1. Introduction
Ovarian cancer remains a significant global health challenge, ranking as the second most common gynecologically malignancy in developed nations and the third in developing countries. Despite advances in treatment, it remains the deadliest gynecologically cancer, with approximately 230,000 new cases diagnosed and 150,000 deaths annually worldwide [
1,
2].
Several factors have been identified as key prognostic indicators for ovarian cancer, including age, residual tumour volume, performance status, and histological type. Additionally, post-operative CA-125 levels, progesterone receptor status, and HER2 expression have emerged as important prognostic markers in recent studies [
3,
4,
5].
This retrospective study aimed to investigate the impact of clinicopathological factors and pre-treatment systemic inflammatory markers on progression-free survival (PFS) and overall survival (OS) in patients with epithelial ovarian cancer (EOC). By analysing these data, we sought to identify factors associated with both patient survival and platinum resistance, a critical determinant of treatment response and prognosis in ovarian cancer.
2. Materials and Methods
2.1. Study Design
We included 154 patients who were at the ages between 21 and 88 to this study who took/didn’t take neoadjuvant chemotherapy, were/weren’t operated, took/didn’t take adjuvant chemotherapy were followed up in Medipol University, Department of Oncology between the years 2013 and 2022.
We determined patient’s stages by using AJCC/UICC 8th edition with clinical and radiological findings at the time when they were first diagnosed. Information were included about age at the diagnosed time, information about operations, FIGO stages, ECOG PS scores, diagnose and operation dates, surgical types, histological type, localizations, grades, lymph nodes that had detected metastasis, lymph nodes that excised, took/didn’t take neoadjuvant chemotherapy (NAC)/adjuvant chemotherapy (AC), types of NAC/AC, dates of treatments, cycles of treatments, CA-125 values and BRCA status if there was, complet CBC results before treatment, relapse localizations, were/weren’t operated when relapses happened, chemotherapy types that was used after relapse, progression status, progression dates, patient’s last status, dead or alive.
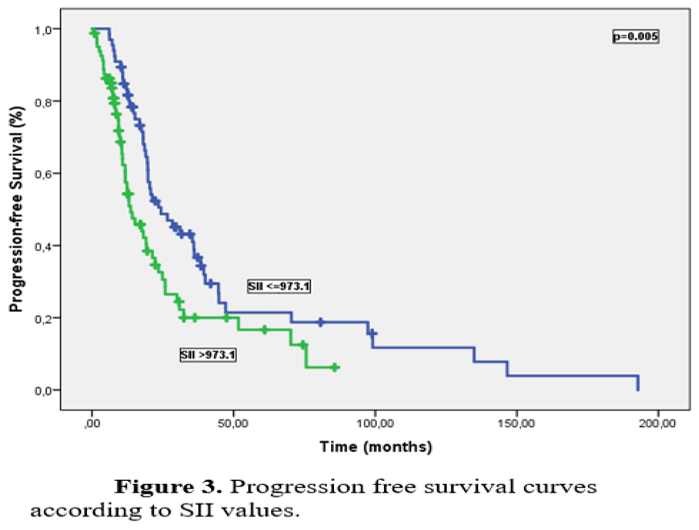
Neutrophil-to-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) and systemic inflammatory index (SII) were calculated, and then their median cut-off values were determined and categorized by using before treatment results of neutrophil, lymphocyte, thrombocyte values from CBCs. SII rates were calculated from Platelet x NLR formula. Patients were classified as low and high NLR (≤ 3 vs. > 3), PLR (≤ 194 vs. > 194), and SII (≤ 973.1 vs. > 973.1) respectively. Of the 154 patients, 47.4% (n=73) had low NLR (≤3). Similarly, 46.8% (n=72) had low PLR (≤194) and 44.9% (n=69) had low SII (≤973.1).
Written informed consent was obtained from all participating patients, or their designated relatives. This study was approved by the Local Ethics Committee at the Medipol University (Istanbul, Turkey), on May 5, 2022 (decision number: 383). Patients have lack of clinicopathological information were excluded from this study.
2.2. Baseline Characteristics of Patients
A total of 154 patients with epithelial ovarian cancer (EOC) were enrolled in this study. The median age of patients was 57 years. Most patients presented with advanced-stage disease. Most patients had a good performance status (ECOG PS 0 or 1). Regarding tumour histology, the most common subtype was serous papillary carcinoma (88.3%). Most tumours were high-grade (89.6%). The majority of patients (29.2%) received NAC prior to surgery due to advanced disease. Most patients underwent cytoreductive surgery, with 78.6% achieving maximal debulking. In terms of platinum sensitivity, 71.4% of patients were platinum sensitive (
Table 1).
2.3. Statistical Analysis
All statistical analyzes were done by using SPSS, version 24.0 (SPSS Inc., Chicago, IL, USA) Descriptive statistics were stated in 95% confidence interval and median for continuous variables. Survival analyses and survival curves were cooperated with log-rank tests by using Kaplan-Meier method. PFS was described as time from operation date or medical treatment respond date to first relapse/progression dates or last doctor visit dates for non-relapsed cases. OS was described as time from diagnose date to last doctor visit date or death date. Univariate Analysis and Multivariate Analysis and Cox Proportional Hazards Model was used to explain survival and clinicopathological futures, NLR, PLR, SII. Binary Logistic Regression Analysis was used to determine parameters that affect platin resistance. 95% Confidence Interval CI was used for determining relation between OS and every independent parameter. All p values were two-sided, and they were accepted significant equals 0.05 or lower.
3. Results
Prognostic Factors and Survival Outcomes
Median PFS and OS times were 19.6 months (95% CI: 17.0-22.2) and 59.6 months (95% CI: 49.2-69.6), respectively, with a median follow-up of 31.5 months. Univariate analysis for PFS identified ECOG PS, primary tumor localization, surgical procedure, platinum resistance status, NLR, PLR, and SII were significant prognostic factors. Median PFS was significantly longer in platinum-sensitive patients compared to platinum-resistant patients (21.3 months vs. 9.3 months, p<0.001). PFS was significantly worse in patients with high NLR, PLR, and SII compared to those with low levels, (23.5 months and 14.2 months for NLR, p=0.003; 23.5 months and 13 months for PLR, p=0.005; 24.3 months and 13.6 months for SII, p=0.005 respectively) (Figures 1–3).
Table 2 summarized the results of univariate analysis of PFS.
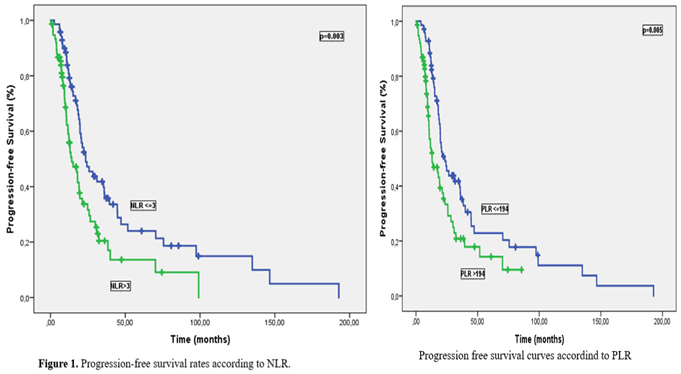
Univariate Analysis for OS showed age, stage, ECOG PS, surgical procedure, platinum resistance status, NLR and PLR were significant prognostic indicators. Median OS time was significantly better in patients with low NLR and PLR compared to those with high levels (61.1 months vs. 37.4 months, p=0.02 and 64.4 months vs 37.4 months, p=0.008, respectively), (Figures 4 and 5). Despite median OS was numerically better in SII≤973.1 patients than SII>973.1 patients (60.7 months vs. 46.1 months), the difference wasn’t statistically significant (p=0.082). Univariate analysis results for OS are showed in
Table 3.
We performed multivariate analysis to show independent prognostic factors when we found significant prognostic factors affecting PFS and OS in univariate analysis. FIGO stage (p<0.001, HR:1.89, 95% CI:1.33-2.67), histopathological type (p=0.006, HR:1.69, 95% CI:1.16-2.46), surgical type (p=0.04, HR:1.34, 95% CI:0.96-1.88) and presence of platinum resistance (p<0.001, HR:4.7, 95% CI: 2.42-9.12) were found as independent prognostic factors for PFS (
Table 2). On the other hand, ECOG PS (p=0.008, HR:1.50, 95% CI:1.11-2.02), surgical type (p=0.045, HR:1.62, 95% CI: 1.01-2.62) and presence of platinum resistance (p=0.004, HR:3.02, 95% CI:1.42-6.42) were independent prognostic factors for OS (
Table 3). We showed NLR, PLR and SII’s prognostic affects for survival in univariate analysis, however we couldn’t show them on multivariate analysis.
Logistic regression analysis to show prediction of platinum resistance which affects survivals directly. It demonstrated that histopathological type (p=0.004, OR:4.06, 95% CI:1.57-10.54), PLR (p=0.03, OR:4.08, 95% CI:0.83-20.07) and surgical type (p=0.032, OR:1.84, 95% CI:0.90-3.76) were independent predictors of platinum resistance (
Table 4).
4. Discussion
Recent studies have established a strong link between inflammation and cancer. Elevated levels of cytokines and chemokines have been implicated in tumour growth, angiogenesis, and metastasis. Novel prognostic factors, such as NLR, PLR, and SII, derived from peripheral blood counts, reflect systemic inflammation and have shown prognostic significance in various solid tumours, including hepatocellular carcinoma, small cell lung carcinoma, and other lung cancers[
6,
7,
8,
9,
10,
11].
Ovarian cancer remains a significant health concern. While traditional prognostic factors like age, residual disease, ECOG PS, and histological type are well-established, newer biomarkers like CA125, progesterone receptor status, and HER2 expression have gained importance[
2,
3,
4,
5].
Zhe Zhao et al. detected that high NLR is related with poor OS and poor PFS (HR:1.70, %95 CI:1.35-2.15 and HR:1.77, %95 CI:1.48-2.12, respectively) and high PLR affects badly to OS and PFS in a meta-analysis which was included 13 studies and 3467 patients who had ovarian cancer. Similarly, Anastasia Prodromidou et al. have pointed out NLR and PLR are promising prognostic factors in a meta-analysis which was included 18 studies and 3453 patients who had ovarian cancer[
12].
It was detected that high NLR values were related with poorer OS (HR:1.34, 95% CI:1.16-1.54) and poorer PFS (HR:1.36, 95% CI:1.17-1.57), high PLR values were related with poorer OS (HR:1.97, 95% CI:1.61-2.40) and poorer PFS (HR:1.79, 95% CI:1.46-2.20) in a meta-analysis which includes 10 studies and 2019 patients who had ovarian cancer. The higher NLR and PLR cut-off values, the more powerful predictive affects in the same study[
13]. On the other hand, Gatot Nyarumenteng et al. researched NLR and PLR’s affect about response to chemotherapy in their study which include 116 patients who has ovarian cancer. Authors detected patients has high NLR (p=0.026) and high PLR (p=0.003) responded better in that study.
Our study aligns with the literature, demonstrating that high NLR and PLR are associated with poorer outcomes. However, the lack of a significant association in multivariate analysis may be attributed to the relatively small sample size and heterogeneity of our patient population. While our study did not find a significant association between NAC and changes in NLR or PLR, this may be due to the limited number of patients treated with NAC.
It is also researched that inflammation parameters with prognosis and prediction of treatment, especially early predictors of platinum resistance. It was showed both NLR and PLR are related with survivals[
12,
13,
14,
15]
Our study is coherent with literature about prognostic futures. In other words, patients who have high NLR and high PLR have poorer PFS and OS. It is showed that high NLR (>3) was related with PFS (p=0.003) and OS (p=0.02) in univariate analysis, however it couldn’t be showed a relation in multivariate analysis. The reasons for this could be low number of our patients or our patient population was heterogenous.
M.Liontos et al. found a significant link between a high NLR and poor OS in ovarian cancer patients treated with NAC treatment. While a decrease in NLR after NAC was predictive of a good response, the initial NLR level did not correlate with response. Patients with high pre-NAC NLR and a decrease in NLR post-NAC had better PFS compared to those with no decrease[
16,
17].
Recent studies have demonstrated that a high peritoneal lavage fluid (PLF) ratio (PLR) is associated with worse overall survival (OS) and progression-free survival (PFS) in ovarian cancer patients. Our study further supports this finding, showing that a high PLR (>194) was significantly associated with worse PFS, and a low PLR (≤194) was associated with better OS in univariate analysis. However, PLR was not found to be an independent prognostic factor in multivariate analysis[
18,
19,
20].
The SII has been studied as a potential biomarker for cancer prognosis, particularly in ovarian and breast cancer. Research by Yongfang Ji and Haiyan Wang has shown a correlation between elevated SII levels and poorer OS in cervical, ovarian, and breast cancer. Furthermore, higher SII levels have been linked to worse disease-free survival (DFS) and PFS in ovarian and triple-negative breast cancer.
A meta-analysis of 68 studies involving patients with peritoneal carcinomatosis post-surgery confirmed the prognostic significance of SII, NLR, and PLR. High SII was found to be an independent risk factor for poor OS, while high PLR was associated with worse DFS in multivariate analysis. These findings suggest that SII, NLR, and PLR could serve as valuable prognostic markers in gynecologically and breast cancer[
21].
Dan Nie et al.'s study revealed a strong correlation between high SII values and advanced disease stages, lymph node metastasis, and tumor recurrence in EOC. High SII was associated with significantly worse PFS and OS in both univariate and multivariate analyses[
22].
The MITO24 study further explored the prognostic significance of SII and NLR in EOC patients. High NLR was identified as an independent risk factor for 6-month PFS in patients treated with chemotherapy but not bevacizumab. Additionally, both high NLR and SII were associated with poorer OS in a multivariate analysis, independent of other clinical factors[
23,
24].
We confirmed the prognostic significance of established factors like FIGO stage, ECOG PS, surgical type, and platinum sensitivity. Our findings support the notion that elevated NLR and PLR are associated with poorer outcomes in ovarian cancer patients. However, their independent prognostic significance in multivariate analysis was not as strong as expected, possibly due to the relatively small sample size and heterogeneity of our patient population. While SII showed a trend towards worse outcomes in univariate analysis, it did not reach statistical significance in multivariate analysis. This may be due to the limited sample size, or the specific patient population studied. Our study identified histopathological type, PLR, and surgical type as independent predictors of platinum resistance. This finding is consistent with previous research suggesting that these factors can influence treatment response.
Our limitations include its retrospective design, relatively small sample size, and short follow-up period. However, a unique aspect of this study is its focus on the predictive value of PLR, NLR, and SII individually for platinum sensitivity, which is a novel contribution to the literature. We believe this research can contribute to the early detection of platinum resistance in patients with EOC.
5. Conclusions
In conclusion, this retrospective study highlights the prognostic significance of systemic inflammatory markers, including NLR, PLR, and SII in EOC patients. Elevated levels of these markers were associated with poorer PFS and OS. While traditional prognostic factors such as FIGO stage, ECOG PS, surgical type, and platinum sensitivity remain crucial, the incorporation of inflammatory markers into clinical practice may provide additional prognostic information. Additionally, our study identified histopathological type, PLR, and surgical type as independent predictors of platinum resistance. This finding underscores the importance of considering these factors in treatment planning and decision-making for ovarian cancer patients. However, further prospective studies with larger sample sizes are needed to validate these findings and to investigate the potential clinical utility of these markers in predicting treatment response and guiding individualized treatment strategies.
Author Contributions
Conceptualization, C.I. and H.M.; methodology, C.I,H.M.; software, C.I, HM.; validation, H.M.,A.B. and O.F.O.; formal analysis, A.B.; investigation, C.I, H.M.; resources, C.I, H.M.; data curation, C.I, H.M, B.M, O.A, O.O, J.H, O.Y, O.FO, .; writing—original draft preparation, C.I, H.M.; writing—review and editing, C.I, H.M.; visualization, C.I, H.M.; supervision, A.B.; project administration, A.B, H.M.; funding acquisition:no funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Istanbul Medipol University (Istanbul, Turkey), approval code: 383; approval date: 5 May 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Torre, L.A. Torre, L.A., et al., Global cancer statistics, 2012. CA: a cancer journal for clinicians, 2015. 65(2): p. 87-108. [CrossRef]
- Lheureux, S. Lheureux, S., et al., Epithelial ovarian cancer. The Lancet, 2019. 393(10177): p. 1240-1253.
- Winter, W.E., 3rd; Maxwell, G.L.; Tian, C.; Carlson, J.W.; Ozols, R.F.; Rose, P.G.; Markman, M.; Armstrong, D.K.; Muggia, F.; McGuire, W.P. Winter, W.E., 3rd; Maxwell, G.L.; Tian, C.; Carlson, J.W.; Ozols, R.F.; Rose, P.G.; Markman, M.; Armstrong, D.K.; Muggia, F.; McGuire, W.P., et al. Prognostic Factors for Stage III Epithelial Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 3621–3627. [CrossRef]
- Hoskins, W.J.; Bundy, B.N.; Thigpen, J.; Omura, G.A. Hoskins, W.J.; Bundy, B.N.; Thigpen, J.; Omura, G.A. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume Stage III epithelial ovarian cancer: A gynecologic oncology group study. Gynecol. Oncol. 1992, 47, 159–166. [CrossRef]
- Crawford, S.C.; Vasey, P.A.; Paul, J.; Hay, A.; Davis, J.A.; Kaye, S.B. Crawford, S.C.; Vasey, P.A.; Paul, J.; Hay, A.; Davis, J.A.; Kaye, S.B. Does Aggressive Surgery Only Benefit Patients With Less Advanced Ovarian Cancer? Results From an International Comparison Within the SCOTROC-1 Trial. J. Clin. Oncol. 2005, 23, 8802–8811. [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [CrossRef]
- Tong, Y.-S.; Tan, J.; Zhou, X.-L.; Song, Y.-Q.; Song, Y.-J. Tong, Y.-S.; Tan, J.; Zhou, X.-L.; Song, Y.-Q.; Song, Y.-J. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J. Transl. Med. 2017, 15, 221. [CrossRef]
- Hong, X.; Cui, B.; Wang, M.; Yang, Z.; Wang, L.; Xu, Q. Hong, X.; Cui, B.; Wang, M.; Yang, Z.; Wang, L.; Xu, Q. Systemic Immune-inflammation Index, Based on Platelet Counts and Neutrophil-Lymphocyte Ratio, Is Useful for Predicting Prognosis in Small Cell Lung Cancer. Tohoku J. Exp. Med. 2015, 236, 297–304. [CrossRef]
- Feng, J.-F.; Chen, S.; Yang, X. Feng, J.-F.; Chen, S.; Yang, X. Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine 2017, 96, e5886. [CrossRef]
- Lolli, C.; Basso, U.; Derosa, L.; Scarpi, E.; Sava, T.; Santoni, M.; Crabb, S.J.; Massari, F.; Aieta, M.; Conteduca, V.; et al. Lolli, C.; Basso, U.; Derosa, L.; Scarpi, E.; Sava, T.; Santoni, M.; Crabb, S.J.; Massari, F.; Aieta, M.; Conteduca, V.; et al. Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget 2016, 7, 54564–54571. [CrossRef]
- Lolli, C.; Caffo, O.; Scarpi, E.; Aieta, M.; Conteduca, V.; Maines, F.; Bianchi, E.; Massari, F.; Veccia, A.; Chiuri, V.E.; et al. Lolli, C.; Caffo, O.; Scarpi, E.; Aieta, M.; Conteduca, V.; Maines, F.; Bianchi, E.; Massari, F.; Veccia, A.; Chiuri, V.E.; et al. Systemic Immune-Inflammation Index Predicts the Clinical Outcome in Patients with mCRPC Treated with Abiraterone. Front. Pharmacol. 2016, 7, 376. [CrossRef]
- Prodromidou, A.; Andreakos, P.; Kazakos, C.; Vlachos, D.E.; Perrea, D.; Pergialiotis, V. Prodromidou, A.; Andreakos, P.; Kazakos, C.; Vlachos, D.E.; Perrea, D.; Pergialiotis, V. The diagnostic efficacy of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in ovarian cancer. Inflamm. Res. 2017, 66, 467–475. [CrossRef]
- Yin, X. Yin, X., et al., Prognostic significance of neutrophil–lymphocyte ratio (NLR) in patients with ovarian cancer: A systematic review and meta-analysis. Medicine, 2019. 98(45): p. e17475. [CrossRef]
- Zhao, Z.; Zhao, X.; Lu, J.; Xue, J.; Liu, P.; Mao, H. Zhao, Z.; Zhao, X.; Lu, J.; Xue, J.; Liu, P.; Mao, H. Prognostic roles of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in ovarian cancer: a meta-analysis of retrospective studies. Arch. Gynecol. Obstet. 2018, 297, 849–857. [CrossRef]
- Winarno, G.N.A.; Pasaribu, M.; Susanto, H.; Nisa, A.S.; Harsono, A.B.; Yuseran, H.; Suardi, D.; Trianasari, N. Winarno, G.N.A.; Pasaribu, M.; Susanto, H.; Nisa, A.S.; Harsono, A.B.; Yuseran, H.; Suardi, D.; Trianasari, N. The Platelet to Lymphocyte and Neutrophil to Lymphocyte Ratios in Predicting Response to Platinum-based Chemotherapy for Epithelial Ovarian Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 1561–1566. [CrossRef]
- Liontos, M.; Andrikopoulou, A.; Koutsoukos, K.; Markellos, C.; Skafida, E.; Fiste, O.; Kaparelou, M.; Thomakos, N.; Haidopoulos, D.; Rodolakis, A.; et al. Liontos, M.; Andrikopoulou, A.; Koutsoukos, K.; Markellos, C.; Skafida, E.; Fiste, O.; Kaparelou, M.; Thomakos, N.; Haidopoulos, D.; Rodolakis, A.; et al. Neutrophil-to-lymphocyte ratio and chemotherapy response score as prognostic markers in ovarian cancer patients treated with neoadjuvant chemotherapy. J. Ovarian Res. 2021, 14, 1–10. [CrossRef]
- Sanna, E.; Tanca, L.; Cherchi, C.; Gramignano, G.; Oppi, S.; Chiai, M.G.; Macciò, A.; Madeddu, C. Sanna, E.; Tanca, L.; Cherchi, C.; Gramignano, G.; Oppi, S.; Chiai, M.G.; Macciò, A.; Madeddu, C. Decrease in Neutrophil-to-Lymphocyte Ratio during Neoadjuvant Chemotherapy as a Predictive and Prognostic Marker in Advanced Ovarian Cancer. Diagnostics 2021, 11, 1298. [CrossRef]
- Tian, C. Tian, C., et al., Prognostic significance of platelet-to-lymphocyte ratio in patients with ovarian cancer: a meta-analysis. European Journal of Clinical Investigation, 2018. 48(5): p. e12917. [CrossRef]
- Jiang, S.; Liu, J.; Chen, X.; Zheng, X.; Ruan, J.; Ye, A.; Zhang, S.; Zhang, L.; Kuang, Z.; Liu, R. Jiang, S.; Liu, J.; Chen, X.; Zheng, X.; Ruan, J.; Ye, A.; Zhang, S.; Zhang, L.; Kuang, Z.; Liu, R. Platelet-lymphocyte ratio as a potential prognostic factor in gynecologic cancers: a meta-analysis. Arch. Gynecol. Obstet. 2019, 300, 829–839. [CrossRef]
- Chon, S.; Lee, S.; Jeong, D.; Lim, S.; Lee, K.; Shin, J. Chon, S.; Lee, S.; Jeong, D.; Lim, S.; Lee, K.; Shin, J. Elevated platelet lymphocyte ratio is a poor prognostic factor in advanced epithelial ovarian cancer. J. Gynecol. Obstet. Hum. Reprod. 2020, 50, 101849. [CrossRef]
- Ji, Y.; Wang, H. Ji, Y.; Wang, H. Prognostic prediction of systemic immune-inflammation index for patients with gynecological and breast cancers: a meta-analysis. World J. Surg. Oncol. 2020, 18, 1–11. [CrossRef]
- Nie, D.; Gong, H.; Mao, X.; Li, Z. Nie, D.; Gong, H.; Mao, X.; Li, Z. Systemic immune-inflammation index predicts prognosis in patients with epithelial ovarian cancer: A retrospective study. Gynecol. Oncol. 2019, 152, 259–264. [CrossRef]
- Ramón-Rodríguez, J.; De-Armas-Conde, N.; Jaén-Torrejimeno, I.; Prada-Villaverde, A.; Rojas-Holguín, A.; López-Guerra, D.; Blanco-Fernández, G. Ramón-Rodríguez, J.; De-Armas-Conde, N.; Jaén-Torrejimeno, I.; Prada-Villaverde, A.; Rojas-Holguín, A.; López-Guerra, D.; Blanco-Fernández, G. Prognostic value of pre-operative systemic immune-inflammation index and platelet to lymphocyte ratio in peritoneal carcinomatosis of ovarian origin. Surg. Oncol. 2022, 42, 101750. [CrossRef]
- Farolfi, A.; Scarpi, E.; Greco, F.; Bergamini, A.; Longo, L.; Pignata, S.; Casanova, C.; Cormio, G.; Bologna, A.; Orditura, M.; et al. Farolfi, A.; Scarpi, E.; Greco, F.; Bergamini, A.; Longo, L.; Pignata, S.; Casanova, C.; Cormio, G.; Bologna, A.; Orditura, M.; et al. Inflammatory indexes as predictive factors for platinum sensitivity and as prognostic factors in recurrent epithelial ovarian cancer patients: a MITO24 retrospective study. Sci. Rep. 2020, 10, 1–8. [CrossRef]
Table 1.
Clinicopathological characteristics of patients.
Table 1.
Clinicopathological characteristics of patients.
| Characteristic |
n (%) |
Age, Year
Interval
Median |
21-88
57 |
Stage
Stage I
Stage II
Stage III
Stage IV |
21(13.6)
6 (3.9)
111 (72.1)
16 (10.4) |
Eastern Cooperative Oncology Group Performance Status
0
I
II
III |
94 (61.0)
41 (26.6)
13 (8.4)
6 (3.9) |
Primary Localization
Ovary
Fallopian Tube
Primary Peritoneal |
127 (82.5)
18 (11.7)
9 (5.8) |
Histopatological Type
Serous Papillary
Clear Cell
Mucinous
Borderline |
136 (88.3)
10 (6.5)
6 (3.9)
2 (1.3) |
Grade
Low
High
Unknown |
8 (5.2)
138 (89.6)
8 (5.2) |
NAC
Yes
No |
45 (29.2)
109 (70.8) |
Surgical Type
Maximal debulking
Optimal debulking
Suboptimal debulking
İnoperable
|
121 (78.6)
20 (13.0)
4 (2.6)
3 (1.9) |
Adjuvant Chemotherapy
Yes
No |
143 (92.9)
11 (7.1) |
Relapse Status
Yes
No |
105 (68.1)
49 (31.9) |
Platinum Resistance
Partially Sensitive
Sensitive |
11 (7.2)
110 (71.4) |
| Resistant |
33 (21.4) |
Table 2.
The results of univariate and multivariate analysis for PFS.
Table 2.
The results of univariate and multivariate analysis for PFS.
| Characteristics |
n (%) |
Median PFS
(month) |
Univariate
P Value |
HR
CI 95% |
Multivariate p Value |
Age, Year
<60
>60 |
86 (55.8)
68 (44.2) |
19.5
19.7 |
0.64 |
|
|
Stage
Stage I
Stage II
Stage III
Stage IV |
21(13.6)
6 (3.9)
111 (72.1)
16 (10.4) |
51.7
17.5
19.2
13.0 |
0.015 |
1.89
1.33-2.67 |
<0.001 |
ECOG PS
0-I
II-III |
135 (87.6)
19 (22.4) |
21.2
18.0 |
0.029 |
1.15
0.89-1.48 |
0.27 |
Primary Localization
Ovary
Fallopian Tube
Primary Peritoneal |
127 (82.5)
18 (11.7)
9 (5.8) |
19.7
13.5
NR |
0.024 |
1.44
0.90-2.31
|
0.11 |
Histopatological Type
Serous Papillary
Clear Cell
Mucinous
Borderline |
136 (88.3)
10 (6.5)
6 (3.9)
2 (1.3) |
20.3
6.9
6.2
12.6 |
0.09 |
1.69
1.16-2.46
|
0.006 |
Grade
Low
High
Unknown |
8 (5.2)
138 (89.6)
8 (5.2) |
NR
19.7
NR |
0.42 |
|
|
NAC
Yes
No |
45 (29.2)
109 (70.8) |
19.0
20.3 |
0.54 |
|
|
Surgical Type
Maximal debulking
Optimal debulking
Suboptimal debulking
İnoperable |
121 (78.6)
20 (13.0)
4 (2.6)
3 (1.9) |
21.2
19.5
13.3
7.4 |
0.003 |
1.34
0.96-1.88
|
0.04 |
Platinum Resistance
Sensitive |
121 (78.6) |
21.3 |
<0.001
|
4.70
2.42-9.12 |
<0.001
|
| Resistant |
33 (21.4) |
9.3 |
|
|
|
NLR
<3
>3 |
73 (47.4)
81 (52.6) |
23.5
14.2 |
0.003 |
1.53
0.81-2.91 |
0.18 |
PLR
<194
>194 |
72 (46.8)
82 (53.2) |
23.5
13.0 |
0.005 |
0.87
0.46-1.64 |
0.67 |
SII
>973.1
>973.1 |
69 (44.9)
85 (55.1) |
24.3
13.6 |
0.005 |
1.67
0.79-3.53 |
0.17 |
Table 3.
Univariate and multivariate analysis for OS.
Table 3.
Univariate and multivariate analysis for OS.
| Characteristics |
n (%) |
Median OS
(month) |
Univariate p Value |
HR
CI 95% |
Multivariate p Value |
Age, Year
<60
>60 |
86 (55.8)
68 (44.2) |
57.1
55.8 |
0.50 |
|
|
Stage
Stage I
Stage II
Stage III
Stage IV |
21(13.6)
6 (3.9)
111 (72.1)
16 (10.4) |
NR
NR
51.7
29.1 |
0.04 |
1.33
0.82-2.15 |
0.23
|
ECOG PS
0-I
II-III |
135 (87.6)
19 (22.4) |
62.0
29.1 |
<0.001 |
1.50
1.11-2.02 |
0.008 |
Primary Localization
Ovary
Fallopian Tube
Primary Peritoneal |
127 (82.5)
18 (11.7)
9 (5.8) |
NR
NR
NR |
0.90 |
|
|
Histopatological Type
Serous Papillary
Clear Cell
Mucinous
Borderline |
136 (88.3)
10 (6.5)
6 (3.9)
2 (1.3) |
51.7
NR
NR
NA |
0.48 |
|
|
Grade
Low
High
Unknown |
8 (5.2)
138 (89.6)
8 (5.2) |
NR
57.1
NR |
0.30 |
|
|
NAC
Yes
No |
45 (29.2)
109 (70.8) |
38.5
56.9 |
0.30 |
|
|
Surgical Type
Maximal debulking
Optimal debulking
Suboptimal debulking
İnoperable |
121 (78.6)
20 (13.0)
4 (2.6)
3 (1.9) |
62.0
56.9
45.5
10.4 |
0.001 |
1.62
1.01-2.62 |
0.045 |
Platinum Resistance
Sensitive |
121 (78.6) |
64.6 |
<0.001 |
3.02
1.42-6.42 |
0.004 |
| Resistant |
33 (21.4) |
25.8 |
|
|
|
NLR
<3
<3 |
73 (47.4)
81 (52.6) |
61.1
37.4 |
0.02 |
1.08
0.42-2.80 |
0.87 |
PLR
<194
>194 |
72 (46.8)
82 (53.2) |
64.6
37.4 |
0.008 |
1.46
0.59-3.61 |
0.40 |
SII
>973.1
>973.1 |
69 (44.9)
85 (55.1) |
60.7
46.1 |
0.082 |
1.08
0.34-3.34 |
0.89 |
Table 4.
The analysis of predictors of platinum resistance.
Table 4.
The analysis of predictors of platinum resistance.
| Factors |
β |
X2
|
p |
OR |
95% CI |
| FIGO stage |
0.20 |
0.21 |
0.64 |
1.22 |
0.51-2.89 |
| EGOG PS |
0.24 |
0.09 |
0.75 |
1.28 |
0.27-5.99 |
| Histopathology |
1.40 |
8.35 |
0.004 |
4.06 |
1.57-10.54 |
| Surgical type |
0.61 |
2.84 |
0.032 |
1.84 |
0.90-3.76 |
| NLR |
-0.27 |
0.08 |
0.77 |
0.75 |
0.11-5.14 |
| PLR |
1.40 |
2.99 |
0.03 |
4.08 |
0.83-20.07 |
| SII |
-1.42 |
1.48 |
0.22 |
0.24 |
0.02-2.37 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).







