Submitted:
06 April 2025
Posted:
08 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
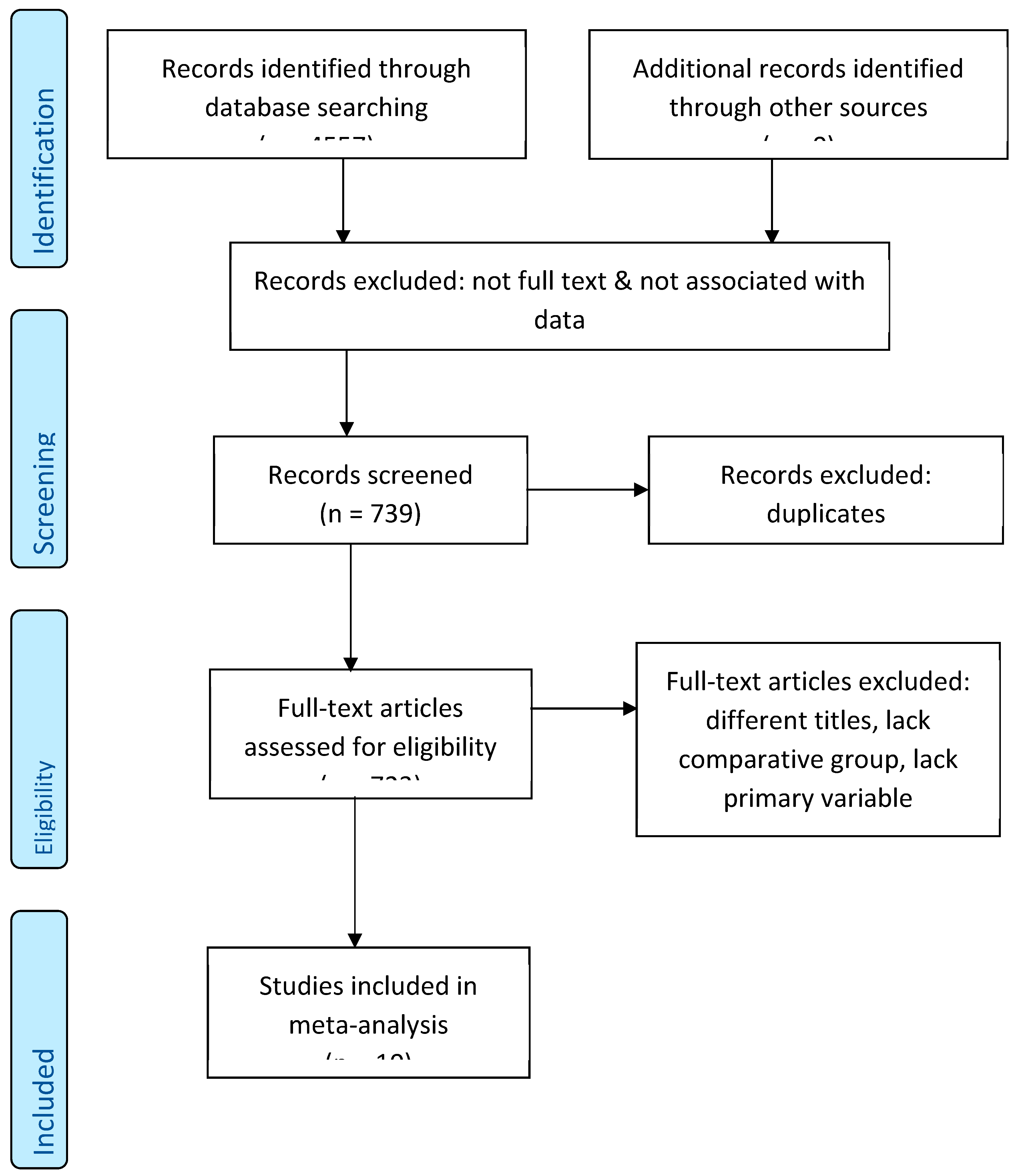
2. Methods
- COVID-19-related terms: “COVID-19,” “SARS-CoV-2,” “coronavirus infection”
- Surgical terms: “emergency surgery,” “urgent surgery,” “general surgery”
- Outcome measures: “mortality,” “complications,” “postoperative outcomes,” “ICU admission,” “mechanical ventilation”
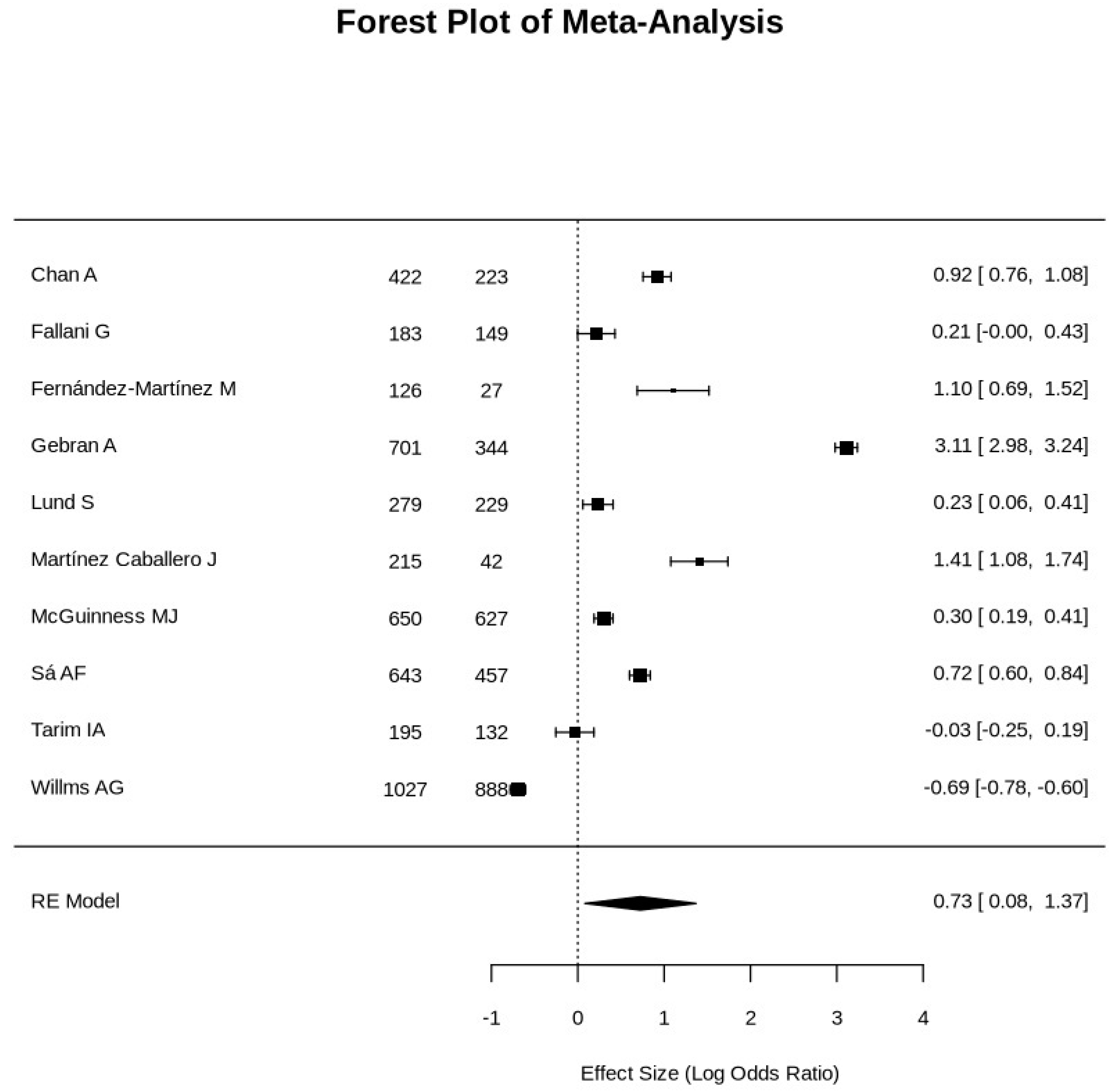
3. Results
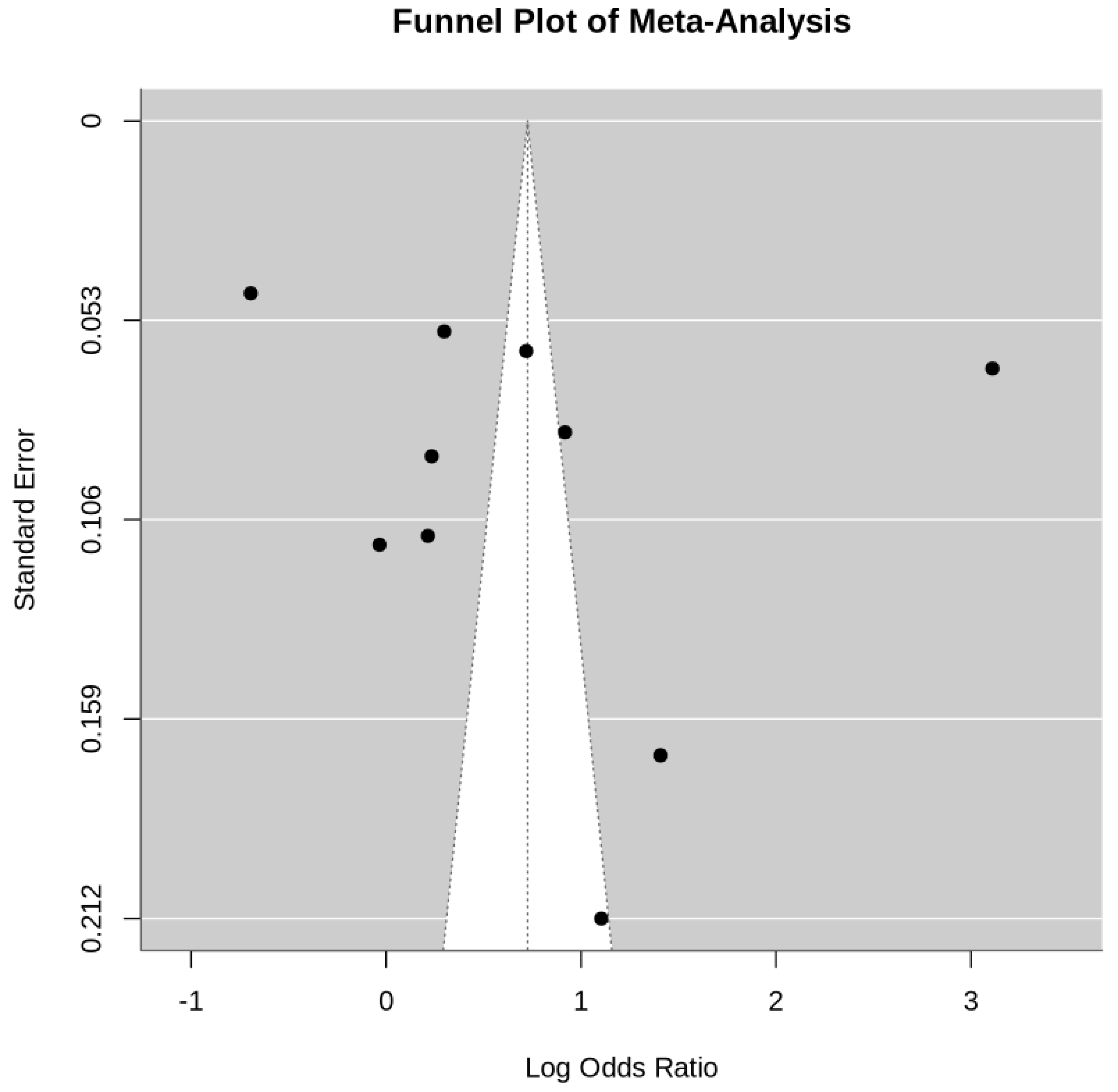
4. Discussion
- Comprehensive and rigorous methodology adhering to PRISMA guidelines.
- Inclusion of diverse study designs across multiple geographical regions, enhancing generalizability.
- Robust statistical analyses to ensure result reliability.
- Markedly high heterogeneity (I² = 99.86%), likely reflecting variations in healthcare infrastructure, patient populations, and surgical practices.
- Potential publication bias, as suggested by funnel plot asymmetry.
- Limited availability of high-quality randomized controlled trials, with most data derived from observational studies.
- Prospective, multicenter studies to validate these findings and elucidate underlying mechanisms.
- Development of perioperative protocols tailored to COVID-19 patients, emphasizing preoperative optimization and postoperative care.
- Exploration of long-term outcomes, including quality of life and functional recovery in COVID-19 surgical patients.
5. Conclusions
Acknowledgement
Conflict of Interest
Abbreviations
| COVID-19 | Coronavirus disease of 2019 |
| ICU | Intensive Care Unit |
| I2 | Statistical measure of inconsistency or heterogeneity between studies |
| OR | Odds ratio |
| 95% CI | 95% Confidence Interval |
| RR | Risk ratio |
| EGS | Emergency general surgery |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PCR | Polymerase Chain Reaction |
| RCT | Randomized controlled trial |
| NOS | Newcastle-Ottawa Scale |
| NOM | Non-Operative Management |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| COPD | Chronic Obstructive Pulmonary Disease |
References
- Abate SM, Mantefardo B, & Basu B. (2020). Postoperative mortality among surgical patients with COVID-19: a systematic review and meta-analysis. Patient Safety in Surgery, 14(1), 1–14. [CrossRef]
- Alazawi W, Pirmadjid N, Lahiri R, & Bhattacharya S. (2016). Inflammatory and Immune Responses to Surgery and Their Clinical Impact. Annals of surgery, 264(1), 73–80. [CrossRef]
- Alelyani RH, Alghamdi AH, Mahrous SM, Alamri BM, Alhiniah MH, Abduh MS, et al. (2022). Impact of COVID-19 Pandemic Lockdown on the Prognosis, Morbidity, and Mortality of Patients Undergoing Elective and Emergency Abdominal Surgery: A Retrospective Cohort Study in a Tertiary Center, Saudi Arabia. International Journal of Environmental Research and Public Health, 19(23), 15660. [CrossRef]
- Aloyan K, Harutyunyan H, & Voskanyan A. (2021). Virology: Current Research Early and Late Complications after Abdominal Surgery in Patients with COVID-19 in Armenia. In Virol Curr Res (Vol. 5).
- Ball L, Costantino F, Fiorito M, Amodio S, & Pelosi P. (2018). Respiratory mechanics during general anaesthesia. Annals of translational medicine, 6(19), 379. [CrossRef]
- Brown WA, Moore EM, & Watters DAK. (2021). Mortality of patients with COVID-19 who undergo an elective or emergency surgical procedure: a systematic review and meta-analysis. Anz Journal of Surgery, 91, 33–41. [CrossRef]
- Carrier F M, Amzallag É, Lecluyse V, Côté G, Couture EJ, D’Aragon F, et al. (2021). Postoperative outcomes in surgical COVID-19 patients: a multicenter cohort study. BMC Anesthesiology, 21(1), 15. [CrossRef]
- Cascella M, Rajnik M, Aleem A, Dulebohn SC, & Di Napoli R. (2024). Features, Evaluation, and Treatment of Coronavirus (COVID-19). StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK554776/.
- Chan A, Stathakis P, Goldsmith P, Smith SJ, & Macutkiewicz C. (2022). The reorganisation of emergency general surgery services during the COVID-19 pandemic in the UK: outcomes of delayed presentation, socio-economic deprivation and Black, Asian and Minority Ethnic patients. Annals of The Royal College of Surgeons of England. [CrossRef]
- Ciarleglio FA, Rigoni M, Rigoni M, Mereu L, Tommaso C, Carrara A, et al. (2021). The negative effects of COVID-19 and national lockdown on emergency surgery morbidity due to delayed access. World Journal of Emergency Surgery, 16(1), 37. [CrossRef]
- Collaborative Covids. (2020). Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: An international cohort study. The Lancet, 396(10243), 27–38. [CrossRef]
- Doglietto F, Vezzoli M, Gheza F, Lussardi GL, Domenicucci M, Vecchiarelli L, et al. (2020). Factors Associated With Surgical Mortality and Complications Among Patients With and Without Coronavirus Disease 2019 (COVID-19) in Italy. JAMA Surgery, 155(8), 691–702. [CrossRef]
- Duggan M, & Kavanagh BP. (2007). Atelectasis in the perioperative patient. Current opinion in anaesthesiology, 20(1), 37–42. [CrossRef]
- Fallani G, Lombardi R, Masetti M, Chisari M, Zanini N, Cattaneo GM, et al. (2021). Urgent and emergency surgery for secondary peritonitis during the COVID-19 outbreak: an unseen burden of a healthcare crisis. Updates in Surgery, 73(2), 753–762. [CrossRef]
- Fernández-Martínez M, Martín-Román L, Fernández-Vázquez ML, Rey-Valcarcel C, Pérez-Díaz D, & Turégano-Fuentes F. (2021). Overall management of emergency general surgery patients during the surge of the COVID-19 pandemic: an analysis of procedures and outcomes from a teaching hospital at the worst hit area in Spain. European Journal of Trauma and Emergency Surgery, 47(3), 693–702. [CrossRef]
- Gebran A, Gaitanidis A, Argandykov D, Maurer LR, Gallastegi AD, Bokenkamp M, et al. (2022). Mortality and pulmonary complications in emergency general surgery patients with COVID-19: A large international multicenter study. The Journal of Trauma and Acute Care Surgery, 93, 59–65. [CrossRef]
- Gerstein NS, Venkataramani R, Goumas AM, Chapman NN, & Deriy L. (2020). COVID-19-Related Cardiovascular Disease and Practical Considerations for Perioperative Clinicians. Seminars in cardiothoracic and vascular anesthesia, 24(4), 293–303. [CrossRef]
- Hedenstierna G, & Edmark L. (2010). Mechanisms of atelectasis in the perioperative period. Best practice & research. Clinical anaesthesiology, 24(2), 157–169. [CrossRef]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497–506. [CrossRef]
- Lazzati A, Rousseau MR, Bartier S, Dabi Y, Challine A, Haddad B, et al. (2021). Impact of COVID-19 on surgical emergencies: nationwide analysis. 5(3). [CrossRef]
- Lescure F-X, Bouadma L, Nguyen DB, Parisey M, Wicky P-H, Behillil S, et al. (2020). Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infectious Diseases, 20(6), 697–706. [CrossRef]
- Luca M De, Sartori A, Vitiello A, Piatto G, Noaro G, Olmi S, et al. (2021). Complications and mortality in a cohort of patients undergoing emergency and elective surgery with perioperative SARS-CoV-2 infection: an Italian multicenter study. Teachings of Phase 1 to be brought in Phase 2 pandemic. Updates in Surgery, 73(2), 745–752. [CrossRef]
- Lund S, MacArthur TA, Fischmann MM, Maroun JW, Dang J, Markos JR, et al. (2021). Impact of COVID-19 Governmental Restrictions on Emergency General Surgery Operative Volume and Severity. American Surgeon, 31348211011113. [CrossRef]
- Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, et al. (2020). The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. Journal of Neuroimmune Pharmacology, 15(3), 359–386. [CrossRef]
- Martínez Caballero J, González González L, Rodríguez Cuéllar E, Ferrero Herrero E, Pérez Algar C, Vaello Jodra V, et al. (2021). Multicentre cohort study of acute cholecystitis management during the COVID-19 pandemic. European journal of trauma and emergency surgery : official publication of the European Trauma Society, 47(3), 683–692. [CrossRef]
- McGuinness MJ, & Harmston C. (2021). The effect of national public health interventions for COVID-19 on emergency general surgery in Northland, New Zealand. Anz Journal of Surgery, 91(3), 329–334. [CrossRef]
- Nandy K, Salunke AA, Pathak SK, Pandey A, Doctor C, Puj K, et al. (2020). Coronavirus disease (COVID-19): A systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetes and Metabolic Syndrome: Clinical Research and Reviews, 14(5), 1017–1025. [CrossRef]
- Pratha AR, Pustela MK, Kaniti VK, Shaik S, & Pecheti T. (2023). Retrospective analysis of outcome of COVID positive patients undergoing emergency surgeries for acute general surgical conditions. International Surgery Journal, 10(3), 432–436. [CrossRef]
- Prasad NK, Çınar S, Lake R, Englum BR, Turner DJ, Siddiqui T, et al. (2021). Increased complications in patients who test COVID-19 positive after elective surgery and implications for pre and postoperative screening. American Journal of Surgery. [CrossRef]
- Rieder M, Goller I, Jeserich M, Baldus N, Pollmeier L, Wirth L, et al. (2020). Rate of venous thromboembolism in a prospective all-comers cohort with COVID-19. Journal of Thrombosis and Thrombolysis, 50(3), 558–566. [CrossRef]
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. (2020). Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Medicine and Infectious Disease, 34, 101623. [CrossRef]
- Sá AF, Lourenço SF, da Silva Teixeira R, Barros F, Costa APN, & Lemos P. (2021). Urgent/emergency surgery during COVID-19 state of emergency in Portugal: a retrospective and observational study. Revista Brasileira De Anestesiologia, 71(2), 123–128. [CrossRef]
- Saleh SK, Oraii A, Soleimani A, Hadadi A, Shajari Z, Montazeri M, et al. (2020). The association between cardiac injury and outcomes in hospitalized patients with COVID-19. Internal and Emergency Medicine, 15(8), 1415–1424. [CrossRef]
- Scholten R, Leijtens B, Hannink G, Kamphuis ET, Somford MP, & van Susante JLC. (2019). General anesthesia might be associated with early periprosthetic joint infection: an observational study of 3,909 arthroplasties. Acta Orthopaedica, 90(6), 554–558. [CrossRef]
- Singh C, Kaman L, Shah A, Thakur UK, Ramavath K, Jaideep B, et al. (2021). Surgical outcome of COVID-19 infected patients: experience in a tertiary care hospital in India. International Surgery Journal, 8(3), 899–903. [CrossRef]
- Stawicki SP, Jeanmonod R, Miller AC, Paladino L, Gaieski DF, Yaffee AQ, et al. (2020). The 2019-2020 novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic: A joint american college of academic international medicine-world academic council of emergency medicine multidisciplinary COVID-19 working group consensus paper. Journal of Global Infectious Diseases, 12(2), 47–93. [CrossRef]
- Tadesse S and Muluye W. (2020) The Impact of COVID-19 Pandemic on Education System in Developing Countries: A Review. Open Journal of Social Sciences, 8, 159-170. [CrossRef]
- Tarim IA, Derebey M, Ozbalci GS, Ozsay O, Yüksek MA, Buyukakincak S, et al. (2021). The impact of the COVID-19 pandemic on emergency general surgery: a retrospective study. Sao Paulo Medical Journal, 139(1), 53–57. [CrossRef]
- Wang B, Li R, Lu Z, & Huang Y. (2020). Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY), 12(7), 6049–6057. [CrossRef]
- Willms AG, Oldhafer KJ, Conze S, Thasler WE, von Schassen C, Hauer T, et al. (2020). Appendicitis during the COVID-19 lockdown: results of a multicenter analysis in Germany. [CrossRef]
- Yuki K, Fujiogi M, & Koutsogiannaki S. (2020). COVID-19 pathophysiology: A review. Clinical Immunology, 215, 108427. [CrossRef]
| First Author | Country | Year | Type of Surgery | Comparative/Control Group |
|---|---|---|---|---|
| Chan A | UK | 2022 | Emergency General Surgery | Present |
| Fallani G | Italy | 2021 | Emergency Abdominal Surgery | Present |
| Fernández-Martínez M | Spain | 2021 | Emergency General Surgery | Present |
| Gebran A | UK | 2022 | Emergency General Surgery | Present |
| Lund S | USA | 2021 | Emergency General Surgery | Present |
| Martínez Caballero J | Spain | 2021 | Acute Cholecystitis | Present |
| McGuinness MJ | New Zealand | 2021 | Emergency General Surgery | Present |
| Sá AF | Portugal | 2021 | Urgent/Emergency Surgery | Present |
| Tarim IA | Turkey | 2021 | Emergency General Surgery | Present |
| Willms AG | Germany | 2021 | Acute Appendicitis | Present |
| First Author | Total patients Covid vs. Non-covid |
Patients’ Age (year) Covid vs. Non-covid |
Gender M:F Covid vs. Non-covid |
Mortality Rates Covid vs. Non-covid |
Morbidity Rates Covid vs. Non-covid |
Mechanical Ventilation Covid vs. Non-covid |
ICU Admission Covid vs. Non-covid |
|---|---|---|---|---|---|---|---|
| Chan A | 223, 422 | 48.6, 48.5 (Mean) | 114:109, 191:231 | 5.8%, 2.4% | 5.6%, 4.8% | Not specified | 8.5%, 7.1% |
| Fallani G. | 149, 183 | 49 (26.5-70), 44 [24-61] | 94:55, 97:86 | 6%, 4.9% | 35.6%, 18% | Not specified | Not specified |
| Fernández-Martínez M | 27, 126 | 57.5 ± 21 (total) | 91:62 (total) | 18.5%, 7% | 85.7%, 26.7% | 66%, 0 | 36%, 14% |
| Gebran A | 344, 701 | 17-70 (total) | 220:124, 406:295) | 40.1%, 2.9% | 72.7%, 0 | 23.9%, 0 | Not specified |
| Lund S | 229, 279 | 59.3, 56.7 (Mean) | 102:127, 121:158 | 5%, 4% | 25%, 29% | Not specified | Not specified |
| Martínez Caballero J | 257, 215 | 69 (52-80), 68 (50-80) (Median) | 146:111, 118:97 | 11.9%, 3.2% | 100%, 26% | Not specified | Not specified |
| McGuinness MJ | 627, 650 | 57, 57 (Median) |
327:300, 314:336 | 4%, 3% | Not specified | Not specified | Not specified |
| Sá AF | 457, 643 | 67, 63 (Median) | 261:196, 368:275 |
11.40%, 5.9% | Not specified | Not specified | Not specified |
| Tarim IA | 132, 195 | 50, 53 (Median) | 74:58, 82:113 | 3%, 3.1% | 7%, 1.5% | Not specified | Not specified |
| Willms AG | 888, 1027 | 36 ± 20, 35 ± 19 (Mean) | 468:420, 510:517 | 0.1%, 0.2% | 14.3%, 13.3% | Not specified | 4.5%, 3.9% |
| First Author | Effect Size (Cohen's d) |
Odds Ratio (OR) | OR, 95% CI | Risk Ratio (RR) | RR, 95% CI |
|---|---|---|---|---|---|
| Chan A | 1.383370166 | 2.5039 | 1.082, 5.7941 | 2.4167 | 1.0792, 5.4119 |
| Fallani G. | 0.68441989 | 1.2388 | 0.4778, 3.2118 | 1.2245 | 0.4975, 3.014 |
| Fernández-Martínez M | 1.666187845 | 3.0158 | 0.9191, 9.8958 | 2.6429 | 0.957, 7.2894 |
| Gebran A | 12.3839779 | 22.415 | 13.718, 36.6256 | 13.8276 | 8.8398, 21.6296 |
| Lund S | 0.697900552 | 1.2632 | 0.5433, 2.9366 | 1.25 | 0.5585, 2.7979 |
| Martínez Caballero J | 2.257458564 | 4.086 | 1.226, 13.6181 | 3.7188 | 1.2336, 11.2104 |
| McGuinness MJ | 0.744309392 | 1.3472 | 0.7377, 2.4602 | 1.3333 | 0.7454, 2.3849 |
| Sá AF | 1.133756906 | 2.0521 | 1.3258, 3.1763 | 1.9322 | 1.2942, 2.8847 |
| Tarim IA | 0.534088398 | 0.9667 | 0.267, 3.5008 | 0.9677 | 0.2779, 3.37 |
| Willms AG | 0.275966851 | 0.4995 | 0.0414, 6.0297 | 0.5 | 0.0416, 6.0162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





