Submitted:
16 December 2024
Posted:
17 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
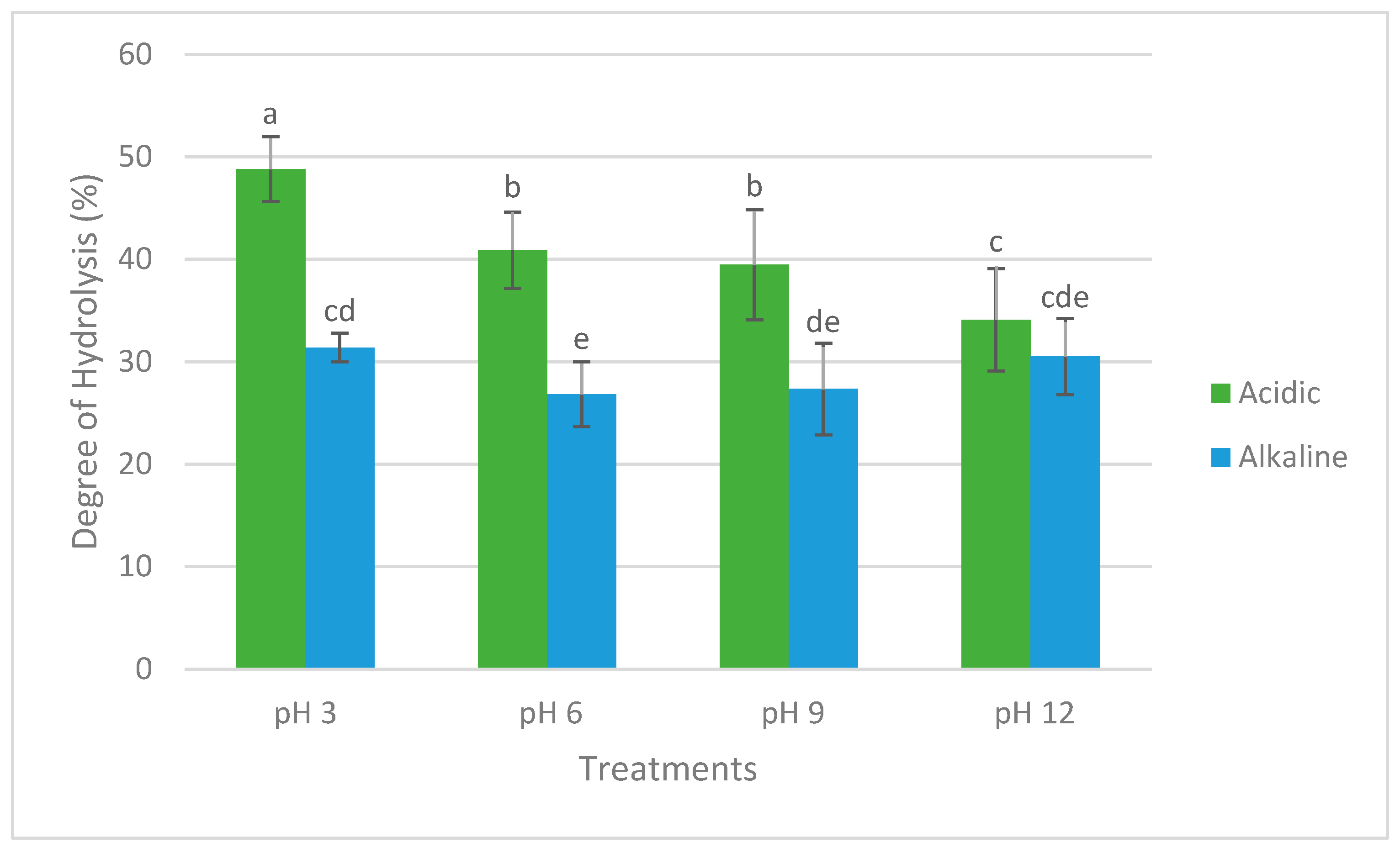
2.1. Protein Content, Protein Recovery, and Degree of Hydrolysis (DH)
2.2. Amino Acid Composition
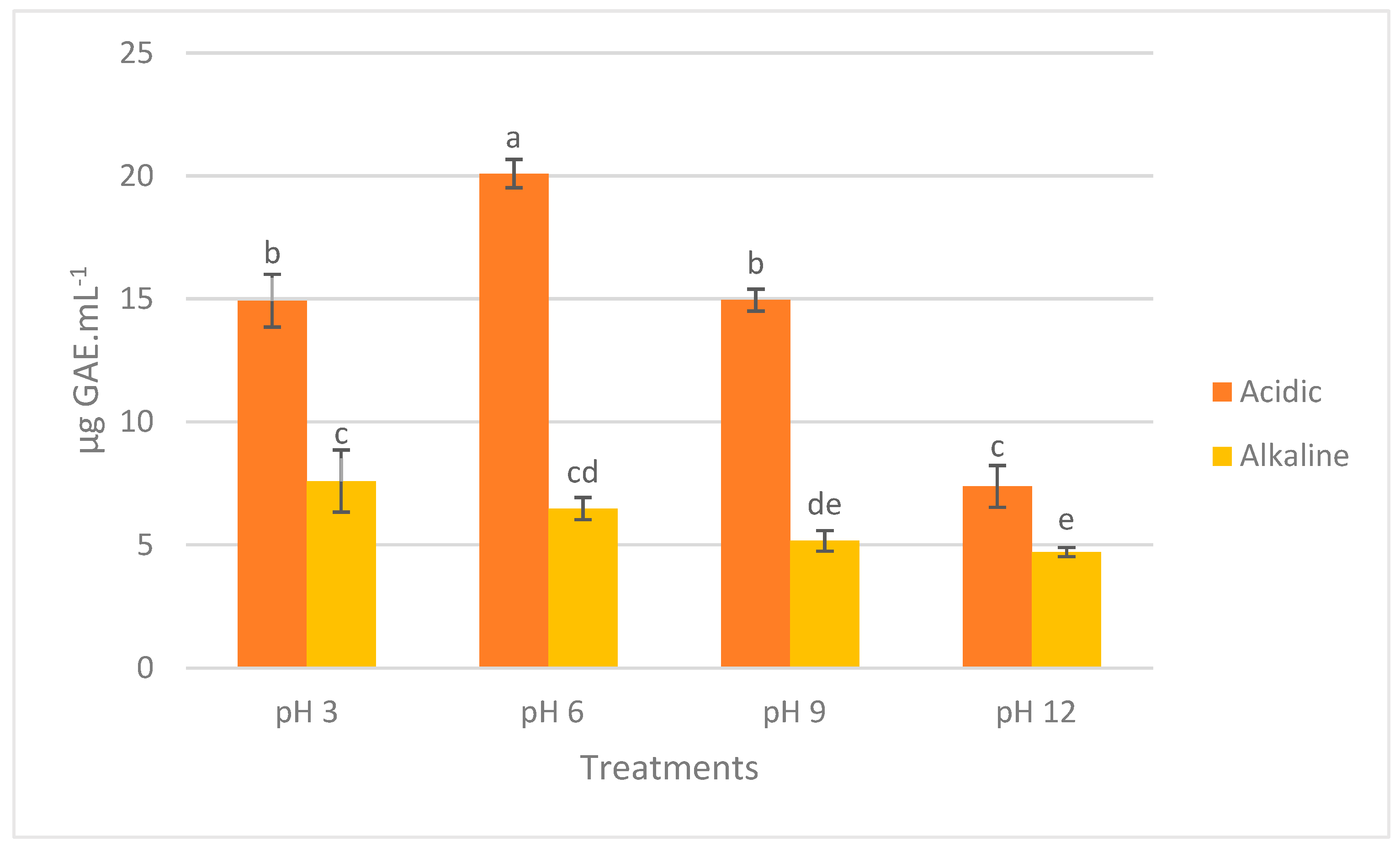
2.3. Total Phenolic Content
2.4. Antioxidant Properties
2.5. Anti-Obesity Properties
3. Discussion
3.1. Protein Content, Protein Recovery, and Degree of Hydrolysis
3.2. Amino Acid Composition
3.3. Total Phenolic Content
3.4. Antioxidant Properties
3.5. Anti-Obesity Properties
4. Materials and Methods
4.1. Seaweed Biomass Preparation
4.2. Chemicals and Enzymes
4.3. Extraction Procedure
4.5. Protein Content and Recovery
4.6. Degree of Hydrolysis (DH)
4.7. Amino Acid Profile
4.8. Total Phenolic Content (TPC)
4.9. DPPH Radical Scavenging Activity
4.10. Fe2+ Chelating Activity
4.12. Porcine Pancreatic Lipase Inhibition Activity
4.13. Porcine Pancreatic α-Amylase Inhibition Activity
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nature Reviews Drug Discovery 2021 20:9 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Lim, D.W.; Lee, J.-E.; Lee, C.; Kim, Y.T. Natural Products and Their Neuroprotective Effects in Degenerative Brain Diseases: A Comprehensive Review. International Journal of Molecular Sciences 2024, 25, 11223. [Google Scholar] [CrossRef]
- Jacobsen, C. Some Strategies for the Stabilization of Long Chain N-3 PUFA-Enriched Foods: A Review. European Journal of Lipid Science and Technology 2015, 117, 1853–1866. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Joshua Ashaolu, T.; Joshua Olatunji, O.; Can Karaca, A.; Lee, C.C.; Mahdi Jafari, S. Anti-Obesity and Anti-Diabetic Bioactive Peptides: A Comprehensive Review of Their Sources, Properties, and Techno-Functional Challenges. Food Research International 2024, 187, 114427. [Google Scholar] [CrossRef] [PubMed]
- Suryaningtyas, I.T.; Je, J.Y. Bioactive Peptides from Food Proteins as Potential Anti-Obesity Agents: Mechanisms of Action and Future Perspectives. Trends Food Sci Technol 2023, 138, 141–152. [Google Scholar] [CrossRef]
- Acquah, C.; Dzuvor, C.K.O.; Tosh, S.; Agyei, D. Anti-Diabetic Effects of Bioactive Peptides: Recent Advances and Clinical Implications. Crit Rev Food Sci Nutr 2022, 62, 2158–2171. [Google Scholar] [CrossRef] [PubMed]
- Aloo, S.O.; Ofosu, F.K.; Kim, N.H.; Kilonzi, S.M.; Oh, D.H. Insights on Dietary Polyphenols as Agents against Metabolic Disorders: Obesity as a Target Disease. Antioxidants 2023, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Marine Drugs 2022, 20, 141. [Google Scholar] [CrossRef]
- Raja, K.; Kadirvel, V.; Subramaniyan, T. Seaweeds, an Aquatic Plant-Based Protein for Sustainable Nutrition - A Review. Future Foods 2022, 5, 100142. [Google Scholar] [CrossRef]
- Echave, J.; Fraga-Corral, M.; Garcia-Perez, P.; Popović-Djordjević, J.; Avdović, E.H.; Radulović, M.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification, and Applications. Marine Drugs 2021, 19, 500. [Google Scholar] [CrossRef]
- de Souza Celente, G.; Sui, Y.; Acharya, P. Seaweed as an Alternative Protein Source: Prospective Protein Extraction Technologies. Innovative Food Science & Emerging Technologies 2023, 86, 103374. [Google Scholar] [CrossRef]
- Sun, Z.; Chi, Q.; Sun, L.; Liu, Y. Protein Extraction from Microalgae Residue and Nutritional Assessment. Bioprocess Biosyst Eng 2022, 45, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2 - Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing, 2019; pp. 33–50 ISBN 978-0-12-814774-0.
- Bock, A.; Kieserling, H.; Steinhäuser, U.; Rohn, S. Impact of Phenolic Acid Derivatives on the Oxidative Stability of β-Lactoglobulin-Stabilized Emulsions. Antioxidants 2023, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Akter, A.; Sobuj, M.K.A.; Islam, M.S.; Chakroborty, K.; Tasnim, N.; Ayon, M.H.; Hossain, M.F.; Rafiquzzaman, S.M. Seaweed Polysaccharides: Sources, Structure and Biomedical Applications with Special Emphasis on Antiviral Potentials. Future Foods 2024, 10, 100440. [Google Scholar] [CrossRef]
- Sellimi, S.; Benslima, A.; Barragan-Montero, V.; Hajji, M.; Nasri, M. Polyphenolic-Protein-Polysaccharide Ternary Conjugates from Cystoseira Barbata Tunisian Seaweed as Potential Biopreservatives: Chemical, Antioxidant and Antimicrobial Properties. Int J Biol Macromol 2017, 105, 1375–1383. [Google Scholar] [CrossRef]
- Yan, S.; Regenstein, J.M.; Qi, B.; Li, Y. Construction of Protein-, Polysaccharide- and Polyphenol-Based Conjugates as Delivery Systems. Crit Rev Food Sci Nutr 2023. [Google Scholar] [CrossRef]
- Naseri, A.; Marinho, G.S.; Holdt, S.L.; Bartela, J.M.; Jacobsen, C. Enzyme-Assisted Extraction and Characterization of Protein from Red Seaweed Palmaria Palmata. Algal Res 2020, 47, 101849. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Enhanced Alkaline Extraction Techniques for Isolating and Modifying Plant-Based Proteins. Food Hydrocoll 2023, 145, 109132. [Google Scholar] [CrossRef]
- Ghelichi, S.; Sørensen, A.-D.M.; Náthia-Neves, G.; Jacobsen, C. PH-Dependent Extraction of Antioxidant Peptides from Red Seaweed Palmaria Palmata: A Sequential Approach. Marine Drugs 2024, 22, 413. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Cabezas, D.M.; Palazolo, G.G. Effects of PH, Protein:Polysaccharide Ratio, and NaCl-Added Concentration on Whey Protein Isolate and Soluble Soybean Polysaccharides Electrostatic-Complexes Formation. Food Chemistry Advances 2022, 1, 100123. [Google Scholar] [CrossRef]
- Momen, S.; Alavi, F.; Aider, M. Alkali-Mediated Treatments for Extraction and Functional Modification of Proteins: Critical and Application Review. Trends Food Sci Technol 2021, 110, 778–797. [Google Scholar] [CrossRef]
- Ruiz, G.A.; Xiao, W.; Van Boekel, M.; Minor, M.; Stieger, M. Effect of Extraction PH on Heat-Induced Aggregation, Gelation and Microstructure of Protein Isolate from Quinoa (Chenopodium Quinoa Willd). Food Chem 2016, 209, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, L.; McClements, D.J. Current Insights into Protein Solubility: A Review of Its Importance for Alternative Proteins. Food Hydrocoll 2023, 137, 108416. [Google Scholar] [CrossRef]
- Pei, J.; Gao, X.; Pan, D.; Hua, Y.; He, J.; Liu, Z.; Dang, Y. Advances in the Stability Challenges of Bioactive Peptides and Improvement Strategies. Curr Res Food Sci 2022, 5, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Calinsky, R.; Levy, Y. Histidine in Proteins: PH-Dependent Interplay between π-π, Cation-π, and CH-π Interactions. J Chem Theory Comput 2024, 20, 6930–6945. [Google Scholar] [CrossRef]
- Tabandeh, S.; Lemus, C.E.; Leon, L. Deciphering the Role of π-Interactions in Polyelectrolyte Complexes Using Rationally Designed Peptides. Polymers (Basel) 2021, 13, 2074. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Solis Irma and Ibarra-Herrera, C.C. and R.-P.M. del R. and L.-V.D. Alkaline Extraction–Isoelectric Precipitation of Plant Proteins. In Green Protein Processing Technologies from Plants: Novel Extraction and Purification Methods for Product Development; Hernández-Álvarez Alan Javier and Mondor, M. and N.M.G., Ed.; Springer International Publishing: Cham, 2023; pp. 1–29. ISBN 978-3-031-16968-7. [Google Scholar]
- Ouyang, L.; Hansen, H.H.W.B.; Cha, H.; Ji, X.; Zhang, J.; Li, Q.; Tan, B.H.; Trinh, Q.T.; Nguyen, N.T.; An, H. A Novel Approach for Nanobubble Generation toward Biomedical Applications. Colloids Surf A Physicochem Eng Asp 2024, 700, 134773. [Google Scholar] [CrossRef]
- Ghelichi, S.; Sørensen, A.-D.M.; Hajfathalian, M.; Jacobsen, C. Effect of Post-Extraction Ultrasonication on Compositional Features and Antioxidant Activities of Enzymatic/Alkaline Extracts of Palmaria Palmata. Mar Drugs 2024, 22. [Google Scholar] [CrossRef] [PubMed]
- Carballal, S.; Banerjee, R. Chapter 19 - Overview of Cysteine Metabolism. In Redox Chemistry and Biology of Thiols; Alvarez, B., Comini, M.A., Salinas, G., Trujillo, M., Eds.; Academic Press, 2022; pp. 423–450 ISBN 978-0-323-90219-9.
- Garrido Ruiz, D.; Sandoval-Perez, A.; Rangarajan, A.V.; Gunderson, E.L.; Jacobson, M.P. Cysteine Oxidation in Proteins: Structure, Biophysics, and Simulation. Biochemistry 2022, 61, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, K.; Michalak, I.; Tuhy, Ł.; Chojnacka, K. The Influence of PH of Extracting Water on the Composition of Seaweed Extracts and Their Beneficial Properties on Lepidium Sativum. Biomed Res Int 2017, 2017, 7248634. [Google Scholar] [CrossRef]
- Pasquet, P.L.; Julien-David, D.; Zhao, M.; Villain-Gambier, M.; Trébouet, D. Stability and Preservation of Phenolic Compounds and Related Antioxidant Capacity from Agro-Food Matrix: Effect of PH and Atmosphere. Food Biosci 2024, 57, 103586. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A Review on Protein–Phenolic Interactions and Associated Changes. Food Research International 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Siemińska-Kuczer, A.; Szymańska-Chargot, M.; Zdunek, A. Recent Advances in Interactions between Polyphenols and Plant Cell Wall Polysaccharides as Studied Using an Adsorption Technique. Food Chem 2022, 373, 131487. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, D.; Li, B.; Lund, M.N.; Xing, Y.; Wang, Y.; Li, F.; Cao, X.; Liu, Y.; Chen, X.; et al. Engineering Polyphenols with Biological Functions via Polyphenol-Protein Interactions as Additives for Functional Foods. Trends Food Sci Technol 2021, 110, 470–482. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–Polyphenol Conjugates: Antioxidant Property, Functionalities and Their Applications. Trends Food Sci Technol 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Hejna, M.; Dell’Anno, M.; Liu, Y.; Rossi, L.; Aksmann, A.; Pogorzelski, G.; Jóźwik, A. Assessment of the Antibacterial and Antioxidant Activities of Seaweed-Derived Extracts. Scientific Reports 2024 14:1 2024, 14, 1–15. [Google Scholar] [CrossRef]
- Mahendran, S.; Maheswari, P.; Sasikala, V.; Rubika, J. jaya; Pandiarajan, J. In Vitro Antioxidant Study of Polyphenol from Red Seaweeds Dichotomously Branched Gracilaria Gracilaria Edulis and Robust Sea Moss Hypnea Valentiae. Toxicol Rep 2021, 8, 1404–1411. [Google Scholar] [CrossRef]
- Yesiltas, B.; García-Moreno, P.J.; Gregersen, S.; Olsen, T.H.; Jones, N.C.; Hoffmann, S. V.; Marcatili, P.; Overgaard, M.T.; Hansen, E.B.; Jacobsen, C. Antioxidant Peptides Derived from Potato, Seaweed, Microbial and Spinach Proteins: Oxidative Stability of 5% Fish Oil-in-Water Emulsions. Food Chem 2022, 385, 132699. [Google Scholar] [CrossRef]
- Gentile, L. Protein–Polysaccharide Interactions and Aggregates in Food Formulations. Curr Opin Colloid Interface Sci 2020, 48, 18–27. [Google Scholar] [CrossRef]
- Feng, Y.; Jin, C.; Lv, S.; Zhang, H.; Ren, F.; Wang, J. Molecular Mechanisms and Applications of Polyphenol-Protein Complexes with Antioxidant Properties: A Review. Antioxidants 2023, 12, 1577. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, M.; Zhang, X.; Li, Z.; Guo, Y.; He, H.; Liang, B.; Li, X.; Ji, C. Design of Protein-Polysaccharide Multi-Scale Composite Interfaces to Modify Lipid Digestion. Trends Food Sci Technol 2022, 127, 38–48. [Google Scholar] [CrossRef]
- Zaharudin, N.; Staerk, D.; Dragsted, L.O. Inhibition of α-Glucosidase Activity by Selected Edible Seaweeds and Fucoxanthin. Food Chem 2019, 270, 481–486. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Paul Ross, R. The α-Amylase and α-Glucosidase Inhibitory Effects of Irish Seaweed Extracts. Food Chem 2013, 141, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, C.; Fu, X. Screening α-Glucosidase Inhibitors from Four Edible Brown Seaweed Extracts by Ultra-Filtration and Molecular Docking. LWT 2021, 138, 110654. [Google Scholar] [CrossRef]
- Austin, C.; Stewart, D.; Allwood, J.W.; McDougall, G.J. Extracts from the Edible Seaweed, Ascophyllum Nodosum, Inhibit Lipase Activity in Vitro: Contributions of Phenolic and Polysaccharide Components. Food Funct 2018, 9, 502–510. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Zhang, L.; Wan, S.; Li, C.; Liu, S. Solvents Effect on Phenolics, Iridoids, Antioxidant Activity, Antibacterial Activity, and Pancreatic Lipase Inhibition Activity of Noni (Morinda Citrifolia L.) Fruit Extract. Food Chem 2022, 377, 131989. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Miao, M. Inhibition of α-Amylase by Polyphenolic Compounds: Substrate Digestion, Binding Interactions and Nutritional Intervention. Trends Food Sci Technol 2020, 104, 190–207. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, G.; Liang, Y.; Song, T.; Zhu, Y.; Ni, H.; Yamaguchi, K.; Oda, T. Inhibitory Effects of a Sulfated Polysaccharide Isolated from Edible Red Alga Bangia Fusco-Purpurea on α-Amylase and α-Glucosidase. Biosci Biotechnol Biochem 2019, 83, 2065–2074. [Google Scholar] [CrossRef]
- Fu, X.; Yang, H.; Ma, C.; Li, X.; Li, D.; Yang, Y.; Xu, Y.; Wang, L. Characterization and Inhibitory Activities on α-Amylase and α-Glucosidase of the Polysaccharide from Blue Honeysuckle Berries. Int J Biol Macromol 2020, 163, 414–422. [Google Scholar] [CrossRef]
- Bjørlie, M.; Hartmann, J.C.; Rasmussen, L.H.; Yesiltas, B.; Sørensen, A.D.M.; Gregersen Echers, S.; Jacobsen, C. Screening for Metal-Chelating Activity in Potato Protein Hydrolysates Using Surface Plasmon Resonance and Peptidomics. Antioxidants 2024, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am J Enol Vitic 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative Properties of Xanthan on the Autoxidation of Soybean Oil in Cyclodextrin Emulsion. J Agric Food Chem 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch Biochem Biophys 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Van Quan, N.; Xuan, T.D.; Tran, H.D.; Thuy, N.T.D.; Trang, L.T.; Huong, C.T.; Andriana, Y.; Tuyen, P.T. Antioxidant, α-Amylase and α-Glucosidase Inhibitory Activities and Potential Constituents of Canarium Tramdenum Bark. Molecules 2019, 24, 605. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Assessing the In Vitro Inhibitory Effects on Key Enzymes Linked to Type-2 Diabetes and Obesity and Protein Glycation by Phenolic Compounds of Lauraceae Plant Species Endemic to the Laurisilva Forest. Molecules 2021, 26, 2023. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, V.; Mustar, S.; Mustafa Khalid, N.; Abd Rashed, A.; Mohd Noh, M.F.; Wilcox, M.D.; Chater, P.I.; Brownlee, I.A.; Pearson, J.P. Inhibitory Activities of Three Malaysian Edible Seaweeds on Lipase and α-Amylase. J Appl Phycol 2013, 25, 1405–1412. [Google Scholar] [CrossRef]
| Protein (%) | Protein recovery (%) | Washed-out protein (%) after pH adjustment* | |
|---|---|---|---|
| Ac-3 | 3.55 ± 0.38b | 24.52 ± 1.11b | 17.79 ± 0.79b |
| Ac-6 | 4.11 ± 0.02b | 25.15 ± 0.23b | 14.91 ± 0.43c |
| Ac-9 | 4.15 ± 0.39b | 25.37 ± 2.31b | 12.23 ± 0.84d |
| Ac-12 | 3.50 ± 0.08b | 26.68 ± 0.92b | 11.90 ± 0.06e |
| Ak-12 | 6.88 ± 1.62a | 38.00 ± 1.59a | - |
| Ak-9 | 6.96 ± 0.20a | 37.71 ± 0.61a | - |
| Ak-6 | 6.20 ± 0.49a | 39.80 ± 1.76a | - |
| Ak-3 | 6.10 ± 0.08a | 40.26 ± 0.50a | 38.70 ± 0.72a |
| Ac-3 | Ac-6 | Ac-9 | Ac-12 | Ak-12 | Ak-9 | Ak-6 | Ak-3 | |
|---|---|---|---|---|---|---|---|---|
| Phenylalanine* | 0.26 ± 0.01c | 0.27 ± 0.07c | 0.29 ± 0.01c | 0.26 ± 0.03c | 0.73 ± 0.09b | 0.98 ± 0.02a | 0.76 ± 0.02b | 0.75 ± 0.24b |
| Leucine* | 0.43 ± 0.01c | 0.43 ± 0.12c | 0.40 ± 0.01c | 0.40 ± 0.07c | 1.39 ± 0.19b | 1.90 ± 0.10a | 1.35 ± 0.05b | 1.37 ± 0.45b |
| Isoleucine* | 0.26 ± 0.01c | 0.26 ± 0.07c | 0.24 ± 0.01c | 0.26 ± 0.04c | 0.81 ± 0.08b | 1.11 ± 0.06a | 0.84 ± 0.02b | 0.82 ± 0.27b |
| Methionine* | 0.32 ± 0.12c | 0.02 ± 0.05d | 0.02 ± 0.05d | ND** | 0.55 ± 0.19ab | 0.70 ± 0.18a | 0.42 ± 0.11bc | 0.36 ± 0.05bc |
| Tyrosine* | 0.18 ± 0.05c | ND | ND | ND | 0.61 ± 0.10b | 0.89 ± 0.14a | 0.53 ± 0.01b | 0.54 ± 0.16b |
| Proline | 1.84 ± 0.02de | 1.79 ± 0.19e | 1.95 ± 0.03cde | 1.73 ± 0.19e | 2.11 ± 0.20cd | 2.96 ± 0.10a | 2.19 ± 0.06c | 2.61 ± 0.25b |
| Valine* | 1.09 ± 0.23c | 0.76 ± 0.16c | 0.74 ± 0.05c | 0.68 ± 0.12c | 2.16 ± 0.49b | 2.73 ± 0.17a | 2.08 ± 0.17b | 2.19 ± 0.43b |
| Alanine | 1.60 ± 0.16c | 1.42 ± 0.15c | 1.52 ± 0.04c | 1.53 ± 0.15c | 2.67 ± 0.28b | 3.71 ± 0.20a | 2.84 ± 0.45b | 3.08 ± 0.67b |
| Threonine* | 0.73 ± 0.19d | 0.51 ± 0.14de | 0.55 ± 0.08de | 0.41 ± 0.08e | 1.04 ± 0.14c | 1.78 ± 0.18a | 1.37 ± 0.19b | 1.20 ± 0.18bc |
| Glycine | 1.58 ± 0.21c | 1.02 ± 0.20cd | 0.98 ± 0.11cd | 0.82 ± 0.15d | 2.61 ± 0.42b | 3.38 ± 0.33a | 2.61 ± 0.13b | 3.10 ± 0.69ab |
| Serine | 1.09 ± 0.27c | 0.59 ± 0.20c | 0.57 ± 0.11c | 0.63 ± 0.15c | 2.36 ± 0.40b | 3.34 ± 0.33a | 2.59 ± 0.20b | 2.86 ± 0.66ab |
| Arginine | 0.43 ± 0.04c | 0.34 ± 0.10c | 0.33 ± 0.01c | 0.32 ± 0.04c | 0.82 ± 0.09b | 1.25 ± 0.03a | 1.00 ± 0.13b | 0.88 ± 0.29b |
| Histidine* | 0.24 ± 0.17ab | ND | ND | ND | 0.20 ± 0.14ab | 0.38 ± 0.11a | 0.11 ± 0.05bc | 0.18 ± 0.10b |
| Lysine* | 3.07 ± 0.75b | ND | ND | ND | 4.39 ± 1.15a | 3.11 ± 0.68b | 2.77 ± 0.48b | 2.99 ± 0.57b |
| Glutamic acid | 6.18 ± 0.35c | 5.69 ± 0.46c | 6.12 ± 0.18c | 5.61 ± 0.60c | 7.09 ± 0.57b | 9.70 ± 0.20a | 7.14 ± 0.19b | 8.92 ± 0.86a |
| Cystine* | 5.57 ± 0.61d | 5.19 ± 0.80d | 8.69 ± 1.41c | 5.53 ± 1.18d | 4.27 ± 0.71d | 24.73 ± 2.15a | 17.41 ± 1.59b | 23.08 ± 2.25a |
| Aspartic acid | 6.25 ± 0.70c | 5.37 ± 0.62c | 6.08 ± 0.63c | 5.51 ± 0.69c | 8.35 ± 0.80b | 10.02 ± 0.77a | 7.86 ± 0.30b | 8.90 ± 0.82ab |
| TAA*** | 31.12 ± 1.92e | 23.66 ± 2.54f | 28.49 ± 1.91ef | 23.70 ± 2.48f | 42.16 ± 5.18d | 72.65 ± 3.48a | 53.88 ± 1.10c | 63.82 ± 5.34b |
| EAA | 12.14 ± 1.17de | 7.45 ± 1.04f | 10.92 ± 1.36ef | 7.54 ± 1.03f | 16.13 ± 2.73d | 39.75 ± 4.74a | 27.66 ± 1.04c | 33.48 ± 2.10b |
| EAA/TAA | 0.39 ± 0.02b | 0.31 ± 0.02c | 0.38 ± 0.02b | 0.32 ± 0.02c | 0.38 ± 0.02b | 0.52 ± 0.01a | 0.52 ± 0.02a | 0.53 ± 0.03a |
| IC50 (mg.mL-1) for DPPH radical scavenging | IC50 (mg.mL-1) for Fe2+ chelating | IC50 (mg.mL-1) for α-glucosidase inhibition | IC50 (mg.mL-1) for pancreatic lipase inhibition | IC50 (mg.mL-1) for α-amylase inhibition | |
|---|---|---|---|---|---|
| Ac-3 | 0.72 ± 0.04b | NR* | NR | NR | NR |
| Ac-6 | 0.30 ± 0.04a | 6.82 ± 0.76d | NR | 7.10 ± 0.29b | 5.97 ± 0.73b |
| Ac-9 | 0.55 ± 0.03ab | 4.13 ± 0.36c | NR | 5.38 ± 0.34a | 5.79 ± 0.30b |
| Ac-12 | 6.49 ± 0.32c | 1.10 ± 0.23ab | NR | NR | 7.39 ± 0.89c |
| Ak-12 | NR | 0.65 ± 0.03a | NR | NR | 3.05 ± 0.66a |
| Ak-9 | NR | 1.64 ± 0.24b | NR | NR | NR |
| Ak-6 | 6.38 ± 0.34c | 3.43 ± 0.95c | NR | NR | NR |
| Ak-3 | 0.58 ± 0.02ab | NR | NR | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





