Submitted:
24 December 2024
Posted:
24 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Preparation for In Vitro Assays
2.2. CD4+ T Cell Isolation
2.3. CD4+ T Cell Infection
2.4. Data Collection and Boolean Model Design for Bioinformatic Simulations
2.5. Assessment of AKT Phosphorylation and Coreceptors Expression
2.6. qPCR Gene Expression Assay
2.7. Glucose Uptake Assay
2.8. Statistical Analysis
3. Results
3.1. Effect of VitD on HIV-1 Infection
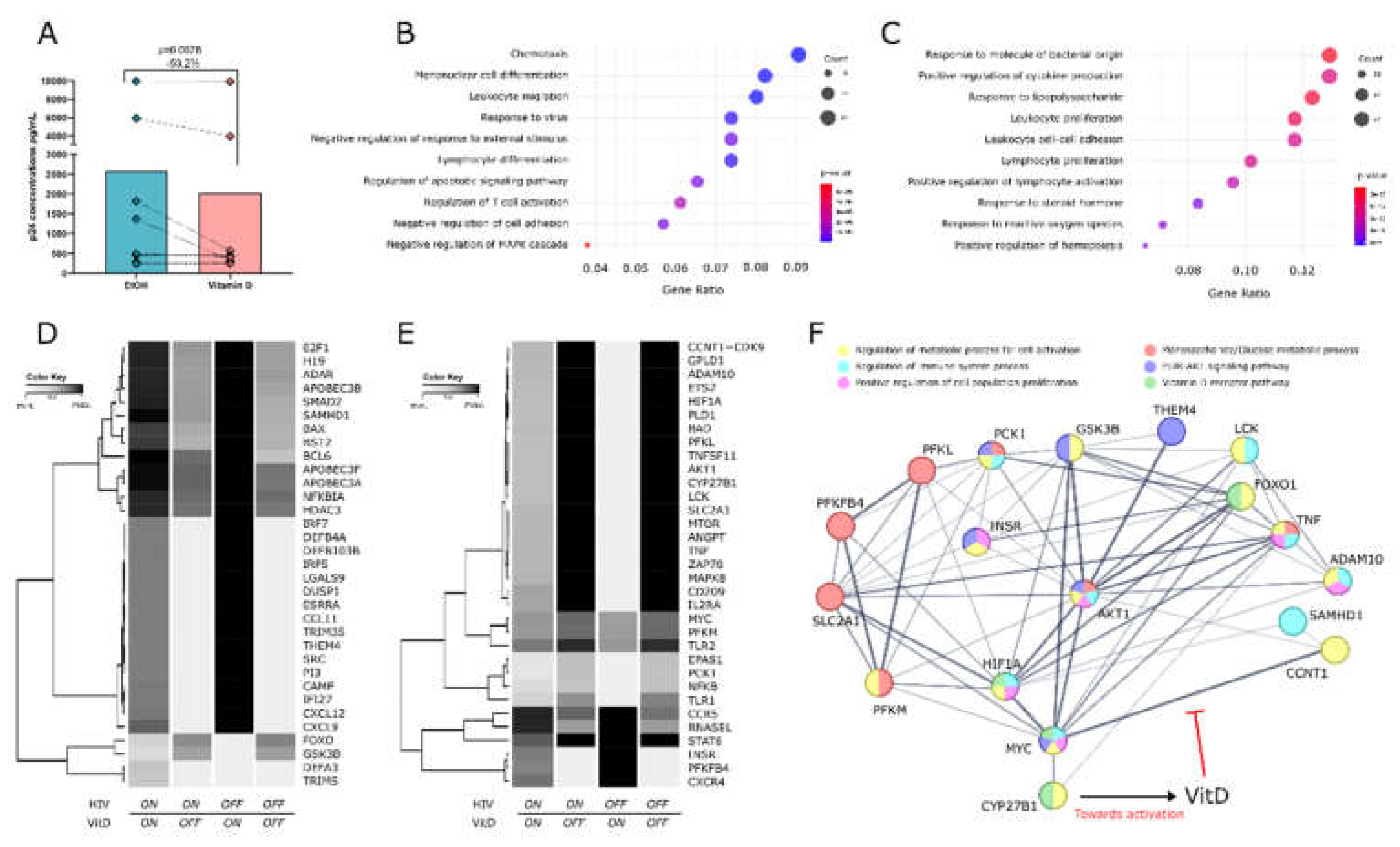
3.2. Boolean Modeling of VitD Effects in Regulating Host Pro- and Antiviral Genes
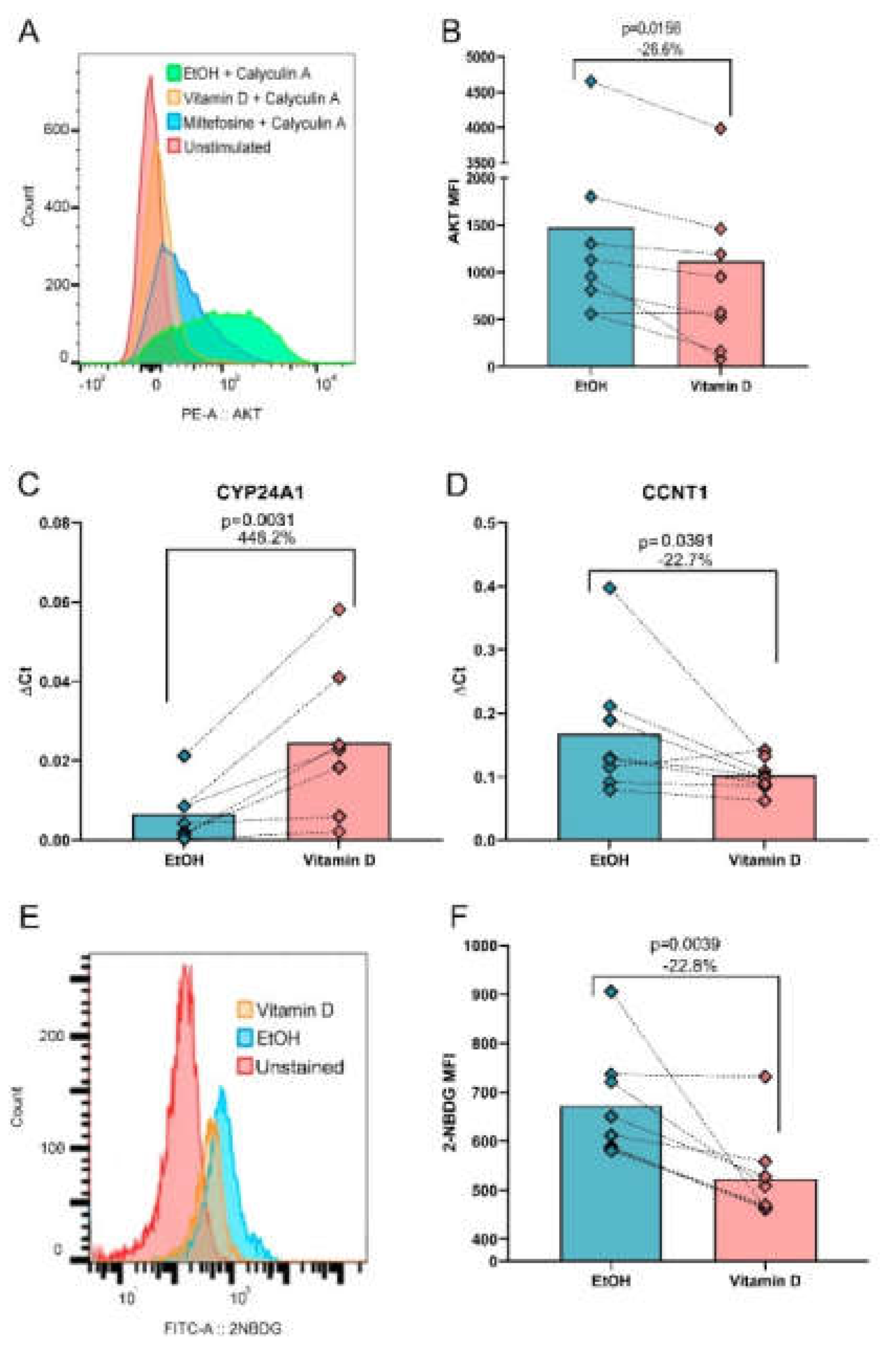
3.3. VitD Reduces AKT Phosphorylation, CCNT1 Expression and Glucose Uptake In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-NBDG | 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose |
| AIDS | Acquired Immunodeficiency Syndrome |
| AKT, PKB | Protein Kinase B |
| APOBEC | Apolipoprotein B mRNA Editing Enzyme, Catalytic Polypeptide |
| BST2 | Tetherin |
| CCR5 | C-C chemokine receptor type 5 |
| CD | Cluster of Differentiation |
| cDNA | Complementary DNA |
| CO2 | Carbon dioxide |
| CXCR4 | C-X-C chemokine receptor type 4 |
| DNA | Deoxyribonucleic Acid |
| dNTP | Deoxynucleotide Triphosphate |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EtOH | Ethanol |
| FBS | Fetal Bovine Serum FBS |
| FOXP3 | Forkhead Box P3 |
| Glut1 | Glucose transporter 1 |
| GO | Gene Ontology |
| HESNs | HIV-Exposed Seronegative individuals |
| HIV | Human Immunodeficiency Virus. |
| HK2 | Hexokinase 2 |
| INSR | Insulin Receptor |
| MFI | Median Fluorescence Intensity |
| mRNA | messenger Ribonucleic Acid |
| mTOR | mechanistic Target of Rapamycin |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| pAKT | phosphorylated AKT |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PCK1 | Phosphoenolpyruvate Carboxykinase |
| PFK | Phosphofructokinase |
| PFK2 | phosphofructokinase 2 |
| PHA | Phytohemagglutinin |
| PI3 | Elafin |
| PI3K | Phosphoinositide 3-kinase |
| P-TEFb | Positive Transcription Elongation Factor b |
| qPCR | quantitative Polymerase Chain Reaction |
| RNA | Ribonucleic Acid |
| RU | Relative expression units of mRNA |
| SDF1, CXCL12 | Stromal Cell-Derived Factor 1 |
| TCR | T Cell Receptor |
| Th | Helper T cell |
| Treg | Regulatory T cell |
| VitD | Vitamin D |
References
- UNAIDS. Global HIV & AIDS statistics — Fact sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 31 October 2024).
- Palmer, C.S.; Duette, G.A.; Wagner, M.C.E.; Henstridge, D.C.; Saleh, S.; Pereira, C.; Zhou, J.; Simar, D.; Lewin, S.R.; Ostrowski, M.; et al. Metabolically active CD4+ T cells expressing Glut1 and OX40 preferentially harbor HIV during in vitro infection. FEBS Lett 2017, 591, 3319–3332. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, J.E.; Pinney, J.W.; Robertson, D.L. The biological context of HIV-1 host interactions reveals subtle insights into a system hijack. BMC Syst Biol 2010, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Valle-Casuso, J.C.; Angin, M.; Volant, S.; Passaes, C.; Monceaux, V.; Mikhailova, A.; Bourdic, K.; Avettand-Fenoel, V.; Boufassa, F.; Sitbon, M.; et al. Cellular Metabolism Is a Major Determinant of HIV-1 Reservoir Seeding in CD4(+) T Cells and Offers an Opportunity to Tackle Infection. Cell Metab 2019, 29, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.M.; Taborda, N.A.; Feria, M.G.; Arcia, D.; Aguilar-Jimenez, W.; Zapata, W.; Rugeles, M.T. High Expression of Antiviral Proteins in Mucosa from Individuals Exhibiting Resistance to Human Immunodeficiency Virus. PLoS ONE 2015, 10, e0131139. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.; Juno, J.; Burgener, A.; Rahman, S.; Mogk, K.; Wachihi, C.; Mwanjewe, J.; Plummer, F.A.; Kimani, J.; Ball, T.B.; Fowke, K.R. A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal Immunol 2012, 5, 277–287. [Google Scholar] [CrossRef]
- Yao, X.D.; Omange, R.W.; Henrick, B.M.; Lester, R.T.; Kimani, J.; Ball, T.B.; Plummer, F.A.; Rosenthal, K.L. Acting locally: innate mucosal immunity in resistance to HIV-1 infection in Kenyan commercial sex workers. Mucosal Immunol 2014, 7, 268–279. [Google Scholar] [CrossRef]
- Miyazawa, M.; Lopalco, L.; Mazzotta, F.; Lo Caputo, S.; Veas, F.; Clerici, M.; Group, E.S.N.S. The 'immunologic advantage' of HIV-exposed seronegative individuals. AIDS 2009, 23, 161–175. [Google Scholar] [CrossRef]
- von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Odum, N.; Geisler, C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol 2010, 11, 344–349. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M.; Lovan, N.; Powers, L.; Gerke, A.; Hunninghake, G.W. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol 2010, 184, 965–974. [Google Scholar] [CrossRef]
- Korf, H.; Wenes, M.; Stijlemans, B.; Takiishi, T.; Robert, S.; Miani, M.; Eizirik, D.L.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology 2012, 217, 1292–1300. [Google Scholar] [CrossRef]
- McMahon, L.; Schwartz, K.; Yilmaz, O.; Brown, E.; Ryan, L.K.; Diamond, G. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect Immun 2011, 79, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; White, J.H. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 2004, 173, 2909–2912. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; Spector, S.A. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J Biol Chem 2011, 286, 18890–18902. [Google Scholar] [CrossRef] [PubMed]
- Coussens, A.K.; Naude, C.E.; Goliath, R.; Chaplin, G.; Wilkinson, R.J.; Jablonski, N.G. High-dose vitamin D3 reduces deficiency caused by low UVB exposure and limits HIV-1 replication in urban Southern Africans. Proc Natl Acad Sci U S A 2015, 112, 8052–8057. [Google Scholar] [CrossRef]
- Stallings, V.A.; Schall, J.I.; Hediger, M.L.; Zemel, B.S.; Tuluc, F.; Dougherty, K.A.; Samuel, J.L.; Rutstein, R.M. High-dose vitamin D3 supplementation in children and young adults with HIV: a randomized, placebo-controlled trial. Pediatr Infect Dis J 2015, 34, e32–40. [Google Scholar] [CrossRef]
- Aguilar-Jimenez, W.; Villegas-Ospina, S.; Gonzalez, S.; Zapata, W.; Saulle, I.; Garziano, M.; Biasin, M.; Clerici, M.; Rugeles, M.T. Precursor Forms of Vitamin D Reduce HIV-1 Infection In Vitro. J Acquir Immune Defic Syndr 2016, 73, 497–506. [Google Scholar] [CrossRef]
- Aguilar-Jimenez, W.; Zapata, W.; Caruz, A.; Rugeles, M.T. High transcript levels of vitamin D receptor are correlated with higher mRNA expression of human beta defensins and IL-10 in mucosa of HIV-1-exposed seronegative individuals. PLoS ONE 2013, 8, e82717. [Google Scholar] [CrossRef]
- Yiyan, S.; Yang, S.; Li, D.; Li, W. Vitamin D Affects the Warburg Effect and Stemness Maintenance of Non- Small-Cell Lung Cancer Cells by Regulating the PI3K/AKT/mTOR Signaling Pathway. Curr Cancer Drug Targets 2022, 22, 86–95. [Google Scholar] [CrossRef]
- Mutt, S.J.; Raza, G.S.; Makinen, M.J.; Keinanen-Kiukaanniemi, S.; Jarvelin, M.R.; Herzig, K.H. Vitamin D Deficiency Induces Insulin Resistance and Re-Supplementation Attenuates Hepatic Glucose Output via the PI3K-AKT-FOXO1 Mediated Pathway. Mol Nutr Food Res 2020, 64, e1900728. [Google Scholar] [CrossRef]
- Pasquereau, S.; Herbein, G. CounterAKTing HIV: Toward a "Block and Clear" Strategy? Front Cell Infect Microbiol 2022, 12, 827717. [Google Scholar] [CrossRef]
- Beer, T.M.; Munar, M.; Henner, W.D. A Phase I trial of pulse calcitriol in patients with refractory malignancies: pulse dosing permits substantial dose escalation. Cancer 2001, 91, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Trump, D.L.; Muindi, J.R.; Black, J.D.; Bernardi, R.J.; Creaven, P.J.; Schwartz, J.; Brattain, M.G.; Hutson, A.; French, R.; Johnson, C.S. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res 2007, 13, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Sigmundsdottir, H.; Pan, J.; Debes, G.F.; Alt, C.; Habtezion, A.; Soler, D.; Butcher, E.C. DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. Nat Immunol 2007, 8, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, J.; Mondal, K.; Ehrnsperger, A.; Andreesen, R.; Kreutz, M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood 2003, 102, 3314–3316. [Google Scholar] [CrossRef] [PubMed]
- O'Doherty, U.; Swiggard, W.J.; Malim, M.H. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol 2000, 74, 10074–10080. [Google Scholar] [CrossRef]
- Han, H.; Cho, J.W.; Lee, S.; Yun, A.; Kim, H.; Bae, D.; Yang, S.; Kim, C.Y.; Lee, M.; Kim, E.; et al. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res 2018, 46, D380–D386. [Google Scholar] [CrossRef]
- Ako-Adjei, D.; Fu, W.; Wallin, C.; Katz, K.S.; Song, G.; Darji, D.; Brister, J.R.; Ptak, R.G.; Pruitt, K.D. HIV-1, human interaction database: current status and new features. Nucleic Acids Res 2015, 43, D566–570. [Google Scholar] [CrossRef]
- Kachalo, S.; Zhang, R.; Sontag, E.; Albert, R.; DasGupta, B. NET-SYNTHESIS: a software for synthesis, inference and simplification of signal transduction networks. Bioinformatics 2008, 24, 293–295. [Google Scholar] [CrossRef]
- Albert, I. Ialbert/Booleannet: Boolean Network Modeling. Available online: https://github.com/ialbert/booleannet (accessed on 19 November 2024).
- Pozuelo-Rubio, M.; Leslie, N.R.; Murphy, J.; Mackintosh, C. Mechanism of activation of PKB/Akt by the protein phosphatase inhibitor Calyculin A. Cell Biochem Biophys 2010, 58, 147–156. [Google Scholar] [CrossRef]
- Cheshenko, N.; Trepanier, J.B.; Stefanidou, M.; Buckley, N.; Gonzalez, P.; Jacobs, W.; Herold, B.C. HSV activates Akt to trigger calcium release and promote viral entry: novel candidate target for treatment and suppression. FASEB J 2013, 27, 2584–2599. [Google Scholar] [CrossRef]
- Walker, N.J. Tech.Sight. A technique whose time has come. Science 2002, 296, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, B.M.; Dziuba, N.; Li, G.; Endsley, M.A.; Murray, J.L.; Ferguson, M.R. Host factors mediating HIV-1 replication. Virus Res 2011, 161, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Korf, H.; Overbergh, L.; van Etten, E.; Verstuyf, A.; Gysemans, C.; Mathieu, C. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol 2010, 121, 221–227. [Google Scholar] [CrossRef]
- Gonzalez, S.M.; Aguilar-Jimenez, W.; Trujillo-Gil, E.; Zapata, W.; Su, R.C.; Ball, T.B.; Rugeles, M.T. Vitamin D treatment of peripheral blood mononuclear cells modulated immune activation and reduced susceptibility to HIV-1 infection of CD4+ T lymphocytes. PLoS ONE 2019, 14, e0222878. [Google Scholar] [CrossRef]
- Abdullah, L.; Hills, L.B.; Winter, E.B.; Huang, Y.H. Diverse Roles of Akt in T cells. Immunometabolism 2021, 3. [Google Scholar] [CrossRef]
- Maira, S.M.; Galetic, I.; Brazil, D.P.; Kaech, S.; Ingley, E.; Thelen, M.; Hemmings, B.A. Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science 2001, 294, 374–380. [Google Scholar] [CrossRef]
- Wang, Q.; He, Y.; Shen, Y.; Zhang, Q.; Chen, D.; Zuo, C.; Qin, J.; Wang, H.; Wang, J.; Yu, Y. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J Biol Chem 2014, 289, 11681–11694. [Google Scholar] [CrossRef]
- Pearce, E.L.; Poffenberger, M.C.; Chang, C.H.; Jones, R.G. Fueling immunity: insights into metabolism and lymphocyte function. Science 2013, 342, 1242454. [Google Scholar] [CrossRef]
- Pan, X.; Baldauf, H.M.; Keppler, O.T.; Fackler, O.T. Restrictions to HIV-1 replication in resting CD4+ T lymphocytes. Cell Res 2013, 23, 876–885. [Google Scholar] [CrossRef]
- Lever, A.M.; Jeang, K.T. Insights into cellular factors that regulate HIV-1 replication in human cells. Biochemistry 2011, 50, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liao, S.; Liang, L.; Deng, J.; Zhou, Y. The relationship between CD4(+) T cell glycolysis and their functions. Trends Endocrinol Metab 2023, 34, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.S.; Ostrowski, M.; Gouillou, M.; Tsai, L.; Yu, D.; Zhou, J.; Henstridge, D.C.; Maisa, A.; Hearps, A.C.; Lewin, S.R.; et al. Increased glucose metabolic activity is associated with CD4+ T-cell activation and depletion during chronic HIV infection. AIDS 2014, 28, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takita, J.; Miyazaki, T.; Nakajima, M.; Fukai, Y.; Masuda, N.; Fukuchi, M.; Manda, R.; Ojima, H.; Tsukada, K.; Kuwano, H. Glut-1 glucose transporter expression in esophageal squamous cell carcinoma is associated with tumor aggressiveness. Anticancer Res 2002, 22, 2635–2639. [Google Scholar]
- Xiang, J.; Wang, K.; Tang, N. PCK1 dysregulation in cancer: Metabolic reprogramming, oncogenic activation, and therapeutic opportunities. Genes Dis 2023, 10, 101–112. [Google Scholar] [CrossRef]
- Ho, P.C.; Bihuniak, J.D.; Macintyre, A.N.; Staron, M.; Liu, X.; Amezquita, R.; Tsui, Y.C.; Cui, G.; Micevic, G.; Perales, J.C.; et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell 2015, 162, 1217–1228. [Google Scholar] [CrossRef]
- Toledano Zur, R.; Atar, O.; Barliya, T.; Hoogi, S.; Abramovich, I.; Gottlieb, E.; Ron-Harel, N.; Cohen, C.J. Genetically engineering glycolysis in T cells increases their antitumor function. J Immunother Cancer 2024, 12. [Google Scholar] [CrossRef]
- Guo, M.; Abd-Rabbo, D.; Bertol, B.C.; Carew, M.; Lukhele, S.; Snell, L.M.; Xu, W.; Boukhaled, G.M.; Elsaesser, H.; Halaby, M.J.; et al. Molecular, metabolic, and functional CD4 T cell paralysis in the lymph node impedes tumor control. Cell Rep 2023, 42, 113047. [Google Scholar] [CrossRef]
- Fisher, S.A.; Rahimzadeh, M.; Brierley, C.; Gration, B.; Doree, C.; Kimber, C.E.; Plaza Cajide, A.; Lamikanra, A.A.; Roberts, D.J. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS ONE 2019, 14, e0222313. [Google Scholar] [CrossRef]
- Pompura, S.L.; Dominguez-Villar, M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J Leukoc Biol 2018. [Google Scholar] [CrossRef]
- Hawse, W.F.; Boggess, W.C.; Morel, P.A. TCR Signal Strength Regulates Akt Substrate Specificity To Induce Alternate Murine Th and T Regulatory Cell Differentiation Programs. J Immunol 2017, 199, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Gerriets, V.A.; Kishton, R.J.; Johnson, M.O.; Cohen, S.; Siska, P.J.; Nichols, A.G.; Warmoes, M.O.; de Cubas, A.A.; MacIver, N.J.; Locasale, J.W.; et al. Foxp3 and Toll-like receptor signaling balance T(reg) cell anabolic metabolism for suppression. Nat Immunol 2016, 17, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, A.N.; Gerriets, V.A.; Nichols, A.G.; Michalek, R.D.; Rudolph, M.C.; Deoliveira, D.; Anderson, S.M.; Abel, E.D.; Chen, B.J.; Hale, L.P.; Rathmell, J.C. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab 2014, 20, 61–72. [Google Scholar] [CrossRef]
- Kempkes, R.W.M.; Joosten, I.; Koenen, H.; He, X. Metabolic Pathways Involved in Regulatory T Cell Functionality. Front Immunol 2019, 10, 2839. [Google Scholar] [CrossRef]
- Steele, L.; Mannion, A.J.; Shaw, G.; Maclennan, K.A.; Cook, G.P.; Rudd, C.E.; Taylor, A. Non-redundant activity of GSK-3alpha and GSK-3beta in T cell-mediated tumor rejection. iScience 2021, 24, 102555. [Google Scholar] [CrossRef]
- Jendrossek, V.; Henkel, M.; Hennenlotter, J.; Vogel, U.; Ganswindt, U.; Muller, I.; Handrick, R.; Anastasiadis, A.G.; Kuczyk, M.; Stenzl, A.; Belka, C. Analysis of complex protein kinase B signalling pathways in human prostate cancer samples. BJU Int 2008, 102, 371–382. [Google Scholar] [CrossRef]
- Xia, Y.; Zhuo, H.; Lu, Y.; Deng, L.; Jiang, R.; Zhang, L.; Zhu, Q.; Pu, L.; Wang, X.; Lu, L. Glycogen synthase kinase 3beta inhibition promotes human iTreg differentiation and suppressive function. Immunol Res 2015, 62, 60–70. [Google Scholar] [CrossRef]
- Wang, R.; Dillon, C.P.; Shi, L.Z.; Milasta, S.; Carter, R.; Finkelstein, D.; McCormick, L.L.; Fitzgerald, P.; Chi, H.; Munger, J.; Green, D.R. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 2011, 35, 871–882. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, L.; Bretones, G.; Molina, E.; Arechaga, I.; Symonds, C.; Acosta, J.C.; Blanco, R.; Fernandez, A.; Alonso, L.; Sicinski, P.; et al. Myc stimulates cell cycle progression through the activation of Cdk1 and phosphorylation of p27. Sci Rep 2019, 9, 18693. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Lan, X.; Cheng, K.; Chandel, N.; Lederman, R.; Jhaveri, A.; Husain, M.; Malhotra, A.; Singhal, P.C. High glucose enhances HIV entry into T cells through upregulation of CXCR4. J Leukoc Biol 2013, 94, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Fujinaga, K.; Huang, F.; Peterlin, B.M. P-TEFb: The master regulator of transcription elongation. Mol Cell 2023, 83, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Hafer, T.L.; Felton, A.; Delgado, Y.; Srinivasan, H.; Emerman, M. A CRISPR Screen of HIV Dependency Factors Reveals That CCNT1 Is Non-Essential in T Cells but Required for HIV-1 Reactivation from Latency. Viruses 2023, 15, 1863. [Google Scholar] [CrossRef] [PubMed]
- Mbonye, U.; Karn, J. The cell biology of HIV-1 latency and rebound. Retrovirology 2024, 21, 6. [Google Scholar] [CrossRef]
- Rice, A.P.; Herrmann, C.H. Regulation of TAK/P-TEFb in CD4+ T lymphocytes and macrophages. Curr HIV Res 2003, 1, 395–404. [Google Scholar] [CrossRef]
- Ellegard, R.; Shankar, E.M.; Larsson, M. Targeting HIV-1 innate immune responses therapeutically. Curr Opin HIV AIDS 2011, 6, 435–443. [Google Scholar] [CrossRef]
- Ruffin, N.; Brezar, V.; Ayinde, D.; Lefebvre, C.; Schulze Zur Wiesch, J.; van Lunzen, J.; Bockhorn, M.; Schwartz, O.; Hocini, H.; Lelievre, J.D.; et al. Low SAMHD1 expression following T-cell activation and proliferation renders CD4+ T cells susceptible to HIV-1. AIDS 2015, 29, 519–530. [Google Scholar] [CrossRef]
- Blanco, R.; Gomez de Cedron, M.; Gamez-Reche, L.; Martin-Leal, A.; Gonzalez-Martin, A.; Lacalle, R.A.; Ramirez de Molina, A.; Manes, S. The Chemokine Receptor CCR5 Links Memory CD4(+) T Cell Metabolism to T Cell Antigen Receptor Nanoclustering. Front Immunol 2021, 12, 722320. [Google Scholar] [CrossRef]
- Jacobs, E.S.; Keating, S.M.; Abdel-Mohsen, M.; Gibb, S.L.; Heitman, J.W.; Inglis, H.C.; Martin, J.N.; Zhang, J.; Kaidarova, Z.; Deng, X.; et al. Cytokines Elevated in HIV Elite Controllers Reduce HIV Replication In Vitro and Modulate HIV Restriction Factor Expression. J Virol 2017, 91. [Google Scholar] [CrossRef]
- Schwab, J.D.; Kuhlwein, S.D.; Ikonomi, N.; Kuhl, M.; Kestler, H.A. Concepts in Boolean network modeling: What do they all mean? Comput Struct Biotechnol J 2020, 18, 571–582. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




