Submitted:
24 December 2024
Posted:
25 December 2024
You are already at the latest version
Abstract
Keywords:
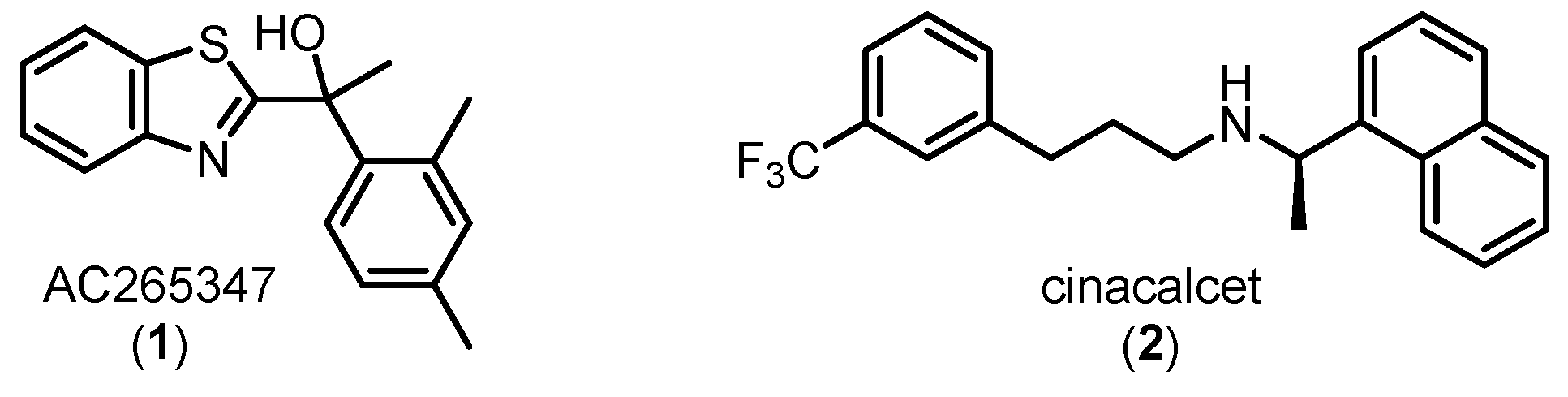
1. Introduction
2. Results and Discussion
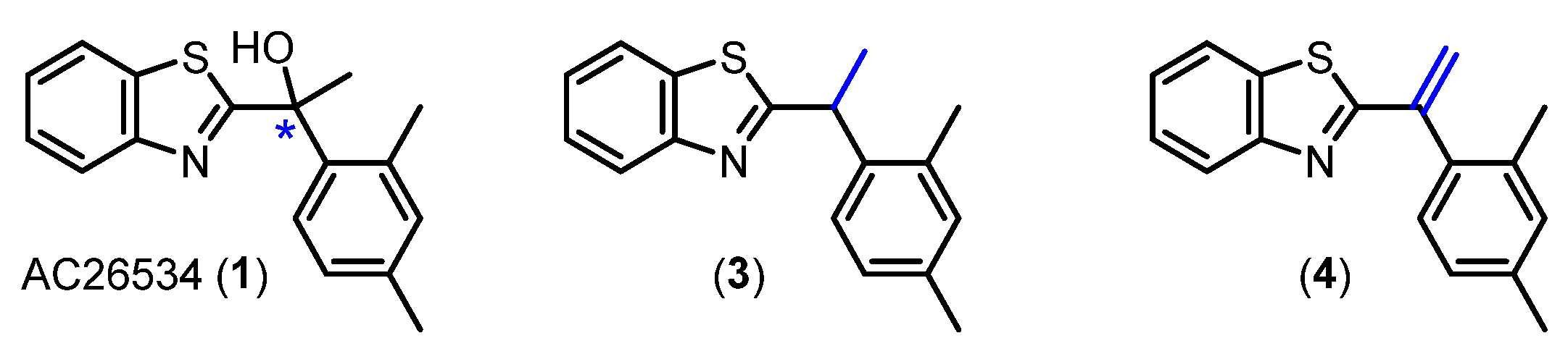
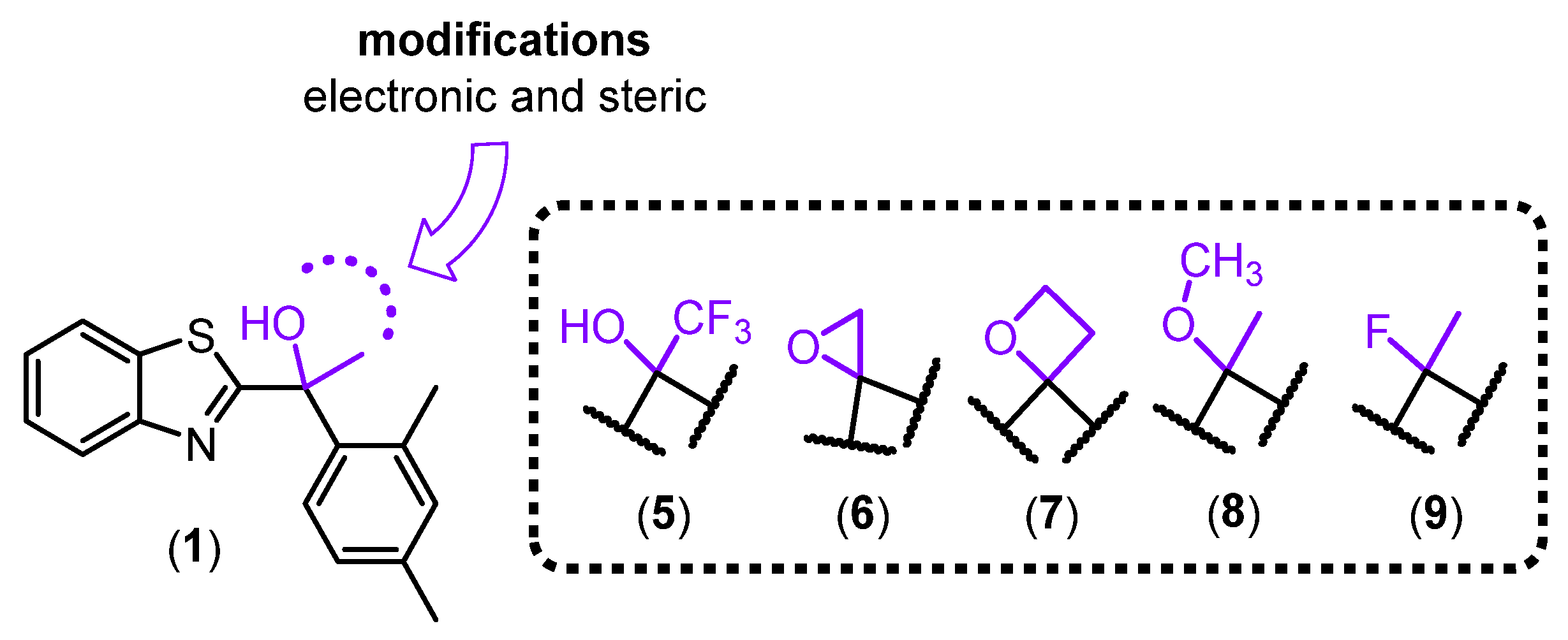
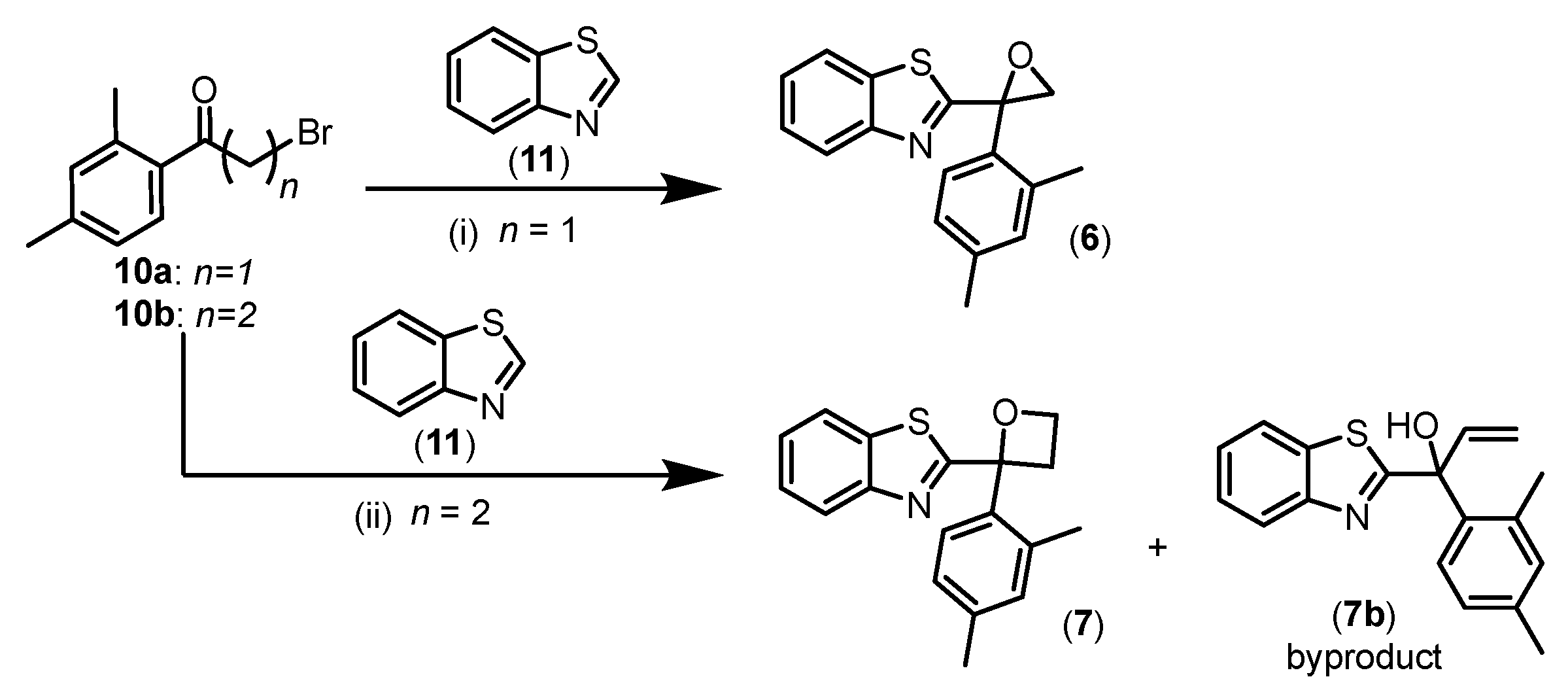
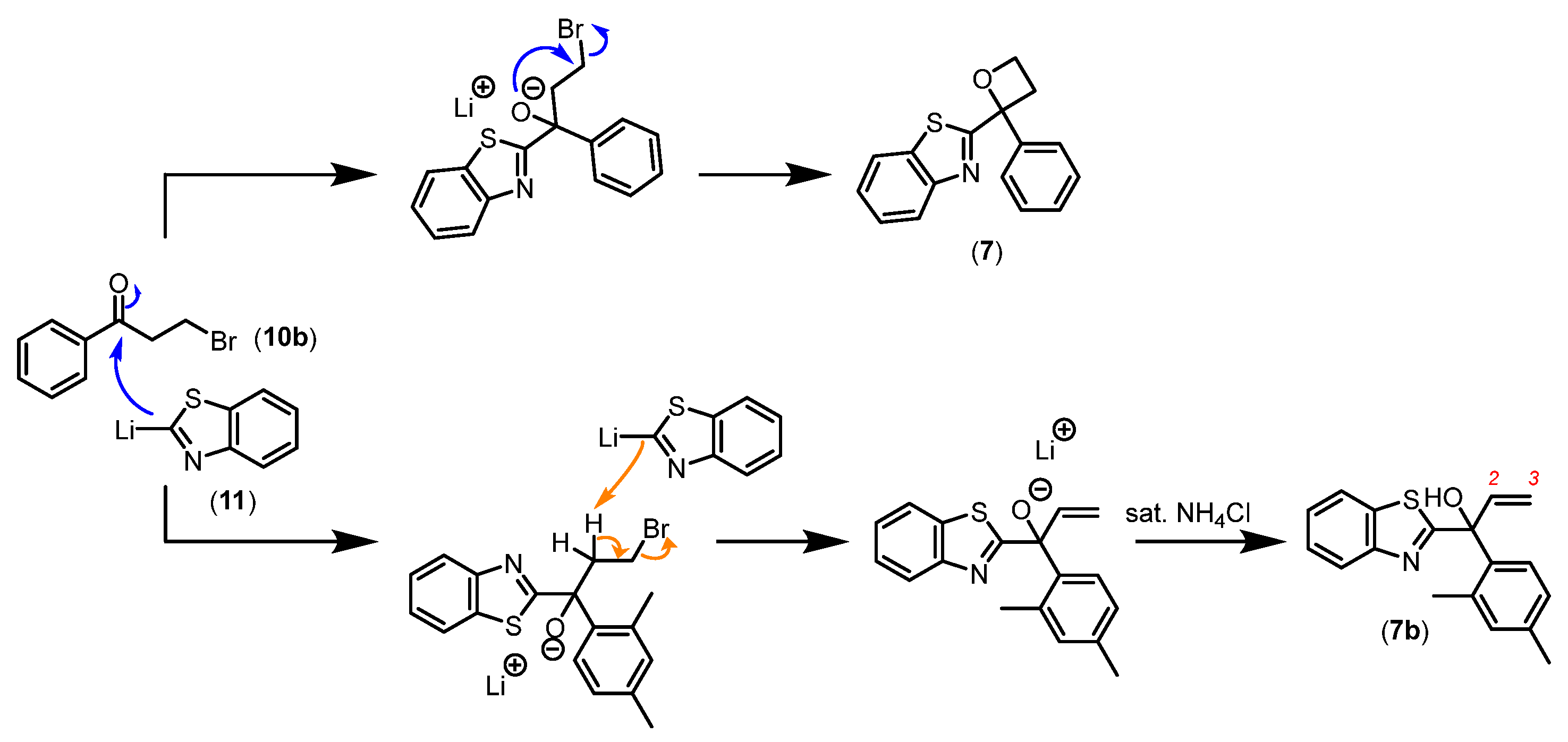
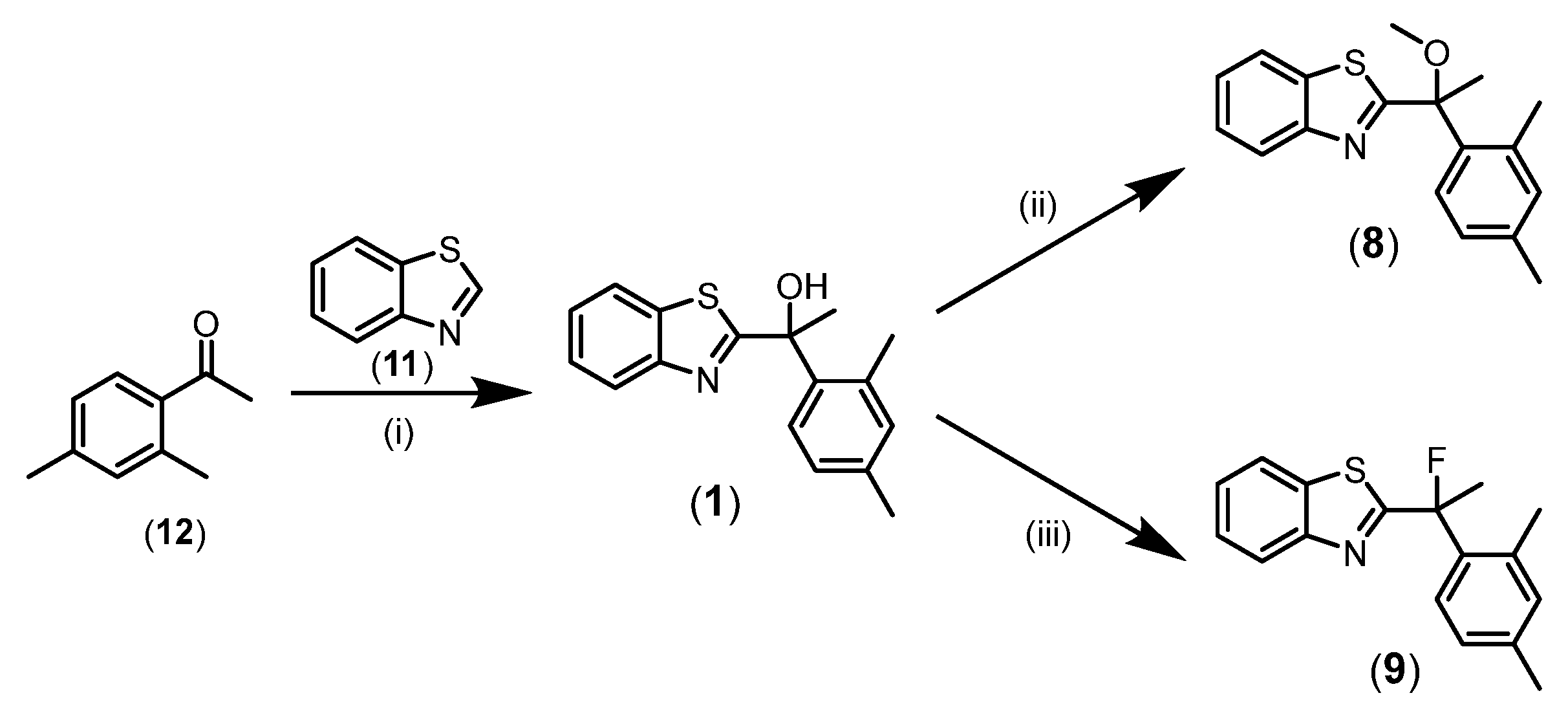
2.1. Chemistry
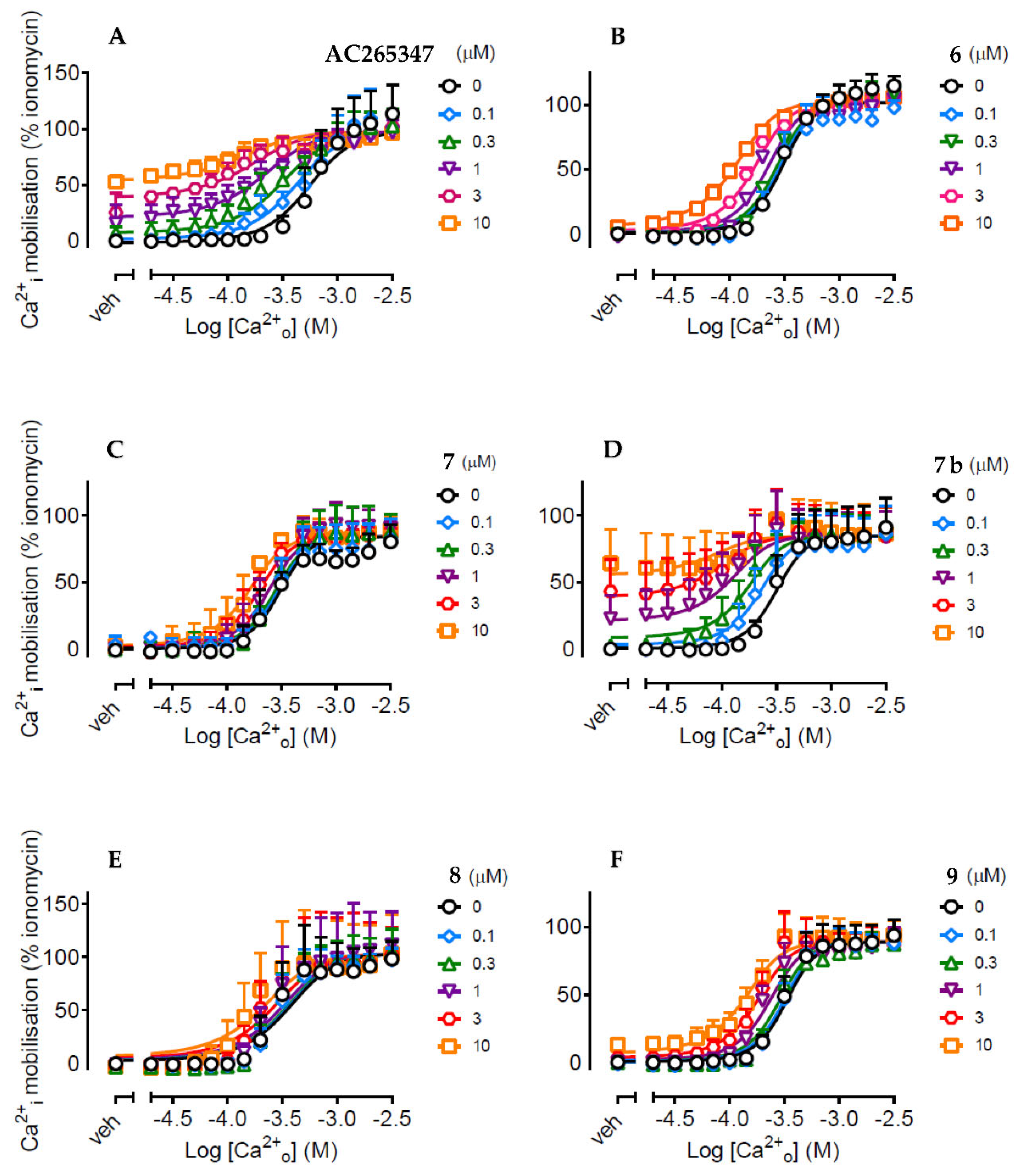
2.2. Pharmacology
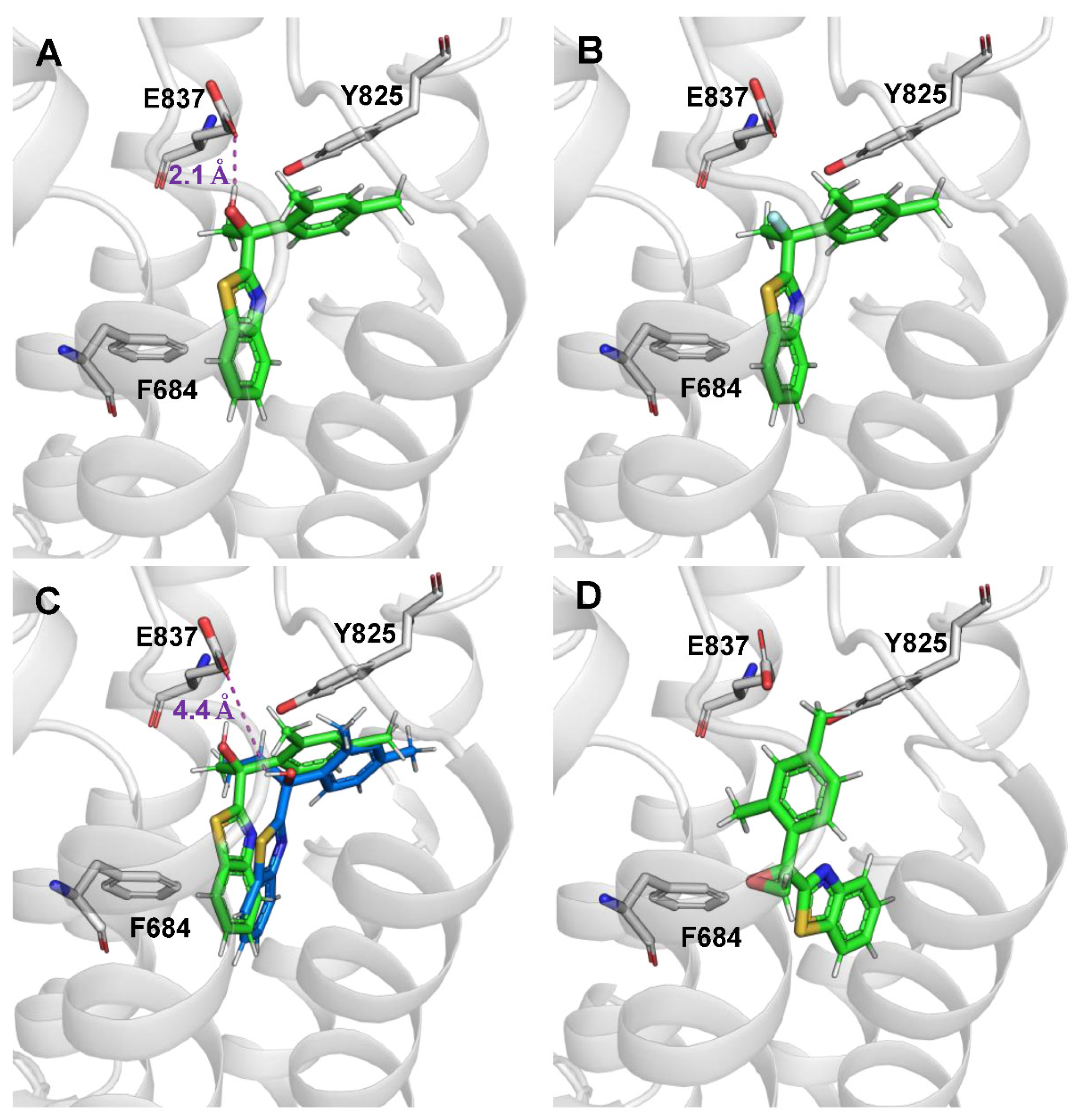
2.3. Molecular Docking Study
3. Materials and Methods
3.1. Materials
3.1.1. Chemistry
3.1.2. Pharmacology
3.2. Chemical Synthesis of Compounds
General Procedure A [16] (lithiation)
General Procedure B [7] (O-cyclisation)
Synthesis of 1-(Benzo[d]thiazol-2-yl)-1-(2,4-dimethylphenyl)ethan-1-ol (1) [3]
Synthesis of 2-(2-(2,4-Dimethylphenyl)oxiran-2-yl)benzo[d]thiazole (6)
Synthesis of 2-(2-(2,4-Dimethylphenyl)oxetan-2-yl)benzo[d]thiazole (7)
Synthesis of 2-(1-(2,4-Dimethylphenyl)-1-methoxyethyl)benzo[d]thiazole (8)
Synthesis of 2-(1-(2,4-Dimethylphenyl)-1-fluoroethyl)benzo[d]thiazole (9)
3.3. Pharmacological Evaluation of Compounds
3.3.1. Functional Assay - Ca2+i Mobilisation
3.3.2. Data Statistical Analysis
3.4. Computational Modelling of Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, J.-N.; Owens, M.; Gustafsson, M.; Jensen, J.; Tabatabaei, A.; Schmelzer, K.; Olsson, R.; Burstein, E.S. Characterization of highly efficacious allosteric agonists of the human calcium-sensing receptor. J Pharmacol Exp Ther 2011, 337, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.; Jensen, J.; Bertozzi, S.M.; Currier, E.A.; Ma, J.-N.; Burstein, E.S.; Olsson, R. Discovery of a class of calcium sensing receptor positive allosteric modulators 1-(benzothiazol-2-yl)-1-phenylethanols. Bioorg Med Chem Lett 2010, 20, 5918–5921. [Google Scholar] [CrossRef] [PubMed]
- Dinh, L.V.; DeBono, A.; Keller, A.N.; Josephs, T.M.; Gregory, K.J.; Leach, K.; Capuano, B. Development of AC265347-Inspired Calcium-Sensing Receptor Ago-Positive Allosteric Modulators. ChemMedChem 2021, 16, 3451–3462. [Google Scholar] [CrossRef]
- Leach, K.; Sexton, P.M.; Christopoulos, A. Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci 2007, 28, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K.L. Selective Fluorination in Drug Design and Development: An Overview of Biochemical Rationales. CTMC 2006, 6, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Welch, J. T. Advances in the preparation of biologically active organofluorine compounds. Tetrahedron 1987, 43, 3123–3197. [Google Scholar] [CrossRef]
- Chikashita, H.; Ishibaba, M.; Ori, K.; Itoh, K. General Reactivity of 2-Lithiobenzothiazole to Various Electrophiles and the Use as a Formyl Anion Equivalent in the Synthesis of α-Hydroxy Carbonyl Compounds. Bull Chem Soc Jpn 1988, 61, 3637–3648. [Google Scholar] [CrossRef]
- Morrill, C.; Morrill, C.; Grubbs, R.H. Highly Selective 1,3-Isomerization of Allylic Alcohols via Rhenium Oxo Catalysis. J Am Chem Soc 2005, 127, 2842–2843. [Google Scholar] [CrossRef] [PubMed]
- Bird, T.G.C.; Bruneau, P.; Crawley, G.C.; Edwards, M.P.; Foster, S.J.; Girodeau, J.M.; Kingston, J.F.; McMillan, R.M. (Methoxyalkyl)thiazoles: a new series of potent, selective, and orally active 5-lipoxygenase inhibitors displaying high enantioselectivity. J Med Chem 1991, 34, 2176–2186. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, A.; Beaulieu, F.; Bennett, C.; Bill, D.R.; Clayton, S.; LaFlamme, F.; Mirmehrabi, M.; Tadayon, S.; Tovell, D.; Couturier, M. Aminodifluorosulfinium Salts: Selective Fluorination Reagents with Enhanced Thermal Stability and Ease of Handling. J Org Chem 2010, 75, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. van der Waals Volumes and Radii. J Phys Chem 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Hevey, R. Bioisosteres of carbohydrate functional groups in glycomimetic design. Biomimetics 2019, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Rychlewski, J.; Rychlewska, U. Effects of Substitution of OH Group by F Atom for Conformational Preferences of Fluorine-Substituted Analogues of (R,R)-Tartaric Acid, Its Dimethyl Diester, Diamide, and N,N,N‘,N‘-Tetramethyl Diamide. Ab Initio Conformational Analysis. J Am Chem Soc 1999, 121, 1912–1921. [Google Scholar] [CrossRef]
- Leach, K.; Gregory, K.J.; Kufareva, I.; Khajehali, E.; Cook, A.E.; Abagyan, R.; Conigrave, A.D.; Sexton, P.M.; Christopoulos, A. Towards a structural understanding of allosteric drugs at the human calcium-sensing receptor. Cell Res 2016, 26, 574–592. [Google Scholar] [CrossRef]
- Gao, Y.; Robertson, M.J.; Rahman, S.N.; et al. Asymmetric activation of the calcium-sensing receptor homodimer. Nature 2021, 595, 455–459. [Google Scholar] [CrossRef]
- Anthony, N.J.; Andresen, B.M.; Northrup, A.B.; Childers, K.K.; Donofrio, A.; Miller, T.A.; Liu, Y.; Machacek, M.R.; Woo, H.C.; Spencer, K.B.; Ellis, J. M.; Altman, M. D.; Romeo, E.T.; Guay, D.; Grimm, J.; Lebrun, M.; Robichaud, J.S.; Wang, L.; Dubois, B.; Deng, Q. Thiazole-substituted Aminoheteroaryls as spleen Tyrosine Kinase Inhibitors, in WO 2014/176210 Al, 2014.
- Keller, A.N.; Kufareva, I.; Josephs, T.M.; Diao, J.; Mai, V.T.; Conigrave, A.D.; Christopoulos, A.; Gregory, K.J.; Leach, K. Identification of global and ligand-specific calcium sensing receptor activation mechanisms. Mol Endocrinol 2018, 93, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Hellyer, S.D.; Albold, S.; Wang, T.; Chen, A.; May, L.; Leach, K.; Gregory, K.J. Selective" Class C G Protein-Coupled Receptor Modulators Are Neutral or Biased mGlu(5) Allosteric Ligands. Mol Pharmacol 2018, 93, 504–514. [Google Scholar] [CrossRef]
- Leach, K.; Loiacono, R.E.; Felder, C.C.; McKinzie, D.L.; Mogg, A.; Shaw, D.B.; Sexton, P.M.; Christopoulos, A. Molecular mechanisms of action and in vivo validation of an M4 muscarinic acetylcholine receptor allosteric modulator with potential antipsychotic properties. Neuropsychopharmacol 2010, 35, 855–869. [Google Scholar] [CrossRef]
- Gregory, K.J.; Giraldo, J.; Diao, J.; Christopoulos, A.; Leach, K. Evaluation of operational models of agonism and allosterism at receptors with multiple orthosteric binding sites. Mol Pharmacol 2020, 97, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Davey, A.E.; Leach, K.; Valant, C.; Conigrave, A.D.; Sexton, P.M.; Christopoulos, A. Positive and Negative Allosteric Modulators Promote Biased Signaling at the Calcium-Sensing Receptor. Endocrinology 2012, 153, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, A. Assessing the distribution of parameters in models of ligand–receptor interaction: to log or not to log. Trends Pharmacol Sci 1998, 19, 351–357. [Google Scholar] [CrossRef]
- Totrov, M.; Abagyan, R. (1999). Derivation of sensitive discrimination potential for virtual ligand screening. RECOMB99 3rd International Conference on Computational Molecular Biology (p. 312). Lyon, France.
- Abagyan, R.; Totrov, M. Biased probability Monte Carlo conformational searches and electrostatic calculations for peptides and proteins. J Mol Biol 1994, 235, 983–1002. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.A.; Totrov, M.; Abagyan, R. Docking and scoring with ICM: the benchmarking results and strategies for improvement. J Comput Aided Mol Des 2012, 26, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Schapira, M.; Totrov, M.; Abagyan, R. Prediction of the binding energy for small molecules, peptides and proteins. J Mol Recognit 1999, 12, 177–190. [Google Scholar] [CrossRef]
| Entry | Structure | pKB ± SD KB, μM) |
Logαβ ± SD (αβ) |
LogτB ± SD (τB) |
n |
|---|---|---|---|---|---|
| AC265347 (1)a | 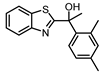 |
5.1 ± 0.09 (7.9) |
2.5 ± 0.9 (316) |
0.5 ± 0.2 (3.2) |
5 |
| 5a | 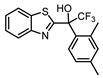 |
5.2 ± 0.2 (6.3) |
1.6 ± 0.9 (40) |
0.7 ± 0.2 (5.0) |
3 |
| 6 | 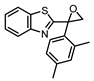 |
4.7 ± 0.4 (20) |
1.5 ± 0.4 (32) |
ND | 3 |
| 7 | 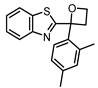 |
4.6 ± 0.2 (25) |
0.9 ± 0.1 (7.9) |
ND | 3 |
| 7b | 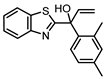 |
4.7 ± 0.4 (20) |
2.5 ± 0.4 (316) |
0.6 ± 0.4 (4.0) |
4 |
| 8 | 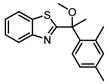 |
4.8 ± 0.6 (16) |
1.1 ± 0.5 (16) |
ND | 3 |
| 9 | 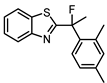 |
4.8 ± 0.5 (16) |
1.6 ± 0.3 (40) |
ND | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





