Submitted:
19 December 2024
Posted:
20 December 2024
You are already at the latest version
Abstract
In recent years, fungal infections have emerged as a significant health concern across veterinary species, especially in livestock such as cattle, where fungal diseases can result in considerable economic losses. Aspergillus species, notably Aspergillus flavus and Aspergillus versicolor, are opportunistic pathogens that pose a threat to both animals and humans. In cattle, Aspergillus infections can lead to mycotic abortion, primarily in late pregnancy, and cause respiratory and gastrointestinal issues. As the incidence of such infections rises, effective antifungal therapies are needed, particularly in the face of increasing antifungal resistance. This study focuses on the synthesis and antifungal evaluation of novel Fmoc-protected 1,2,4-triazolyl-α-amino acids (5 and 6) and their dipeptides (8 and 9), designed to combat fungal pathogens with a targeted and po-tentially safer profile compared to traditional antifungal agents like fluconazole. Through opti-mized precipitation techniques and the activated ester method, we synthesized the compounds and evaluated their antifungal activity against Aspergillus versicolor (ATCC 12134) and Aspergillus flavus (ATCC 10567). The results showed that dipeptide (8) demonstrated promising antifungal activity at 0.16 mL, comparable to fluconazole, a standard treatment for fungal infections. Notably, increasing the concentration of fluconazole to 0.33 mL did not improve its antifungal efficacy, while the dipeptide's effect was enhanced with increased dosage, suggesting the potential of these dipeptides as more potent or selective antifungal agents. In contrast, the protected amino acid (5) exhibited consistent inhibition across all tested concentrations, aligning with the effects observed for fluconazole at higher concentrations. The increasing prevalence of fungal infections in im-munocompromised animals, coupled with the limited number of effective antifungal drugs, underscores the need for innovative therapeutic approaches. In cattle, Aspergillus infections can have devastating consequences for both animal health and agricultural productivity, with affected herds experiencing reduced milk production, poor fertility, and in severe cases, abortion. As conventional antifungal treatments may pose risks of resistance development or adverse side effects, novel antifungal peptides, such as the synthesized dipeptides in this study, present a promising alternative. These compounds offer the dual benefits of high bioactivity against pathogens like Aspergillus species and the potential for reduced resistance due to their distinct mechanisms of action. Our findings indicate that the synthesized 1,2,4-triazolyl dipeptides (8 and 9) hold significant promise as potential antifungal agents, not only for veterinary use in treating Aspergillus-induced diseases in cattle but also for broader applications in human and animal health. Given the rising concerns of antifungal resistance, these peptides, with their unique bio-active structures, could serve as a novel class of therapeutic agents with enhanced specificity and fewer side effects. Further research into their mechanism of action, in vivo efficacy, and safety profiles is warranted to fully realize their potential as antifungal drugs in clinical and agricultural settings.
Keywords:
1. Introduction
1.1. Fungal Strains Affecting Animal Species
1.2.Antifungal Drugs
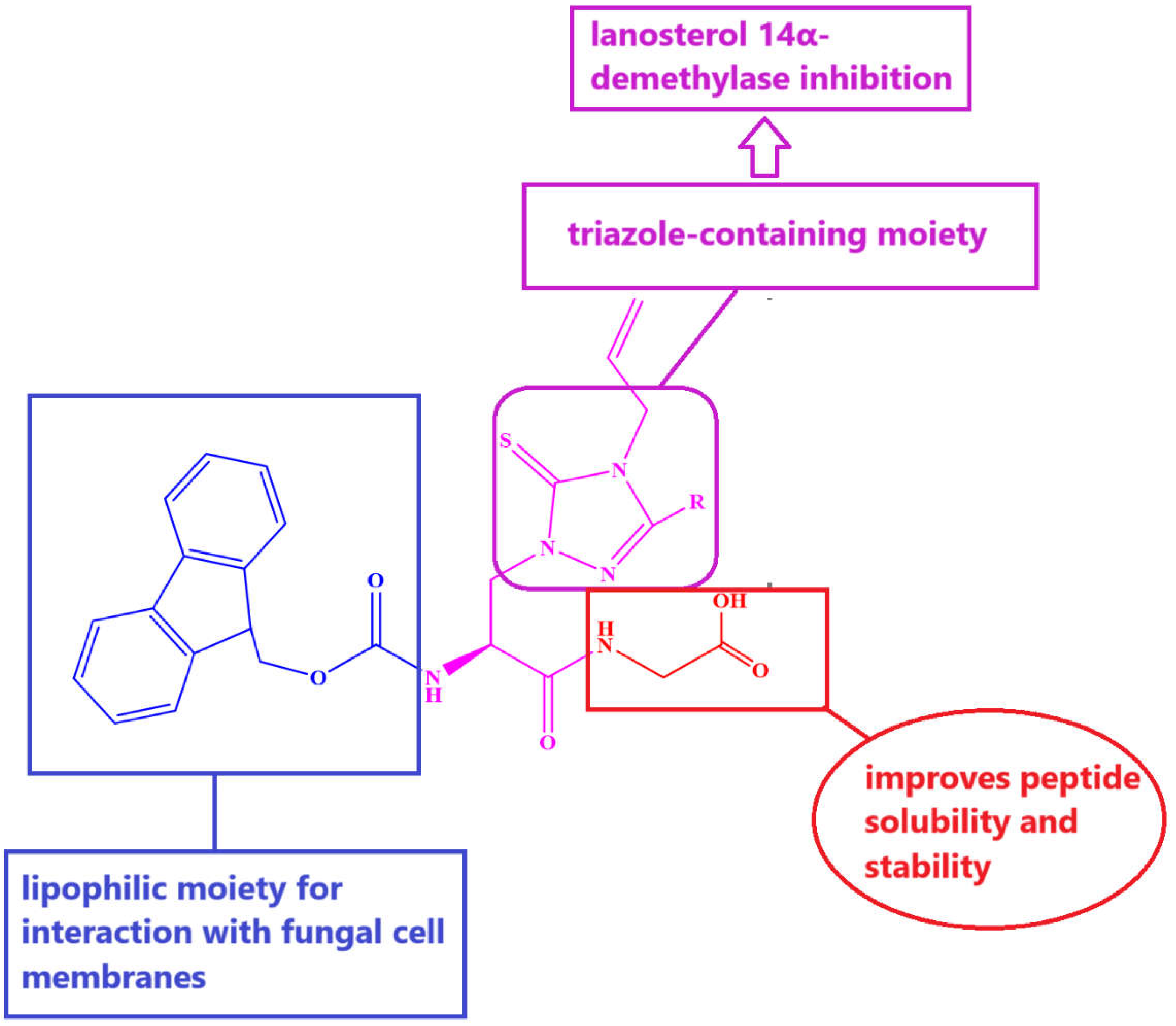
1.3. Aim of This Work
- (i)
- membrane disruption: the triazole ring can insert into fungal membranes, disrupting their integrity, causing leakage of cellular contents, and leading to cell death;
- (ii)
- inhibition of ergosterol biosynthesis: similar to other triazole drugs, these pep tides may inhibit ergosterol synthesis, a key component of fungal cell membranes, impairing membrane function;
- (iii)
- cell wall disruption: triazole peptides may interfere with the synthesis of fungal cell wall components (e.g., chitin, β-glucan), weakening the cell wall and causing osmotic lysis;
- (iv)
- DNA and protein synthesis inhibition: the triazole ring can bind to metal ions involved in DNA replication and interfere with enzymes crucial for protein synthesis;
- (v)
- increased stability and bioavailability: the incorporation of triazole increases the peptide's stability, bioavailability, and resistance to enzymatic degradation;
- (vi)
- immune modulation: some peptides may enhance immune recognition and responses, aiding in fungal clearance.
2.1. Materials
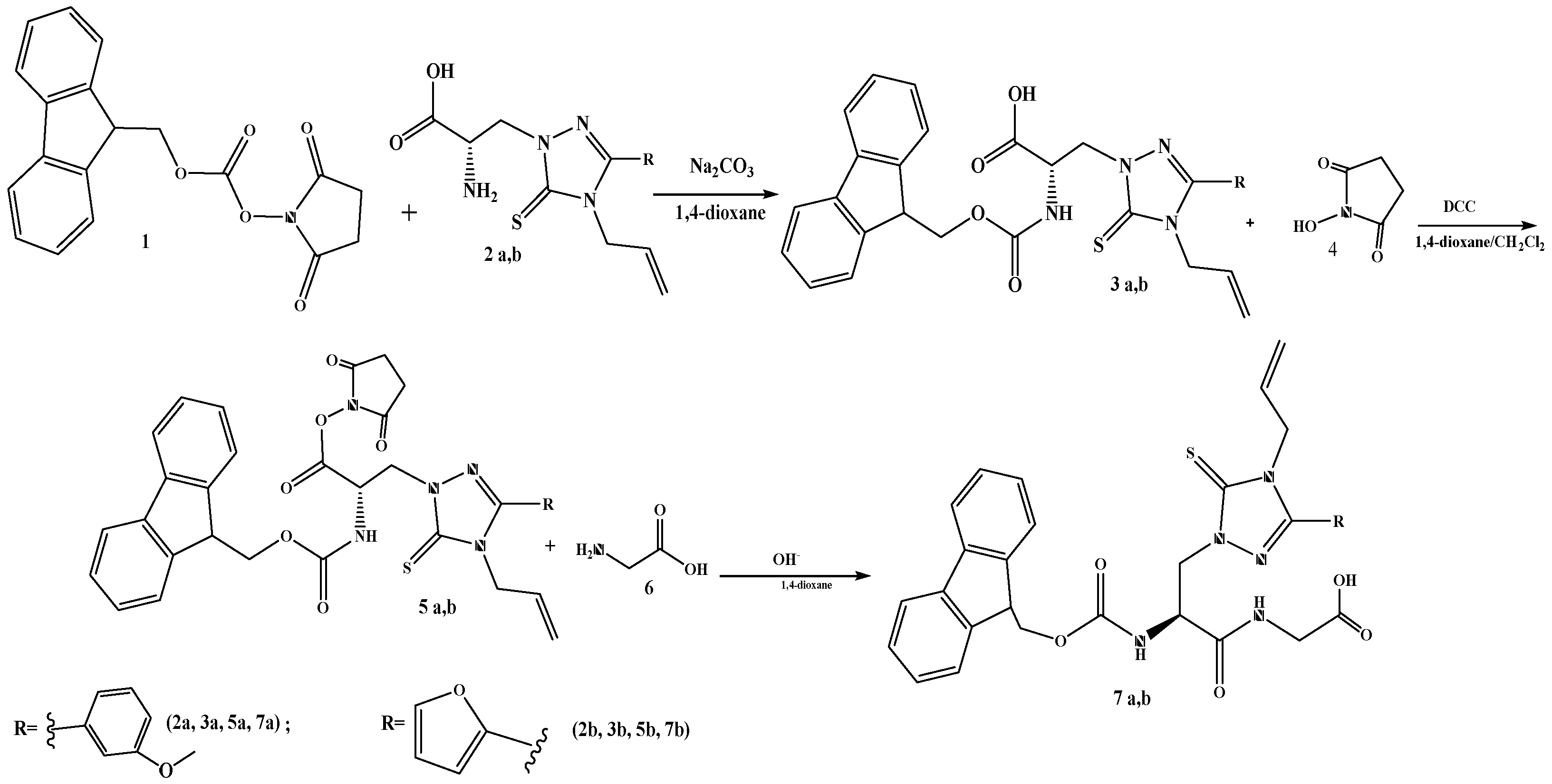
2.2. Synthesis of Derivatives of 9-Fluorenylmethoxycarbonyl Protected α-Amino Acids
2.3. Synthesis of N-Oxysuccinimide Esters
2.4. Synthesis of Dipeptides
2.5. Antifungal Activity Assessment
2.6. Molecular Docking
3. Results
3.1. Synthesis of Dipeptides
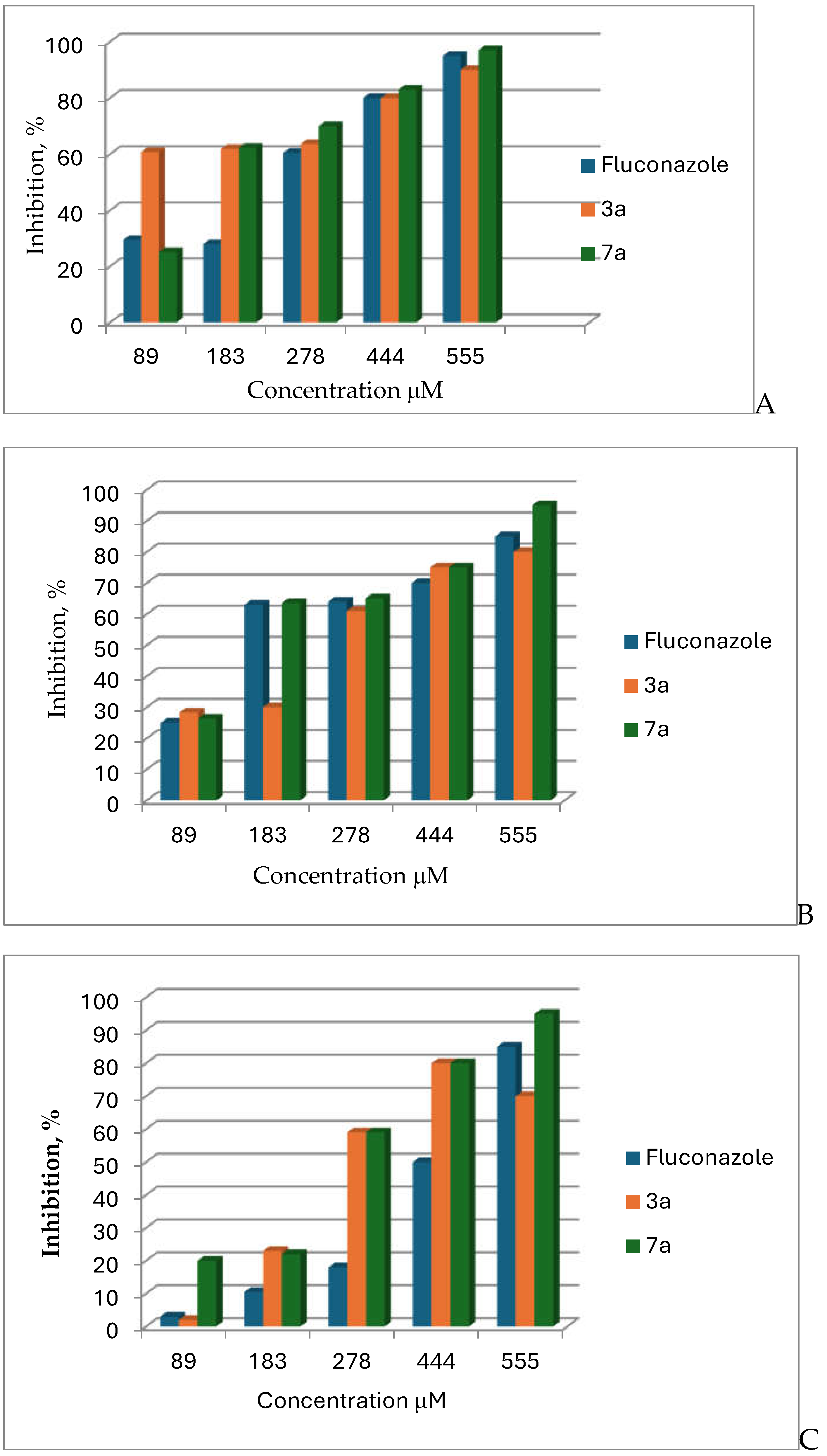
3.2. Antifungal Activity
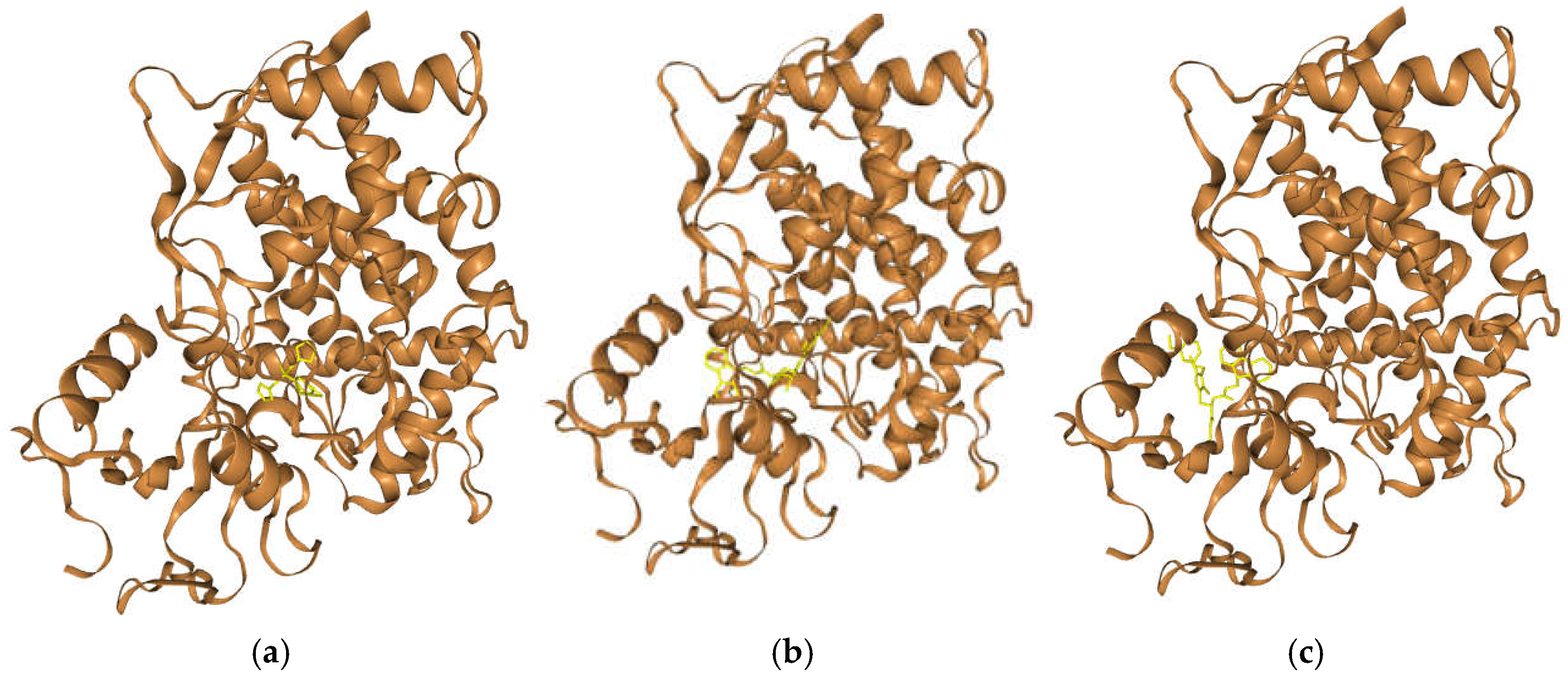
3.4. In Silico Exploration of the Mechanism Behind the Activity of Compounds 5 and 8 Against Aspergillus Species: Molecular Docking of the Antifungal Molecules with Sterol 14-Alpha Demethylase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Enaud, R.; Vandenborght, LE.; Coron, N.; Bazin, T.; Prevel, R.; Schaeverbeke, T.; Berger, P.; Fayon, M.; Lamireau, T.; Delhaes, L. The Mycobiome: A Neglected Component in the Microbiota-Gut-Brain Axis. Microorganisms. 2018, 6(1), 22. [Google Scholar] [CrossRef] [PubMed]
- Kapitan, M.; Niemiec, MJ.; Steimle, A.; Frick, JS.; Jacobsen, ID. Fungi as Part of the Microbiota and Interactions with Intestinal Bacteria. Curr Top Microbiol Immunol. 2019, 422, 265–301. [Google Scholar] [CrossRef] [PubMed]
- Money, N.P. Fungi and biotechnology. The Fungi (Third Edition), Watkinson, S.C., Boddy, L., Money, N.P., Eds.; Academic Press: United States, 2016; 401- 424.
- Mukherjee, D.; Singh, S.; Kumar, M.; Kumar, V.; Datta, S.; Dhanjal, DS. Fungal biotechnology: role and aspects, in Fungi and their Role in Sustainable Development: Current Perspectives, Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2018; 91-103.
- Troise, A.D.; Dathan, N.A.; Fiore, A.; Roviello, G.; Di Fiore, A.; Caira, S.; Cuollo, M.; De Simone, G.; Fogliano, V.; Monti, S.M. Faox Enzymes Inhibited Maillard Reaction Development during Storage Both in Protein Glucose Model System and Low Lactose UHT Milk. Amino Acids 2013, 46, 279–288. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012, 11. 484, 186–194. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Bosco, SMG.; de Hoog, S.; Ebel, F.; Elad, D.; Gomes, RR.; Jacobsen, ID.; Jensen, HE.; Martel, A.; Mignon, B.; Pasmans, F.; Piecková, E.; Rodrigues, AM.; Singh, K.; Vicente, VA.; Wibbelt, G.; Wiederhold, NP.; Guillot, J. Fungal infections in animals: a patchwork of different situations. Med Mycology. 2018, 56(1), 165–187. [CrossRef]
- Casadevall, A.; Pirofski, LA. Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect Immun. 2000, 68(12), 6511–6518. [Google Scholar] [CrossRef]
- Guarro, J.; Gené, J.; Stchigel, A.M. Developments in fungal taxonomy. Clinical Microbiology Reviews. 1999, 12(3), 454–500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seyedmousavi, S.; Guillot, J.; Tolooe, A.; Verweij, PE.; de Hoog, G.S. Neglected fungal zoonoses: hidden threats to man and animals. Clin Microbiol Infect. 2015, 21(5), 416–25. [Google Scholar] [CrossRef] [PubMed]
- Desoubeaux, G.; Bailly, É.; Chandenier, J. Diagnosis of invasive pulmonary aspergillosis: updates and recommendations. Med Mal Infect. 2014, 44(3), 89–101. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999, 12(2), 310–50. [Google Scholar] [CrossRef]
- Paulussen, C.; Hallsworth, J.E.; Álvarez-Pérez, S.; Nierman, W.C.; Hamill, P.G.; Blain, D.; Rediers, H.; Lievens, B. Ecology of aspergillosis: insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb Biotechnol. 2017, 10(2), 296–322. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Guillot, J.; Arné, P.; de Hoog, G.S.; Mouton, J.W.; Melchers, W.J.; Verweij, P.E. Aspergillus and aspergilloses in wild and domestic animals: a global health concern with parallels to human disease. Med Mycology. 2015, 53(8), 765–797. [Google Scholar] [CrossRef] [PubMed]
- Desoubeaux, G.; Cray, C. Animal Models of Aspergillosis. Comp Med. 2018, 68(2), 109–123. [Google Scholar] [PubMed]
- Henker, L.C.; Lorenzett, M.P.; Lopes B.C.; Dos Santos, I.R.; Bandinelli, M.B.; Bassuino, D.M.; Juffo, G.D.; Antoniassi. N.A.B.; Pescador, C.A.; Sonne, L.; Driemeier, D.; Pavarini, S.P. Pathological and etiological characterization of cases of bovine abortion due to sporadic bacterial and mycotic infections. Braz J Microbiol. 2022, 53(4), 2251-2262. [CrossRef]
- Elad, D.; Segal, E. Diagnostic Aspects of Veterinary and Human Aspergillosis. Frontiers in Microbiology 2018, 9. [Google Scholar] [CrossRef]
- Mittal, J.; Szymczak, W.A.; Pirofski, L.A.; Galen, B.T. Fungemia caused by Aureobasidium pullulans in a patient with advanced AIDS: a case report and review of the medical literature. JMM Case Rep. 2018, 5(4), 1–5. [Google Scholar] [CrossRef]
- Verdecia, J.; Jankowski, C. A.; Reynolds, M. L.; McCarter, Y.; Ravi, M. Fungemia due to Aureobasidium pullulans. Med. Mycol. Case Rep. 2022, 37, 26–28. [Google Scholar] [CrossRef]
- Mehta, S.R.; Johns, S.; Stark, P.; Fierer, J. Successful treatment of Aureobasidium pullulans central catheter-related fungemia and septic pulmonary emboli. IDCases. 2017, 10, 65–67. [Google Scholar] [CrossRef]
- Kaur, R.; Wadhwa, A.; Gulati, A.; Agrawal, A. An unusual phaeoid fungi: Ulocladium, as a cause of chronic allergic fungal sinusitis. Iran J Microbiol. 2010, 2(2), 95–97. [Google Scholar]
- Badenoch, P.R.; Halliday, C.L.; Ellis, D.H.; Billing, K.J.; Mills, R.A. Ulocladium atrum keratitis. J Clin Microbiol. 2006, 44(3), 1190–1193. [Google Scholar] [CrossRef]
- Durán, M.T.; Del Pozo, J.; Yebra, M.T.; Crespo, M.G.; Paniagua, M.J.; Cabezón, M.A.; Guarro, J. Cutaneous infection caused by Ulocladium chartarum in a heart transplant recipient: case report and review. Acta Derm Venereol. 2003, 83(3), 218–221. [Google Scholar] [CrossRef] [PubMed]
- Martins, LML. Allergy to Fungi in Veterinary Medicine: Alternaria, Dermatophytes and Malassezia Pay the Bill! Journal of Fungi. 2022, 27. 8(3), 235. [Google Scholar] [CrossRef]
- Fernandes, Ch.; Casadevall, A.; Gonçalves, T. ; Mechanisms of Alternaria pathogenesis in animals and plants. FEMS Microbiology Reviews. 2023, 47(6), 1–25. [Google Scholar] [CrossRef]
- Roemer, T.; Krysan, D.J. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med. 2014, 4(5), 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Liu, J.; Zhang, G.; Li, J.; Zhang, S.; Guo, H. Design, Synthesis, and Antifungal Activities of Novel 1,2,4-Triazole Schiff Base Derivatives. Chem Biodivers. 2018, 15(9), 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, W-N.; Jiang,Y-M.; Du, H-T.; Mao-Fa Yang, M-F.; Synthesis and antifungal activity of novel 1,2,4-triazole derivatives containing an amide moiety. J Heterocycl Chem. 2019, 1–8. [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.H.; Sono, M. Cytochrome P-450 and chloroperoxidase: thiolate-ligated heme enzymes. Spectroscopic determination of their active-site structures and mechanistic implications of thiolate ligation. Chemical Reviews, 1987, 87(5), 1255–1276. [CrossRef]
- Kazeminejad, Z.; Marzi, M.; Shiroudi, A.; Kouhpayeh, S.A.; Farjam, M.; Zarenezhad, E. Novel 1, 2, 4-Triazoles as Antifungal Agents. Biomed Res Int. 2022, 22, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Vicidomini, C.; Palumbo, R.; Moccia, M.; Roviello, G.N. Oxidative Processes and Xenobiotic Metabolism in Plants: Mechanisms of Defense and Potential Therapeutic Implications. Journal of Xenobiotics 2024, 14, 1541–1569. [Google Scholar] [CrossRef]
- Vicidomini, C.; Fontanella, F.; Tiziana D’Alessandro; Roviello, G.N. A Survey on Computational Methods in Drug Discovery for Neurodegenerative Diseases. Biomolecules 2024, 14, 1330–1330. [CrossRef] [PubMed]
- Costanzo, M.; De Giglio, M.A.R.; Gilhen-Baker, M.; Roviello, G.N. The Chemical Basis of Seawater Therapies: A Review. Environmental Chemistry Letters 2024, 22, 2133–2149. [Google Scholar] [CrossRef]
- Gamberi, C.; Leverette, C.L.; Davis, A.C.; Ismail, M.; Piccialli, I.; Borbone, N.; Oliviero, G.; Vicidomini, C.; Palumbo, R.; Roviello, G.N. Oceanic Breakthroughs: Marine-Derived Innovations in Vaccination, Therapy, and Immune Health. Vaccines 2024, 12, 1263. [Google Scholar] [CrossRef] [PubMed]
- Marasco, D.; Vicidomini, C.; Krupa, P.; Cioffi, F.; Huy, P. D. Q.; Li, M. S.; Florio, D.; Broersen, K.; De Pandis, M. F.; Roviello, G. N. Plant isoquinoline alkaloids as potential neurodrugs: A comparative study of the effects of benzo[c]phenanthridine and berberine-based compounds on β-amyloid aggregation. Chemico-Biological Interactions, 2020, 330, 109300. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, D.; Oliviero, G.; Roviello, G. N.; Bucci, E. M.; Piccialli, G. G-quadruplex-forming oligonucleotide conjugated to magnetic nanoparticles: synthesis, characterization, and enzymatic stability assays. Bioconjugate Chemistry, 2011, 22(6), 1251–1258. [CrossRef]
- Sharma, K.K.; Ravi, R.; Maurya, I.K.; Kapadia, A.; Khan, S.I.; Kumar, V.; Tikoo, K.; Jain, R. Modified histidine containing amphipathic ultrashort antifungal peptide, His[2-p-(n-butyl)phenyl]-Trp-Arg-OMe exhibits potent anticryptococcal activity. Eur J Med Chem. 2021, 223, 113635. [Google Scholar] [CrossRef]
- Sharma, K.K.; Maurya, I.K.; Khan, S.I.; Jacob, M.R.; Kumar, V.; Tikoo, K.; Jain, R. Discovery of a Membrane-Active, Ring-Modified Histidine Containing Ultrashort Amphiphilic Peptide That Exhibits Potent Inhibition of Cryptococcus neoformans. J Med Chem. 2017, 60(15), 6607–6621. [Google Scholar] [CrossRef]
- Tivari S., R.; Kokate S., V.; Sobhia E., M.; Kumar S., G.; Shelar U., B.; Jadeja Y., S. A Series of Novel Bioactive Cyclic Peptides: Synthesis by Head-to-Tail Cyclization Approach, Antimicrobial Activity and Molecular Docking Studies. ChemistrySelect 2022, 7, e202201481. [Google Scholar] [CrossRef]
- Tivari, S.R.; Kokate, S.V.; Belmonte-Vázquez, J.L.; Pawar, T.J.; Patel, H.; Ahmad, I.; Gayke, M.S.; Bhosale. R.S.; Jain, V.D.; Muteeb, G.; Delgado-Alvarado, E.; Jadeja, Y. Synthesis and Evaluation of Biological Activities for a Novel 1,2,3,4-Tetrahydroisoquinoline Conjugate with Dipeptide Derivatives: Insights from Molecular Docking and Molecular Dynamics Simulations. ACS Omega. 2023, 8(51), 48843-48854. [CrossRef]
- De Lucca, A.J. ; Antifungal Peptides: Potential Candidates for the Treatment of Fungal Infections. Expert Opinion on Investigational Drugs 2000, 9, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Skwarecki, A.S.; Schielmann, M., Martynow, D.; Kawczyński, M; Wiśniewska, A; Milewska, M.J.; Milewski S. Antifungal dipeptides incorporating an inhibitor of homoserine dehydrogenase. J Pept Sci. 2018, 24(1). [CrossRef]
- Saghyan, A. S.; Simonyan, H. M.; Petrosyan, S. G.; Geolchanyan, A. V.; Roviello, G. N.; Musumeci, D.; Roviello, V. Thiophenyl-substituted triazolyl-thione L-alanine: asymmetric synthesis, aggregation, and biological properties. Amino Acids, 2014, 46(3), 695–702. [CrossRef]
- Roviello, G.N.; Ricci, A.; Bucci, E.M.; Pedone, C. Synthesis, Biological Evaluation and Supramolecular Assembly of Novel Analogues of Peptidyl Nucleosides. Molecular BioSystems 2011, 7, 1773. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.N.; Gaetano, S.D.; Capasso, D.; Cesarani, A.; Bucci, E.M.; Pedone, C. Synthesis, Spectroscopic Studies and Biological Activity of a Novel Nucleopeptide with Moloney Murine Leukemia Virus Reverse Transcriptase Inhibitory Activity. Amino Acids 2009, 38, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Emri, T.; Majoros, L.; Tóth, V.; Pócsi, I. Echinocandins: production and applications. Applied Microbiology and Biotechnology, 2013, 97(8), 3267–3284. [CrossRef]
- Duncan, V.M.S.; O’Neil, D.A. Commercialization of Antifungal Peptides. Fungal Biology Reviews 2013, 26, 156–165. [Google Scholar] [CrossRef]
- Agrawal, P.; Bhalla, S.; Chaudhary, K.; Kumar, R.; Sharma, M.K.; Gajendra P., S. Raghava In Silico Approach for Prediction of Antifungal Peptides. Frontiers in Microbiology 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Frontiers in Cellular and Infection Microbiology 2020, 10. [Google Scholar] [CrossRef]
- El-Bahnsawye, M.; Hussein, M.K.A.; Elmongy, E.I.; Awad, H.M.; Tolan, A.A.E.; Moemen, Y.S.; El-Shaarawy, A.; El-Sayed, I.E. Design, Synthesis, and Antiproliferative Activity of Novel Neocryptolepine-Rhodanine Hybrids. Molecules. 2022, 27(21), 7599. [Google Scholar] [CrossRef]
- Grabeck, J.; Mayer, J.; Miltz, A.; Casoria, M.; Quagliata, M.; Meinberger, D.; Klatt, AR.; Wielert, I.; Maier, B.; Papini, AM.; Neundorf, I. Triazole-Bridged Peptides with Enhanced Antimicrobial Activity and Potency against Pathogenic Bacteria. ACS Infect Dis. 2024, 9;10(8):2717-2727. [CrossRef]
- Staśkiewicz, A.; Ledwoń, P.; Rovero, P.; Papini, A.M.; Latajka, R. Triazole-Modified Peptidomimetics: An Opportunity for Drug Discovery and Development. Frontiers in Chemistry 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Righetto, G.M.; Lopes, J.L.d.S.; Bispo, P.J.M.; André, C.; Souza, J.M.; Andricopulo, A.D.; Beltramini, L.M.; Camargo, I.L.B.d.C. Antimicrobial Activity of an Fmoc-Plantaricin 149 Derivative Peptide against Multidrug-Resistant Bacteria. Antibiotics 2023, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthi Pinelopi Apostolidou; Chrysoula Kokotidou; Platania, V.; Nikolaou, V.; Georgios Landrou; Emmanouil Nikoloudakis; Georgios Charalambidis; Chatzinikolaidou, M.; Coutsolelos, A.G.; Mitraki, A. Antimicrobial Potency of Fmoc-Phe-Phe Dipeptide Hydrogels with Encapsulated Porphyrin Chromophores Is a Promising Alternative in Antimicrobial Resistance. Biomolecules 2024, 14, 226–226. [CrossRef]
- Misra, S.; Mukherjee, S.; Ghosh, A.; Singh, P.; Mondal, S.; Ray, D.; Bhattacharya, G.; Ganguly, D.; Ghosh, A.; Aswal, V.K.; et al. Single Amino-Acid Based Self-Assembled Biomaterials with Potent Antimicrobial Activity. Chemistry - A European Journal 2021, 27, 16744–16753. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.S.; Rohane, S.H. Review on Discovery Studio: An Important Tool for Molecular Docking. Asian Journal Of Research in Chemistry 2021, 14, 1–3. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, D.; Zhou, P.; Li, B.; & Huang, S.-Y. ; & Huang, S. Nucleic Acids Research 2017, 45(W1), W365–W373. [Google Scholar] [CrossRef]
- Pasqualina Liana, Scognamiglio; Riccardi, C.; Palumbo, R.; Gale, T.F.; Musumeci, D.; Roviello, G.N. Pasqualina Liana Scognamiglio; Riccardi, C.; Palumbo, R.; Gale, T.F.; Musumeci, D.; Roviello, G.N. Self-Assembly of Thyminyl L-Tryptophanamide (TrpT) Building Blocks for the Potential Development of Drug Delivery Nanosystems. Journal of nanostructure in chemistry 2023. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Zou, X. An Iterative Knowledge-Based Scoring Function for Protein-Protein Recognition. Proteins: Structure, Function, and Bioinformatics 2008, 72, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Sargsyan, T.H.; Stepanyan, L.A.; Israyelyan, M.H.; Gasparyan, A.A.; Saghyan, A.S.The Synthesis and in vitro Study of 9-fluorenylmethoxycarbonyl Protected Non-Protein Amino Acids Antimicrobial Activity Eurasian Chemico-Technological Journal, 2023, 25(4), 235–240. [CrossRef]
- Sargsyan, A.; Hakobyan, H.; Mardiyan, Z.; Jamharyan, S.; Dadayan, A.; Sargsyan, T.; Hovhannisyan, N. Modeling, Synthesis, and In Vitro Screening of Unusual Amino Acids and Peptides as Protease Inhibitors. J. Chem. Technol. Metall. 2023; 58, 3. [Google Scholar] [CrossRef]
- Hargrove, T.Y.; Garvey, E.P.; Hoekstra, W.J.; Yates, C.M.; Wawrzak, Z.; Rachakonda, G.; Villalta, F.; Lepesheva, G.I. Crystal Structure of the New Investigational Drug Candidate VT-1598 in Complex with Aspergillus Fumigatus Sterol 14α-Demethylase Provides Insights into Its Broad-Spectrum Antifungal Activity. Antimicrobial Agents and Chemotherapy, 2017, 61(7). [Google Scholar] [CrossRef]
- Sagatova, A.A.; Keniya, M.V.; Wilson, R.K.; Monk, B.C.; Tyndall, J.D.A. Structural Insights into Binding of the Antifungal Drug Fluconazole to Saccharomyces Cerevisiae Lanosterol 14α-Demethylase. Antimicrobial Agents and Chemotherapy. 2015, 59, 4982–4989. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, E.; Zhang, Y.; Huang, S.-Y.; Xiao, Y. HDOCK Update for Modeling Protein- RNA / DNA Complex Structures. 2022, 31(11),:e4441. [CrossRef]
- Roviello, V.; Musumeci, D.; Mokhir, A.; Roviello, G.N. Evidence of Protein Binding by a Nucleopeptide Based on a Thymine-Decorated L-Diaminopropanoic Acid through CD and In Silico Studies. Curr. Med. Chem. 2021, 28(24), 5004–5015. [Google Scholar] [CrossRef]
- Roviello, G.N.; Ricci, A.; Bucci, E.M.; Pedone, C. Synthesis, Biological Evaluation, and Supramolecular Assembly of Novel Analogues of Peptidyl Nucleosides. Mol. Biosyst. 2011, 7(5), 1773–1778. [Google Scholar] [CrossRef]
- Hughes, A.B. (Ed.). Amino Acids, Peptides and Proteins in Organic Chemistry: Building Blocks, Catalysis and Coupling Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, 2011. Print ISBN: 9783527321025; Online ISBN: 9783527631803. [CrossRef]
- Warrilow, A.G.S.; Melo, N.; Martel, C.M.; Parker, J.E.; Nes, W.D.; Kelly, S.L.; Kelly, D.E. Expression, Purification, and Characterization of Aspergillus fumigatus Sterol 14-Alpha Demethylase (CYP51) Isoenzymes A and B. Antimicrob. Agents Chemother. 2010, 54(10), 4225–4234. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Lockhart, S.R.; Najvar, L.K.; Berkow, E.L.; Jaramillo, R.; Olivo, M.; Garvey, E.P.; Yates, C.M.; Schotzinger, R.J.; Catano, G.; Patterson, T.F. The Fungal Cyp51-Specific Inhibitor VT-1598 Demonstrates In Vitro and In Vivo Activity against Candida auris. Antimicrob. Agents Chemother. 2019, 63(3), e02233–18. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Gresnigt, M.S.; Romani, L.; Netea, M.G.; Latgé, J.-P. Aspergillus fumigatus Morphology and Dynamic Host Interactions. Nat. Rev. Microbiol. 2017, 15(11), 661–674. [Google Scholar] [CrossRef]
- De Hoog, G.S.; Horré, R. Molecular Taxonomy of the Alternaria and Ulocladium Species from Humans and Their Identification in the Routine Laboratory. Mycoses 2002, 45(8), 259–276. [Google Scholar] [CrossRef] [PubMed]
- Zalar, P.; Gostincar, C.; de Hoog, G.S.; Ursic, V.; Sudhadham, M.; Gunde-Cimerman, N. Redefinition of Aureobasidium pullulans and Its Varieties. Stud. Mycol. 2008, 61, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, J.A.; Hu, C.; Aitken, S.L.; Beyda, N. Antifungal Resistance: A Concerning Trend for the Present and Future. Curr. Infect. Dis. Rep. 2019, 21(12), 47. [Google Scholar] [CrossRef] [PubMed]
| Species | Aspergillus-related conditions | Clinical Manifestations | Affected Systems/Organs |
|---|---|---|---|
| Birds | Aspergillosis | Respiratory distress, weight loss, lethargy | Primarily lungs (respiratory system) |
| Cattle | Mycotic abortion | Abortion or stillbirth, particularly in late pregnancy | Uterus, placenta |
| Horses | Laryngeal pouch mycosis | Respiratory issues, coughing, nasal discharge | Laryngeal pouch, respiratory system |
| Mycotic keratitis | Eye infections, corneal ulcers, vision impairment | Eyes (cornea) | |
| Dogs | Nasal/paranasal aspergillosis | Nasal discharge, facial pain, sinus issues | Nasal and paranasal tissues |
| Intervertebral space infection | Neurological symptoms (e.g., ataxia, paralysis) | Spine (intervertebral spaces) | |
| Renal aspergillosis | Kidney dysfunction, potential kidney failure | Kidneys | |
| Cats | Sinonasal, sino-orbital aspergillosis | Respiratory distress, nasal discharge, eye problems (e.g., conjunctivitis, exophthalmos) | Sinuses, orbital region, lungs (pulmonary system) |
| Concentration of test compounds | Names and numbers of strains according to the Microbial Depository Center | ||||||
|---|---|---|---|---|---|---|---|
| Aspergillus versicolor 12134 | A.flavus 10567 | A.candidus 10711 | Alternaria alternata 8126 |
Ulocladium botrytis 12027 |
Aureobasidium pullulans 8269 |
||
| Fluconazole | 89 μM | + | + | - | ++ | ++ | +++ |
| 183 μM | + | ++ | + | ++ | +++ | +++ | |
| 278 μM | ++ | ++ | + | +++ | +++ | +++ | |
| 2a | 89 μM | - | - | - | - | - | - |
| 183 μM | + | - | - | + | - | + | |
| 278 μM | + | + | - | + | + | + | |
| 3a | 89 μM | ++ | + | - | - | + | - |
| 183 μM | ++ | + | + | + | + | + | |
| 278 μM | ++ | ++ | ++ | ++ | ++ | ++ | |
| 7a | 89 μM | + | + | + | + | + | - |
| 183 μM | ++ | ++ | + | ++ | ++ | - | |
| 278 μM | ++ | ++ | ++ | ++ | ++ | - | |
| 2b | 89 μM | - | - | - | - | - | - |
| 183 μM | + | + | - | - | - | - | |
| 278 μM | + | + | + | + | + | - | |
| 3b | 89 μM | + | + | - | + | + | - |
| 183 μM | + | + | + | + | + | + | |
| 278 μM | - | ++ | + | ++ | ++ | ++ | |
| 7b | 89 μM | - | + | - | - | - | - |
| 183 μM | - | + | + | - | - | - | |
| 278 μM | + | ++ | + | + | + | - | |
| Aspergillus Species | Compound | Inhibition at 555 μM (%) | IC50 (μM) | SD |
|---|---|---|---|---|
| Aspergillus versicolor (12134) | fluconazole | 95 | 254.01 | 0.05 |
| 3a | 90 | - | 0.09 | |
| 7a | 97 | 169.94 | 0.09 | |
| Aspergillus flavus (10567) | fluconazole | 85 | 184.64 | 0.09 |
| 3a | 80 | 267.86 | 0.06 | |
| 7a | 95 | 176.69 | 0.1 | |
| Aspergillus candidus (10711) | fluconazole | 85 | 476.20 | 0.08 |
| 3a | 70 | 240.35 | 0.04 | |
| 7a | 95 | 248.94 | 0.04 |
| Compound | HDOCK Score (Top 1 Pose) | HDOCK Score (Avg. Top 1-3 ± SD) | Receptor Interface Residues for the Top 1 Poses (residue—ligand distance/Å) | Common Residues |
|---|---|---|---|---|
| Fluconazole | -193.30 | -183.27 ± 11.13 | TYR 122 (3.460 Å), LEU 125 (4.013 Å), THR 126 (3.484 Å), PHE 130 (3.176 Å), VAL 135 (3.295 Å), TYR 136 (3.261 Å), ALA 307 (2.987 Å), GLY 308 (4.884 Å), SER 311 (3.536 Å), ILE 373 (3.680 Å), LEU 503 (4.080 Å), PHE 504 (2.951 Å), HEM 580 (2.982 Å) | TYR 122, LEU 125, THR 126, PHE 130, VAL 135, TYR 136, ALA 307, GLY 308, SER 311, ILE 373, LEU 503, PHE 504, HEM 580 |
| 3a | -207.97 | -192.63 ± 11.86 | TYR 68 (2.876 Å), LEU 91 (3.496 Å), VAL 121 (3.717 Å), TYR 122 (1.493 Å), LEU 125 (2.678 Å), THR 126 (1.979 Å), TYR 136 (4.045 Å), PHE 229 (4.538 Å), PHE 234 (2.725 Å), ALA 307 (2.652 Å), GLY 308 (4.381 Å), SER 311 (3.302 Å), ILE 373 (2.803 Å), HIS 374 (3.010 Å), SER 375 (3.111 Å), ILE 376 (3.278 Å), ILE 377 (3.117 Å), ARG 378 (4.992 Å), ASN 398 (4.823 Å), TYR 500 (4.472 Å), SER 502 (4.805 Å), LEU 503 (2.285 Å), PHE 504 (3.955 Å), HEM 580 (3.095 Å) | TYR 122, LEU 125, THR 126, TYR 136, ALA 307, GLY 308, SER 311, ILE 373, LEU 503, PHE 504, HEM 580 |
| 7a | -206.62 | -190.42 ± 11.51 | THR 65 (3.017 Å), ILE 66 (3.865 Å), TYR 68 (3.957 Å), GLY 69 (2.937 Å), ILE 70 (3.821 Å), LEU 91 (2.091 Å), LEU 92 (3.069 Å), GLY 93 (3.464 Å), LYS 94 (2.725 Å), THR 96 (4.979 Å), TYR 122 (4.669 Å), PRO 231 (3.321 Å), ILE 232 (3.429 Å), PHE 234 (2.594 Å), MET 235 (2.112 Å), ILE 373 (4.311 Å), HIS 374 (3.240 Å), SER 375 (2.883 Å), ILE 377 (3.910 Å), TYR 500 (2.672 Å), SER 501 (3.521 Å), SER 502 (2.803 Å), LEU 503 (3.029 Å), PHE 504 (4.955 Å) | TYR 122, ILE 373, LEU 503, PHE 504 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





