1. Introduction
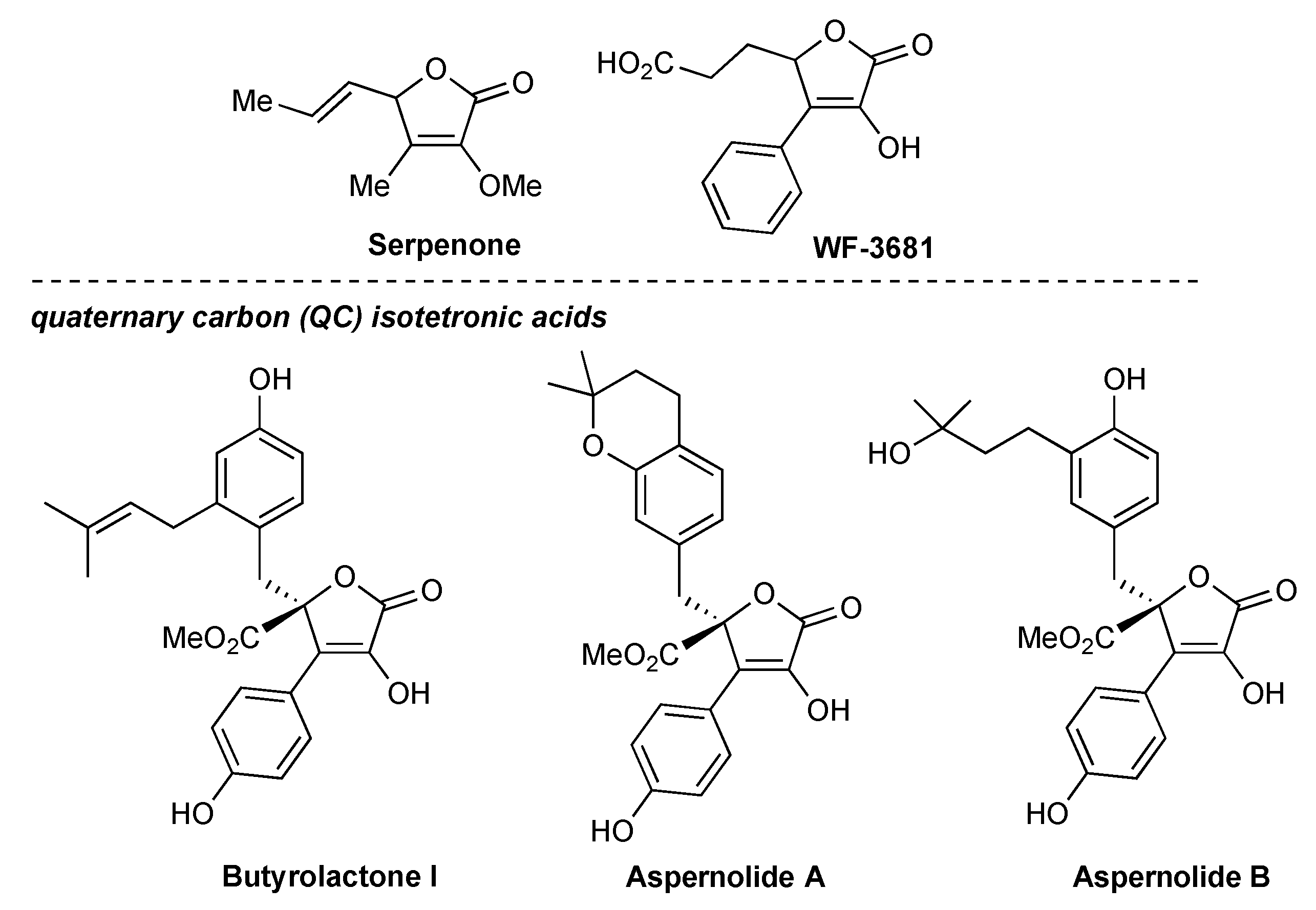
Isotetronic acids are classified as butenolides and are significant for their synthetic applications and inherent biological properties.[
1] Serpenone was recognized as a metabolite of the higher fungus Hypoxylon serpens and demonstrated efficacy in the treatment of diabetic neuropathy.[
2] WF-3681, an additional fungal metabolite extracted from Chaetomella raphigera, was reported to possess aldose reductase inhibitory action (
Figure 1).[
3] A particular class of optically active isotetronic acids displaying a quaternary carbon (QC) functionalized with an ester group and an alkyl substituent (QC-isotetronic acids) has been shown to have exceptional bioactivity. This is the case of butyrolactone I and Aspernolides A/B, natural products isolated from the fungi Aspergillus terreus, which exhibit very promising antitumor activity against colon, lung, prostatic, and pancreatic carcinoma (
Figure 1).[
4,
5,
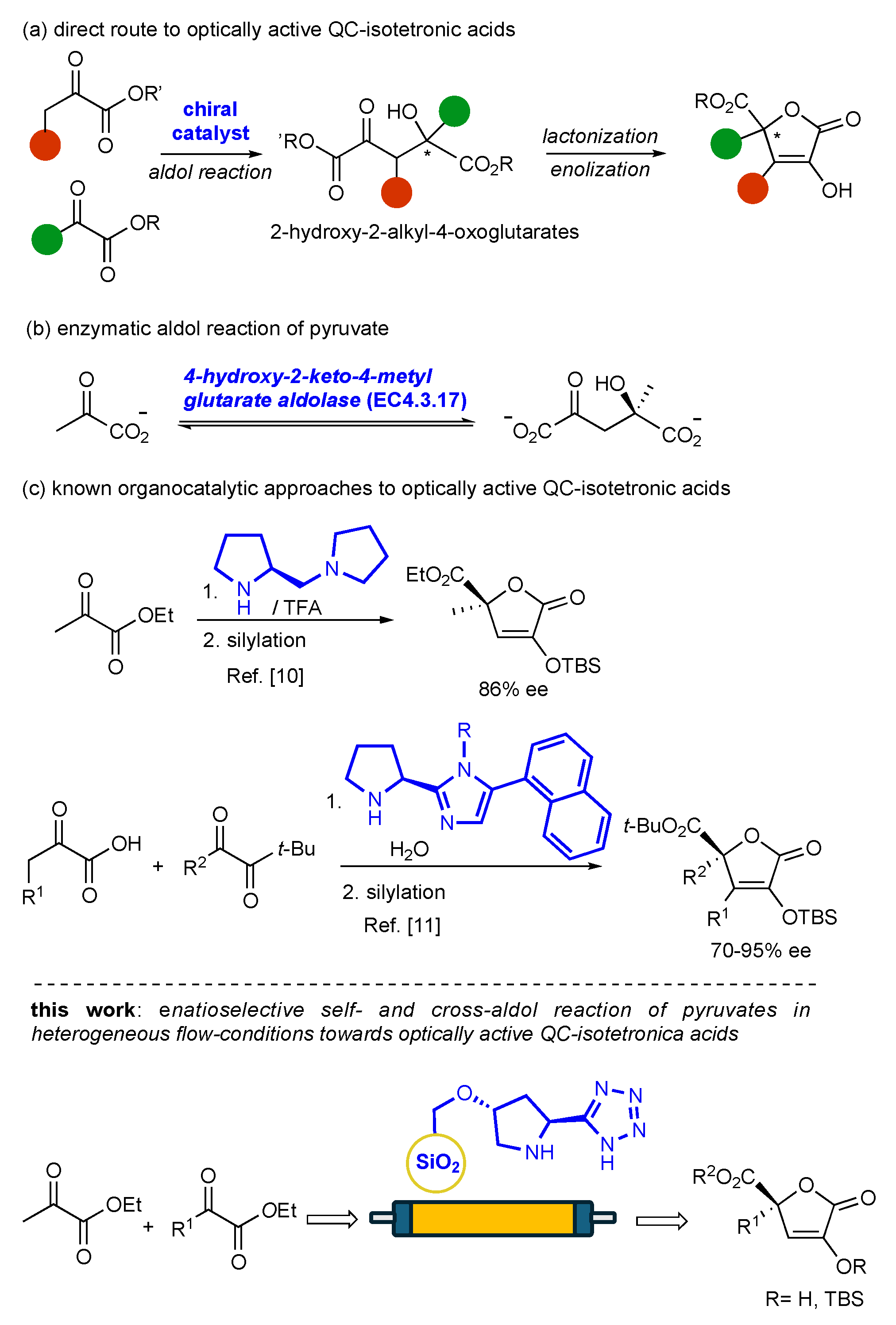
6] Consequently, the development of an efficient and stereoselective method to access this bioactive scaffold would be highly desirable for new lead identification and optimization. The enantioselective catalytic self- and cross-aldol reactions of pyruvates leading to chiral quaternary α-hydroxy esters (2-hydroxy-2-alkyl-4-oxoglutarates) followed by a lactonization/enolization sequence certainly represents the most straightforward route to explore the (stereo)chemical diversity of optically active QC-isotetronic acids (
Scheme 1a). The natural enzyme 4-hydroxy-2-oxo-4-methyl-glutarate aldolase (EC4.1.3.17) effectively promotes the self-condensation of pyruvate with complete stereocontrol in the biosynthesis of the corresponding α-hydroxy acid (
Scheme 1b).[
7] To the best of our knowledge, however, enzymatic aldol reactions based on different α-keto ester/acid combinations have not been documented in the literature yet, thus demonstrating how enzyme substrate specificity may represent a limitation of biocatalysis on some occasions. Indeed, the first asymmetric homo- and cross-aldol condensations of α-keto esters leading to protected QC-isotetronic acids (O-
t-butyldimethylsilyl derivatives) were described by the group of Jørgensen using a chiral copper(II)/bisoxazoline metal catalyst.[
8,
9] Our group also contributed to this area of research, describing in 2004 the first organocatalytic approach to the enantioselective self-aldol reaction of ethyl pyruvate promoted by a proline-like catalyst; by this strategy, the corresponding isotetronic acid was obtained in good yield and enantioselectivity (
Scheme 1c).[
10] More recently, Liu, Li, and their co-workers showed the ability of water in the presence of bulky proline-imidazole amphiphilic organocatalysts to differentiate the nucleophilicity between α-keto esters and α-keto acids and thus obtain a range of protected, optically active QC-isotetronic acids (
Scheme 1c).[
11]. Today, organocatalysis in heterogeneous conditions has proven to be a robust methodology for highly selective, metal-free syntheses of biologically relevant molecules and active pharmaceutical ingredients (APIs) with advantages compared to traditional solution-phase approaches in terms of product isolation, catalyst recycling, and process intensification using continuous flow reactors.[
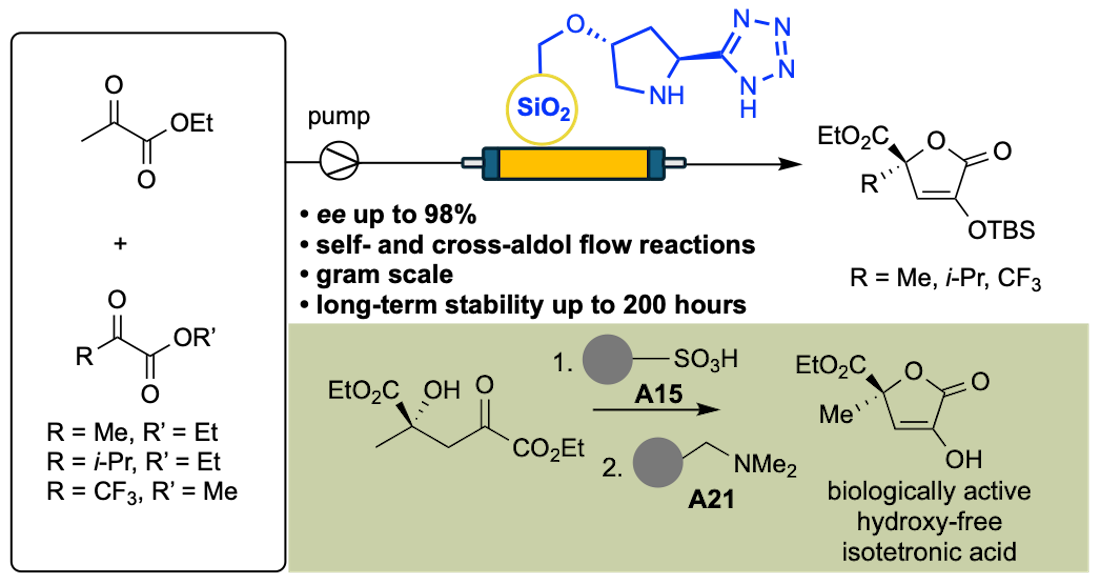
12] Accordingly, following our interest in the application of flow chemistry as an enabling technology for the efficient and sustainable production of valuable (bio)molecules,[
13,
14,
15,
16] we report herein on model self- and cross-aldol flow reactions of pyruvates by operating packed-bed reactors functionalized with the silica supported 5-(pyrrolidin-2-yl)tetrazole organocatalyst, which is a ‘privileged’ catalytic structure deeply investigated by our group in its heterogenous versions.[
13],[
17,
18] We also outline in this contribution the possible application of a set of polymer-supported reagents and sequestrants to facilitate the isolation of QC-isotetronic acids in their biologically active hydroxy-free form.
2. Results and Discussion
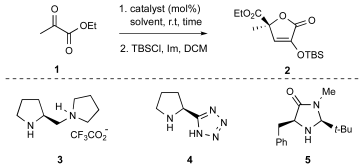
In our previous study,[
10] we evidenced that the self-aldol reaction of ethyl pyruvate 1 occurs effectively under the action of a diamine-protic acid catalyst, namely the (S)-(+)-1-(2-pyrrolidinylmethyl)pyrrolidine/trifluoroacetic acid (TFA) couple
3, in i-PrOH at room temperature. Hence, the behavior of
1 in the presence of other highly active organocatalysts operating via either HOMO or LUMO activation mechanisms [
19] was examined to gather further data on this transformation in the solution phase before moving to heterogeneous conditions. Accordingly, the Arvidsson-Ley-Yamamoto (S)-2-pyrrolidine-tetrazole
4 (often simply referred to as Ley catalyst), [
20,
21] and the MacMillan imidazolidinone
5 [
22] were considered and their activity evaluated in different solvents (
Table 1) after conversion of the crude aldol reaction mixture into the protected O-TBS isotetronic acid
2 (TBSCl, Imidazole, DCM). Indeed, it is important to emphasize that the purification by standard procedures (aqueous workup and chromatography) of unprotected QC-isotetronic acids in real isolated yields is reported to be precluded due to the lability of this class of molecules under aqueous and acid conditions.[
1],[
10,
11] Catalyst
4 proved to be less effective than
3 as the best yield (46%) and ee (62%) values of
2 (entry 4) were obtained in i-PrOH and with a 10 mol% optimized loading of the catalyst. On the other hand, the imidazolidinone
5 (30 mol%) in the presence of 1 equiv. of TFA or AcOH in different solvents (i-PrOH, DMSO, MeCN) did not promote at all the homoaldolization of
1.
To create a model cross-aldol reaction of pyruvates that was selectively controlled by steric effects, we then proceeded our propaedeutic solution phase study by identifying a suitable α-keto ester that could serve as the electrophilic coupling partner of ethyl pyruvate
1. After a brief screening of substituted α-keto esters (Supporting Materials), this reagent, which needed to be scarcely or not at all enolizable, was identified in the ethyl 3-methyl-2-oxobutyrate
6 (
Table 2). Therefore, the target aldol reaction of
1 with
6 was studied in i-PrOH at room temperature by changing the molar ratio of the reactants, the catalyst, and the loading of the latter. The reaction outcome was monitored through the formation of the chiral O-silyl isotetronic acids
2 and
7 as detected by NMR analysis. A first indication on the occurrence of the cross-aldol reaction was obtained by mixing the two α-keto esters in 1:1 molar ratio in the presence of the diamine/TFA couple
3 or the pyrrolidine-tetrazole
4 with a 30 mol% loading of each catalyst (
Table 2, entries 1 and 2). However, under these conditions, the homo-aldol reaction of
1 still prevailed as shown by the higher yields of the isotetronic acid
2 with respect to
7. This indicates that the intrinsic acceptor ability of ethyl pyruvate
1 is superior to that of the alkyl branched α-keto ester 6, as it could be expected based on steric considerations. Hence, attempts to affecting the kinetic of the system were carried out by employing an excess of
6 with respect to
1 and by slowly adding the latter to the solution of the former and the catalyst using a syringe pump. Better results were obtained with the catalyst
4 with respect to
3, whereas
5/TFA was again ineffective (entry 7). In fact, with a 30 mol% loading of
4 the QC-isotetronic acid
7 was isolated in very high enantiomeric purity (98% ee) and in good yet superior yield (40%) to that of
2 (34%; entry 4). A lower loading (20 mol%) of the catalyst
4 resulted in lower yield and optical purity of both products
2 and
7 (entry 5). As detected in control experiments, the ratio of
2 and
7 was the same at a shorter reaction time, i.e.
3 instead of
7 days (entry 6), thus indicating that the self- and cross-aldol intermediates (not shown) were stable under the conditions employed.
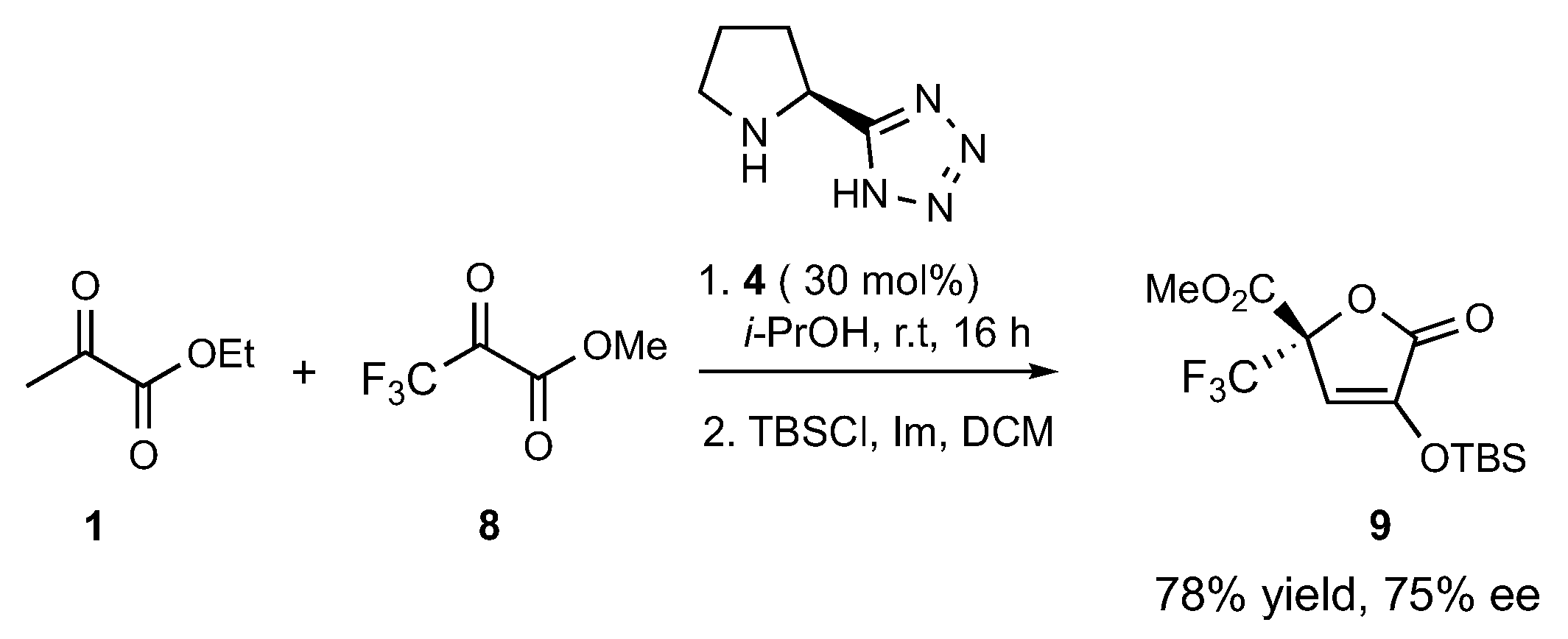
The optimal conditions of entry 4 (30 mol% of
4, i-PrOH, r.t.) were then applied to the cross-aldol reaction of ethyl pyruvate
1 with the non-enolizable and highly electrophilic methyl 3,3,3-trifluoropyruvate
8 (
Scheme 2). The selectivity of this model coupling was controlled by favorable electronic effects; specifically, the protected isotetronic acid
9 was obtained in good yield (78%) and ee (75%) in a shorter reaction time (16 h) with only 2 equivalents of acceptor
8, while also detecting the formation of a minor amount of
2 (14%). Of note, the registered enantioselectivity was much higher than that recently observed for the same cross-aldol process catalyzed by a primary amine-derived catalyst (48% ee).[
23]
As far as the stereoselectivity of the aldol processes is concerned, the absolute configuration of isotetronic acid
2 was determined to be (S) by comparison of the optical rotation value with that previously reported for the same compound, [
8,
9] while that of isotetronic acids
7 and
9 was assigned by analogy and a common mechanistic hypothesis for the key aldol step (vide infra).
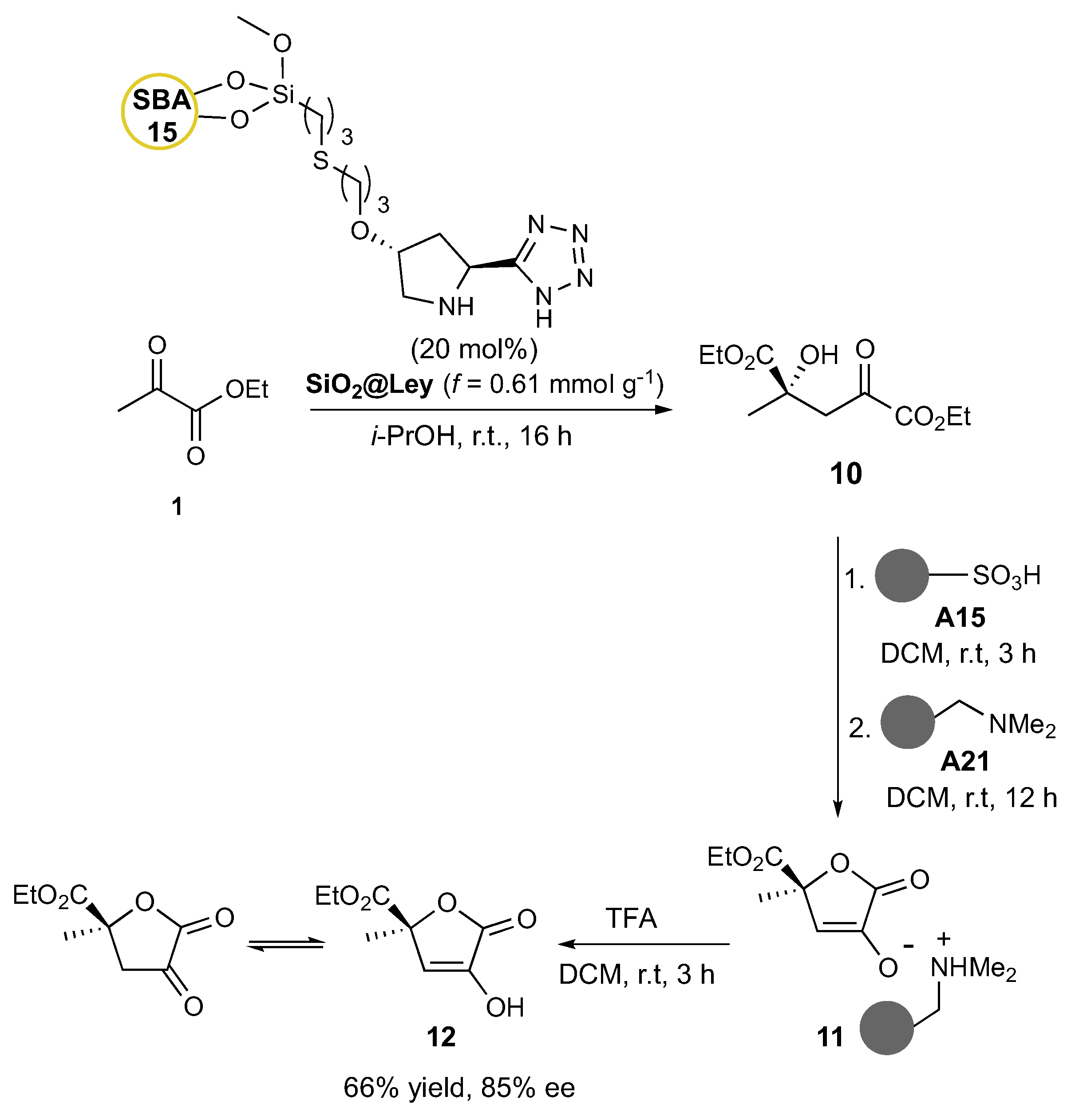
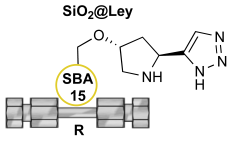
With the above data in hand and the goal of improving the efficiency of QC-isotetronic acid production, the best performing tetrazole organocatalyst
4 was supported on SBA-15 silica material to give the heterogeneous
SiO2@Ley catalyst following the procedure previously described by our group (loading f = 0.61 mmol g
-1; median pore size = 5.36 nm).[
13,
17] After a brief screening of different polarity solvents aimed at maximizing conversion efficiency and enantioselectivity (i-PrOH, MeCN, DMSO, DCM, Et
2O, Toluene), the self-aldol of ethyl pyruvate
1 was optimized in i-PrOH with 20 mol% of
SiO2@Ley, furnishing the 2-hydroxy-2-alkyl-4-oxoglutarate
10 in 76% yield and almost pure form (
1H NMR analysis) after catalyst filtration and evaporation of unreacted
1 (
Scheme 3). At this stage, our approach to the biologically active hydroxy-free isotetronic acid
12 was attempted by applying the so-called catch-and-release technique.[
24] Accordingly, the self-aldol mixture was first treated with the acidic resin A15 to promote the lactonization of the functionalized glutarate
10 to the isotetronic acid
12, which in turn was sequestered by the basic resin A21 as the corresponding ammonium salt
11. After filtration of soluble by-products, the solid material was treated with TFA to release in solution the QC-isotetronic acid
12 in equilibrium with its dicarbonyl form in 66% overall yield from
1 and 85% ee. Notably, the
SiO2@Ley catalyst outperformed its homogeneous counterpart
4 in terms of enantioselectivity (85% vs. 62% ee), suggesting a matrix effect on transition state geometry possibly due to favorable hydrogen bonding interactions. An in-depth examination of the impact of the silica support on the stereochemical outcome of aldol reactions of pyruvates promoted by the pyrrolidine-tetrazole unit is currently underway in our laboratories. Unfortunately, the direct lactonization in basic conditions with A21 mainly resulted in the enolization of
10 and the formation of a complex reaction mixture that could not be elaborated into the target butenolide
12.
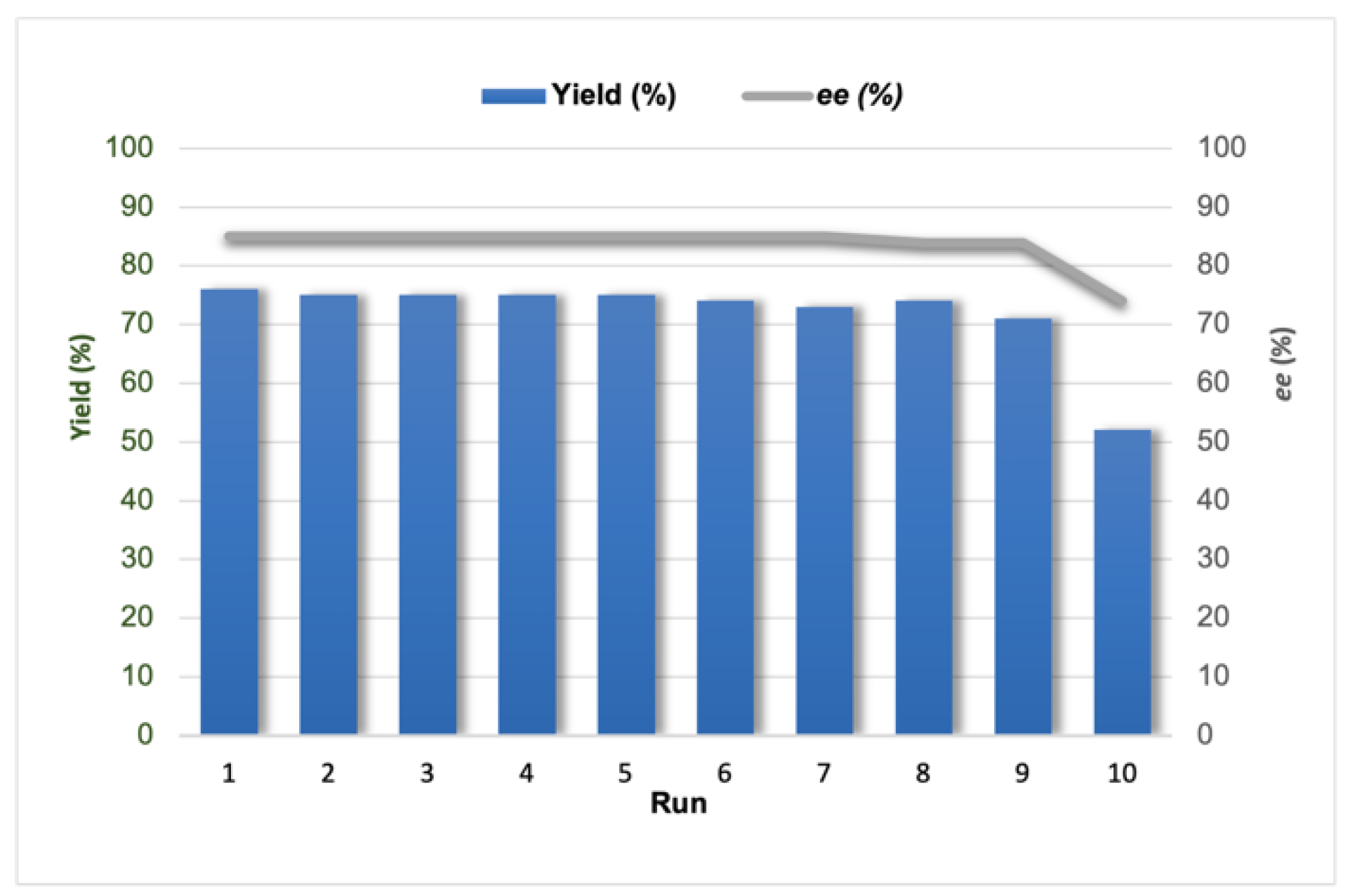
Alongside, the recyclability of the
SiO2@Ley catalyst was also investigated (
Figure 2), observing the maintenance of conversion efficiency and a small drop in enantioselectivity only after the tenth run of the aldol reaction.
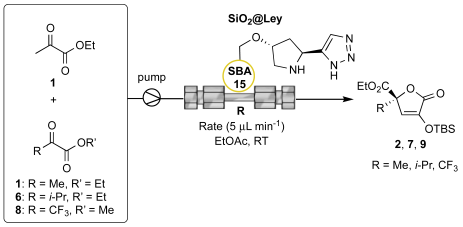
The asymmetric self- and cross-aldol reactions of pyruvates promoted by heterogeneous (organo)catalysts in continuous-flow regime is a completely unexplored research domain, potentially providing novel avenues for the intensification of isotetronic acid synthesis. Therefore, based on our experience on the operation of organocatalytic fixed-bed reactors[
13,
17,
18,
25,
26] we next investigated the transition of the
1 homocoupling from batch to continuous-flow conditions. Initially, the mesoreactor R was fabricated by slurry packing the
SiO2@Ley catalyst (i-PrOH as solvent) into a stainless-steel column (10 cm length, 2.1 mm internal diameter). Then, the dead volume (V
0) and the total porosity (ε
tot) of the reactor were determined by pycnometry for the calculation of the residence time at different flow rates (
Table 3).
The first goal of the flow studies was to completely convert the ethyl pyruvate
1 in a single pass-flow mode to facilitate the post reaction phase and the subsequent elaboration of the crude aldol mixture into the protected isotetronic acid
2 (
Table 4). After some experimentation (only selected data are reported), full conversion was achieved by pumping a 0.14 M solution of
1 with a flow rate of 5 μL min
-1 (residence time = 49 min). Under these conditions (entry 3), the steady-state regime was reached in approximately one hour and a turnover frequency (TOF) value of 4.8 mmol
prod d
-1 mmol
cat-1 was recorded. Importantly, the mesoreactor
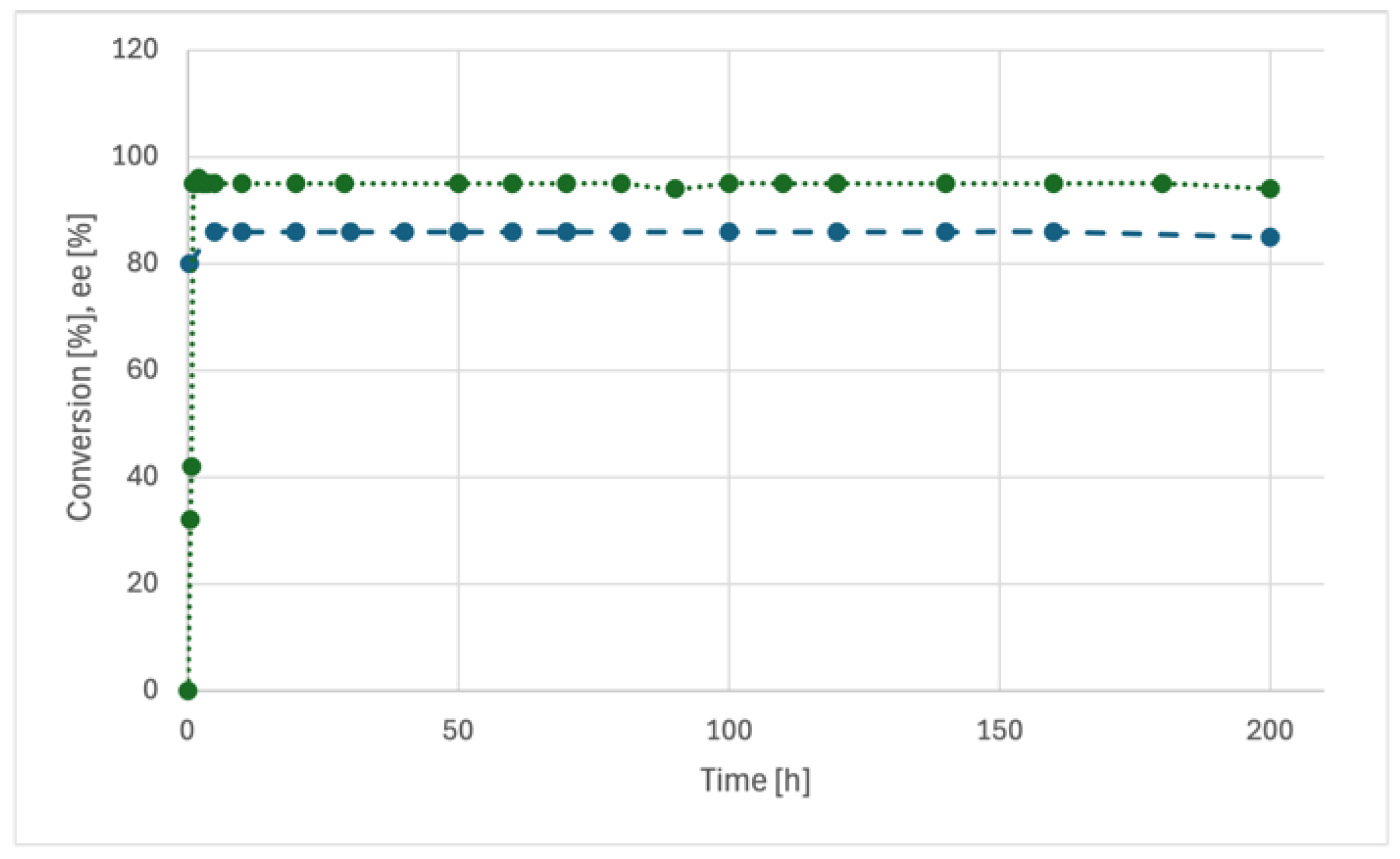
R could be operated for ca. 200 hours with unaltered values of conversion efficiency (>95%) and enantioselectivity (85% ee;
Figure 3), thereby confirming the already noted long-term stability of the catalytic
SiO2@Ley packing material in a flow regime.[
13,
17] Gratifyingly, by suitably adjusting the initial reagent concentrations (entries 4-5), the selected acceptors
6 and
8 effectively reacted with the donor
1, achieving complete instant conversions at a flow rate of 5 μL min
-1. In the
1/
6 coupling, the excess of unreacted acceptor
6 could be conveniently recovered by fractional distillation at reduced pressure for minimal waste generation. Significantly, the
SiO2@Ley catalyst could reproduce the level of stereoselectivity imparted by the homogeneous
4, yielding the target isotetronic acids
7 and
9 in 98% and 75% ee, respectively.
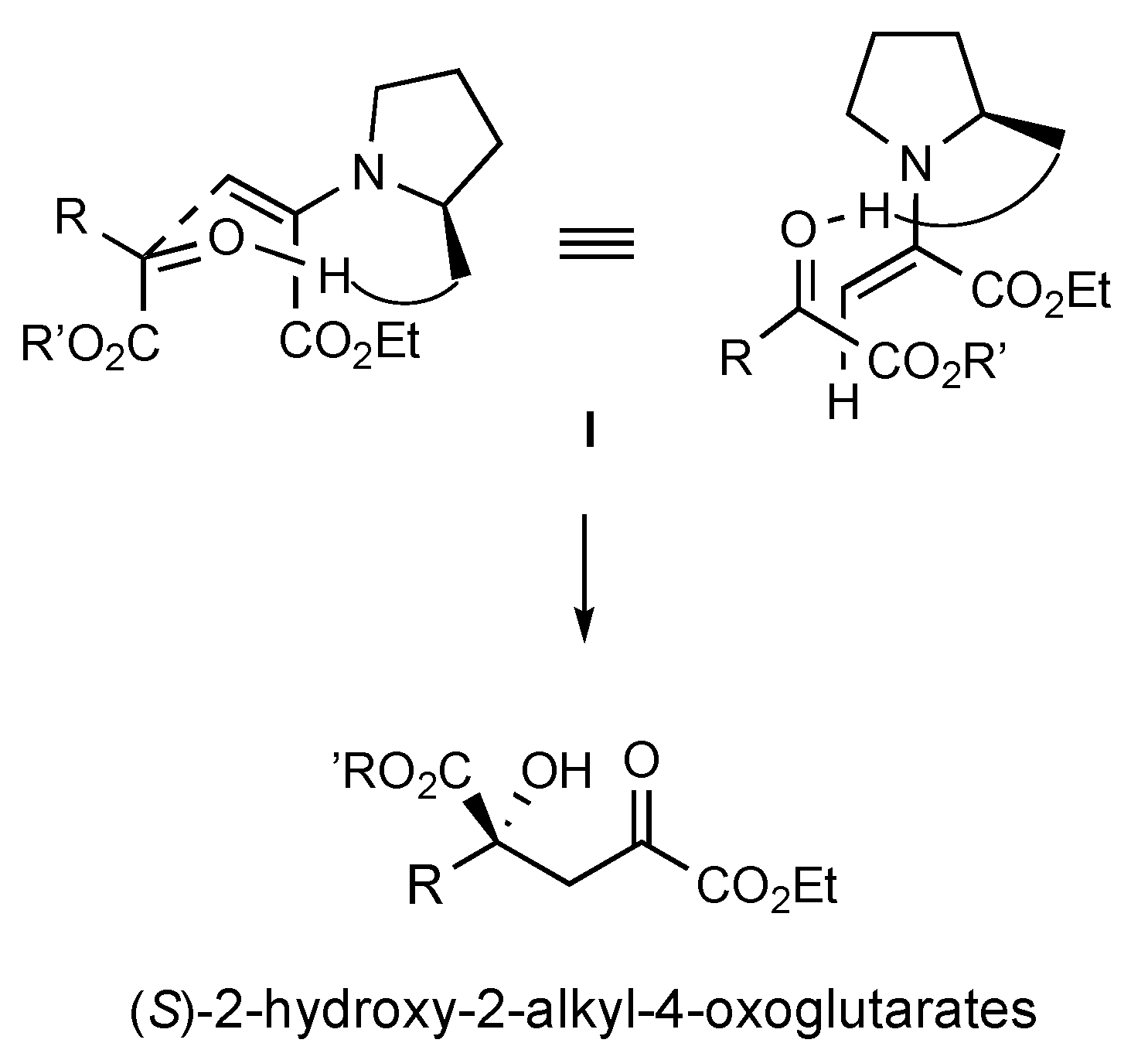
To account for the formation of S-configured isotetronic acids
2,
7, and
9 in the aldol reaction of pyruvates under pyrrolidine-tetrazole catalysis, we propose the chairlike transition state (TS)
I, in analogy with standard proline-catalyzed transformations (
Scheme 4).[
27,
28,
29] Accordingly, the (S)-2-pyrrolidine-tetrazole moiety is expected to direct a nucleophilic si-facial attack of the α-keto ester acceptor by the enamine intermediate generated in situ from the pyruvate donor.
3. Materials and Methods
Commercially available reagents were purchased from commercial sources and used without any subsequent purification. The solvents used for starting preparations were distilled from appropriate drying agents and stored over 3 Å molecular sieves. 1H, 13C, and 19F NMR spectra were recorded on Varian Mercury Plus 400 and Brucker Magnet System Ascend 500 MHz spectrometers in CDCl3 at room temperature. 13C{1H} NMR spectra were recorded in 1H broad-band decoupled mode, and chemical shifts (δ) are reported in parts per million (ppm) relatives to the residual solvent peak. Reactions were monitored by TLC on silica gel 60 F254. Flash column chromatography was performed on silica gel 60 (230−400 mesh). High-resolution mass spectra (HRMS) were recorded in positive ion mode by an Agilent 6520 HPLC-Chip Q/TF-MS nanospray instrument using a time-of-flight, a quadrupole, or a hexapole unit to produce spectra. Enantiomeric excesses were measured with HPLC DAD/RID, Agilent technologies series 1260 Infiniti II. Flow reactions are performed using an HPLC pump (Agilent Technologies, series 1100) and syringe pump (Harvard Apparatus, syringe pump model 22).
3.1. Self-Aldol Reaction of Ethyl Pyruvate 1 Promoted by the Ley Catalyst 4 (Table 1, entry 4)
Ethyl (S)-4-((tert-butyldimethylsilyl)oxy)-2-methyl-5-oxo-2,5-dihydrofuran-2-carboxylat
e (2). To a stirred solution of organocatalyst 4 (12 mg, 0.09 mmol) in
i-PrOH (1 mL) ethyl pyruvate 1 (100 μL, 0.90 mmol) was added dropwise. The mixture was stirred at room temperature for 18 h then concentrated. The resulting residue was dissolved in anhydrous CH
2Cl
2 (4 mL) then imidazole (184 mg, 2.70 mmol) and tert-butyldimethylsilyl chloride (271 mg, 1.80 mmol) were added sequentially. The mixture was stirred at room temperature for 18 h, then concentrated, and eluted from a column of silica gel with 6:1 cyclohexane-Et
2O to give 2 (124 mg, 46%;
ee = 62%) as a clear colorless oil.
1H NMR (400 MHz, CDCl
3) δ = 6.22 (s, 1H), 4.21 (q,
J = 7.1 Hz, 2H), 1.68 (s, 3H), 1.27 (t,
J = 7.1 Hz, 3H), 0.95 (s, 9H), 0.24 (s, 6H).
13C{
1H} NMR (101 MHz, CDCl
3) δ = 169.0, 168.1, 143.2, 124.4, 81.6, 62.4, 25.4, 22.9, 18.3, 14.0, -4.8. Spectroscopic data are in accordance with literature. [
8,
9,
10]
3.2. Cross-Aldol Reaction of Ethyl Pyruvate 1 with Ethyl 3-methyl-2-oxobutyrate 6 Promoted by the Ley Catalyst 4 (Table 2, Entry 4)
Ethyl (S)-4-((tert-butyldimethylsilyl)oxy)-2-isopropyl-5-oxo-2,5-dihydrofuran-2-carboxylate (7). To a stirred solution of the organocatalyst 4 (21 mg, 0.15 mmol) in i-PrOH (1 mL) ethyl 3-methyl-2-oxobutyrate 6 (655 μL, 4.50 mmol) was added in one portion. To the resulting mixture a solution of ethyl pyruvate 1 (50 μL, 0.45 mmol) in i-PrOH (2 mL) was added in 36 h by means of a syringe pump. The mixture was stirred at room temperature for an additional 132 h then concentrated. The resulting residue was dissolved in anhydrous CH2Cl2 (4 mL) then imidazole (92 mg, 1.35 mmol) and tert-butyldimethylsilyl chloride (136 mg, 0.90 mmol) were added sequentially. The mixture was stirred at room temperature for 18 h, then concentrated and eluted from a column of silica gel with 9:1 cyclohexane-AcOEt to give 7 (59 mg, 40%; ee = 98%) as a clear colorless oil. [α]D20 = –48.5 (c 0.8, CHCl3). HPLC analysis Chiralpak® IA (n-Hexane:i-PrOH 97:3, flow rate 1 mL min−1, 242 nm, 20 °C) t1 = 4.2 min (minor), t2 = 4.4 min (major), 99.08:0.92 er. 1H NMR (400 MHz, CDCl3) δ = 6.16 (s, 1H), 4.30 – 4.18 (m, 2H), 2.45 (p, J = 6.9 Hz, 1H), 1.56 (s, 1H), 1.29 (t, J = 6.9 Hz, 3H), 1.02 (d, J = 6.9 Hz, 3H), 0.96 (s, 9H), 0.88 (d, J = 6.9 Hz, 3H), 0.24 (d, J = 4.2 Hz, 6H). 13C{1H} NMR (101 MHz, CDCl3): δ = 169.0, 168.3, 143.2, 123.1, 87.4, 62.1, 33.7, 25.4, 18.3, 17.0, 15.7, 14.1, -4.9. HRMS (ESI) m/z: [M + H]+ calcd for C16H29O5Si+ 329.4880, found 329.4801.
3.3. Cross-Aldol Reaction of Ethyl Pyruvate 1 with Methyl Trifluoropyruvate 8 Promoted by the Ley Catalyst 4 (Scheme 2)
Methyl (S)-4-((tert-butyldimethylsilyl)oxy)-5-oxo-2-(trifluoromethyl)-2,5-dihydrofuran-2-carboxylate (9). To a stirred solution of organocatalyst 4 (21 mg, 0.15 mmol) and methyl 3,3,3-trifluoropyruvate 8 (92 μL, 0.90 mmol) in i-PrOH (2 mL) ethyl pyruvate 1 (50 μL, 0.45 mmol) was added dropwise. The mixture was stirred at room temperature for 16 h then concentrated. The resulting residue was dissolved in anhydrous CH2Cl2 (4 mL) then imidazole (184 mg, 2.70 mmol) and tert-butyldimethylsilyl chloride (271 mg, 1.80 mmol) were added sequentially. The mixture was stirred at room temperature for 18 h, then concentrated, and eluted from a column of silica gel with 6:4 pentane-toluene to give 9 (119 mg, 78%; ee = 75%) as a clear colorless oil. [α]D20 = +9.6 (c 1.2, CHCl3). HPLC analysis Chiralpak® IA (n-Hexane:i-PrOH 98:2, flow rate 1 mL min−1, 242 nm, 20 °C) t1 = 10.8 min (major), t2 = 11.3 min (minor), 87.51:12.49 er. 1H NMR (400 MHz, CDCl3) δ = 5.81 (s, 1H), 3.80 (s, 3H), 0.90 (s, 9H), 0.18 (s, 6H). 13C{1H} NMR (126 MHz, CDCl3) δ = 166.4, 163.9, 144.0, 123.3 (q, J = 286.8 Hz), 111.9, 94.4 (q, J = 33.3 Hz), 61.9, 26.4, 14.1, -3.0. 19F NMR (471 MHz, CDCl3) δ = -77.71. HRMS (ESI) m/z: [M + H]+ calcd for C13H20F3O5Si+ 341.1027, found 341.1032.
3.4. Procedure for the Heterogeneous Synthesis of the Hydroxy-Free QC-Isotetronic Acid 12 (Scheme 3) and Protected Isotetronic Acid 2 Under Batch Conditions
Diethyl (
S)-2-hydroxy-2-methyl-4-oxopentanedioate (10). To a stirred suspension of SiO
2@Ley catalyst (725 mg, 0.45 mmol;
f = 0.61 mmolg
-1) in
i-PrOH (5 mL) ethyl pyruvate 1 (500 μL, 4.50 mmol) was added dropwise. The mixture was stirred for an additional 16 h then filtered, and concentrated to give 10 (397 mg, 76%) at least 95% pure as judged by
1H NMR analysis.
1H NMR (400 MHz, CDCl
3) δ = 4.32 (q,
J = 7.2 Hz, 2H), 4.25 (q,
J = 7.2 Hz, 2H), 3.54 (broad s, 1H), 3.35 (d,
J = 2.8 Hz, 2H), 1.46 (s, 3H), 1.36 (t,
J = 7.2 Hz, 3H), 1.28 (t,
J = 7.2 Hz, 3H).
13C{
1H} NMR (126 MHz, CDCl
3) δ = 191.9, 175.4, 160.3, 72.1, 62.8, 62.3, 48.2, 26.7, 14.1, 14.0. Spectroscopic data are in accordance with literature.[
10]
The filtered SiO
2@Ley catalyst was thoroughly washed with fresh portions of Et
2O, dried, and then subjected to the recycle experiments (
Figure 3).
Ethyl (
S)-4-hydroxy-2-methyl-5-oxo-2,5-dihydrofuran-2-carboxylate (12). The above crude 10 (397 mg) was dissolved in CH
2Cl
2 (8 mL) then Amberlyst 15 (1.5 g) was added in one portion. The suspension was shaken at room temperature for 3 h and then the resin was filtered off and washed thoroughly with CH
2Cl
2. The combined filtrates were concentrated and dried under high vacuum. The resulting crude material was dissolved in CH
2Cl
2 (12 mL) then Amberlyst 21 (2.5 g) was added in one portion. The suspension was shaken at room temperature for 12 h and then the resin was filtered off and washed thoroughly with CH
2Cl
2. Next, the resin was suspended in CH
2Cl
2 (8 mL) and then TFA (4 mL) was added in one portion. The resulting mixture was shaken at room temperature for 2 h. The resin was then filtered off and washed thoroughly with CH
2Cl
2. The combined filtrates were concentrated to give the isotetronic acid 12 in equilibrium with its dicarbonyl form (276 mg, 66% from 1; 85%
ee) at least 95% pure as determined by
1H NMR analysis. Selected date for 12
1H NMR (400 MHz, CDCl
3) δ = 6.25 (s, 1H), 5.20 (s, 1H), 4.23 (s, 2H), 1.73 (s, 3H), 1.29 (t,
J = 7.1 Hz, 3H).
13C{
1H} NMR (126 MHz, CDCl
3) δ = 169.1, 168.6, 142.3, 119.0, 83.3, 62.7, 22.8, 14.0. Spectroscopic data are in accordance with literature.[
10]
For the determination of 12 enantiomeric excess, the crude 10 was silylated in a parallel experiment as described in section 3.1. to give 2 (ee = 85%) as a clear colorless oil. [α]D20 = –30.6 (c 1.2, CHCl3). HPLC analysis Chiralpak® IA (n-Hexane:i-PrOH 98:2, flow rate 1 mL min−1, 242 nm, 20 °C) t1 = 4.7 min (minor), t2 = 5.1min (major), 92.51:7.49 er.
3.5. Fabrication of Mesoreactor R
A slurry solution was prepared suspending an excess of catalyst SiO2@Ley in i-PrOH. A stainless-steel column (10 × 0.21 cm) was filled with the slurry solution under constant pressure (300 bars, 30 min, i-PrOH as solvent) by using an air driven liquid pump. Microreactor void volume (V0) was determined by pycnometry, filling and weighting accurately the mesoreactor with two different solvents (solvent 1: i-PrOH; solvent 2: n-hexane). V0 was calculated according to the following formula where w1 and w2 are the weights of the microreactor filled respectively with solvents 1 and 2 and δ1 and δ2 are the solvent densities: V0 = (w1- w2)/( δ1- δ2)
3.6. Experimental Set-Up for Flow Experiments and Continuous-Flow Synthesis of Isotetronic Acids 2, 7, and 9 (Table 4)
The system used for the flow reaction was made of one pump (Agilent 1100 micro series) connected to the reactor by a feeding channel that was used to deliver a solution of 1 (0.14 M) and either acceptor 6 (0.28 M) or 8 (2.67 M) in i-PrOH. The feed solution was pumped with a flow rate of 5 μL min-1 into R for ca. 1 h for reaching the optimal steady-state regime, and then for additional 24 h. The outlet stream was frequently analyzed for the evaluation of 1 conversion by 1H NMR analysis. The collected solution of the 24 h experiment was concentrated and subjected to the silylation procedure as described before for the determination of the yield and enantiomeric excesses of isotetronic acids 2, 7, and 9.
3.7. Long-Term Stability Experiment (Figure 4)
The long-term stability investigation was performed by feeding the mesoreactor R with a solution of ethyl pyruvate 1 (0.14 M) for 200 h. The outlet stream was analyzed every 12 h for the evaluation of 1 conversion by 1H NMR analysis. Periodically, fractions were collected to determine the enantiomeric excesses of isotetronic acid 2.