Submitted:
18 December 2024
Posted:
18 December 2024
You are already at the latest version
Abstract
The effects of hexanal supplementation in the storage atmosphere of ‘Fuji Kiku’ apples were in-vestigated. Contents of volatile compounds (VC) in the headspace emitted by apple fruit during cold storage and in the headspace of apple fruit and juice during shelf life were determined. Hexanal treatment during storage significantly affected the VC profile by stimulating the pro-duction or retention of key esters, including hexyl acetate, ethyl acetate, and butyl 2-methylbutanoate, during cold storage. Supplementation of hexanal also increased the produc-tion of linear esters, especially hexyl acetate, and promoted the formation of branched esters such as ethyl 2-methylbutanoate and hexyl 2-methylbutanoate during shelf life. Hexanal also increased the alcohol concentrations, with a significant increase in hexanol and 2-pentanol. Partial least squares discriminant analysis showed clear separation between control and hexanal-treated samples, with compounds like butyl hexanoate and 2-methyl-1-butanol being the most influential. Apple juice extracted from the flesh of hexanal-treated apples exhibited higher concentrations of key VCs, including 2-methylbutyl acetate, hexyl acetate, and 2-methyl-1-butanol. No significant differences in firmness were observed, however, hexanal showed an inhibitory effect on color development of fruit. This study highlights the potential of hexanal in influencing aroma-related compounds and provides insight into strategies to improve postharvest aroma in apples.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material, Treatments, and Storage
2.2. Flesh Firmness
2.3. Colour Measurements
2.4. Extraction of the VCs from the Apple Fruit Headspace in the Storage Chambers During the Cold Storage
2.5. Extraction of VCs from the Apple Fruit Headspace Durning Shelf Life
2.6. Extraction of VCs from the Apple Juice Headspace Durning Shelf Life
2.7. Determination of VCs
2.8. Data Analysis
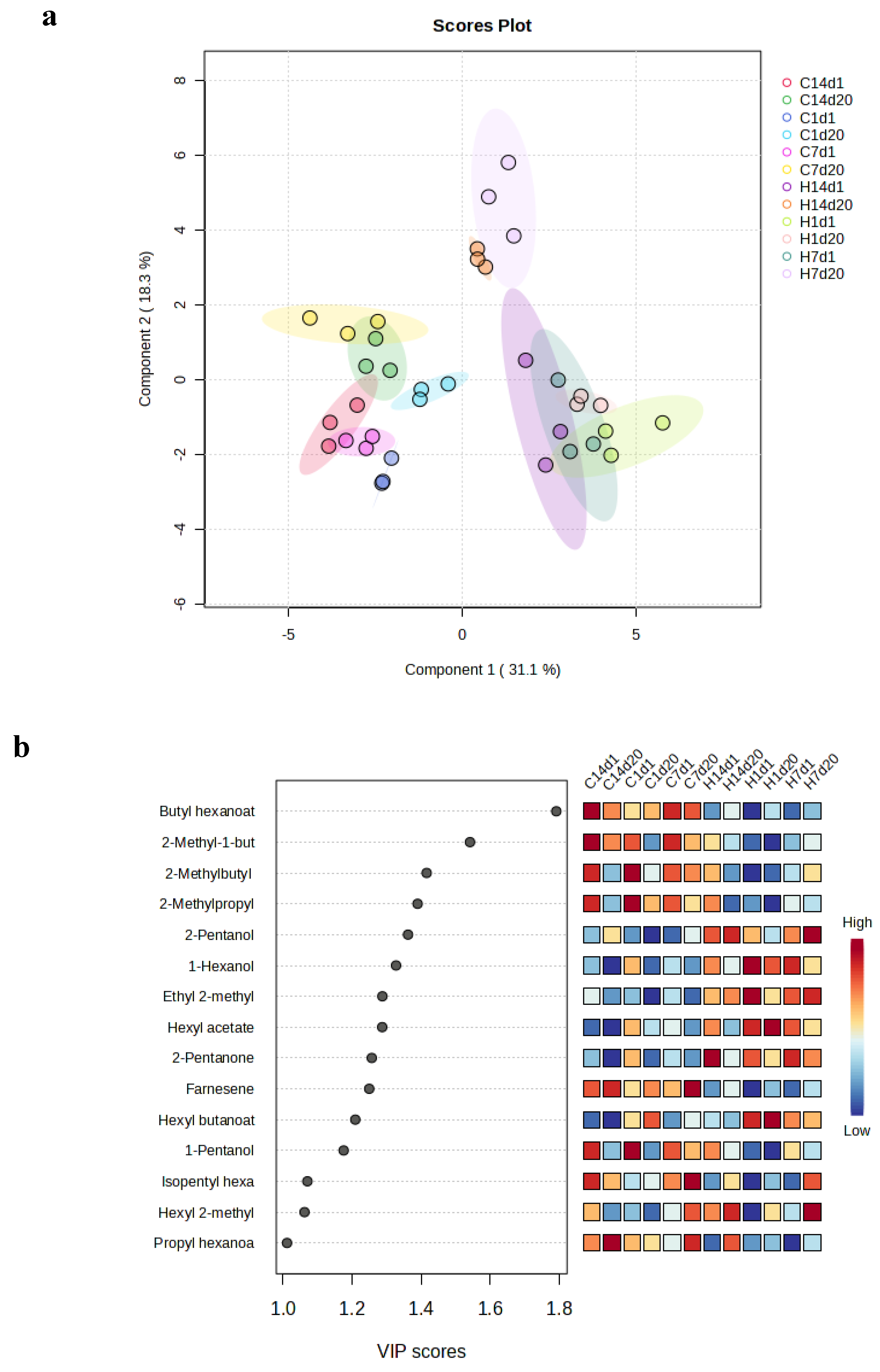
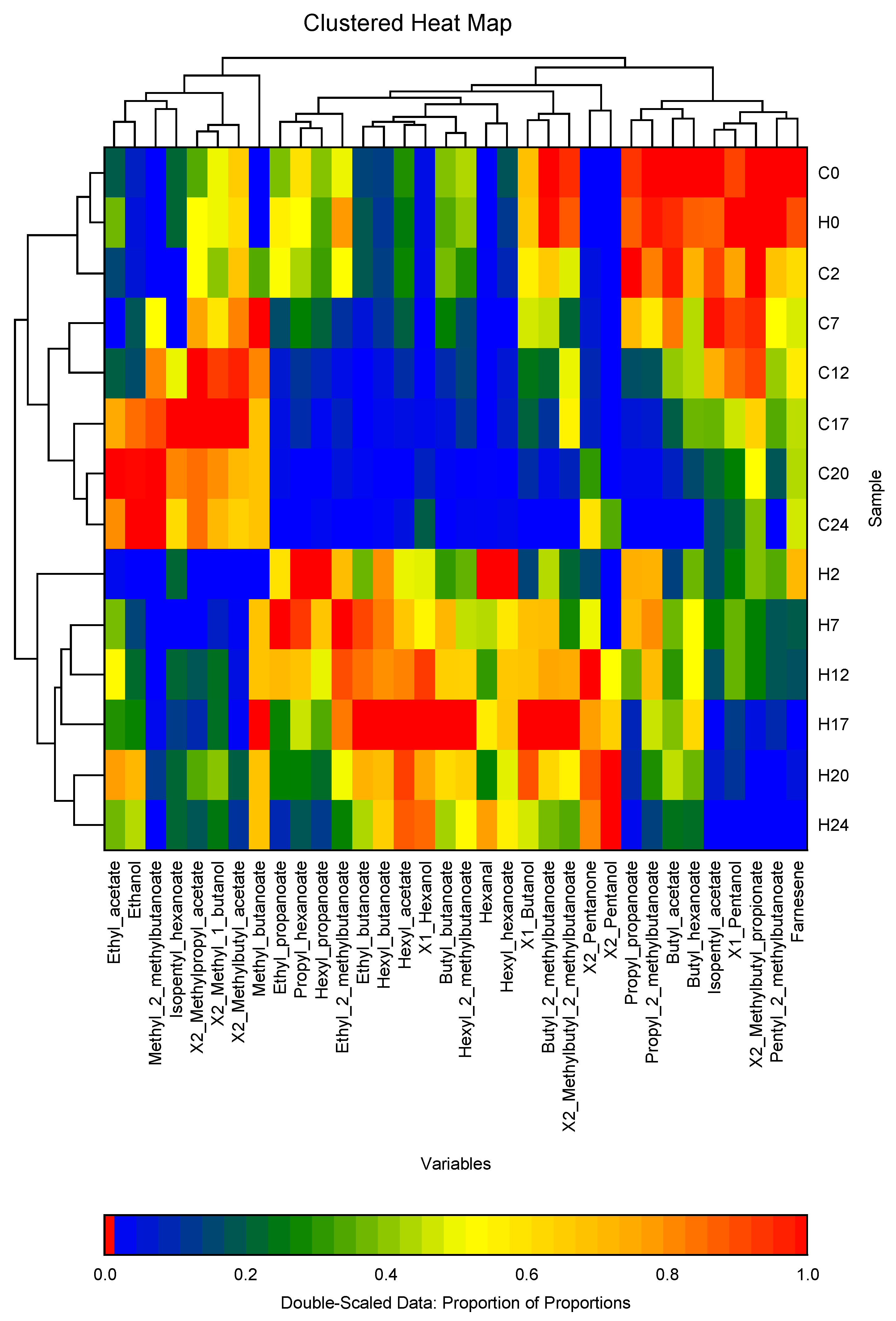
3. Results
3.1. Flesh Firmness and Colour
| Control | Hexanal | |
|---|---|---|
| L | 75,08 ± 2,40 | 75,51 ± 2,64 |
| a* | -4,42 ± 3,65 | -6,35 ±2,86 |
| b* | 45,55 ± 1,99 | 44,39 ± 2,29 |
| ΔL | 2,24 ± 1,97 | |
| Δa* | 2,99 ± 2,10 | |
| Δb* | 1,16 ± 2,01 | |
| ΔE | 5,62 ± 2,95 | |
| Firmness (kg/cm2) | 6,92± 0,71 | 6,59 ± 0,66 |
3.2. VCs Profile from the from the Apple Fruit Headspace in the storage Chambers During the Cold Storage
3.3. VCs Profile from the from the Apple Fruit Headspace During the Shelf Life
| Plus 1 day of shelf life | Plus 7 days of shelf life | Plus 14 days of shelf life | ||||||||||||||||||||||
| +1 °C | +20 °C | +1 °C | +20 °C | +1 °C | +20 °C | |||||||||||||||||||
| Control | Hexanal | Control | Hexanal | Control | Hexanal | Control | Hexanal | Control | Hexanal | Control | Hexanal | |||||||||||||
| VC (OT (mg/L)) | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd | avg | sd |
| Ethyl acetate (3280a) | /a | / | 0.09b | 0.01 | /a | / | 0.18b | 0.16 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / |
| Ethyl butanoate (9b) | /a | / | 0.11b | 0.07 | /a | / | 0.08b | 0.01 | / | / | 0.05 | 0.04 | / | / | 0.01 | 0.01 | / | / | 0.02 | 0.01 | / | / | 0.01 | 0.00 |
| Propyl propanoate (880a) | 0.02 | 0.01 | 0.06 | 0.06 | 0.13 | 0.03 | 0.17 | 0.01 | / | / | 0.06 | 0.06 | 0.03 | 0.02 | 0.02 | 0.02 | / | / | 0.03 | 0.03 | 0.03 | 0.02 | 0.06 | 0.00 |
| Butyl acetate (66a) | 0.26a | 0.06 | 0.97b | 0.03 | 0.46a | 0.06 | 5.71b | 0.23 | 0.15a | 0.01 | 1.21b | 0.62 | 0.08 | 0.03 | 0.13 | 0.07 | 0.07 | 0.01 | 0.81 | 0.52 | 0.06 | 0.03 | 0.18 | 0.01 |
| Butyl butanoate (100c) | 0.02a | 0.00 | 0.21b | 0.11 | 0.20a | 0.03 | 0.87b | 0.07 | 0.02 | / | 0.21 | 0.14 | 0.03 | 0.01 | 0.04 | 0.03 | 0.01 | / | 0.11 | 0.09 | 0.03 | 0.01 | 0.08 | 0.01 |
| Hexyl acetate (2c) | 0.93a | 0.17 | 7.07b | 2.06 | 2.25a | 0.19 | 28.56b | 1.24 | 0.38a | 0.04 | 6.26b | 1.34 | 0.40a | 0.07 | 1.74b | 0.44 | 0.14a | 0.02 | 3.03b | 1.58 | 0.12a | 0.05 | 1.55b | 0.29 |
| Propyl hexanoate (nf) | 0.01 | 0.00 | 0.01 | 0.01 | 0.04 | 0.01 | 0.04 | 0.01 | 0.01 | / | 0.01 | 0.01 | 0.03 | 0.01 | 0.02 | 0.03 | 0.01 | 0.01 | / | / | 0.03 | 0.02 | 0.07 | 0.01 |
| Hexyl propanoate (8e) | 0.09 | 0.02 | 0.10 | 0.02 | 0.91a | 0.21 | 2.11b | 0.49 | 0.03 | 0.01 | 0.11 | 0.06 | 0.06a | 0.02 | 0.22b | 0.08 | 0.02 | / | 0.05 | 0.03 | 0.02a | 0.01 | 0.25b | 0.02 |
| Butyl hexanoate (700b) | 0.05 | 0.01 | 0.04 | 0.01 | 0.30 | 0.07 | 0.33 | 0.03 | 0.07a | 0.01 | 0.04b | 0.01 | 0.10 | 0.04 | 0.10 | 0.10 | 0.05 | 0.02 | 0.03 | 0.01 | 0.08 | 0.04 | 0.31 | 0.37 |
| Hexyl butanoate (250b) | 0.05a | 0.01 | 0.41b | 0.09 | 0.54a | 0.24 | 2.28b | 0.21 | 0.03a | / | 0.23b | 0.10 | 0.08 | 0.03 | 0.30 | 0.20 | 0.02 | / | 0.09 | 0.07 | 0.03 | 0.01 | 0.19 | 0.02 |
| Hexyl hexanoate (6400a) | 0.14 | 0.03 | 0.22 | 0.10 | 0.76a | 0.37 | 1.89b | 0.12 | 0.10a | 0.01 | 0.15b | 0.01 | 0.19 | 0.08 | 0.58 | 0.38 | 0.07 | 0.01 | 0.08 | 0.01 | 0.07 | 0.03 | 0.27 | 0.02 |
| Total linear estres | 1.56a | 0.15 | 9.29b | 1.91 | 5.58a | 1.08 | 42.23b | 1.41 | 0.79a | 0.06 | 8.33b | 2.12 | 1.01a | 0.32 | 3.16b | 0.96 | 0.39a | 0.03 | 4.25b | 2.28 | 0.46 | 0.21 | 3.14 | 0.21 |
| Methyl 2-methylbutanoate (0.048d) | / | / | / | / | 0.01a | 0.00 | /b | / | / | / | / | / | 0.04 | 0.02 | 0.09 | 0.08 | / | / | / | / | 0.04 | 0.02 | 0.32 | 0.49 |
| 2-Methylpropyl acetate (25a) | 0.06a | 0.01 | 0.02b | 0.01 | 0.09a | 0.01 | 0.05b | 0.01 | 0.04 | 0.00 | 0.04 | 0.01 | 0.03 | 0.01 | 0.04 | 0.01 | 0.03 | 0.00 | 0.03 | 0.01 | 0.01 | 0.00 | 0.07 | 0.00 |
| Ethyl 2-methylbutanoate (0.13f) | /a | / | 0.19b | 0.08 | /a | / | 0.12b | 0.03 | / | / | 0.08 | 0.07 | / | / | 0.21 | 0.36 | / | / | 0.03 | 0.03 | / | / | 0.14 | 0.03 |
| 2-Methylbutyl acetate (11c) | 9.30a | 1.67 | 4.57b | 1.59 | 19.20 | 0.66 | 18.64 | 0.99 | 7.26 | 1.34 | 7.91 | 0.53 | 7.77 | 1.80 | 11.84 | 2.47 | 5.23 | 0.94 | 6.90 | 0.98 | 3.35a | 0.99 | 12.65b | 1.16 |
| Propyl 2-methylbutanoate (0.02d) | 0.02 | 0.00 | 0.17 | 0.11 | 0.07a | 0.01 | 0.30b | 0.05 | 0.01 | 0.00 | 0.18 | 0.15 | 0.07 | 0.03 | 0.05 | 0.05 | 0.01 | 0.01 | 0.10 | 0.09 | 0.14 | 0.09 | 0.26 | 0.04 |
| Isopentyl acetate (7.2d) | 0.04a | 0.00 | 0.03b | 0.00 | 0.13a | 0.01 | 0.21b | 0.02 | 0.02a | 0.00 | 0.05b | 0.01 | 0.03 | 0.01 | 0.04 | 0.01 | 0.01 | 0.00 | 0.03 | 0.02 | 0.01 | 0.01 | 0.05 | 0.00 |
| 2-Methylbutyl propionate (nf) | 0.03a | 0.02 | /b | / | 0.65a | 0.12 | 0.13b | 0.03 | 0.04a | 0.01 | 0.01b | 0.01 | 0.20 | 0.05 | 0.23 | 0.09 | 0.02a | 0.01 | /b | / | 0.08a | 0.03 | 0.32b | 0.02 |
| Butyl 2-methylbutanoate (17b) | 0.05a | 0.01 | 0.26b | 0.09 | 0.26a | 0.06 | 0.94b | 0.14 | 0.07 | 0.01 | 0.32 | 0.19 | 0.19 | 0.09 | 0.29 | 0.23 | 0.05 | 0.01 | 0.22 | 0.15 | 0.25 | 0.14 | 0.73 | 0.16 |
| 2-Methylbutyl 2-methylbutanoate (nf) | 0.07 | 0.03 | 0.12 | 0.03 | 0.45 | 0.08 | 0.54 | 0.07 | 0.12 | 0.03 | 0.17 | 0.08 | 0.89a | 0.30 | 2.20b | 0.76 | 0.13 | 0.03 | 0.14 | 0.06 | 0.72 | 0.29 | 3.42 | 0.70 |
| Pentyl 2-methylbutanoate (nf) | 0.01a | 0.00 | /b | / | 0.06 | 0.01 | 0.02 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.07 | 0.04 | 0.10 | 0.06 | 0.01a | 0.00 | /b | 0.00 | 0.06 | 0.03 | 0.21 | 0.05 |
| Hexyl 2-methylbutanoate (22c) | 0.73a | 0.03 | 1.98b | 0.08 | 3.66a | 0.58 | 8.97b | 0.78 | 0.70a | 0.11 | 2.34b | 0.28 | 1.94a | 0.84 | 8.81b | 1.69 | 0.56a | 0.07 | 1.73b | 0.36 | 1.00a | 0.47 | 11.58b | 0.91 |
| Isopentyl hexanoate (nf) | 0.04a | 0.02 | 0.02b | 0.00 | 0.44a | 0.10 | 0.14b | 0.02 | 0.08a | 0.02 | 0.02b | 0.00 | 0.30 | 0.10 | 0.43 | 0.21 | 0.10a | 0.02 | 0.02b | 0.00 | 0.12 | 0.04 | 0.48 | 0.09 |
| Total branched estres | 10.35 | 1.76 | 7.36 | 1.27 | 25.03a | 1.22 | 30.06b | 0.52 | 8.36a | 1.49 | 11.12b | 0.24 | 11.53a | 3.17 | 24.32b | 2.89 | 6.15 | 1.07 | 9.21 | 1.65 | 5.78 | 1.87 | 29.95 | 0.79 |
| Ethanol (10000a) | 0.22 | 0.10 | 0.52 | 0.26 | 0.76 | 1.09 | 0.91 | 0.56 | 0.23 | 0.16 | 0.13 | 0.03 | 0.30 | 0.40 | 0.37 | 0.57 | 0.11 | 0.09 | 0.09 | 0.02 | 0.03 | 0.01 | 0.22 | 0.08 |
| 2-Pentanol (nf) | 0.01a | 0.00 | 0.07b | 0.00 | 0.03a | 0.01 | 0.15b | 0.03 | 0.01a | 0.00 | 0.09b | 0.01 | 0.03a | 0.01 | 0.28b | 0.05 | 0.01a | 0.00 | 0.07b | 0.00 | 0.02a | 0.00 | 0.42b | 0.02 |
| 1-Butanol (492a) | 0.25a | 0.06 | 1.82b | 0.85 | 0.71a | 0.13 | 3.33b | 0.27 | 0.15 | 0.03 | 1.65 | 0.95 | 0.14 | 0.02 | 0.20 | 0.11 | 0.05 | 0.02 | 0.94 | 0.67 | 0.08 | 0.04 | 0.35 | 0.03 |
| 2-Methyl-1-butanol (1200a) | 7.76 | 0.40 | 5.35 | 0.86 | 10.28a | 2.47 | 8.97b | 0.52 | 8.08 | 1.91 | 7.89 | 1.24 | 9.56 | 1.57 | 11.22 | 1.24 | 6.89 | 0.62 | 6.97 | 1.61 | 7.66 | 1.58 | 17.60 | 1.70 |
| 1-Pentanol (150a) | 0.05 | 0.01 | 0.05 | 0.02 | 0.10 | 0.02 | 0.12 | 0.02 | 0.03a | 0.01 | 0.07b | 0.02 | 0.04 | 0.01 | 0.07 | 0.02 | 0.02a | 0.00 | 0.05b | 0.02 | 0.03 | 0.01 | 0.12 | 0.03 |
| 1-Hexanol (2500a) | 0.74a | 0.16 | 7.31b | 0.44 | 1.01a | 0.17 | 12.84b | 0.69 | 0.29a | 0.10 | 4.65b | 0.83 | 0.35a | 0.09 | 1.85b | 0.02 | 0.14 | 0.03 | 2.25 | 0.89 | 0.13a | 0.04 | 2.59b | 0.00 |
| Total alcohols | 9.03a | 0.35 | 15.12b | 1.99 | 12.89 | 3.64 | 26.31 | 0.83 | 8.78 | 2.00 | 14.47 | 2.94 | 10.42a | 1.87 | 13.99b | 0.49 | 7.22 | 0.73 | 10.37 | 3.20 | 7.94 | 1.51 | 20.81 | 0.64 |
| 2-Pentanone (2300d) | 0.03a | 0.01 | 0.19b | 0.02 | 0.03a | 0.01 | 0.30b | 0.13 | 0.02a | 0.00 | 0.17b | 0.02 | 0.01a | 0.01 | 0.13b | 0.05 | 0.01a | / | 0.13b | 0.02 | /a | / | 0.17b | 0.01 |
| Hexanal (5a) | /a | / | 11.72b | 2.34 | /a | / | 0.67b | 0.10 | /a | / | 0.55b | 0.06 | / | / | / | / | /a | / | 0.14b | 0.01 | / | / | / | / |
| α -Farnesene (87a) | 1.33a | 0.02 | 0.21b | 0.00 | 9.63a | 0.32 | 1.92b | 0.26 | 1.21 | 0.20 | 0.24 | 0.01 | 6.79 | 1.32 | 4.30 | 1.91 | 1.00a | 0.05 | 0.18b | 0.03 | 4.20 | 1.51 | 7.01 | 4.95 |
| Total other | 1.36a | 0.01 | 12.13b | 2.36 | 9.66a | 0.33 | 2.89b | 0.15 | 1.22 | 0.20 | 0.96 | 0.08 | 6.80 | 1.32 | 4.43 | 1.95 | 1.01a | 0.05 | 0.44b | 0.05 | 4.21 | 1.51 | 6.45 | 4.96 |
3.4. VCs from the Apple Juice Headspace Durning Shelf Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| RT | ION | |
| Ethyl Acetate | 10.04 | 61.0, 70.1, 88.1 |
| Ethanol | 11.81 | 31.1. 45.1, 43.0 |
| Ethyl propanoate | 12.78 | 102.1. 75.00, 87.1 |
| Propyl acetate | 13.49 | 61.10, 73.10 |
| 2-Pentanone | 13.682 | 86.1, 71.1, 58.1 |
| Methyl butanoate | 13.97 | 74.0, 87.10, 58.90 |
| Methyl 2-methylbutanoate | 14.92 | 88.1, 101.10, 69.00 |
| 2-Methylpropyl acetate | 15.04 | 73.00, 61.10, 101.10 |
| Ethyl butanoate | 16.04 | 71.10, 88.10, 101.00 |
| Propyl propanoate | 16.37 | 75.00, 86.90, 59.10 |
| Ethyl 2-methylbutanoate | 16.66 | 102.10, 85.10, 115.10 |
| Butyl acetate | 17.6 | 73.10, 61.10, 87.10 |
| Hexanal | 18.12 | 72.10, 82.00, 67.10 |
| 2-Pentanol | 19.29 | 73.10, 87.00, 40.90 |
| 2-Methylbutyl acetate | 19.64 | 70.10, 61.10, 85.10 |
| Propyl 2-methylbutanoate | 20.26 | 85.10, 103.10, 74.10 |
| 1-Butanol | 20.4 | 56.10, 41.10, 75.10 |
| Isopentyl acetate | 21.76 | 70.10, 61.00, 73.10 |
| 2-Methylbutyl propionate | 22.32 | 70.1, 87.00, 75.10 |
| 3-Hexanol | 22.36 | 59.10, 73.10, 55.10 |
| 2-Methyl-1-butanol | 22.88 | 70.10, 57.10, 53.10 |
| Butyl butanoate | 23.49 | 71.10, 89.10, 101.1 |
| Trans-2-hexenal | 23.94 | 69.10, 83.10, 98.20 |
| Butyl 2-methylbutanoate | 23.99 | 103.1, 85.10, 130.00 |
| 1-Pentanol | 24.57 | 55.10, 70.10, 31.10 |
| Hexyl acetate | 25.6 | 84.10, 69.10, 101.10 |
| 2-Methylbutyl 2-methylbutanoate | 25.84 | 70.10, 85.00, 103.10 |
| 2-Heptanol | 27.05 | 83.10, 70.10, 98.10 |
| Propyl hexanoate | 27.3 | 99.10, 117310, 61.00 |
| 2-Methyl-2-butenol | 27.34 | 71.10, 86.10, 53.10 |
| Pentyl 2-methylbutanoate | 27.61 | 103.00, 85.10, 70.10 |
| Hexyl propanoate | 28.06 | 75.10, 84.20, 69.10 |
| 1-Hexanol | 28.41 | 56.10, 69.10, 84.10 |
| Butyl hexanoate | 30.67 | 117.10, 99.10, 71.10 |
| Hexyl butanoate | 30.76 | 89.10, 84.10, 71.10 |
| Hexyl 2-methylbutanoate | 31.11 | 103.10, 85.10, 74.10 |
| Heptanol | 31.96 | 70.1, 56.20, 83.10 |
| Isopentyl hexanoate | 32.01 | 70.10, 99.10, 117.10 |
| 6-Methyl-5-hepten-2-ol | 32.2 | 95.10, 69.10, 111.10 |
| Ethylhexanol | 33.09 | 57.2, 83.20, 112.20 |
| Hexyl hexanoate | 37.11 | 117.10, 99.10, 84.10 |
| α-Farnesene | 41.33 | 93.10, 107.10, 119.10 |
References
- Fedrigotti, V. B., & Fischer, C. (2020). Why per capita apple consumption is falling: Insights from the literature and case evidence from south tyrol. Horticulturae, 6(4), 1–22. [CrossRef]
- Espino-Díaz, M., Sepúlveda, D. R., González-Aguilar, G., & Olivas, G. I. (2016). Biochemistry of apple aroma: A review. Food Technology and Biotechnology, 54(4).
- Mostafa, S., Wang, Y., Zeng, W., & Jin, B. (2022). Floral Scents and Fruit Aromas: Functions, Compositions, Biosynthesis, and Regulation. In Frontiers in Plant Science (Vol. 13). Frontiers Media S.A. [CrossRef]
- Roberts, G., & Spadafora, N. D. (2020). Analysis of Apple Flavours: The Use of Volatile Organic Compounds to Address Cultivar Differences and the Correlation between Consumer Appreciation and Aroma Profiling. Journal of Food Quality, 2020. [CrossRef]
- Yang, S., Hao, N., Meng, Z., Li, Y., & Zhao, Z. (2021). Identification, comparison and classification of volatile compounds in peels of 40 apple cultivars by hs–spme with gc–ms. Foods, 10(5). [CrossRef]
- Chen, J., Zhang, D., Mi, H., Pristijono, P., Ge, Y., Lv, J., Li, Y., & Liu, B. (2022). Tissue-Specific Recovery Capability of Aroma Biosynthesis in ‘Golden Delicious’ Apple Fruit after Low Oxygen Storage. Agronomy, 12(11), 2794. [CrossRef]
- Ashitha, G. N., Sunny, A. C., & Nisha, R. (2020). Effect of Pre-harvest and Post-harvest Hexanal Treatments on Fruits and Vegetables: A Review. Agricultural Reviews, OF. [CrossRef]
- FDA. (2019). NOF/\LAB Laboratories voe (Volatile organic compounds).
- Sulaimankhil, Z., Sethi, S., Sharma, R. R., Verma, M. K., & Bhowmik, A. (2021a). Influence of hexanal concentration and exposure time on quality of cold stored apples (Malus domestica). In Indian Journal of Agricultural Sciences (Vol. 91, Issue 5). [CrossRef]
- Vasil’ev, V. G., Bykova, T. A., & Lebedev, B. V. (1991). Thermodynamics of hexanal at 0-330K. 65(1), 51–54.
- Yumbya, P., Ambuko, J., Hutchinson, M., Owino, W., Juma, J., Machuka, E., & Mutuku, J. M. (2021). Transcriptome analysis to elucidate hexanal’s mode of action in preserving the post-harvest shelf life and quality of banana fruits (Musa acuminata). Journal of Agriculture and Food Research, 3. [CrossRef]
- Ranjan, S., Chandrasekaran, R., Paliyath, G., Lim, L. T., & Subramanian, J. (2020). Effect of hexanal loaded electrospun fiber in fruit packaging to enhance the post harvest quality of peach. Food Packaging and Shelf Life, 23. [CrossRef]
- Fan, L., Song, J., Beaudry, R. M., & Hildebrand, P. D. (2006). Effect of hexanal vapor on spore viability of Penicillin expansum, lesion development on whole apples and fruit volatile biosynthesis. Journal of Food Science, 71(3). [CrossRef]
- Song, J., & Bangerth, F. (2003). Fatty acids as precursors for aroma volatile biosynthesis in pre-climacteric and climacteric apple fruit. Postharvest Biology and Technology, 30(2), 113–121. [CrossRef]
- Sulaimankhil, Z., Sethi, S., Sharma, R. R., Verma, M. K., Dahuja, A., & Bhowmik, A. (2021b). Influence of aqueous hexanal on quality of ‘Royal Delicious’ apple during cold storage. Acta Physiologiae Plantarum, 43(9).
- Nagarajan, V., Kizhaeral S, S., Subramanian, M., Rajendran, S., & Ranjan, J. (2021). Encapsulation of a Volatile Biomolecule (Hexanal) in Cyclodextrin Metal-Organic Frameworks for Slow Release and Its Effect on Preservation of Mangoes. ACS Food Science and Technology, 1(10). [CrossRef]
- Hutchinson, M. J., Ouko, J. R., Yumbya, P. M., Ambuko, J. L., Owino, W. O., & Subramanian, J. (2022). Efficacy of Hexanal Field Spray on the Postharvest Life and Quality of Papaya Fruit (Carica papaya L.) in Kenya. Advances in Agriculture, 2022. [CrossRef]
- Öz, A. T., & Kafkas, E. (2022). Volatile compositions of strawberry fruit during shelf life using pre and postharvest hexanal treatment. Journal of Food Processing and Preservation, 46(6).
- Kim, K., Chun, I. J., Suh, J. H., & Sung, J. (2023). Relationships between sensory properties and metabolomic profiles of different apple cultivars. Food Chemistry: X, 18. [CrossRef]
- Ma, N., Zhu, J., Wang, H., Qian, M. C., & Xiao, Z. (2024). Comparative Investigation of Aroma-Active Volatiles in (“Ruixue”, “Liangzhi”, “Crystal Fuji,” and “Guifei”) Apples by Application of Gas Chromatography-Mass Spectrometry-Olfactometry (GC-MS-O) and Two-Dimensional Gas Chromatography-Quadrupole Mass Spectrometry (GC × GC-qMS) Coupled with Sensory Molecular Science. Journal of Agricultural and Food Chemistry, 72(45), 25229–25250.
- Wu, X., Bi, J., & Fauconnier, M. L. (2022). Characteristic Volatiles and Cultivar Classification in 35 Apple Varieties: A Case Study of Two Harvest Years. Foods, 11(5). [CrossRef]
- Kreissl J, Mall V, Steinhaus P, Steinhaus M. (2022).Leibniz-LSB@TUM Odorant Database, Version 1.2. Leibniz Institute for Food Systems Biology at the Technical University of Munich: Freising, Germany.
- Cheema, A., Padmanabhan, P., Amer, A., Parry, M. J., Lim, L.-T., Subramanian, J., & Paliyath, G. (2017). Postharvest hexanal vapor treatment delays ripening and enhances shelf life of greenhouse grown sweet bell pepper (Capsicum annum L.). Postharvest Biology and Technology, 136, 80–89. [CrossRef]
- Cheema, A., Padmanabhan, P., Subramanian, J., Blom, T., & Paliyath, G. (2014). Improving quality of greenhouse tomato (Solanum lycopersicum L.) by pre- and postharvest applications of hexanal-containing formulations. Postharvest Biology and Technology, 95, 13–19. [CrossRef]
- Öz, A. T., Eryol, B., & Ali, M. A. (2023). Postharvest hexanal application delays senescence and maintains quality in persimmon fruit during low temperature storage. Journal of the Science of Food and Agriculture, 103(15), 7653–7663.
- Tiwari, K., & Paliyath, G. (2011). Microarray analysis of ripening-regulated gene expression and its modulation by 1-MCP and hexanal. Plant Physiology and Biochemistry, 49(3), 329–340. [CrossRef]
- Soares, C. G., Do Prado, S. B. R., Andrade, S. C. S., & Fabi, J. P. (2021). Systems biology applied to the study of papaya fruit ripening: The influence of ethylene on pulp softening. Cells, 10(9). [CrossRef]
- Sriskantharajah, K., El Kayal, W., Ayyanath, M. M., Saxena, P. K., Sullivan, A. J., Paliyath, G., & Subramanian, J. (2021). Preharvest spray hexanal formulation enhances postharvest quality in ‘Honeycrisp’ apples by regulating phospholipase d and calcium sensor proteins genes. Plants, 10(11). [CrossRef]
- Li, C. X., Zhao, X. H., Zuo, W. F., Zhang, T. L., Zhang, Z. Y., & Chen, X. Sen. (2020). The effects of simultaneous and sequential inoculation of yeast and autochthonous Oenococcus oeni on the chemical composition of red-fleshed apple cider. LWT, 124, 109184. [CrossRef]
- Zhou, X., Dong, L., Zhou, Q., Wang, J. wei, Chang, N., Liu, Z. yong, & Ji, S. juan. (2015). Effects of intermittent warming on aroma-related esters of 1-methylcyclopropene-treated “Nanguo” pears during ripening at room temperature. Scientia Horticulturae, 185, 82–89. [CrossRef]
- Zhu, D., Ren, X., Wei, L., Cao, X., Ge, Y., Liu, H., & Li, J. (2020). Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Scientia Horticulturae, 260, 108879. [CrossRef]
- Pontesegger, N., Rühmer, T., & Siegmund, B. (2023). Physicochemical Attributes, Aroma Profile, and Sensory Quality of Organic Crimson Crisp Apples after Storage. Foods, 12(9). [CrossRef]
- Dixon, J., & Hewett, E. W. (2000). Factors affecting apple aroma/flavour volatile concentration: A review. In New Zealand Journal of Crop and Horticultural Science (Vol. 28, Issue 3, pp. 155–173). [CrossRef]
- Contreras, C., & Beaudry, R. (2013). Lipoxygenase-associated apple volatiles and their relationship with aroma perception during ripening. Postharvest Biology and Technology, 82, 28–38. [CrossRef]
- Li, D., Guo, J., Ma, H., Pei, L., Liu, X., Wang, H., Chen, R., Zhao, Z., & Gao, H. (2023). Changes in the VOC of Fruits at Different Refrigeration Stages of ‘Ruixue’ and the Participation of Carboxylesterase MdCXE20 in the Catabolism of Volatile Esters. Foods, 12(10). [CrossRef]
- Ferenczi, A., Sugimoto, N., & Beaudry, R. M. (2021). Emission patterns of esters and their precursors throughout ripening and senescence in ‘Redchief Delicious’ apple fruit and implications regarding biosynthesis and aroma perception. Journal of the American Society for Horticultural Science, 146(5), 297–328. [CrossRef]
- Yang, S., Yu, J., Yang, H., & Zhao, Z. (2023). Genetic analysis and QTL mapping of aroma volatile compounds in the apple progeny ‘Fuji’ × ‘Cripps Pink.’ Frontiers in Plant Science, 14.
- Donadel, J. Z., Thewes, F. R., Anese, R. de O., Schultz, E. E., Berghetti, M. R. P., Ludwig, V., Klein, B., Cichoski, A. J., Barin, J. S., Both, V., Brackmann, A., & Wagner, R. (2019). Key volatile compounds of ‘Fuji Kiku’ apples as affected by the storage conditions and shelf life: Correlation between volatile emission by intact fruit and juice extracted from the fruit. Food Research International, 125. [CrossRef]
- Souleyre, E. J. F., Greenwood, D. R., Friel, E. N., Karunairetnam, S., & Newcomb, R. D. (2005). An alcohol acyl transferase from apple (cv. Royal Gala), MpAAT1, produces esters involved in apple fruit flavor. FEBS Journal, 272(12), 3132–3144. [CrossRef]
- Qi, W., Wang, H., Zhou, Z., Yang, P., Wu, W., Li, Z., & Li, X. (2020). Ethylene Emission as a Potential Indicator of Fuji Apple Flavor Quality Evaluation Under Low Temperature. Horticultural Plant Journal, 6(4), 231–239. [CrossRef]
- Song, J., Leepipattanawit, R., Deng, W., & Beaudry, R. M. (1996). Hexanal Vapor Is a Natural, Metabolizable Fungicide: Inhibition of Fungal Activity and Enhancement of Aroma Biosynthesis in Apple Slices. In J. AMER. SOC. HORT. SCI (Vol. 121, Issue 5). [CrossRef]
- Rowan, D. D., Allen, J. M., Fielder, S., & Hunt, M. B. (1999). Biosynthesis of straight-chain ester volatiles in Red Delicious and Granny Smith apples using deuterium-labeled precursors. Journal of Agricultural and Food Chemistry, 47(7), 2553–2562. [CrossRef]
| Plus 1 day shelf life | Plus 7 days of shelf life | |||
| Control | Hexanal | Control | Hexanal | |
| 2-Pentanone | /a | 0.04 ± 0.01b | /a | 0.01 ± 0.00b |
| 2-Methylpropyl acetate | 0.01 ± 0.01a | 0.03 ± 0.00b | / | / |
| Butyl acetate | 0.02 ± 0.03a | 0.20 ± 0.12b | / | / |
| Hexanal | 1.61 ± 0.59a | 1.89 ± 0.68a | 1.24 ± 0.46a | 1.38 ± 0.57a |
| 2-Pentanol | 0.02 ± 0.00a | 0.19 ± 0.03b | 0.02 ± 0.00a | 0.14 ± 0.02b |
| 2-Methylbutyl acetate | 2.70 ± 1.50a | 7.19 ± 1.81b | 0.99 ± 0.66a | 0.95 ± 0.36a |
| 1-Butanol | 0.04 ± 0.03a | 0.12 ± 0.12a | 0.04 ± 0.03a | 0.02 ± 0.01a |
| Isopentyl acetate | /a | 0.01 ± 0.01b | / | / |
| 2-Methylbutyl propionate | 0.01 ± 0.00a | 0.01 ± 0.00a | / | / |
| 3-Hexanol | /a | 0.04 ± 0.00b | /a | 0.02 ± 0.00b |
| 2-Methyl-1-butanol | 2.03 ± 0.70a | 5.80 ± 2.25b | 1.16 ± 0.45a | 1.25 ± 0.80a |
| trans-2-Hexenal | 0.34 ± 0.08a | 0.32 ± 0.07a | 0.29 ± 0.05a | 0.29 ± 0.12a |
| Hexyl acetate | 0.02 ± 0.01a | 0.16 ± 0.09b | /a | 0.01 ± 0.01a |
| 2-Methylbutyl 2-methylbutanoate | /a | 0.01 ± 0.00b | / | / |
| 2-Methyl-2-butenol | 0.03 ± 0.01a | 0.03 ± 0.01a | 0.02 ± 0.01a | 0.02 ± 0.01a |
| 1-Hexanol | 0.12 ± 0.06a | 0.41 ± 0.13b | 0.15 ± 0.04a | 0.18 ± 0.12a |
| Heptanol | 0.02 ± 0.00a | 0.02 ± 0.00a | 0.02 ± 0.00a | 0.02 ± 0.00a |
| 6-Methyl-5-hepten-2-ol | 0.03 ± 0.02a | 0.06 ± 0.02b | 0.01 ± 0.00a | 0.01 ± 0.00a |
| Ethylhexanol | 0.10 ± 0.03a | 0.08 ± 0.02a | 0.06 ± 0.03a | 0.04 ± 0.01a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




