Submitted:
23 December 2024
Posted:
24 December 2024
You are already at the latest version
Abstract
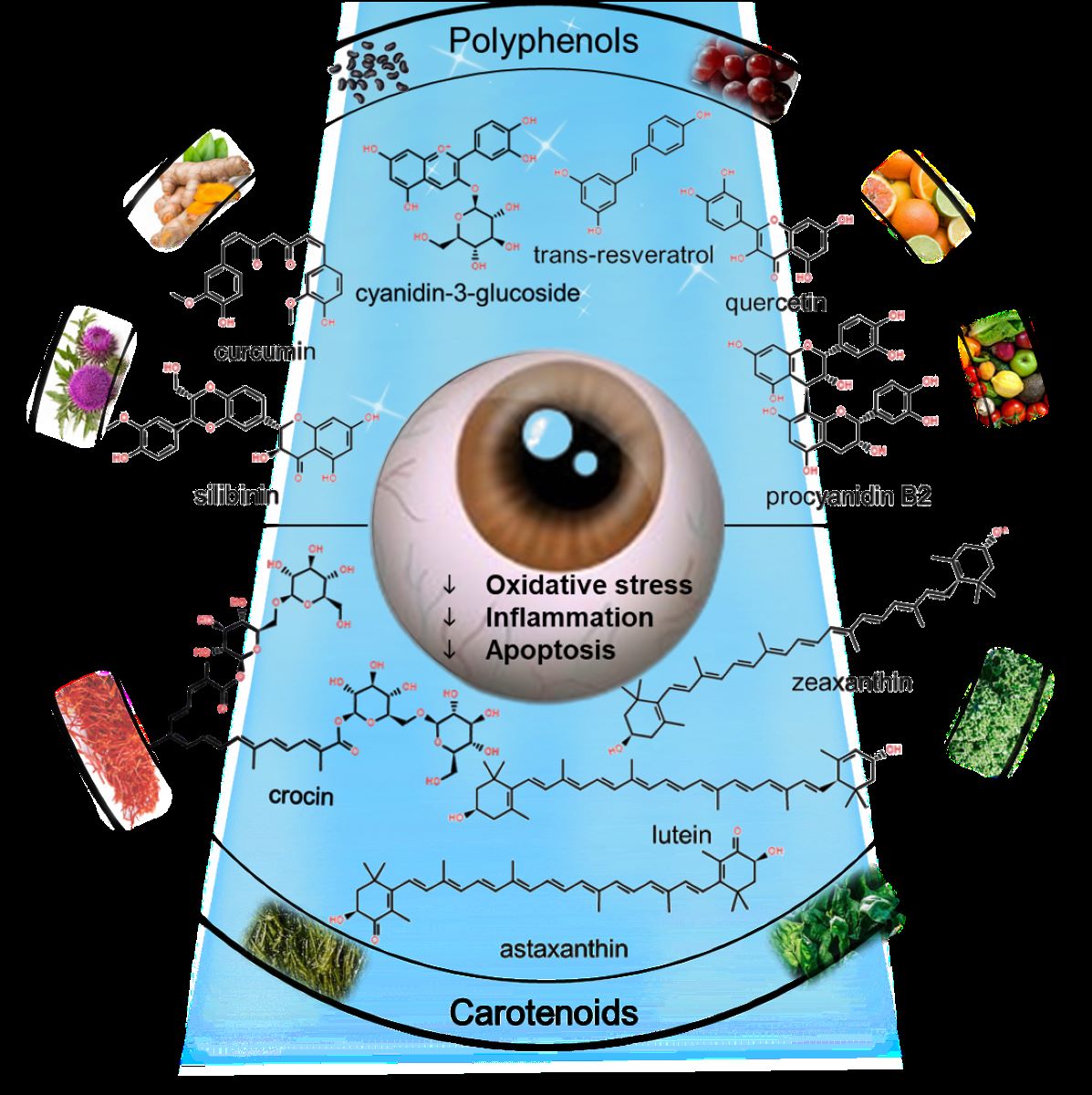
Keywords:
1. Introduction
2. Naturally Occurring Phytochemicals and Plant Extracts with Protective Potential Against BL-Induced Retinal Damage
2.1. Polyphenols
2.1.1. Resveratrol and Resveratrol Analogs
2.1.2. Curcumin
2.1.3. Anthocyanins
2.1.4. Quercetin, quercetin-3-O-α-L-arabinopyranoside, and myricetin
2.1.5. Cynaroside
2.1.6. Procyanidin B2
2.1.7. Phloroglucinol
2.1.8. Silibinin
| Compounds | Phototoxic lighting | Experimental models | Effects | References |
|---|---|---|---|---|
| Resveratrol | A2E (10 or 20 µM) + BL (430 nm, 4.02 J/cm2, 7 min for 3 d) | ARPE-19 cells | ↓ Oxidized A2E formation and intracellular A2E accumulation | [22] |
| Curcumin | Cool white LED (1,000 lux, 3 h, 09:00–12:00) | Wistar rats | Inhibits NF-κB activation and downregulates the inflammatory gene expression | [28] |
| A2E (20 µM) + BL (450 nm, 9.4 mW/cm2) | ARPE-19 cells | Inhibits c-Abl and p53 mRNA expression to prevent apoptosis | [29] | |
| Cyanidin-3-glucoside | A2E (3 μM) + BL (430 nm, 1.5 mW/cm2, 30 min) | ARPE-19 cells | Scavenges ROS and inhibits A2E photooxidation | [45] |
| Malvidin-3-glucoside | BL for 30 min | ARPE-19 cells | Inhibits mitochondrial damage and ROS production | [47] |
| Quercetin | White fluorescent light (3,000 lux) | Sprague–Dawley rats | ↓ 8-OHdG level & TUNEL-positive cells | [56] |
| A2E (3 μM) + BL (430 nm, 1.5 mW/cm2, 30 min) | ARPE-19 cells | Inhibits proinflammatory cytokines release and cell apoptosis Inhibits MG-H1 levels & RAGE mRNA expression |
[45] | |
| Quercetin-3-O-α-L-arabinopyranoside | Blue-green light (480 nm, 4,000 lux, 10 min) | ARPE-19 cells Balb-c mice |
Inhibits NF-κB, AP-1, and C3 expression Inhibits the pyrolysis of poly polymerases Inhibits the thinning of the INL, OPL, and ONL in the mouse retina |
[60] |
| Myricetin and quercetin | Bright light (10,000 lux, 45 min) | Abca4−/−Rdh8−/− mice | Inhibits inflammatory responses and photoreceptor apoptosis | [63] |
| Myricetin | BL (420 nm, 500 lux, 17.4 W/m2, 20 h); A2E (30 μM) | Primary bovine retinal cells | Prevents BL-induced and A2E-induced death of primary photoreceptor cells in the retina | [66] |
| Cynaroside | A2E (25 μM) + BL (430 nm, 2500 lux, 2 h) | ARPE-19 cells | Impedes NF-κB signaling and NLRP3 inflammasome activation Normalizes oxidative stress-related markers Inhibits apoptotic cell death |
[69] |
| Procyanidin B2 | A2E (25 μM) + BL (2000 lux, 30 min) | ARPE-19 cells | Prevents ROS generation, ER stress, and mitochondria-dependent apoptosis | [74] |
| Silibinin | BL (530 nm with a peak of 470 nm, 12.08 W/m2, 24 h) | Retinal ganglion cells | Inhibits apoptosis | [87] |
2.2. Lipoic Acid
2.3. Prunella vulgaris L. Extracts
| Plant extracts | Phototoxic lighting | Experimental models | Effects | References |
|---|---|---|---|---|
| Blueberry anthocyanin-rich extracts | White LED (420–800 nm, 2,500 lux for 12 h) | ARPE-19 cells | Inhibits cellular senescence | [48] |
| Vaccinium myrtillus L. (bilberry) and Vaccinium vitis-idaea (lingonberry) | BL (460–470 nm, 2500 lux, 6 h) | Photoreceptor 661W cells | Inhibits the generation of ROS and regulates the activation of NF-κB, p38, MAPK, and caspase-3/7 to protect retinal photoreceptor cells | [49] |
| Grape skin | BL (2000 lux, 30 min) | ARPE-19 cells | Inhibits the ER-stress-mediated intrinsic apoptotic pathway | [51] |
| Prunella vulgaris var. L | A2E (20 μM) + BL (430 nm, 4000 lux, 10 min) | ARPE-19 cells | Activates Nrf2/HO-1 signaling, inhibits ROS and MDA production ↓ inflammation |
[97] |
| BL (430 nm, 10000 lux, 1 h/d for 14 d) | BALB/c mice |
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cougnard-Gregoire, A.; Merle, B.M.J.; Aslam, T.; Seddon, J.M.; Aknin, I.; Klaver, C.C.W.; Garhöfer, G.; Layana, A.G.; Minnella, A.M.; Silva, R.; Delcourt, C. Blue light exposure: ocular hazards and prevention-A narrative review. Ophthalmol. Ther. 2023, 12, 755–788. [Google Scholar] [CrossRef]
- Behar-Cohen, F.; Martinsons, C.; Viénot, F.; Zissis, G.; Barlier-Salsi, A.; Cesarini, J.P.; Enouf, O.; Garcia, M.; Picaud, S.; Attia, D. Light-emitting diodes (LED) for domestic lighting: any risks for the eye? Prog. Retin. Eye Res. 2011, 30, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.A.; Bahmani, H. A review of the current state of research on artificial blue light safety as it applies to digital devices. Heliyon 2022, 8, e10282. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.J.; Chien, P.T.; Wen, Y.T.; Wu, C.H. A comprehensive review of experimental models for investigating blue light-induced ocular damage: Insights into parameters, limitations, and new opportunities. Exp. Eye Res. 2024, 249, 110142. [Google Scholar] [CrossRef]
- Ratnayake, K.; Payton, J.L.; Meger, M.E.; Godage, N.H.; Gionfriddo, E.; Karunarathne, A. Blue light-triggered photochemistry and cytotoxicity of retinal. Cell. Signal. 2020, 69, 109547. [Google Scholar] [CrossRef] [PubMed]
- Marie, M.; Bigot, K.; Angebault, C.; Barrau, C.; Gondouin, P.; Pagan, D.; Fouquet, S.; Villette, T.; Sahel, J.A.; Lenaers, G.; Picaud, S. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell. Death. Dis. 2018, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Bao, X.L.; Cong, Y.Y.; Fan, B.; Li, G.Y. Autophagy in age-related macular degeneration: A regulatory mechanism of oxidative stress. Oxid. Med. Cell. Longev. 2020, 2020, 2896036. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, D.; Zhang, Y.; Zhang, L.; Liao, Z.; Aihemaitijiang, S.; Hou, Y.; Zhan, Z.; Xie, K.; Zhang, Z. Lutein protected the retina from light induced retinal damage by inhibiting increasing oxidative stress and inflammation. J. Funct. Foods 2020, 73, 104107. [Google Scholar] [CrossRef]
- Ouyang, X.; Yang, J.; Hong, Z.; Wu, Y.; Xie, Y.; Wang, G. Mechanisms of blue light-induced eye hazard and protective measures: a review. Biomed. Pharmacother. 2020, 130, 110577. [Google Scholar] [CrossRef]
- Cabrera, M.P.; Chihuailaf, R.H. Antioxidants and the integrity of ocular tissues. Vet. Med. Int. 2011, 2011, 905153. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxid. Med. Cell Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research, G. The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1. Control Clin. Trials 1999, 20, 573–600. [Google Scholar]
- Group, A.R.; Chew, E.Y.; Clemons, T.; SanGiovanni, J.P.; Danis, R.; Domalpally, A.; McBee, W.; Sperduto, R.; Ferris, F.L. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012, 119, 2282–2289. [Google Scholar]
- Choo, P.P.; Woi, P.J.; Bastion, M.C.; Omar, R.; Mustapha, M.; Md Din, N. Review of evidence for the usage of antioxidants for eye aging. Biomed. Res. Int. 2022, 2022, 5810373. [Google Scholar] [CrossRef]
- Pintea, A.; Rugină, D.: Resveratrol and the human retina. In Handbook of Nutrition, Diet, and the Eye. Elsevier; 2019: 127-145.
- Alarcon De La Lastra, C.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, C.H.; Li, H.J.; Hsiao, C.Y.; Su, C.C.; Lee, P.L.; Hung, C.F. Protective effects of resveratrol against UVA-induced damage in ARPE19 cells. Int. J. Mol. Sci. 2015, 16, 5789–5802. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chan, F.; Sun, H.; Yan, J.; Fan, D.; Zhao, D.; An, J.; Zhou, D. Resveratrol protects human keratinocytes HaCaT cells from UVA-induced oxidative stress damage by downregulating Keap1 expression. Eur. J. Pharmacol. 2011, 650, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Choung, S.Y. Protective effects of resveratrol and its analogs on age-related macular degeneration in vitro. Arch. Pharm. Res. 2016, 39, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, R.; Bertelli, M.; Scaffidi, E.; Bumah, V.V.; Biagioni, F.; Busceti, C.L.; Puglisi-Allegra, S.; Fornai, F. The neurobiology of nutraceuticals combined with light exposure, a case report in the course of retinal degeneration. Arch. Ital. Biol. 2021, 159, 134–150. [Google Scholar] [CrossRef]
- Richer, S.; Stiles, W.; Ulanski, L.; Carroll, D.; Podella, C. Observation of human retinal remodeling in octogenarians with a resveratrol based nutritional supplement. Nutrients 2013, 5, 1989–2005. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.H.; Zhang, X.Y.; Leung, K.W.; Duan, R.; Dong, T.T.; Qin, Q.W.; Tsim, K.W. Resveratrol, an inhibitor binding to VEGF, restores the pathology of abnormal angiogenesis in retinopathy of prematurity (ROP) in mice: Application by intravitreal and topical instillation. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Shakibaei, M.; Harikumar, K.B.; Aggarwal, B.B. Resveratrol addiction: to die or not to die. Mol. Nutr. Food Res. 2009, 53, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.P.; Soares Lopes, D.P.; de Moraes, R.C.J.; Vieira Goncalves, C.; Pereira Rosa, L.; da Silva Rosa, F.C.; da Silva, R.A.A. Photoactivated resveratrol against Staphylococcus aureus infection in mice. Photodiagnosis Photodyn. Ther. 2019, 25, 227–236. [Google Scholar] [CrossRef]
- Mandal, M.N.; Patlolla, J.M.; Zheng, L.; Agbaga, M.P.; Tran, J.T.; Wicker, L.; Kasus-Jacobi, A.; Elliott, M.H.; Rao, C.V.; Anderson, R.E. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic. Biol. Med. 2009, 46, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Lee, E.H.; Kim, S.R.; Jang, Y.P. Anti-apoptotic effects of Curcuma longa L. extract and its curcuminoids against blue light-induced cytotoxicity in A2E-laden human retinal pigment epithelial cells. J. Pharm. Pharmacol. 2017, 69, 334–340. [Google Scholar] [PubMed]
- Souza, E.Q.M.; da Rocha, T.E.; Toro, L.F.; Guiati, I.Z.; Freire, J.d.O.A.; Ervolino, E.; Brandini, D.A.; Garcia, V.G.; Theodoro, L.H. Adjuvant effects of curcumin as a photoantimicrobial or irrigant in the non-surgical treatment of periodontitis: Systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2021, 34, 102265. [Google Scholar] [CrossRef] [PubMed]
- Muniz, I.P.R.; Galantini, M.P.L.; Ribeiro, I.S.; Goncalves, C.V.; Dos Santos, D.P.; Moura, T.C.; Silva, E.S.; Silva, N.R.; Cipriano, B.P.; Correia, T.M.L.; et al. Antimicrobial photodynamic therapy (aPDT) with curcumin controls intradermal infection by Staphylococcus aureus in mice with type 1 diabetes mellitus: a pilot study. J. Photochem. Photobiol. B. 2021, 224, 112325. [Google Scholar] [CrossRef] [PubMed]
- Cossu, M.; Ledda, L.; Cossu, A. Emerging trends in the photodynamic inactivation (PDI) applied to the food decontamination. Food. Res. Int. 2021, 144, 110358. [Google Scholar] [CrossRef] [PubMed]
- Polat, E.; Kang, K. Natural photosensitizers in antimicrobial photodynamic therapy. Biomedicines 2021, 9. [Google Scholar] [CrossRef]
- Dahl, T.A.; Bilski, P.; Reszka, K.J.; Chignell, C.F. Photocytotoxicity of curcumin. Photochem. Photobiol. 1994, 59, 290–294. [Google Scholar] [CrossRef]
- Banerjee, S.; Prasad, P.; Hussain, A.; Khan, I.; Kondaiah, P.; Chakravarty, A.R. Remarkable photocytotoxicity of curcumin in HeLa cells in visible light and arresting its degradation on oxovanadium(IV) complex formation. Chem. Commun. (Camb) 2012, 48, 7702–7704. [Google Scholar] [CrossRef]
- Seidi Damyeh, M.; Mereddy, R.; Netzel, M.E.; Sultanbawa, Y. An insight into curcumin-based photosensitization as a promising and green food preservation technology. Compr. Rev. Food Sci. 2020, 19, 1727–1759. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, S.A.; El Shishtawy, R.M.; Al Footy, K.O. Curcumin analogues and their hybrid molecules as multifunctional drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef]
- Huang, Q.; Braffett, B.H.; Simmens, S.J.; Young, H.A.; Ogden, C.L. Dietary polyphenol intake in US adults and 10-year trends: 2007-2016. J. Acad. Nutr. Diet 2020, 120, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Igwe, E.O.; Charlton, K.E.; Probst, Y.C. Usual dietary anthocyanin intake, sources and their association with blood pressure in a representative sample of Australian adults. J. Hum. Nutr. Diet 2019, 32, 578–590. [Google Scholar] [CrossRef]
- Nomi, Y.; Iwasaki-Kurashige, K.; Matsumoto, H. Therapeutic effects of anthocyanins for vision and eye health. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huo, Y.; Zhao, L.; Lu, F.; Wang, O.; Yang, X.; Ji, B.; Zhou, F. Cyanidin-3-glucoside and its phenolic acid metabolites attenuate visible light-induced retinal degeneration in vivo via activation of Nrf2/HO-1 pathway and NF-kappaB suppression. Mol. Nutr. Food Res. 2016, 60, 1564–1577. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, H.J.; Sparrow, J.R. Quercetin and cyanidin-3-glucoside protect against photooxidation and photodegradation of A2E in retinal pigment epithelial cells. Exp. Eye Res. 2017, 160, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.P.; Zhou, J.; Nakanishi, K.; Sparrow, J.R. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochem. Photobiol. 2005, 81, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Yacout, S.M.; Gaillard, E.R. The Anthocyanins, oenin and callistephin, protect RPE cells against oxidative stress. Photochem. Photobiol. 2017, 93, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, X.; Zhang, D.; Zhou, F.; Wang, D.; Wei, Y.; Gao, F.; Xie, L.; Jia, G.; Wu, W.; Ji, B. Blueberry anthocyanins: protection against ageing and light-induced damage in retinal pigment epithelial cells. Br. J. Nutr. 2012, 108, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Kuse, Y.; Tsuruma, K.; Kobayashi, S.; Shimazawa, M.; Hara, H. Protective effects of bilberry and lingonberry extracts against blue light-emitting diode light-induced retinal photoreceptor cell damage in vitro. BMC Complement. Altern. Med. 2014, 14, 1–11. [Google Scholar] [CrossRef]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; Boban, M. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, T.; Jiang, Y.; Wu, L.; Cai, X.; Sun, X.; Sun, X. Photooxidative damage in retinal pigment epithelial cells via GRP78 and the protective role of grape skin polyphenols. Food Chem. Toxicol. 2014, 74, 216–224. [Google Scholar] [CrossRef]
- Yu, C.C.; Nandrot, E.F.; Dun, Y.; Finnemann, S.C. Dietary antioxidants prevent age-related retinal pigment epithelium actin damage and blindness in mice lacking alphavbeta5 integrin. Free Radic. Biol. Med. 2012, 52, 660–670. [Google Scholar] [CrossRef]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.; Graf, B.A.; O'Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Nakamura, Y.; Iida, H.; Ito, K.; Ohguro, H. Comparative assessment of distribution of blackcurrant anthocyanins in rabbit and rat ocular tissues. Exp. Eye Res. 2006, 83, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Du, X. The therapeutic use of quercetin in ophthalmology: recent applications. Biomed. Pharmacother. 2021, 137, 111371. [Google Scholar] [CrossRef]
- Koyama, Y.; Kaidzu, S.; Kim, Y.C.; Matsuoka, Y.; Ishihara, T.; Ohira, A.; Tanito, M. Suppression of light-induced retinal degeneration by quercetin via the AP-1 pathway in rats. Antioxidants (Basel) 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ueda, K.; Zhao, J.; Sparrow, J.R. Correlations between photodegradation of bisretinoid constituents of retina and dicarbonyl adduct deposition. J. Biol. Chem. 2015, 290, 27215–27227. [Google Scholar] [CrossRef]
- Cao, X.; Liu, M.; Tuo, J.; Shen, D.; Chan, C.C. The effects of quercetin in cultured human RPE cells under oxidative stress and in Ccl2/Cx3cr1 double deficient mice. Exp. Eye Res. 2010, 91, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Maher, P.; Hanneken, A. Flavonoids protect retinal ganglion cells from oxidative stress–induced death. Invest. Ophthalmol. Vis. Sci. 2005, 46, 4796–4803. [Google Scholar] [CrossRef]
- Kim, J.; Jin, H.L.; Jang, D.S.; Jeong, K.W.; Choung, S.Y. Quercetin-3-O-α-l-arabinopyranoside protects against retinal cell death via blue light-induced damage in human RPE cells and Balb-c mice. Food Funct. 2018, 9, 2171–2183. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar]
- Ortega, J.T.; Parmar, T.; Jastrzebska, B. Flavonoids enhance rod opsin stability, folding, and self-association by directly binding to ligand-free opsin and modulating its conformation. J. Biol. Chem. 2019, 294, 8101–8122. [Google Scholar] [CrossRef]
- Ortega, J.T.; Parmar, T.; Golczak, M.; Jastrzebska, B. Protective Effects of flavonoids in acute models of light-induced retinal degeneration. Mol. Pharmacol. 2021, 99, 60–77. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Luo, L. Effect of myricetin on primary open-angle glaucoma. Transl. Neurosci. 2018, 9, 132–141. [Google Scholar] [CrossRef]
- Shin, J.C.; Jung, H.Y.; Harikishore, A.; Kwon, O.D.; Yoon, H.S.; Kim, K.T.; Choi, B.H. The flavonoid myricetin reduces nocturnal melatonin levels in the blood through the inhibition of serotonin N-acetyltransferase. Biochem. Biophys. Res. Commun. 2013, 440, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Laabich, A.; Manmoto, C.C.; Kuksa, V.; Leung, D.W.; Vissvesvaran, G.P.; Karliga, I.; Kamat, M.; Scott, I.L.; Fawzi, A.; Kubota, R. Protective effects of myricetin and related flavonols against A2E and light mediated-cell death in bovine retinal primary cell culture. Exp. Eye Res. 2007, 85, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-inflammatory and active biological properties of the plant-derived bioactive compounds luteolin and luteolin 7-glucoside. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.H.; Dong, X.W.; Yu, H.L.; Shen, W.; Lv, X.Y.; Wang, R.; Cheng, X.X.; Xiong, F.; Hu, X.L.; Wang, H. Cynaroside protects the blue light-induced retinal degeneration through alleviating apoptosis and inducing autophagy in vitro and in vivo. Phytomedicine 2021, 88, 153604. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Hu, X.; Feng, J.; Wang, H.; Xiong, F. Protective effects of cynaroside on oxidative stress in retinal pigment epithelial cells. J. Biochem. Mol. Toxicol. 2019, 33, e22352. [Google Scholar] [CrossRef]
- Rodriguez-Ramiro, I.; Ramos, S.; Bravo, L.; Goya, L.; Martin, M.A. Procyanidin B2 induces Nrf2 translocation and glutathione S-transferase P1 expression via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. Eur J Nutr 2012, 51, 881–892. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Tokutake, S. Procyanidin-rich extract from grape seeds prevents cataract formation in hereditary cataractous (ICR/f) rats. J. Agric. Food Chem. 2002, 50, 4983–4988. [Google Scholar] [CrossRef]
- Reddy, G.B.; Muthenna, P.; Akileshwari, C.; Raghu, G.; Suryanarayana, P. Antiglycating potential of procyanidin-B2 isolated from cinnamon bark: Prevention or treatment of diabetic ocular complications (cataract & retinopathy). Invest. Ophthalmol. Vis. Sci. 2013, 54, 1945–1945. [Google Scholar]
- Li, W.; Jiang, Y.; Sun, T.; Yao, X.; Sun, X. Supplementation of procyanidins B2 attenuates photooxidation-induced apoptosis in ARPE-19 cells. Int. J. Food Sci. Nutr. 2016, 67, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Davis, A.; Rodriguez, M.E.; Agron, S.; Hackam, A.S. Protective effects of a grape-supplemented diet in a mouse model of retinal degeneration. Nutrition 2016, 32, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, Z.; Wang, K.; Zhu, X.; Ali, Y.; Shu, W.; Bao, X.; Zhu, L.; Fan, X.; Murray, M.; Zhou, F. Procyanidin B2 and rutin in Ginkgo biloba extracts protect human retinal pigment epithelial (RPE) cells from oxidative stress by modulating Nrf2 and Erk1/2 signalling. Exp. Eye Res. 2021, 207, 108586. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Huo, Y.; Zhou, F.; Wu, W.; Lu, F.; Yang, X.; Guo, X.; Chen, P.; Deng, Q. Protective effect of proanthocyanidins from sea buckthorn (Hippophae rhamnoides L.) seed against visible light-induced retinal degeneration in vivo. Nutrients 2016, 8, 245. [Google Scholar]
- Stoupi, S.; Williamson, G.; Viton, F.; Barron, D.; King, L.J.; Brown, J.E.; Clifford, M.N. In vivo bioavailability, absorption, excretion, and pharmacokinetics of [14C] procyanidin B2 in male rats. Drug Metab. Dispos. 2010, 38, 287–291. [Google Scholar] [CrossRef]
- Spencer, J.P.; Schroeter, H.; Shenoy, B.; Srai, S.K.; Debnam, E.S.; Rice-Evans, C. Epicatechin is the primary bioavailable form of the procyanidin dimers B2 and B5 after transfer across the small intestine. Biochem. Biophys. Res. Commun. 2001, 285, 588–593. [Google Scholar] [CrossRef]
- Xiao, Y.; Hu, Z.; Yin, Z.; Zhou, Y.; Liu, T.; Zhou, X.; Chang, D. Profiling and distribution of metabolites of procyanidin B2 in mice by UPLC-DAD-ESI-IT-TOF-MSn technique. Front. Pharmacol. 2017, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Zhang, R.; Lee, N.H.; Hyun, J.W. Phloroglucinol attenuates ultraviolet B radiation-induced matrix metalloproteinase-1 production in human keratinocytes via inhibitory actions against mitogen-activated protein kinases and activator protein-1. Photochem. Photobiol. 2012, 88, 381–388. [Google Scholar] [CrossRef]
- Cia, D.; Cubizolle, A.; Crauste, C.; Jacquemot, N.; Guillou, L.; Vigor, C.; Angebault, C.; Hamel, C.P.; Vercauteren, J.; Brabet, P. Phloroglucinol protects retinal pigment epithelium and photoreceptor against all-trans-retinal-induced toxicity and inhibits A2E formation. J. Cell Mol. Med. 2016, 20, 1651–1663. [Google Scholar] [CrossRef] [PubMed]
- Taveau, N.; Cubizolle, A.; Guillou, L.; Pinquier, N.; Moine, E.; Cia, D.; Kalatzis, V.; Vercauteren, J.; Durand, T.; Crauste, C.; Brabet, P. Preclinical pharmacology of a lipophenol in a mouse model of light-induced retinopathy. Exp. Mol. Med. 2020, 52, 1090–1101. [Google Scholar] [CrossRef]
- Zhang, J.; Kiser, P.D.; Badiee, M.; Palczewska, G.; Dong, Z.; Golczak, M.; Tochtrop, G.P.; Palczewski, K. Molecular pharmacodynamics of emixustat in protection against retinal degeneration. J. Clin. Invest. 2015, 125, 2781–2794. [Google Scholar] [CrossRef] [PubMed]
- Kren, V.; Valentova, K. Silybin and its congeners: from traditional medicine to molecular effects. Nat. Prod. Rep. 2022, 39, 1264–1281. [Google Scholar] [CrossRef]
- Lin, C.H.; Li, C.H.; Liao, P.L.; Tse, L.S.; Huang, W.K.; Cheng, H.W.; Cheng, Y.W. Silibinin inhibits VEGF secretion and age-related macular degeneration in a hypoxia-dependent manner through the PI-3 kinase/Akt/mTOR pathway. Br. J. Pharmacol. 2013, 168, 920–931. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, H.; Wang, Z.; Guan, W.; Kang, X.; Tai, X.; Sun, Y. Silibinin declines blue light-induced apoptosis and inflammation through MEK/ERK/CREB of retinal ganglion cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4059–4065. [Google Scholar] [CrossRef] [PubMed]
- Tvrdy, V.; Pourova, J.; Jirkovsky, E.; Kren, V.; Valentova, K.; Mladenka, P. Systematic review of pharmacokinetics and potential pharmacokinetic interactions of flavonolignans from silymarin. Med. Res. Rev. 2021, 41, 2195–2246. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Tziona, P.; Rekka, E.A. Lipoic acid. Kinetics and pluripotent biological properties and derivatives. Mol. Biol. Rep. 2021, 48, 6539–6550. [Google Scholar] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef] [PubMed]
- Demir, U.; Demir, T.; Ilhan, N. The protective effect of alpha-lipoic acid against oxidative damage in rabbit conjunctiva and cornea exposed to ultraviolet radiation. Ophthalmologica 2005, 219, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Lin, D.P.; Chang, L.S.; Huang, T.P.; Liu, H.J.; Luk, C.P.; Lo, Y.L.; Chang, H.H. Dietary alpha-lipoic acid prevents UVB-induced corneal and conjunctival degeneration through multiple effects. Invest. Ophthalmol. Vis. Sci. 2013, 54, 6757–6766. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, B.A.; Roberts, R.; Stemmler, A.; Luan, H.; Gradianu, M. Impaired apparent ion demand in experimental diabetic retinopathy: correction by lipoic acid. Invest. Ophthalmol. Vis. Sci. 2007, 48, 4753–4758. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Luan, H.; Berkowitz, B.A. alpha-lipoic acid corrects late-phase supernormal retinal oxygenation response in experimental diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2006, 47, 4077–4082. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, C.; Song, D.; Li, Y.; Song, Y.; Su, G.; Dunaief, J.L. Systemic administration of the antioxidant/iron chelator alpha-lipoic acid protects against light-induced photoreceptor degeneration in the mouse retina. Invest. Ophthalmol. Vis. Sci. 2014, 55, 5979–5988. [Google Scholar] [CrossRef]
- Psotová, J.; Kolár, M.; Sousek, J.; Svagera, Z.; Vicar, J.; Ulrichová, J. Biological activities of Prunella vulgaris extract. Phytother. Res. 2003, 17, 1082–1087. [Google Scholar] [CrossRef]
- Kim, J.; Cho, K.; Choung, S.Y. Protective effect of Prunella vulgaris var. L extract against blue light induced damages in ARPE-19 cells and mouse retina. Free. Radic. Biol. Med. 2020, 152, 622–631. [Google Scholar] [PubMed]
- Barker, F.M., 2nd; Snodderly, D.M.; Johnson, E.J.; Schalch, W.; Koepcke, W.; Gerss, J.; Neuringer, M. Nutritional manipulation of primate retinas, V: effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Invest. Ophthalmol. Vis. Sci. 2011, 52, 3934–3942. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Gao, S.; Zhou, J.; Qin, J.; Taylor, A.; Johnson, E.J.; Tang, G.; Sparrow, J.R.; Gierhart, D.; Shang, F. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free. Radic. Biol. Med. 2012, 53, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Yang, C.M.; Yang, C.H. Protective Effect of astaxanthin on blue light light-emitting diode-induced retinal cell damage via free radical scavenging and activation of PI3K/Akt/Nrf2 pathway in 661W cell model. Mar. Drugs 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Laabich, A.; Vissvesvaran, G.P.; Lieu, K.L.; Murata, K.; McGinn, T.E.; Manmoto, C.C.; Sinclair, J.R.; Karliga, I.; Leung, D.W.; Fawzi, A.; Kubota, R. Protective effect of crocin against blue light- and white light-mediated photoreceptor cell death in bovine and primate retinal primary cell culture. Invest. Ophthalmol. Vis. Sci. 2006, 47, 3156–3163. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Zhang, X.; Chen, Q.; Chen, H.; Sun, L.; Liu, G. Protective Effect of fucoxanthin isolated from Laminaria japonica against visible light-induced retinal damage both in vitro and in vivo. J. Agric. Food Chem. 2016, 64, 416–424. [Google Scholar] [CrossRef]
- Fontaine, V.; Monteiro, E.; Brazhnikova, E.; Lesage, L.; Balducci, C.; Guibout, L.; Feraille, L.; Elena, P.P.; Sahel, J.A.; Veillet, S.; Lafont, R. Norbixin protects retinal pigmented epithelium cells and photoreceptors against A2E-mediated phototoxicity in vitro and in vivo. PLoS One 2016, 11, e0167793. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





