69% of the patients and was most common in the coronary arteries, similar to results from a previous study. However, contrary to that study, the rates of coronary, femoral, and carotid atherosclerosis were higher in females, possibly because of the higher proportion of females in the definite FH group. Additionally, while 33% of the patients in the previous study had atherosclerosis in all three regions, the rate was 25% in our study. These findings highlight the high prevalence and frequency of subclinical atherosclerosis in patients with FH and provide valuable insights into the occurrence of atherosclerosis in this population.
However, different results have been obtained when examining the regions in which subclinical atherosclerosis develops. In the Aragon Workers' Health Study (AWHS) by Laclaustra et al., 72% of the participants had subclinical atherosclerosis, and the femoral region was the most common site. Active smoking was the strongest predictor of femoral and carotid plaques, whereas hypertension was associated with CAC, the strongest predictor of coronary atherosclerosis [
13]. In our study, hypertension was initially associated with subclinical and coronary atherosclerosis in univariable analysis but lost significance in multivariable analysis. Smoking was an independent predictor of femoral atherosclerosis but not of subclinical atherosclerosis. Interestingly, femoral plaques in the AWHS study showed a higher sensitivity in predicting CAC than carotid plaques. In our analysis, femoral plaques were independent predictors of coronary atherosclerosis, unlike carotid plaques, underscoring their importance in predicting coronary atherosclerosis.
In other studies, the predictors of subclinical atherosclerosis varied. Chan et al. found that advanced age, male sex, hypertension, and pretreatment LDL-C and Lp(a) levels were independent predictors of coronary artery disease (CAD) [
14]. Allard et al. identified male sex, diabetes, a family history of CVD, and high Lp(a) levels as predictors of cardiovascular events (CVE) [
15]. Similarly, in our study, an advanced age, male sex, pretreatment LDL-C levels, and Lp(a) levels ≥30 mg/dL were independent predictors of coronary atherosclerosis. These findings emphasize the importance of considering multiple risk factors when assessing the ASCVD risk in patients with FH.
Conflicting results have been reported regarding the relationship between high plasma Lp(a) levels and CAD in patients with FH. Some studies have reported that a high Lp(a) level is an independent risk factor for CAD in patients with FH, while others have reported no relationship between Lp(a) levels and CAD [
15,
16,
17].
Chan et al. divided 390 patients with FH into two groups based on the presence of CAD. The mean Lp(a) level was 42 mg/dL, and the Lp(a) level was significantly higher in patients with a history of CAD [
14]. In addition, Alonso et al. investigated the relationship between Lp(a) levels and cardiovascular disease (CVD) in patients with FH and their non-FH relatives . The average Lp(a) level was 24 mg/dL, and 30% of patients had levels >50 mg/dL. These higher levels were significantly associated with patients with FH who had a history of CVD, compared to those without. Notably, the Lp(a) levels between males and females were not significantly different. Additionally, among patients with FH and a history of CVD, approximately 46% have Lp(a) levels >50 mg/dL [
18]. Nenseter et al. observed no difference in Lp(a) levels between males and females [
19]. In our study, the mean Lp(a) level was 16 mg/dL, 32% of patients had Lp(a) levels ≥30 mg/dL, and 19.5% had levels ≥50 mg/dL. Higher Lp(a) levels were observed in patients with subclinical atherosclerosis, with significantly higher levels in females, likely due to the sex distribution. Multivariable regression analysis identified an Lp(a) level ≥30 mg/dL as an independent predictor of coronary atherosclerosis.
Advanced age is an important parameter in assessing the risk of ASCVD. The SCAPIS study conducted by Bergström et al. showed that coronary atherosclerosis starts approximately 10 years later in females, and the CAC score is a good indicator of the presence of coronary atherosclerosis [
20]. In a study led by Walus-Miarka et al. that included 154 patients diagnosed with definite and possible FH according to the Simon–Broome clinical score, patients with detectable carotid plaques were older [
21]. In our study, the mean age of individuals with subclinical atherosclerosis was 10 years older than that of other individuals. Therefore, advanced age is an important predictor for the presence of subclinical atherosclerosis.
In both national and international studies, despite the classification of patients with FH as high risk for ASCVD, medication usage rates often fell short of the target values. Ray et al. analyzed 4,112 patients receiving lipid-lowering therapy, and approximately 5% were diagnosed with FH. Only 25% of high-risk primary prevention patients and 11% of very high-risk patients achieved the target LDL-C levels [
22]. Similarly, Kayikcioglu et al. examined 1,071 patients suspected of having FH and found treatment rates at 42% and treatment goal attainment rates of 2.1% [
23]. In our study, medication usage and achievement of treatment goal rates were notably low, similar to those in these studies. However, we expect that these rates and patients’ medication adherence may improve after screening for subclinical atherosclerosis. Demonstrating subclinical atherosclerosis in patients with FH may enhance medication adherence and goal attainment.
Our study had several limitations. Firstly, the cross-sectional design and single-center setting may limit its generalizability to broader populations. Secondly, only 21% of patients had genetic test results. Not conducting genetic testing for all participants deprived us of valuable insights into the genetic basis of FH. Additionally, with only 32% of the population taking medications, the interpretation of the results may be affected. The absence of contrast-enhanced coronary CT angiography increased the risk of missing noncalcified plaques. Future studies should consider multicenter and prospective designs to address these limitations and provide more detailed and reliable results.
5. Conclusions
Familial hypercholesterolemia increases the risk of early cardiovascular events. Subclinical atherosclerosis, as observed in this study, is prevalent among patients with FH. Early diagnosis coupled with the prompt initiation of lipid-lowering therapy is paramount for improving outcomes
Despite the elevated cardiovascular risk in patients with FH, medication adherence remains suboptimal, with few patients achieving treatment goals. Screening for subclinical atherosclerosis may improve medication adherence, adoption of preventive measures, and it may increase physician commitment to treatment protocols. Thus, detecting subclinical atherosclerosis may be pivotal in optimizing treatment strategies for patients with FH
Author Contributions
Conceptualization, Muhammed Deniz and Umit Sinan; Data curation, Abdullah Ebeoglu, Omer Gul, Ali Nayir, Pelinsu Ozkan and Zubeyir Bulat; Formal analysis, Muhammed Deniz, Baris Guven, Ibrahim Turk, Ozlem Demirelce, Husamettin Kimyonok and Dildar Konukoglu; Investigation, Muhammed Deniz, Baris Guven, Habibe Deniz and Dildar Konukoglu; Methodology, Muhammed Deniz, Omer Gul, Zubeyir Bulat, Ibrahim Turk, Ozlem Demirelce, Husamettin Kimyonok and Habibe Deniz; Project administration, Muhammed Deniz and Umit Sinan; Resources, Baris Guven, Abdullah Ebeoglu, Ibrahim Turk and Dildar Konukoglu; Supervision, Murat Ersanli, Veysel Oktay and Umit Sinan; Writing – original draft, Muhammed Deniz and Umit Sinan; Writing – review & editing, Muhammed Deniz, Murat Ersanli, Veysel Oktay and Umit Sinan.
Funding
This study was funded by Istanbul University-Cerrahpasa Scientific Research Projects Coordination Office (Project number: TTU-2023-37495). But the authors received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
The study received ethical approval from the Istanbul University-Cerrahpasa Ethics Committee, with approval granted under decision dated 19.09.2023, bearing reference number E-83045809-604.01.01-789808. The research adhered to the ethical principles outlined in the Declaration of Helsinki, and informed consent was obtained from all participants.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data are available from authors upon request.
Acknowledgments
We acknowledge the support from the Istanbul University-Cerrahpasa Scientific Research Projects Coordination Office.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IMT |
Intima-medial thickness |
| CAC |
Coronary artery calcium |
| LAD |
Left anterior descending coronary artery |
| Cx |
Circumflex artery |
| RCA |
Right coronary artery |
| Lp(a) |
Lipoprotein a |
| Apo-A1 |
Apolipoprotein A1 |
| Apo-B |
Apolipoprotein B |
| PCSK-9 |
Proprotein convertase subtilisin/kexin type 9 |
| BMI |
Body Mass Index |
| CAD |
Coronary Artery Disease |
| BMI |
Body mass index |
| TG |
Triglyceride |
| HU |
Hounsfield Unit. |
| LDL-C |
Low Density Lipoprotein-Cholesterol |
| HDL-C |
High Density Lipoprotein-Cholesterol |
| FH |
Familial hypercholesterolemia |
| DLCN |
Dutch Lipid Clinical Network |
| ASCVD |
Atherosclerotic cardiovascular disease |
| USG |
Ultrasonography |
References
- Mc-Gowan MP, Hosseini-Dehkordi SH, Moriarty PM, Duell PB (2019) Diagnosis and Treatment of Heterozygous Familial Hypercholesterolemia. J Am Heart Assoc 8:e013225. [CrossRef]
- Cuchel M, Raal FJ, Hegele RA, Al-Rasadi K, Arca M, Averna M, Buckert E, Freiberger T, Gaudet T, Harad-Shiba M, Hudgins LC, Kayikcioglu M, Masana L, Parhofer KG, van-Lennep JER, Santos RD, Stroes ESG, Watts GF, Wiegman A, Stock JK, Tokgozoglu LS, Catapano AL, Ray KK (2023) 2023 Update on European Atherosclerosis Society Consensus Statement on Homozygous Familial Hypercholesterolemia: New treatments and clinical guidance. Eur Heart J 44:2277-2291. [CrossRef]
- Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Covington AM, DePalma SM, Minissian MB, Orringer CE, Smith SC Jr, Waring AA, Wilkins JT (2022) 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 80:1366–1418. [CrossRef]
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O (2020) 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 41:111–188. [CrossRef]
- Kawai K, Finn AV, Virmani R; Subclinical Atherosclerosis Collaborative (2024) Subclinical Atherosclerosis: Part 1: What Is it? Can it Be Defined at the Histological Level? Arterioscler Thromb Vasc Biol 44:12–23. [CrossRef]
- Gatto L, Prati F (2020) Subclinical atherosclerosis: how and when to treat it? European Heart Journal Supplements 22:E87–90. [CrossRef]
- Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR, Kronmal R, Lima JAC, Liu KJ, McClelland RL, Michos E, Post WS, Shea S, Watson KE, Wong ND (2018) Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J 39:2401–2408. [CrossRef]
- Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Desvarieux M, Ebrahim S, Fatar M, Hernandez Hernandez R, Kownator S, Prati P, Rundek T, Taylor A, Bornstein N, Csiba L, Vicaut E, Woo KS, Zannad F (2004) Mannheim Intima-Media Thickness Consensus. Cerebrovasc Dis 18:346–349. [CrossRef]
- Park TH (2016) Evaluation of carotid plaque using ultrasound imaging. J Cardiovasc Ultrasound 24:91-95. [CrossRef]
- Duell PB, Gidding SS, Andersen RL, Knickelbine T, Anderson L, Gianos E, Shrader P, Kindt I, O'Brien EC, McCann D, Hemphill LC, Ahmed CD, Martin SS, Larry JA, Ahmad ZS, Kullo IJ, Underberg JA, Guyton J, Thompson P, Wilemon K, Roe MT, Rader DJ, Cuchel M, Linton MF, Shapiro MD, Moriarty PM, Knowles JW (2019) Longitudinal low density lipoprotein cholesterol goal achievement and cardiovascular outcomes among adult patients with familial hypercholesterolemia: The CASCADE FH registry. Atherosclerosis 289:85–93. [CrossRef]
- Pérez de Isla L, Alonso R, Mata N, Fernández-Pérez C, Muñiz O, Díaz-Díaz JL, Saltijeral A, Fuentes-Jiménez F, de Andrés R, Zambón D, Piedecausa M, Cepeda JM, Mauri M, Galiana J, Brea Á, Sanchez Muñoz-Torrero JF, Padró T, Argueso R, Miramontes-González JP, Badimón L, Santos RD, Watts GF, Mata P (2017) Predicting cardiovascular events in familial hypercholesterolemia: The SAFEHEART registry (Spanish Familial Hypercholesterolemia Cohort Study). Circulation 135:2133–2144. [CrossRef]
- Mattina A, Giammanco A, Giral P, Rosenbaum D, Carrié A, Cluzel P, Redheuil A, Bittar R, Béliard S, Noto D, Quartarone A, Averna M, Bruckert É, Gallo A (2019) Polyvascular subclinical atherosclerosis in familial hypercholesterolemia: The role of cholesterol burden and gender. Nutr Metab Cardiovasc Dis 29:1068–1076. [CrossRef]
- Laclaustra M, Casasnovas JA, Fernández-Ortiz A, Fuster V, León-Latre M, Jiménez-Borreguero LJ, Pocovi M, Hurtado-Roca Y, Ordovas JM, Jarauta E, Guallar E, Ibañez B, Civeira F (2016) Femoral and carotid subclinical atherosclerosis association with risk factors and coronary calcium: The AWHS study. J Am Coll Cardiol 67:1263–1274 . [CrossRef]
- Chan DC, Pang J, Hooper AJ, Burnett JR, Bell DA, Bates TR, van Bockxmeer FM, Watts GF (2015) Elevated lipoprotein(a), hypertension and renal insufficiency as predictors of coronary artery disease in patients with genetically confirmed heterozygous familial hypercholesterolemia. Int J Cardiol 201:633–638. [CrossRef]
- Allard MD, Saeedi R, Yousefi M, Frohlich J (2014) Risk stratification of patients with familial hypercholesterolemia in a multi-ethnic cohort. Lipids Health Dis 13:65. [CrossRef]
- Jansen AC, van Aalst-Cohen ES, Tanck MW, Trip MD, Lansberg PJ, Liem AH, van Lennep HW, Sijbrands EJ, Kastelein JJ (2004) The contribution of classical risk factors to cardiovascular disease in familial hypercholesterolemia: data in 2400 patients. J Intern Med 256:482–490. [CrossRef]
- de Sauvage Nolting PR, Defesche JC, Buirma RJ, Hutten BA, Lansberg PJ, Kastelein JJ (2003) Prevalence and significance of cardiovascular risk factors in a large cohort of patients with familial hypercholesterolemia. J Intern Med 253:161–168. [CrossRef]
- Alonso R, Andres E, Mata N, Fuentes-Jiménez F, Badimón L, López-Miranda J, Padró T, Muñiz O, Díaz-Díaz JL, Mauri M, Ordovás JM, Mata P; SAFEHEART Investigators (2014) Lipoprotein(a) Levels in Familial Hypercholesterolemia. J Am Coll Cardiol 63:1982–1989. [CrossRef]
- Nenseter MS, Lindvig HW, Ueland T, Langslet G, Ose L, Holven KB, Retterstøl K (2011) Lipoprotein(a) levels in coronary heart disease susceptible and resistant patients with familial hypercholesterolemia. Atherosclerosis 216:426–432. [CrossRef]
- Bergström G, Persson M, Adiels M, Björnson E, Bonander C, Ahlström H, Alfredsson J, Angerås O, Berglund G, Blomberg A, Brandberg J, Börjesson M, Cederlund K, de Faire U, Duvernoy O, Ekblom Ö, Engström G, Engvall JE, Fagman E, Eriksson M, Erlinge D, Fagerberg B, Flinck A, Gonçalves I, Hagström E, Hjelmgren O, Lind L, Lindberg E, Lindqvist P, Ljungberg J, Magnusson M, Mannila M, Markstad H, Mohammad MA, Nystrom FH, Ostenfeld E, Persson A, Rosengren A, Sandström A, Själander A, Sköld MC, Sundström J, Swahn E, Söderberg S, Torén K, Östgren CJ, Jernberg T (2021) Prevalence of Subclinical Coronary Artery Atherosclerosis in the General Population. Circulation 144:916-929. [CrossRef]
- Waluś-Miarka M, Czarnecka D, Kloch-Badełek M, Wojciechowska W, Kapusta M, Malecki MT (2017) Carotid artery plaques – Are risk factors the same in men and women with familial hypercholesterolemia? Int J Cardiol 244:290–295. [CrossRef]
- Ray KK, Molemans B, Schoonen WM, Giovas P, Bray S, Kiru G, Murphy J, Banach M, De Servi S, Gaita D, Gouni-Berthold I, Hovingh GK, Jozwiak JJ, Jukema JW, Kiss RG, Kownator S, Iversen HK, Maher V, Masana L, Parkhomenko A, Peeters A, Clifford P, Raslova K, Siostrzonek P, Romeo S, Tousoulis D, Vlachopoulos C, Vrablik M, Catapano AL, Poulter NR (2021) EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: the DA VINCI study. Eur J Prev Cardiol 28:1279-1289. [CrossRef]
- Kayikcioglu M, Tokgozoglu L, Dogan V, Ceyhan C, Tuncez A, Kutlu M, Onrat E, Alici G, Akbulut M, Celik A, Yesilbursa D, Sahin T, Sonmez A, Ozdogan O, Temizhan A, Kilic S, Bayram F, Sabuncu T, Coskun FY, Ildizli M, Durakoglugil E, Kirilmaz B, Yilmaz MB, Yigit Z, Yildirim AB, Gedikli O, Topcu S, Oğuz A, Demir M, Yenerçağ M, Yıldırır A, Demircan S, Yilmaz M, Kaynar LG, Aktan M, Durmus RB, Gokce C, Ozcebe Oİ, Akyol TK, Okutan H, Sag S, Gul OO, Salcioglu Z, Altunkeser BB, Kuku I, Yasar HY, Kurtoglu E, Kose MD, Demircioglu S, Pekkolay Z, Ilhan O, Can LH (2018) Atherosclerosis 277:341-346. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).