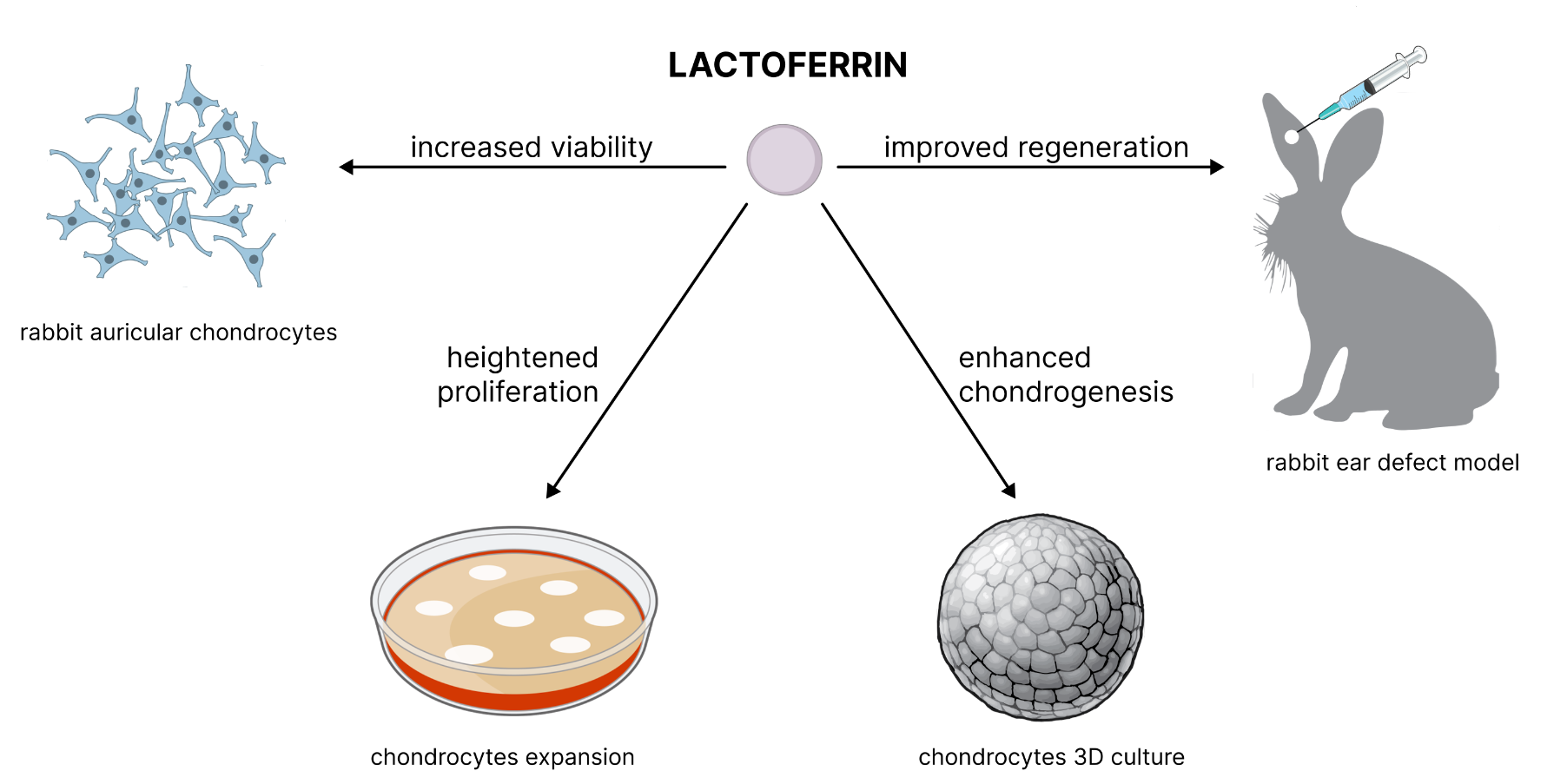
1. Introduction
The auricle is vulnerable to injuries due to its location and the very thin layer of skin covering it. Animal bites, head trauma, burns, and congenital malformations not only result in cosmetic defects but can also impact the functioning of the auditory system. Therefore, the issue of auricular reconstruction remains relevant.
The elastic cartilage, which constitutes the auricle, is an avascular tissue with an extracellular protein matrix reinforced by a three-dimensional network of collagen fibers. It has limited regenerative capacity due to the sparse distribution of highly differentiated, non-dividing chondrocytes, slow matrix turnover, and a low reserve of progenitor cells [
1]. For decades, auricular reconstruction has involved forming an ear scaffolds from autologous costal cartilage [
2]. This technique yields satisfactory results due to the similar properties of the cartilages, which allow the creation of a durable, biocompatible scaffold. However, it has drawbacks such as cartilage transplant deformation over time, the risk of pneumothorax, and infection due to cartilage harvesting [
3].
Nowadays, tissue engineering and the creation of artificial cartilage from the patient's cells have become more preferable, modern, and safer methods. These approaches avoid unnecessary surgical interventions and the use of cartilage analogs [
4]. Nevertheless, challenges include low tissue cellularity [
5], the need for vascularization of the skin and cartilage, limited cell proliferation, risks of oncogenicity and abnormal differentiation, thermal and mechanical forces, and mechanical and immunological complexities in integration with the host tissue [
6].
Since the mid-20th century and to this day, researchers have been interested in the pro-regenerative properties of the iron-binding protein lactoferrin (LF) [
7]. Studies have shown that LF exerts anti-inflammatory and anti-apoptotic effects and stimulates cell proliferation, positively influencing the regeneration of articular hyaline cartilage. In human articular chondrocytes, LF has been shown to reduce apoptosis [
8] and stimulate chondrocyte proliferation [
9], as well as decrease the expression of catabolic metalloproteinases (MMP-1, MMP-3, and MMP-13), destructive cytokines (IL-1β and IL-6), and inflammatory mediators (iNOS, COX-2) during IL-1β-induced inflammation [
10,
11]. LF can induce the protective expression of anti-inflammatory cytokines IL-4, IL-10 [
10], and IL-11 [
12], and activate the expression of BMP7, exerting a pro-chondrogenic effect [
13].
In vivo models have shown that LF improves bone condition and reduces the expression of catabolic metalloproteinases in a mono-iodoacetate-induced temporomandibular joint osteoarthritis model in rats [
14]. In therapy, LF can be used in combination with other anti-inflammatory drugs, such as dexamethasone, as it can reduce the pro-apoptotic effect on articular chondrocytes [
15]
The effects of LF have also been studied in the treatment of intervertebral disc diseases. LF prevents the decrease in proteoglycan synthesis by nucleus pulposus chondrocytes, reduces the expression of catabolic metalloproteinases, and pro-inflammatory factors iNOS, IL-6 during IL-1-induced inflammation [
16]. Additionally, its synergistic effect with pro-chondrogenic BMP7 has been noted in nucleus pulposus chondrocytes [
17].
Given the pronounced anti-inflammatory and pro-regenerative effects of lactoferrin on chondrocytes, we hypothesize that it can stimulate the regeneration of elastic cartilage, making it applicable in reconstructive surgery and tissue engineering of the auricle.
2. Results
2.1. LF increases viability and proliferation of auricular chondrocytes
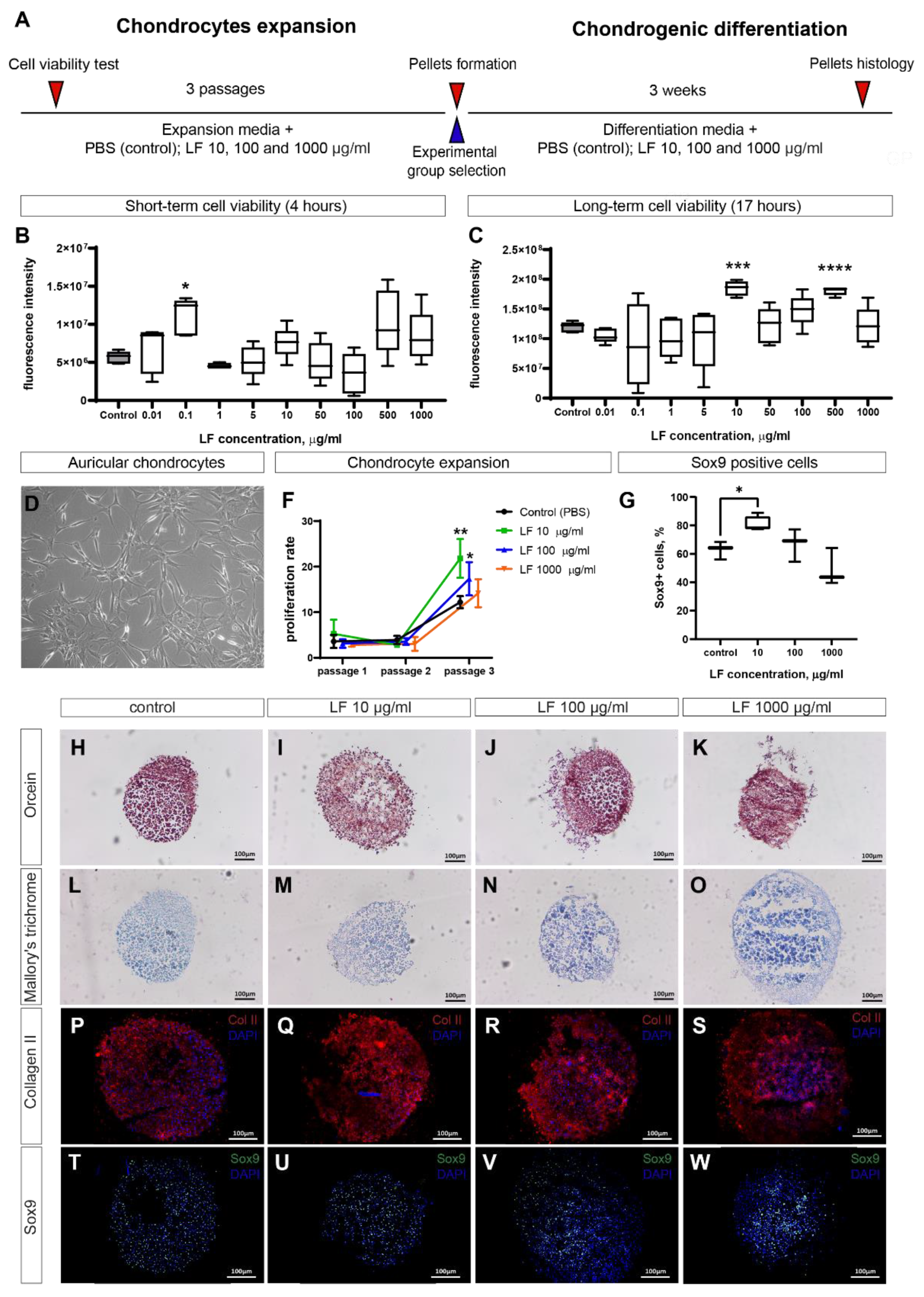
The effect of lactoferrin (LF) on the viability of auricular chondrocytes was evaluated for cells at passage 1 using the Alamar Blue assay. Various concentrations of LF were used to establish a dose-dependent effect. It was shown that short-term cultivation with LF at a concentration of 0.1 µg/ml significantly increased chondrocyte viability, although such a noticeable effect was not observed at other doses (
Figure 1B). During prolonged cultivation, the effects of lactoferrin were more pronounced: at concentrations of 10 and 500 µg/ml, it increased the metabolic activity of the cells (
Figure 1C).
Since the increase in cell metabolic activity might be related to an increase in cell number and proliferation, we further expanded the cells over three passages and recorded the fold increase in cell number – the proliferation rate. The general appearance of auricular chondrocytes in culture is presented in
Figure 1D. The cells were cultured with LF added to the medium at four different concentrations. In the first two passages, LF did not significantly affect cell proliferation, which was generally low. However, in the third passage, a sharp increase in the proliferation rate was observed, with LF at concentrations of 10 and 100 µg/ml significantly stimulating this process.
Thus, LF can stimulate the metabolic activity of cells and their proliferation, although an appropriate dosage needs to be selected. For our studies, the concentration of 10 µg/ml was optimal. Therefore, in further experiments on the effect of LF on chondrogenic differentiation, cells grown in medium supplemented with 10 µg/ml LF were used.
2.2. LF stimulates chondrogenesis in 3D pellets culture of auricular chondrocytes
To evaluate the ability of LF to stimulate chondrogenesis in vitro, we used a 3D pellets culture model. Pellets were formed from cells that had been expanded in a medium containing 10 µg/ml of LF and then cultured in a medium containing three different concentrations of LF: 10, 100 and 1000 µg/ml. Histological analysis with orcein staining showed the presence of elastic fibers in the extracellular matrix in all groups (
Figure 1H-K). Mallory staining did not reveal a significant amount of proteoglycans (
Figure 1L-O), which is consistent with the composition of elastic cartilage in the ear. It is noteworthy that over three weeks in pellets culture, chondrocytes didn’t form a dense extracellular matrix in all pellets, which was easily identifiable by histological staining. Nevertheless, immunohistochemical staining demonstrated the presence of type II collagen in all groups, although its localization was clustered (
Figure 1P-S). Staining for the chondrocyte marker Sox9 showed a high percentage of positive cells in all samples. Quantitative analysis of the percentage of Sox9+ cells showed a significant increase in the group with 10 µg/ml LF (
Figure 1G). Increasing the concentration of LF led to a decrease in the percentage of Sox9+ cells, although this effect wasn’t statistically significant.
Thus, it can be concluded that LF can stimulate chondrogenesis, although dose optimization is required. In our experiments, the optimal concentration of LF was 10 µg/ml.
2.3. LF promotes healing of cartilage defects in rabbit ear
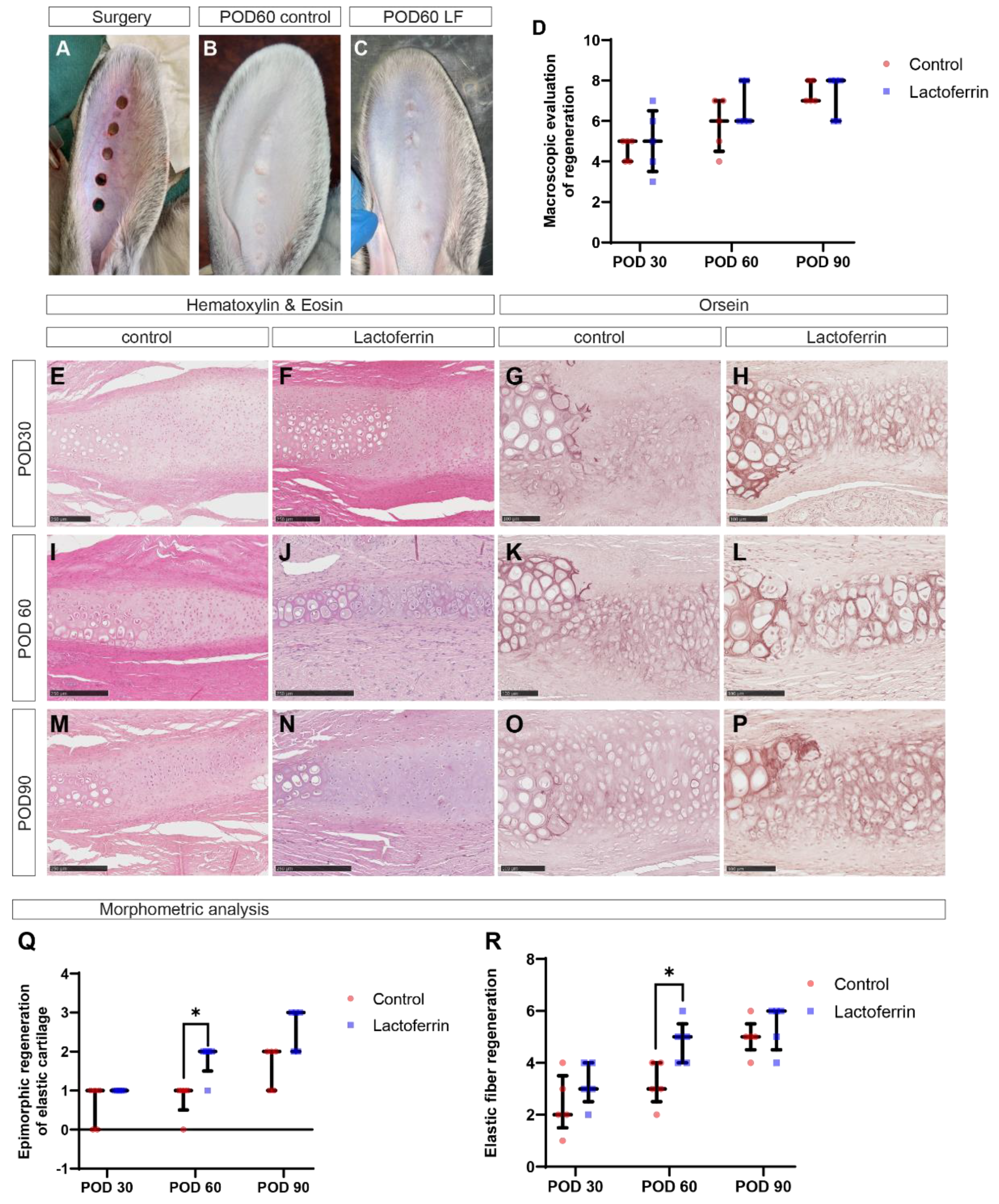
To evaluate the ability of LF to stimulate elastic cartilage regeneration in vivo, we utilized a rabbit ear defect model (
Figure 2A). Macroscopic analysis showed that at POD30, most defects in the control and experimental groups were epithelialized, characterized by a central retraction that pulled and deformed the surrounding tissues, with little difference in color and consistency between them. At POD60, most defects in both groups were completely epithelialized, with newly formed tissue having a smooth, flat surface, gray color, and firm-elastic consistency (
Figure 2B,C). By POD90, complete normalization of wound color was observed, and the newly formed tissue had the same density as the surrounding tissues. Macroscopic scoring showed no statistically significant differences between the groups (
Figure 2D).
At POD30, histological analysis revealed immature fibrous cartilage tissue formed near the edges of the intact cartilage plate in both groups. In the control (
Figure 2, E), the regenerating chondrocytes were predominantly small and round, surrounded by collagen fibers, with only some cells having lacunae. Dense immature fibrous tissue composed of fibroblasts, blood vessels, and thick bundles of collagen fibers filled the space between the edges of the regenerating cartilage plate. In the lactoferrin group (
Figure 2F), larger cartilage regenerates with a high density of proliferating chondrocytes were observed compared to the control, and the volume of fibrous tissue between the edges of the cartilage regenerates was reduced. Orcein staining revealed only small areas of regenerating cartilage with few elastic fibers in the control group (
Figure 2G), whereas extensive layers of elastic cartilage formed in the lactoferrin group (
Figure 2H).
By POD60, cartilage plate regeneration significantly accelerated, leading to a marked increase in the area of fibrous cartilage. Regenerating chondrocytes increased in size and became more dispersed. In the control, less mature regions of cartilage regenerate with small, numerous chondrocytes (
Figure 2I) and low content and uneven distribution of elastic fibers continued to be observed (
Figure 2K). In the lactoferrin group, compared to the control, more mature cartilage regenerate areas with large round chondrocytes and large lacunae (
Figure 2J) predominated, uniformly surrounded by numerous elastic fibers (
Figure 2L).
By POD90, the structure of the cartilage regenerate in both groups resembled normal elastic cartilage (
Figure 2M–P), but only in the lactoferrin group did the cartilage plate regenerate along the entire length of the defect in most samples. The lactoferrin group achieved complete regeneration of the cartilage plate with restoration of the elastic cartilage type, as confirmed by orcein staining (
Figure 2O).
Morphometric analysis of cartilage regeneration quality (
Figure 2Q,R) showed that in the lactoferrin group, the sizes of cartilage regenerates with a high degree of maturity and the content of elastic fibers were significantly higher than in the control at POD60. No statistically significant differences were found between the groups at other time points, although a trend was observed.
Thus, the obtained data suggest that lactoferrin positively influenced the organotypic regeneration of ear cartilage compared to the control group. The extent of highly mature cartilage regenerates with elastic fiber content increased linearly over time and was maximal following lactoferrin treatment at POD60.
Figure 2.
In vivo experiments. A – Appearance of rabbit ear defect and its healing at post-operative day 60 in the control group (B) and the lactoferrin group (C). D – Macroscopic assessment of regeneration. Staining of defect healing on post-operative day 30 (E-H), 60 (I-L), and 90 (M-P), with hematoxylin-eosin and orcein staining. Semi-quantitative morphometric analysis of obtained sections: epimorphic regeneration (Q) and elastic cartilage regeneration (R). LF-lactoferrin; POD – post-operative day. * - p<0.05.
Figure 2.
In vivo experiments. A – Appearance of rabbit ear defect and its healing at post-operative day 60 in the control group (B) and the lactoferrin group (C). D – Macroscopic assessment of regeneration. Staining of defect healing on post-operative day 30 (E-H), 60 (I-L), and 90 (M-P), with hematoxylin-eosin and orcein staining. Semi-quantitative morphometric analysis of obtained sections: epimorphic regeneration (Q) and elastic cartilage regeneration (R). LF-lactoferrin; POD – post-operative day. * - p<0.05.
3. Discussion
A significant amount of research has been dedicated to investigating the effects of lactoferrin on the hyaline cartilage of joints. It has been demonstrated that lactoferrin possesses anti-inflammatory [
10,
11,
12], anti-catabolic [
8,
10] and prochondrogenic effects [
11,
13]. However, such studies have not been previously conducted for the elastic cartilage of the ear.
In this study, we demonstrated the ability of lactoferrin (LF) to stimulate the metabolic activity of auricular chondrocytes. Previous studies have shown similar effects of LF on articular chondrocytes at comparable doses [
9]. It is important to note that viability assays such as Alamar Blue can reflect increases in signal due to either enhanced metabolism of individual cells or changes in cell number due to proliferation and apoptosis [
18]. Our research showed that LF does not affect the proliferation of auricular chondrocytes in early passages; however, its effects become more pronounced by the third passage.
Interestingly, the stimulatory effect of LF is concentration-dependent. We identified 10 µg/ml as the optimal concentration for stimulating proliferation and chondrogenesis and influencing the metabolism of auricular chondrocytes. Increasing the LF concentration to 1 mg/kg did not result in significant effects. Similar findings were observed in intestinal epithelial cells, where low doses of LF stimulated metabolism, whereas higher doses had pro-apoptotic effects [
19]. Thus, the optimal dose of lactoferrin should be empirically determined to avoid adverse effects.
Earlier studies have shown that LF's mechanism for stimulating metabolic activity has some homology with TGF-β1 [
9]. Furthermore, LF appears to partially mediate chondrogenesis via the Smad2/3-Sox9 signaling pathway [
20]. Additionally, LF can also stimulate the BMP signaling pathway to enhance chondrogenesis, as shown for various types of chondrocytes [
13,
17]. It should be noted that these are not the only molecular targets of LF; it can interact with DAMP and PAMP receptors [
21] and modulate the NF-kB pathway [
22], as well as activate ERK, MAPK, and Akt signaling pathways [
10]. Thus, from a molecular perspective, its action on cells is quite complex.
Thus, lactoferrin may be a highly promising supplement to the culture medium, promoting chondrogenesis in elastic cartilage equivalents. This is particularly important for the rapidly advancing field of bioprinting and cultivation of tissue-engineered constructs from various cells [
23].
In in vivo experiments on rabbit ear cartilage healing, LF also demonstrated its pro-regenerative properties. It is known that the rabbit ear cartilage plate can fully regenerate under certain conditions [
24]. The size, type (volume), and location of the defect play significant roles in the healing of full thickness defects in auricular cartilage in rabbits [
25,
26]. The outcome of the wound healing can be either the complete restoration of the tissues of the ear, or the partial replacement of them with a scar. The factors controlling the regeneration of rabbit ear cartilage are understudied [
27].
The results of our study demonstrated that lactoferrin had a beneficial effect on organotypic regeneration of ear cartilage, accelerating the growth and maturation of regenerating cartilage compared with the control group. It is important to note that elastic cartilage formed, replacing fibrous cartilage tissue over the course of regeneration. The number of elastic fibers increased linearly depending on time. In the LF's group, compared to the control, elastic fibers formed faster (~1.5 times) in newly formed cartilage at POD60. It is well known that LF stimulated the release of cytokines and growth factors [
28] that could modulate elastogenesis [
29]. Also, this effect may be mediated by LF well-known anti-inflammatory [
22] and antimicrobial properties [
30].
So LF may be responsible for the additive stimulatory effect on the formation of elastic fibers. As a result, in LF's group in the regenerating cartilage large lacunae were surrounded by numerous elastic fibers, and dystrophic changes were completely absent. The morphological characteristics of the tissue were as close as possible to those of the normal elastic cartilage tissue. Based on our results, we consider that lactoferrin is favorable for the epimorphic regeneration of elastic cartilage in rabbit ear.
4. Materials and Methods
4.1. Animals
All the animal experiments were approved by the bioethics committee of the Sechenov University (№ 13-22 from 22.06.2022, Moscow, Russian Federation). Experiments conducted in accordance with the Directive 2010/63/EU of the European Parliament and of the Council and with FELASA guidelines and recommendations. The study utilized Chinchilla rabbits (males, 2-2.5 kg). The rabbits were housed under standard vivarium conditions, each in an individual pet cage, and were provided with a complex pelleted laboratory diet along with constant access to water.
4.2. Auricular chondrocytes isolation
For cell isolation, rabbit ears were harvested post-euthanasia using Zoletil 100 (Vibrac, Carros, France; 60 mg/kg) followed by cervical dislocation. The skin was separated using a blade, and the obtained cartilage was washed in PBS solution and then in DMEM (Gibco, 41966029) containing gentamicin 20 µg/ml. The cartilage was then cut into smaller fragments, homogenized, and incubated for 15 hours at 37°C in a 0.1% collagenase type II (Worthington, LS004177) solution in DMEM supplemented with 20 µg/ml gentamicin. After incubation, the cells were passed through a 100 µm cell strainer and seeded into culture dish.
4.3. Cell culture
The overall in vitro experimental scheme is shown in
Figure 1, A. Cell expansion was conducted over three passages in DMEM/F12 medium (Gibco, 11320033) containing 10% FBS (Gibco, A3160801) and 10 µg/ml gentamicin (Sigma, G1272-100ML). To study the effect of LF on proliferative activity, it was added to the medium at concentrations of 10, 100, and 1000 µg/ml, while control cells received an equivalent volume of PBS. LF was provided by the Institute of Gene Biology, Russian Academy of Sciences, Moscow (Goldman et al., 2012).
The proliferation rate was determined as the ratio of the number of cells at the end of the culture period to the number of cells initially seeded. Based on the experimental results, chondrogenic differentiation was performed only for the experimental group with the highest proliferation.
After the third passage, cells were collected and 3D pellet cultures were formed for chondrogenic differentiation, according to the protocol previously described (Kurenkova et al., 2023). Briefly, 500,000 cells were collected in tubes, centrifuged at 400g for 7 minutes, and the pellet was maintained in culture conditions upon formation. Differentiation was conducted in chondrogenic medium (DMEM high glucose supplemented with 100 µM 2-mercaptoethanol, 2mM sodium pyruvate, 0.35mM L-proline, 1% ITS+3, 1% PenStrep, 5 µg/ml ascorbic acid, 0.1 µM dexamethasone, 10 ng/ml TGF-β3) for 3 weeks. LF was also added to the medium at concentrations of 10, 100, and 1000 µg/ml, while control pellets received an equivalent volume of PBS.
4.4. Cell viability test
Сell viability was assessed at passage 1. Cells were seeded into 96-well plates and grown in expansion medium until they reached 70-80% confluency. The medium was then replaced with fresh medium containing Alamar Blue (Invitrogen, DAL1025), and lactoferrin at concentrations of 0.01, 0.1, 1, 5, 10, 50, 100, 500, and 1000 µg/ml, or PBS in the control. For short-term viability assessment, cells were cultured for 4 hours, and for long-term effects, cells were cultured for 17 hours. Resorufin fluorescence was measured using a Victor Nivo spectrophotometer (PerkinElmer, USA). The fluorescence of the culture medium with added Alamar Blue was used as background values, which were subtracted from the fluorescence values of each well.
4.5. Surgery
For the surgery, the animals were anesthetized by intramuscular injection of Zoletil 100 (Vibrac, France; 6 mg/kg), supplemented with local anesthesia of the surgical site using a 0.5% solution of novocaine. Full-thickness defects were created using 6 mm diameter biopsy punches (Dermal Punch, Sterylab, Italy). Five punch holes were made in each rabbit's ear, spaced 10 mm apart (
Figure 1A). The wounds were dressed with Cosmopore bandages (Paul Hartmann, Heidenheim an der Brenz, Germany) and treated with a 3% hydrogen peroxide solution for three days.
On the 4th week post-surgery, the animals were divided into two groups (three rabbits each): a control group with no treatment and a treatment group where the peripheral wound areas were injected with 0.1 ml of a lactoferrin solution at a dose of 0.06 mg. On days 30, 60, and 90 post-treatment (POD30, POD60, and POD90), the animals were euthanized by administering Zoletil 100 (Vibrac, Carros, France; 60 mg/kg). The defect areas, along with surrounding tissues approximately 2-3 mm from the edges of the original wounds, were excised and fixed.
4.6. Macroscopic evaluation
Macroscopic evaluation of tissues surrounding the defect area was conducted at predetermined control points (POD30, POD60, and POD90). The visual assessment included the percentage of defect closure, defect density, defect color, and surface texture. A semiquantitative analysis using a scoring system was also performed (
Table 1).
4.7. Histology
All samples were fixed in 10% neutral buffered formalin (BioVitrum, B06-001/L). Pellets were fixed for 3 hours followed by dehydration in 30% sucrose before embedding in OCT. Pellet sections of 16 µm thickness were obtained using a cryostat. Immunohistochemical staining for cartilage markers Sox9 (1:100, Sigma, HPA001758) and collagen II (1:200, Invitrogen, PA1-26206) was performed using a modified rabbit-on-rabbit staining protocol. For Sox9, antigen retrieval was done by boiling in citrate buffer, and for collagen II with 0.5% pepsin in 5 mM HCl for 20 minutes at 37°C. Washing was performed with TBST (Tris-buffered saline with 0.1% Tween 20). Blocking was done in TBS buffer containing 0.1% Tween 20, 0.1% Triton X100, and 6% normal horse serum for 5 hours. Incubation with primary antibodies was performed overnight at 4°C, and with secondary antibodies (1:500, Jackson Immuno Research, 711-165-152) for 1 hour. Nuclei were stained with DAPI. Images were captured using an Olympus FLUOVIEW FV3000 confocal microscope.
Ear defect samples were fixed for 24 hours and underwent standard processing. Briefly, samples were dehydrated using isopropyl alcohol (BioVitrum, 06-002/L) in an Epredia STP120 automated tissue processor (Thermo Fisher Scientific, USA). After dehydration, the samples were embedded into paraffin blocks (BioVitrum, Histomix, 247). Sections (4-μm thick) were prepared from the paraffin blocks using a microtome (Sakura Finetek Japan Co., Ltd.), placed on slides and dried in a thermostat at 37°C for 24 hours before staining.
Histological staining with hematoxylin and eosin (BioVitrum, 05-002), Mallory’s trichrome (BioVitrum, 21-032), and orcein (BioVitrum, 21-034) was performed according to the manufacturer's protocol.
A Hamamatsu Nanozoomer S20 histological scanner (Hamamatsu, Japan) was used for slide imaging. An experienced pathologist described the following morphological findings: distance between defect edges, epithelization, characteristics of subepithelial tissue (regenerated dermis/granulation tissue/scar), characteristics of cartilage plate regeneration (volume of granulation tissue, degree of cell differentiation, thickening of the perichondrium), and presence of dystrophic changes in intact and regenerated cartilage.
4.8. Morphometric analysis
Semiquantitative evaluation of epimorphic regeneration and elastic fiber content was performed using a scoring system reported in a previous study (Valieva et al., 2023) (
Table 2 and
Table 3). Morphometric analysis was conducted using NDP.view 2 software (Hamamatsu, Japan). For each rabbit, multiple sections were assessed to ensure accuracy and reliability of the data. Serial sections were made for each rabbit with a step size of 50 µm, and for each defect, 5 sections were assessed and then averaged.
4.9. Statistical analysis
Statistical analysis of experimental data was performed using GraphPad Prism 8 (GraphPad Software, Inc., USA). The normality of the distribution was determined using the Shapiro-Wilk test. For chondrocyte viability and percent of Sox9+ cells analysis, the Brown-Forsythe ANOVA test was used, followed by multiple comparisons with a single control group using Dunnett's test. For the assessment of proliferative activity, a mixed-effects model (REML) was employed, followed by Dunnett's multiple comparisons test. Data are presented as mean and 95% confidence interval.
For morphometric analysis, the Mann-Whitney test was used for group comparisons within a single time point. Data are presented as median and interquartile range.
In all tests, the significance level was set at α=0.05. Differences were considered statistically significant at p<α.
5. Conclusions
In both in vitro and in vivo models, we demonstrated the stimulatory effects of lactoferrin (LF) on chondrogenesis for rabbit auricular chondrocytes. The results of this study, including the experimentally optimized concentrations, can be utilized for auricular tissue engineering and in clinical applications to stimulate the regeneration of cartilage injuries.
Author Contributions
Conceptualization, A.D.K., A.L.F., A.A.A. and P.S.T.; Methodology A.D.K., N.B.S. and S.A.S.; Validation, A.D.K. and A.L.F.; Formal analysis, A.D.K.; Investigation, A.D.K., N.B.S., S.A.S., N.E.D., A.L.F., A.V.I., E.R.S.; Data curation, A.D.K., N.B. S., E.R.S. and A.L.F.; Writing—original draft preparation, A.D.K., N.B.S., S.A.S.; Writing—review and editing, A.D.K., N.B.S., S.A.S., A.L.F., N.E.D., A.V.I., E.R.S., A.A.A., P.S.T.; Visualization, A.D.K. and A.L.F.; Supervision, A.A.A. and P.S.T.; Project administration, A.D.K., A.L.F., A.A.A. and P.S.T.; Funding acquisition, A.D.K., A.A.A. and P.S.T.
Funding
This research was funded by Russian Science Foundation, grant number 23-75-01066.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Bioethics Committee of the Sechenov University № 13-22 from 22.06.2022, Moscow, Russian Federation.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The preparation of lactoferrin was carried out using the unique scientific facility Transgenebank. The authors also thanks Valieva Y.M. for the initial analysis of the histological samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sterodimas, A.; de Faria, J.; Correa, W.E.; Pitanguy, I. Tissue Engineering and Auricular Reconstruction: A Review. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Humphries, S.; Joshi, A.; Webb, W.R.; Kanegaonkar, R. Auricular Reconstruction: Where Are We Now? A Critical Literature Review. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Bos, E.J.; Doerga, P.; Breugem, C.C.; van Zuijlen, P.P. The Burned Ear; Possibilities and Challenges in Framework Reconstruction and Coverage. Burns 2016, 42, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, H.; Wang, Y.; Bi, S.; Zhou, K.; Li, H.; Zhou, C.; Wang, Y.; Wu, W.; Peng, B.; et al. The Application and Progress of Tissue Engineering and Biomaterial Scaffolds for Total Auricular Reconstruction in Microtia. Front. Bioeng. Biotechnol. 2023, 11, 1–13. [Google Scholar] [CrossRef]
- Dai, W.; Sun, M.; Leng, X.; Hu, X.; Ao, Y. Recent Progress in 3D Printing of Elastic and High-Strength Hydrogels for the Treatment of Osteochondral and Cartilage Diseases. Front. Bioeng. Biotechnol. 2020, 8, 1–19. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Jeyaraman, N.; Nallakumarasamy, A.; Ramasubramanian, S.; Yadav, S. Critical Challenges and Frontiers in Cartilage Tissue Engineering. Cureus 2024, 16, 1–9. [Google Scholar] [CrossRef]
- Antoshin, A.A.; Shpichka, A.I.; Huang, G.; Chen, K.; Lu, P.; Svistunov, A.A.; Lychagin, A. V.; Lipina, M.M.; Sinelnikov, M.Y.; Reshetov, I.V.; et al. Lactoferrin as a Regenerative Agent: The Old-New Panacea? Pharmacol. Res. 2021, 167, 105564. [Google Scholar] [CrossRef]
- Xue, H.; Tu, Y.; Ma, T.; Liu, X.; Wen, T.; Cai, M.; Xia, Z.; Mei, J. Lactoferrin Inhibits IL-1β-Induced Chondrocyte Apoptosis through AKT1-Induced CREB1 Activation. Cell. Physiol. Biochem. 2015, 36, 2456–2465. [Google Scholar] [CrossRef]
- Brandl, N.; Zemann, A.; Kaupe, I.; Marlovits, S.; Huettinger, P.; Goldenberg, H.; Huettinger, M. Signal Transduction and Metabolism in Chondrocytes Is Modulated by Lactoferrin. Osteoarthr. Cartil. 2010, 18, 117–125. [Google Scholar] [CrossRef]
- Yan, D.; Chen, D.; Shen, J.; Xiao, G.; van Wijnen, A.J.; Im, H. Bovine Lactoferricin Is Anti-inflammatory and Anti-catabolic in Human Articular Cartilage and Synovium. J. Cell. Physiol. 2013, 228, 447–456. [Google Scholar] [CrossRef]
- Rasheed, N.; Alghasham, A.; Rasheed, Z. Lactoferrin from Camelus Dromedarius Inhibits Nuclear Transcription Factor-Kappa B Activation, Cyclooxygenase-2 Expression and Prostaglandin E2 Production in Stimulated Human Chondrocytes. Pharmacogn. Res. 2016, 8, 135–141. [Google Scholar] [CrossRef]
- Yan, D.; Kc, R.; Chen, D.; Xiao, G.; Im, H.J. Bovine Lactoferricin-Induced Anti-Inflammation Is, in Part, via up-Regulation of Interleukin-11 by Secondary Activation of STAT3 in Human Articular Cartilage. J. Biol. Chem. 2013, 288, 31655–31669. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Tang, W.; Kamiya, N.; Kim, H. Lactoferrin Activates BMP7 Gene Expression through the Mitogen-Activated Protein Kinase ERK Pathway in Articular Cartilage. Biochem. Biophys. Res. Commun. 2013, 431, 31–35. [Google Scholar] [CrossRef]
- Koca, C.G.; Yıldırım, B.; Özmen, Ö.; Çiçek, M.F.; İğneci, M.; Kırarslan, Ö.; Erdil, A. Comparison of the Efficacy of Intra-Articular Injections of Hyaluronic Acid and Lactoferrin in Mono-Iodoacetate-Induced Temporomandibular Joint Osteoarthritis: A Histomorphometric, Immunohistochemistry, and Micro-Computed Tomography Analysis. Jt. Dis. Relat. Surg. 2023, 34, 166–175. [Google Scholar] [CrossRef]
- Tu, Y.; Xue, H.; Francis, W.; Davies, A.P.; Pallister, I.; Kanamarlapudi, V.; Xia, Z. Lactoferrin Inhibits Dexamethasone-Induced Chondrocyte Impairment from Osteoarthritic Cartilage through up-Regulation of Extracellular Signal-Regulated Kinase 1/2 and Suppression of FASL, FAS, and Caspase 3. Biochem. Biophys. Res. Commun. 2013, 441, 249–255. [Google Scholar] [CrossRef]
- Kim, J.; Ellman, M.B.; Yan, D.; An, H.S.; KC, R.; Li, X.; Chen, D.; Xiao, G.; Cs-Szabo, G.; Hoskin, D.W.; et al. Lactoferricin Mediates Anti-inflammatory and Anti-catabolic Effects via Inhibition of IL-1 and LPS Activity in the Intervertebral Disc. J. Cell. Physiol. 2013, 228, 1884–1896. [Google Scholar] [CrossRef]
- Ellman, M.B.; Kim, J.; An, H.S.; Chen, D.; Kc, R.; Li, X.; Xiao, G.; Yan, D.; Suh, J.; van Wjnen, A.J.; et al. Lactoferricin Enhances BMP7-Stimulated Anabolic Pathways in Intervertebral Disc Cells. Gene 2013, 524, 282–291. [Google Scholar] [CrossRef]
- Longhin, E.M.; El Yamani, N.; Rundén-Pran, E.; Dusinska, M. The Alamar Blue Assay in the Context of Safety Testing of Nanomaterials. Front. Toxicol. 2022, 4, 1–10. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Jiang, P.; Stensballe, A.; Bendixen, E.; Sangild, P.T.; Chatterton, D.E.W. Bovine Lactoferrin Regulates Cell Survival, Apoptosis and Inflammation in Intestinal Epithelial Cells and Preterm Pig Intestine. J. Proteomics 2016, 139, 95–102. [Google Scholar] [CrossRef]
- Takayama, Y.; Mizumachi, K. Inhibitory Effect of Lactoferrin on Hypertrophic Differentiation of ATDC5 Mouse Chondroprogenitor Cells. BioMetals 2010, 23, 477–484. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Yami, H.A.; Tahmoorespur, M.; Javadmanesh, A.; Tazarghi, A.; Sekhavati, M.H. The Immunomodulatory Effects of Lactoferrin and Its Derived Peptides on NF-ΚB Signaling Pathway: A Systematic Review and Meta-Analysis. Immunity Inflamm. Dis. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Yadav, P.K.; Pandey, R.; Mehrotra, D. Auricular Reconstruction via 3D Bioprinting Strategies: An Update. J. Oral Biol. Craniofacial Res. 2022, 12, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Ad-El, D.D.; Selah, J.; Goshen, G.; Dano, I. Induction of Cartilage Growth in a Rabbit Ear Model: A Pilot Study. Eur. J. Plast. Surg. 2006, 28, 513–516. [Google Scholar] [CrossRef]
- Gawriluk, T.R.; Simkin, J.; Thompson, K.L.; Biswas, S.K.; Clare-Salzler, Z.; Kimani, J.M.; Kiama, S.G.; Smith, J.J.; Ezenwa, V.O.; Seifert, A.W. Comparative Analysis of Ear-Hole Closure Identifies Epimorphic Regeneration as a Discrete Trait in Mammals. Nat. Commun. 2016, 7, 1–16. [Google Scholar] [CrossRef]
- Valieva, Y.; Igrunkova, A.; Fayzullin, A.; Serejnikova, N.; Kurkov, A.; Fayzullina, N.; Valishina, D.; Bakulina, A.; Timashev, P.; Shekhter, A. Epimorphic Regeneration of Elastic Cartilage: Morphological Study into the Role of Cellular Senescence. Biology 2023, 12, 565. [Google Scholar] [CrossRef]
- ten Koppel, P. A New in Vivo Model for Testing Cartilage Grafts and Biomaterials: The “rabbit Pinna Punch-Hole” Model. Biomaterials 2001, 22, 1407–1414. [Google Scholar] [CrossRef]
- Presti, S.; Manti, S.; Parisi, G.F.; Papale, M.; Barbagallo, I.A.; Volti, G.L.; Leonardi, S. Lactoferrin: Cytokine Modulation and Application in Clinical Practice. J. Clin. Med. 2021, 10. [Google Scholar] [CrossRef]
- Sproul, E.P.; Argraves, W.S. A Cytokine Axis Regulates Elastin Formation and Degradation. Matrix Biol. 2013, 32, 86–94. [Google Scholar] [CrossRef]
- Gruden, Š.; Ulrih, N.P. Diverse Mechanisms of Antimicrobial Activities of Lactoferrins, Lactoferricins, and Other Lactoferrin-Derived Peptides. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).